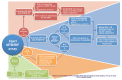
5.1. Selecting individuals to be tested
The decision about whether to perform an Xpert MTB/RIF assay should be made by a health-care professional who conducts a thorough risk assessment for the likelihood of TB for each individual presenting at the health centre. To facilitate the decision on whether the Xpert MTB/RIF should be used and at which stage of the diagnostic process, these individuals can be assigned into several groups presented here. WHO's updated policy document continues to strongly advise that Xpert MTB/RIF be used as the initial diagnostic test in both adults and children who are at risk of MDR-TB or HIV-associated TB, and that these two groups should be prioritized for testing with Xpert MTB/RIF when resources are limited.
Group A
This group includes individuals (both adults and children) suspected of having TB who are considered to be at risk of harbouring drug-resistant TB bacilli (these risk groups should be defined according to national policies or as defined in WHO's Guidelines for the Programmatic Management of Drug-resistant TB18).
It also includes both adults and children who have been treated with anti-TB drugs and in whom TB has again been diagnosed, that is, all retreatment categories (failure, return after loss to follow-up, return after relapse).
Xpert MTB/RIF should be used as the initial diagnostic test in these individuals rather than conventional microscopy, culture and DST.
- 18
Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization; 2008. Emergency Update 2008. (WHO/HTM/TB/2008.402).
A country or setting with high prevalence of rifampicin resistant TB (RR-TB) may also decide to use Xpert MTB/RIF for all smear-positive cases to rapidly detect rifampicin resistance.
Group B
Individuals (adults and children) suspected of having HIV-associated TB should ideally be offered HIV testing routinely, preferably before investigation with Xpert MTB/RIF. HIV testing should be performed according to national guidelines.
Among adults and adolescents living with HIV, a person suspected of having TB is defined as anyone who reports any one of the following symptoms: current cough, fever, weight loss or night sweats.19 Among children living with HIV, TB should be suspected in any child who has any one of the following symptoms: poor weight gain, fever, current cough or a history of contact with someone who has TB.20
Xpert MTB/RIF should be used as the initial diagnostic test rather than conventional microscopy, culture and DST in all persons living with HIV who have signs or symptoms of TB, in persons who are seriously ill and suspected of having TB regardless of their HIV status, and in those whose HIV status is unknown but who present with strong clinical evidence of HIV infection in settings where there is a high prevalence of HIV or among members of a risk group for HIV.
- 19
- 20
HIV prevalent settings are defined as countries, sub-national administration units (e.g., districts, counties), or selected facilities (e.g., referral hospitals, drug rehabilitation centres) where the adult HIV prevalence rate among pregnant women is ⩾ 1% or in which the HIV prevalence among TB patients is ⩾ 5%.
Policy recommendations also allow the use of Xpert MTB/RIF as a follow-on test to microscopy in adults who are not considered to be at risk of either MDR-TB or HIV-associated TB. This recommendation acknowledges that evidence from systematic reviews has shown significant diagnostic superiority of Xpert MTB/RIF over microscopy when compared to culture.21 It is conditional, however, taking into account significant resource implications if all individuals with negative sputum smear results are routinely tested with Xpert MTB/RIF. Thus, this recommendation is not to be used as a rule suggesting that all smear-negative individuals should be tested with Xpert MTB/RIF but rather as a possible, well justified strategy in some settings.
Group C
This group includes adults suspected of having TB but who are not at risk of MDR-TB or HIV-associated TB (that is, adults who are HIV-negative or whose HIV status is unknown and who are not a member of a risk group for HIV or who live in a setting with a low prevalence of HIV).
These individuals may receive an Xpert MTB/RIF test as an initial diagnostic test for TB. When resource limitations do not allow Xpert MTB/RIF to be used for all individuals, sputum-smear examination may be conducted first; using Xpert MTB/RIF for smear-negative individuals will identify TB cases missed by smear microscopy.
The updated WHO policy document recommends using the Xpert MTB/RIF assay for all individuals (adults and children) suspected of having TB. These recommendations are conditional acknowledging significant resource implications should programmes decide to test everyone suspected of having TB (a sizeable group in many countries) using the Xpert MTB/RIF assay. The recommendation for adults is based on stronger body of evidence than for children, however given the diagnostic difficulties in paediatric TB diagnosis, children may be prioritized for Xpert MTB/RIF testing if resources are limited.
