All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: tni.ohw@sredrokoob). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: tni.ohw@snoissimrep).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009.

WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care.
Show details23.1. Glove policies
23.1.1. Reasons for glove use
Prior to the emergence of HIV and the acquired immunodeficiency syndrome (AIDS) epidemic, gloves were essentially worn primarily by HCWs either caring for patients colonized or infected with certain pathogens or exposed to patients with a high risk of hepatitis B. Since 1987, a dramatic increase in glove use has occurred in an effort to prevent the transmission of HIV and other bloodborne pathogens from patients to HCWs.905 The National Institute for Occupational Safety and Health Administration in the USA (NIOSHA) mandates that gloves be worn during all patient-care activities involving exposure to blood or body fluids that may be contaminated with blood,906 including contact with mucous membranes and non-intact skin. In addition, gloves should be worn during outbreak situations, as recommended by specific requirements for Personal Protective Equipment (PPE).58,423,906 The broad scope of these recommendations for glove use potentially leads to inevitable, undesirable consequences, such as the misuse and the overuse of gloves; therefore, there is a need to define glove use indications with greater precision.
Medical glove use by HCWs is recommended for two main reasons: 1) to reduce the risk of contaminating HCWs’ hands with blood and other body fluids; 2) to reduce the risk of germ dissemination to the environment and of transmission from the HCWs to the patient and vice versa, as well as from one patient to another.701,884,907,908
Single-use (also called disposable) examination gloves, either non-sterile or sterile, are usually made of natural rubber latex or synthetic non-latex materials such as vinyl, nitrile and neoprene (polymers and copolymers of chloroprene). Because of the increasing prevalence of latex sensitivity among HCWs and patients, the FDA has approved a variety of powdered and powder-free latex gloves with reduced protein contents, as well as synthetic gloves that can be made available by health-care institutions for use by latex-sensitive HCWs and for patients with latex hypersensitivity.909 Several new technologies are emerging (e.g. impregnated glove materials that release chlorine dioxide when activated by light or moisture to produce a disinfecting micro-atmosphere),910 but none of them has so far led to changes in glove use recommendations.49 The correct and consistent use of existing technologies with documented effectiveness is encouraged before new technologies are introduced. The main feature of examination gloves to bear in mind is that they are meant to be single-use and to be discarded.907,911,912 In most cases, they are non-sterile.
Sterile surgical gloves are required for surgical interventions. Some non-surgical care procedures, such as central vascular catheter insertion, also require surgical glove use. In addition to their sterile properties, these gloves have characteristics of thickness, elasticity and strength that are different from other medical gloves (either sterile or non-sterile).
Medical gloves are designed to serve for care purposes only and are not appropriate for housekeeping activities in health-care facilities. Other specific types of gloves are intended for these types of non-care activities.
In published studies, the barrier integrity of gloves has varied considerably based on the type and quality of glove material, intensity of use, length of time used, manufacturer, whether gloves were tested before or after use, and the method used to detect glove leaks.913–920 In some published studies, vinyl gloves more frequently had defects than did latex gloves, the difference being greatest after use.913,914,917,921 Intact vinyl gloves, however, provide protection comparable to that provided by latex gloves.913 Limited studies suggest that nitrile gloves have leakage rates close to those of latex gloves.922–925 Although recent studies suggest that improvements have been made in the quality of gloves,919 the laboratory and clinical studies cited above provide strong evidence that hands should still be decontaminated or washed after glove removal.73,123,139,204,520,914
23.1.2. Glove efficacy