Group D
This group includes all individuals suspected of having TB (adults and children). Xpert MTB/RIF may be used as an initial diagnostic test for TB. This can result in more bacteriologically confirmed patients and shortened time to treatment22. Resource limitations may affect the ability of national programmes to undertake Xpert MTB/RIF testing in all individuals in this group.
- 22
Theron G, et al. Feasibility, Accuracy, and Clinical Effect of Point-of-care Xpert MTB/RIF Testing for Tuberculosis in Primary-care Settings in Africa: a Multicentre, Randomised, Controlled Trial. Lancet. 2013 [PubMed: 24176144] [CrossRef].
In many settings, the majority of individuals suspected of having TB will not have risk factors for MDR-TB or HIV-associated TB. Therefore, careful consideration should be given to the resource implications and cost effectiveness of routinely offering Xpert MTB/RIF testing. Smear microscopy may be placed first in the diagnostic algorithm, with Xpert MTB/RIF used as a follow-on test for those who have negative smear microscopy results but who are suspected of having TB with the aim of finding TB cases missed by smear microscopy. While Xpert MTB/RIF is more expensive than conventional microscopy, using it as the initial diagnostic test will increase the number of patients with bacteriologically confirmed TB, given the higher sensitivity of Xpert MTB/RIF especially in settings with a high prevalence of HIV. Such an algorithm may require additional screening using either chest X-ray (if it is accessible and affordable) or further clinical assessment as a pre-test screening tool to reduce the numbers of individuals to be tested, given that in most settings the vast majority (e.g. ∼90%) of individuals suspected of having TB would have a negative result from smear microscopy. Setting-specific operational research is needed to understand the cost effectiveness of using smear microscopy or chest X-ray, or both, before Xpert MTB/RIF.
5.2. Test performance
As with any other diagnostic test, the performance of Xpert MTB/RIF depends on the prevalence of the target conditions (TB disease and rifampicin resistance) in the population tested, and on the reference standard used.
5.2.1. Accuracy of the reference standard
Culture is regarded as the best reference standard for active TB, and was the reference standard used in the systematic review on use of Xpert MTB/RIF in pulmonary TB. Phenotypic culture-based DST methods using WHO's recommended critical concentrations were the reference standard for rifampicin resistance.23
Three studies have raised concerns about phenotypic DST methods, in particular using the automated BACTEC MGIT (mycobacterial growth indicator tube) 960 Mycobacterial Detection System (Becton Dickinson, Franklin Lakes, NJ, United States) to detect rifampicin resistance. One study involved several TB Supranational Reference Laboratories (Van Deun 2009) and reported that the BACTEC 460 system and the BACTEC MGIT 960 system missed certain strains associated with low-level rifampicin resistance.24 Another study (Williamson 2012) used Xpert MTB/RIF and gene sequencing, and identified four patients (three with clinical information available) whose TB isolates contained mutations in the rpoB gene but appeared to be rifampicin-susceptible according to MGIT 960 system. In that study, 2/49 (4.1%) patients whose isolates did not have apparent mutations of the rpoB gene, experienced treatment failure compared with 3/3 (100%) patients whose isolates did have the rpoB gene mutations but had been found to be susceptible to rifampicin using phenotypic methods25.
A study involving retreatment patients (Van Deun 2013) found that several rpoB mutations conferring low-grade resistance were often missed by rapid phenotypic DST, particularly with the MGIT 960 system but also to a lesser extent by conventional (solid media) DST. The authors suggested that this may be the reason why molecular DST for rifampicin resistance is perceived to have insufficient specificity.26 Although the study involved retreatment patients, the results appear to hold also for individuals newly diagnosed with TB (Van Deun, personal communication, 2013).
Therefore, determining specificity of a molecular DST method using only phenotypic DST as a reference may underestimate the specificity of the molecular method of DST. In light of these findings, it is unclear whether and to what extent Xpert MTB/RIF might outperform phenotypic DST methods for detecting rifampicin resistance.
WHO will continue to collect and evaluate data on this issue, and will formally review the accuracy of phenotypic resistance standards for DST once sufficient data become available.
5.2.2. Using Xpert MTB/RIF to detect TB
Given the high sensitivity of Xpert MTB/RIF in detecting TB (88%), the negative predictive value (NPV) is greater than 98% both in settings with a low prevalence of TB and in those with a high prevalence of TB – that is, a negative result accurately excludes TB in most situations. Typically, in high-burden settings, between 10% and 20% of persons with respiratory symptoms will have culture-confirmed TB. In such settings the vast majority of patients with a negative result from Xpert MTB/RIF will not have TB. However, the ability of any diagnostic test using sputum specimens to detect TB depends on the quality of the specimen collected; therefore, an individual with a negative result from Xpert MTB/RIF could still have TB. An individual still suspected of having TB after a negative Xpert MTB/RIF test may, therefore, require further clinical management and another diagnostic test, including a repeated Xpert MTB/RIF test using a different sputum specimen.
The specificity of Xpert MTB/RIF for detecting TB is very high (99%), and false-positive results are likely to be linked to the detection by Xpert MTB/RIF of dead M. tuberculosis bacilli that would not be detected by culture, which is the present reference standard. Given that the specificity of Xpert MTB/RIF is not 100%, the positive predictive value (PPV) of Xpert MTB/RIF testing is adversely affected in settings with a low prevalence of disease or in populations with a low prevalence. Testing for TB is not usually implemented in a general, asymptomatic population but in individuals suspected of having TB following some form of screening involving, for example, symptom assessment or chest X-ray. Such screening procedures increase the prevalence of TB in the group tested, and improve the PPV of the test, making concerns related to false-positive results less relevant.
5.2.3. Using Xpert MTB/RIF to detect rifampicin resistance
Given the high sensitivity of Xpert MTB/RIF in detecting rifampicin resistance (95%), the NPV (the NPV for rifampicin resistance is the proportion of cases diagnosed as rifampicin-susceptible that are truly susceptible) is greater than 98% both in settings with a low prevalence of rifampicin resistance and those with a high prevalence of rifampicin resistance. Therefore a negative result accurately excludes the possibility of rifampicin resistance and, usually, no further testing is required to confirm negative results. In rare instances, when a patient is strongly suspected of having MDR-TB even after a negative result from Xpert MTB/RIF, a follow-up test may be done using phenotypic culture-based DST to detect rifampicin resistance that is conferred by regions outside of the rpoB region detected by Xpert MTB/RIF. Administrative errors are often more frequent than technical errors, and an unexpected result suggesting susceptibility to rifampicin could belong to a specimen from a different patient. Follow-up testing using Xpert MTB/RIF on a fresh specimen may be done if in doubt.
The specificity of Xpert MTB/RIF in detecting rifampicin resistance is very high (98%), and increasing evidence has shown that the infrequent occurrence of so-called false-positive results may be linked to the detection by Xpert MTB/RIF of strains that are truly resistant to rifampicin, but which are not detected by the phenotypic culture-based DST, the present reference standard. Such strains appear to have clinically relevant mutations in the region conferring resistance to rifampicin, causing disease for which first-line treatment is likely to fail. A study by Van Deun and colleagues27 showed that an epidemiologically-significant proportion of rifampicin-resistant strains (10-13%) in patients who have experienced their first treatment failure and in relapsed patients may be missed by rapid phenotypic DST.
The PPV for detecting rifampicin resistance (the PPV for rifampicin resistance is the proportion of cases diagnosed as rifampicin-resistant that are truly resistant) using Xpert MTB/RIF exceeds 90% in settings or groups of patients where the underlying prevalence of rifampicin resistance is greater than 15%, and the PPV is probably even higher considering the limitations of the present reference standard, as mentioned above. In settings or groups where rifampicin resistance is rare, the PPV is adversely affected but it can be greatly improved by undertaking a careful risk assessment of individual patients and targeting testing carefully to increase the pre-test probability of rifampicin resistance.
It is important to differentiate between new cases of TB and previously treated cases of TB; previously treated cases are much more likely to have MDR-TB. According to drug resistance surveillance data from 114 countries, the global weighted proportion of MDR-TB among previously treated cases is 20% (95% CI, 13-26%), which is several times higher than the proportion of new cases with MDR-TB (3.7% ; 95% CI, 2.1-5.2%).27
Therefore, even in settings with a low prevalence of MDR-TB, testing previously treated TB cases with Xpert MTB/RIF will result in a high PPV for the detection of rifampicin resistance.
5.3. Interpreting results from Xpert MTB/RIF
To complete any diagnostic algorithm, the test results need to be interpreted appropriately. Accurately interpreting results allows health-care workers and clinicians to make correct decisions about the interventions needed in relation to patient management and registration, and to any additional laboratory work-up that may be required. It is therefore important to train health-care staff how to interpret and follow-up any new test being introduced.
The interpretation of Xpert MTB/RIF results and follow-on steps will depend on both the result and the risk group from which the patient originated, based on the risk assessment as described in section 5.1. All patients identified as having TB by Xpert MTB/RIF should be initiated on the appropriate WHO-recommended treatment regimen as soon as possible. The prompt treatment initiation will have a positive effect on patients' outcomes, and a treatment regimen can be refined later if additional results become available.
As shown in , an Xpert MTB/RIF result can indicate that M. tuberculosis (MTB) was not detected, MTB was detected and was not resistant to rifampicin (that is, it is rifampicin susceptible), or that MTB was detected and it was resistant to rifampicin. A small proportion of tests may result in an error or invalid result; these tests need to be repeated.
Interpreting results from Xpert MTB/RIF tests.
When Xpert MTB/RIF does not detect M. tuberculosis, the disease can be ruled out in most cases unless there is still a strong suspicion of TB (special attention is required in people living with HIV who have signs and symptoms of TB) that may warrant further investigation (such as a chest X-ray, culture, another Xpert MTB/RIF test, or a trial of antibiotics). The ability of any diagnostic test to detect TB depends on the quality of the specimen collected.
When Xpert MTB/RIF detects M. tuberculosis without rifampicin resistance, the patient should be referred for the appropriate WHO-recommended first-line regimen and registered as a case with susceptible bacteriologically confirmed TB. Further testing by phenotypic DST is not required.
When Xpert MTB/RIF detects M. tuberculosis with rifampicin resistance, decisions about subsequent steps depend on the patient's risk group.
In patients from a group considered to be at high risk of MDR-TB, a WHO-recommended regimen for MDR-TB with the addition of isoniazid should be initiated; the patient should be registered as having bacteriologically confirmed rifampicin-resistant TB (RR-TB), and another sputum sample should be taken immediately and prior to starting treatment; these additional specimens should be sent for phenotypic DST for at least isoniazid, fluoroquinolones and second-line injectables. Confirmatory testing of rifampicin resistance using another testing technology is not necessary in such cases (given the high PPV for rifampicin resistance in this group). When the DST results are available, treatment can be modified if necessary and the patient's registration can be updated accordingly. Treatment modifications may include stopping isoniazid if resistance has been found, changing the quinolone and/or second-line injectable, or, in the case of XDR-TB, placing the patient on an appropriately designed regimen that includes group V drugs. The patient's registration should be modified to reflect any new information, and the case should be notified according to national regulations.
In patients considered to be at low risk of MDR-TB, rifampicin resistance may be unexpected and clinicians may be hesitant to enrol patients on a treatment regimen requiring second-line drugs (mostly because of the treatment length and concerns about toxicity). An unexpected Xpert MTB/RIF result may be attributed to the PPV for rifampicin resistance in a group that has a low underlying prevalence, or may result from nonsystematic or random errors at the preanalytical or postanalytical stages of testing (these errors are relatively frequent even in quality-assured laboratories). These include clerical errors made when information about specimens or test results is recorded, or administrative errors that result in specimens being mixed up, etc. An immediately repeated Xpert MTB/RIF test on a fresh specimen can be useful in excluding preanalytical and postanalytical errors and improving a clinician's confidence when deciding on treatment.
When the result of a second Xpert MTB/RIF test identifies TB but not rifampicin resistance (an expected result in an individual at low risk of MDR-TB), a WHO-recommended first-line regimen should be prescribed, and the patient should be registered as having susceptible, bacteriologically confirmed TB. Further testing by phenotypic DST is not required.
When the result of a second Xpert MTB/RIF test on a fresh specimen again shows rifampicin resistance, a WHO-recommended regimen for MDR-TB with the addition of isoniazid may be started without any further delay. In this case the patient should be registered as having bacteriologically confirmed rifampicin-resistant TB, and an additional specimen should be taken for phenotypic DST to re-confirm resistance to rifampicin and also to test for susceptibility to isoniazid, fluoroquinolones and second-line injectables. When DST results are available, the treatment regimen and patient registration should be adjusted as appropriate. Treatment modifications may include stopping isoniazid if resistance has been found, changing quinolone and/or second-line injectable, or, in cases where XDR-TB has been detected, placing the patient on an appropriately designed regimen that includes group V anti-TB agents. The patient's registration should be modified to reflect new information and the case should be notified according to national regulations.
In cases where discordant results are obtained from Xpert MTB/RIF and phenotypic DST or LPA, the available culture isolate should be referred to a reference laboratory for DNA sequencing; while awaiting the results, a clinical decision should be made whether to continue the MDR-TB regimen. The detection of a change in the amino acid sequence of the rifampicin resistance determining region (RRDR) should be considered confirmation of clinically significant rifampicin resistance.
The management of patients with MDR-TB should follow international standards of care as outlined in WHO's Guidelines for Programmatic Management of Drug-resistant Tuberculosis.28 The Xpert MTB/RIF assay is not suitable for monitoring a patient's response to treatment. Conventional microscopy and culture are required for monitoring MDR-TB patients during treatment.
5.4. Diagnostic algorithms
National programmes need to develop setting-specific, evidence-based and cost-optimized algorithms designed to ensure universal access to high quality TB, MDR-TB and HIV-related TB diagnosis. Implementation of Xpert MTB/RIF testing should be managed by Ministries of Health within the context of national plans for the appropriate management of TB, MDR-TB and HIV-associated TB; the implementation should include the development of country-specific screening and diagnostic strategies, means for ensuring timely access to quality-assured first-line and second-line anti-TB drugs, and appropriate care-delivery mechanisms.
The settings in which Xpert MTB/RIF is used and the algorithms for using the test should be guided by the country-specific or region-specific epidemiology of TB, HIV and MDR-TB, by the available resources, anticipated cost effectiveness of the algorithm. The algorithm should also take into account all screening and diagnostic tools available in the country and their characteristics. Testing costs should also be measured against the costs of treatment, the benefits to patient and public health, including direct financial savings associated with decreased delays in diagnosis and reduced transmission associated with providing early and appropriate treatment.
The adoption of Xpert MTB/RIF does not eliminate the need for conventional TB microscopy, culture and DST. Microscopy or culture, or both, remain necessary for monitoring treatment since it is unlikely that any currently available test that uses DNA detection will be suitable for monitoring treatment. In addition, conventional culture and DST will be required to detect resistance to anti-TB agents other than rifampicin. Because Xpert MTB/RIF detects resistance only to rifampicin, countries with documented or suspected cases of XDR-TB should establish or expand their capacity for conventional culture and DST for second-line anti-TB agents, and to ensure that testing for second-line drugs is quality assured and based on WHO's policies and guidance.
Ministries of health and national TB programs should actively obtain information on the adoption of Xpert MTB/RIF by private-sector laboratories and other private health-care providers, seek information about their intended use, and enforce notification of all TB cases detected in the private sector using Xpert MTB/RIF. In settings where private sector providers are widely used by TB patients, these providers should be made aware of the availability of Xpert MTB/RIF, and which groups should have priority for testing using Xpert MTB/RIF; referrals from these providers should be actively monitored. Collaboration among private providers and national TB programmes may be mutually beneficial, allowing private providers to access concessional prices and national TB programmes to ensure that patients detected in the private sector are duly reported and subsequently registered for appropriate treatment.
Careful overview of the advantages and limitations of currently available TB diagnostics and DST methods is necessary in designing the most appropriate diagnostic algorithm. Inclusion of Xpert MTB/RIF into the diagnostic algorithm should take into account both the selection of individuals to test (also in relation to other available TB testing technologies) and the interpretation of the Xpert MTB/RIF results and management decisions that should follow each possible result, therefore mapping and joining these two processes into one diagnostic algorithm. Diagnostic algorithms can be different for each country or particular setting and depend on epidemiology, other available technologies, financial and human resources.
Pre-test screening strategies can be useful to reduce the number of individuals who ultimately undergo Xpert MTB/RIF testing in a TB case finding diagnostic algorithm. Two examples of such strategies are symptom and chest X-ray screening, both of which representing additional costs and requiring additional efforts depending on the setting.29
A brief overview of the advantages and limitations of different TB diagnostics is given below to assist in developing an appropriate diagnostic algorithm.
Sputum-smear microscopy
Microscopy is suitable for laboratories at peripheral and higher levels and it can be done safely under minimal biosafety conditions. It is inexpensive but has limited sensitivity, which is further reduced in HIV-positive individuals. Microscopy identifies acid fast bacilli not M. tuberculosis, which may affect its specificity in settings with a low burden of TB or places with a high prevalence of NTM. Microscopy cannot distinguish between viable and non-viable organisms, or between susceptible organisms and resistant. Microscopy is used to monitor patients' responses to anti-TB therapy and light-emitting diode (LED) fluorescence microscopy is recommended.30 An extensive quality assurance programme must be implemented for microscopy to control for human error and sustain high quality performance.
Culture methods
Conventional culture (either solid or liquid) is suitable for national or regional laboratories. Manipulation of both solid cultures and liquid cultures requires the highest biosafety measures in the TB laboratory, and results are inevitably delayed due to the slow growth of mycobacteria. The use of both solid culture and liquid culture is recommended by WHO, and liquid culture is regarded as the gold standard for detecting TB; liquid culture results are also available more rapidly than results from solid culture. All positive cultures must be speciated to confirm M. tuberculosis. Culture is required to monitor the response of patients with MDR-TB to anti-TB therapy.
Phenotypic drug susceptibility testing
Phenotypic DST is suitable for national or regional laboratories. Being a conventional culture-based method, phenotypic DST requires the highest biosafety measures in the TB laboratory, and therefore is usually available only at national or higher-level regional laboratories. DST for second-line anti-TB agents should be done on all M. tuberculosis isolates with confirmed multidrug resistance. Phenotypic DST for second-line anti-TB agents is required to confirm or exclude XDR-TB.
Molecular line probe assay (LPA)
Due to its complexity and biosafety requirements, LPA is suitable only for national or regional level laboratories. LPA requires at least three separate rooms, and dedicated equipment, consumables and reagents in each room to minimize DNA cross-contamination. WHO recommends LPA to detect resistance to rifampicin and isoniazid only on smear-positive sputum specimens and M. tuberculosis isolates. Its sensitivity for detection of isoniazid resistance is sub-optimal. LPA can be used as a diagnostic test for MDR-TB, however, conventional culture (solid or liquid) is required to monitor treatment response (culture conversion) for patients with MDR-TB. Conventional second-line DST is required to detect XDR-TB because using LPA for detection of resistance to second-line anti-TB agents is not currently recommended by WHO due to sub-optimal performance of the test.
5.5. Monitoring patients during treatment
Molecular tests, including Xpert MTB/RIF, are not suitable for monitoring patients during treatment because these tests detect DNA from both viable and non-viable bacilli. The management of patients with HIV-associated TB and drug-resistant TB also requires concurrent clinical laboratory capacity (for example biochemistry, haematology, general microbiology) to monitor treatment and associated comorbid conditions.
Patients whose diagnosis of TB is confirmed by Xpert MTB/RIF and who have rifampicin-susceptible TB disease should be monitored during treatment with sputum-smear microscopy except in cases of extrapulmonary TB. No additional sputum-smear microscopy examination needs to be performed to establish their baseline status. For these patients, sputum-smear microscopy should be performed when the intensive phase of treatment has been completed, 5 months into treatment and at the end of treatment, following WHO guidelines.
Treatment outcomes for patients with a positive result from smear microscopy, culture or Xpert MTB/RIF at the start of treatment should be categorized according to the current WHO guidelines. All current treatment outcome definitions should be applied, including the outcome “Cured” – that is, a patient with a positive Xpert MTB/RIF test at baseline can be declared cured if a negative smear result is recorded at the end of treatment.
Patients whose TB and rifampicin resistance have been confirmed by Xpert MTB/RIF and who have been placed on an MDR-TB treatment regimen should be monitored by sputum-smear microscopy and culture, following WHO's current guidelines. If resources permit, monthly culture is recommended throughout treatment, given that this has been shown to have the greatest benefit in detecting treatment failure.
5.6. Using Xpert MTB/RIF in drug resistance surveys
Lack of laboratory capacity for culture and DST and the absence of referral systems at lower levels of networks are among the most important problems hindering implementation of drug resistance surveys31, which measure the frequency of drug resistance among a representative sample of TB patients. If carefully planned and implemented, the use of Xpert MTB/RIF in drug resistance surveys could greatly reduce logistical issues, transportation costs, and laboratories' workloads. Though not a complete surrogate for MDR-TB, particularly in settings with low levels of rifampicin resistance32 rifampicin resistance is the most important indicator of MDR-TB.
At least two groups of countries could benefit considerably from the use of Xpert MTB/RIF in drug resistance surveys. The first group is countries in which laboratories would struggle to cope with the workload generated by a survey while managing their routine work and maintaining high standards of quality. The second group is countries where there is no capacity to perform culture and DST. In these settings, instead of relying entirely on testing abroad – usually at a TB Supranational Reference Laboratory, which increases logistical issues and incurs further operational costs – Xpert MTB/RIF could be used to detect rifampicin-resistant specimens requiring further testing in a specialized laboratory.
Most patients enrolled in drug resistance surveys are at a low risk of rifampicin resistance. Given the high NPV of the test for detecting rifampicin resistance in such populations, Xpert MTB/RIF will accurately identify those whose disease is not resistant, reliably screening them out. Patients in whom rifampicin resistance is detected would be a relatively small group, thus presenting a lower workload for the central laboratory or TB Supranational Reference Laboratory. Strains from this group would require further testing to confirm resistance to rifampicin and to detect resistance to isoniazid and selected second-line drugs (fluoroquinolones and injectable agents).
5.7. Using Xpert MTB/RIF in TB prevalence surveys
TB prevalence surveys33 are important for obtaining a direct measurement of the absolute burden of disease caused by TB. TB prevalence surveys are population-based surveys that measure the number of people with TB disease in a sample. The number of people with active TB disease in the general population is relatively low (usually less than 1%), hence surveys typically involve large population sample sizes and require screening participants with interviews and chest X-rays and subsequent bacteriological testing of all participants with symptoms or chest X-ray abnormalities. Therefore, prevalence surveys entail a substantial workload for the laboratories involved, and this capacity is not always available in countries where the survey is planned or the quality of the testing is not assured.
The sensitivity of Xpert MTB/RIF in smear-negative culture-positive specimens is approximately 68%. For the purpose of TB prevalence surveys Xpert MTB/RIF cannot generally be considered a suitable replacement for culture to accurately estimate the prevalence of bacteriologically confirmed pulmonary TB. Nevertheless, when estimating the prevalence of bacteriologically confirmed pulmonary TB in surveys based on Xpert MTB/RIF testing, statistical adjustments can be made to account for the known diagnostic performance of Xpert MTB/RIF. Because Xpert MTB/RIF does not require advanced or additional infrastructure within the culture laboratory supporting the survey, it may facilitate the conduct of TB prevalence surveys. Furthermore, experience in several TB prevalence surveys have revealed several serious challenges that arise when large numbers of smear-positive samples are subsequently not confirmed by culture to be M. tuberculosis. Thus, the use of Xpert MTB/RIF on all smear-positive samples should help to rapidly identify samples with NTM (by identifying and excluding those with M. tuberculosis). This will ensure that individuals with TB receive appropriate treatment and prevent unnecessary treatment of those without TB. Survey participants with prominent symptoms or radiological abnormalities may also benefit from being tested with Xpert MTB/RIF in cases in which the culture has been contaminated or showed no growth. Appropriate operational research is, however, required before any definitive recommendations can be made on the use of Xpert MTB/RIF in TB prevalence surveys.
- 21
Expert Group meeting report: October 2013. Geneva: World Health Organization; 2014. Using the Xpert MTB/RIF assay to detect pulmonary and extrapulmonary tuberculosis and rifampicin resistance in adults and children. (available at http://www.who.int/tb/laboratory/policy_statements/en/)
- 23
Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. Geneva: World Health Organization; 2008. WHO/HTM/TB/2008.392. [PubMed: 26290924].
- 24
- 25
Williamson DA, et al. An evaluation of the Xpert MTB/RIF assay and detection of false-positive rifampicin resistance in Mycobacterium tuberculosis. Diagnostic Microbiology and Infectious Diseases. 2012;74:207–209 [PubMed: 22819240].
- 26
- 27
Global tuberculosis report 2012. Geneva: World Health Organization; (WHO/HTM/TB/2012.6).
- 28
- 29
- 30
- 31
Weyer K, et al. Rapid Molecular TB Diagnosis: Evidence, Policy-making and Global Implementation of Xpert(R)MTB/RIF. The European Respiratory Journal. 2012 [PubMed: 23180585] [CrossRef].
- 32
Smith SE, et al. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. International Journal of Tuberculosis and Lung Disease. 2012;16(2):203–205. [PMC free article: PMC4593497] [PubMed: 22136739]
- 33