The efficacy of gloves in preventing contamination of HCWs’ hands has been confirmed in several clinical studies.72,110,139 One study found that HCWs who wore gloves during patient contact contaminated their hands with an average of only 3 CFUs per minute of patient care, compared with 16 CFUs per minute for those not wearing gloves.72 Two other studies of HCWs caring for patients with C. difficile or VRE found that wearing gloves prevented hand contamination among a majority of those having direct contact with patients.110,139 Wearing gloves also prevented HCWs from acquiring VRE on their hands when touching contaminated environmental surfaces.139 Preventing gross contamination of the hands is considered important because handwashing or hand antisepsis may not remove all potential pathogens when hands are heavily contaminated.88,278 Furthermore, several studies provide evidence that wearing gloves can help reduce transmission of pathogens in health-care settings.701,884 In a prospective controlled trial that required HCWs routinely to wear vinyl gloves when handling any body substances, the incidence of C. difficile diarrhoea among patients decreased from 7.7 cases/1000 patient discharges before the intervention to 1.5 cases/1000 discharges during the intervention.422 The prevalence of asymptomatic C. difficile carriage also decreased significantly on “glove” wards, but not on control wards. In ICUs with VRE or MRSA epidemics, requiring all HCWs to wear gloves to care for all patients in the unit (universal glove use) appeared to contribute to the control of outbreaks.926–928 These data must be interpreted in the light of the actual direct impact on patient care, however, and some additional considerations need to be discussed.49,929 Glove use is not sufficient to prevent germ transmission and infection if not rigorously accompanied by previous and successive further preventive measures.930 The benefit of gloves is strictly related to the conditions of usage; the appropriateness of the latter strongly influences the actual reduction of germ dissemination and infection cross-transmission.
Hand hygiene is the most important measure to protect patients, HCWs and the environment from microbial contamination. Hand hygiene indications exist regardless of glove use, even if they influence glove wearing. A study highlighted the risk related to universal gloving as regards multidrug-resistant organism transmission: universal gloving can lead to a significant increase of device-related infections.884. Furthermore, wearing gloves does not provide complete protection against the acquisition of infections caused by HBV and HSV.913,931,932 These studies provide definitive evidence that gloves must be removed after care of a single patient and during the care of a patient, when moving from any body site to another such as non intact skin, mucous membrane or invasive medical device within the same patient, and that hand cleansing must be performed after glove removal. Bacterial flora colonizing patients may be recovered from the hands of up to 30% of HCWs who wear gloves during patient contact.123,139 Doebbeling and colleagues520 conducted an experimental study in which the artificial contamination of gloves was undertaken with conditions close to clinical practice. The authors cultured the organisms used for artificial contamination from 4–100% of the gloves and observed counts between 0 and 4.7 log on hands after glove removal. In a recent study identifying neonatal-care activities at higher risk for hand contamination, the use of gloves during routine neonatal care did not fully protect HCWs’ hands from bacterial contamination with organisms such as Enterobacteriacae, S. aureus, and fungi.73 In such instances, pathogens presumably gain access to the caregivers’ hands via small defects in gloves or by contamination of hands during glove removal.123,520,913,914
23.1.3. Glove use and hand hygiene
The impact of wearing gloves on compliance with hand hygiene policies has not been definitively established, as published studies have yielded contradictory results.49,216,661,672,739 Several studies found that HCWs who wore gloves were less likely to cleanse their hands upon leaving a patient’s room,661,688,739,908,930 and two established an association between inappropriate glove use and low compliance with hand hygiene.908,930 In contrast, three other studies found that HCWs who wore gloves were significantly more likely to cleanse their hands following patient care.216,672,802,933 Most of these studies were focused on hand hygiene performance after glove removal only and did not consider other indications. One study found that the introduction of gloves increased overall compliance with hand hygiene, but the introduction of isolation precautions did not result in improved compliance.934 For example, compliance with glove changing when moving between different body sites in the same patient was unsatisfactory, as well as compliance with optimal hand hygiene practices. Furthermore, although some studies demonstrated a high compliance with glove use, they did not investigate its possible misuse.683,689,935,936 Surveys conducted at facilities with limited resources showed that low compliance with recommendations for glove use and its misuse is not only associated with shortage of supply, but also with a poor knowledge and perception of the risk of pathogen transmission.695,937–940 Other studies pointed out the practical difficulty to combine hand hygiene and glove use.689,759 In one study, glove use compliance rates were 75% or higher across all HCW groups except doctors, whose compliance was only 27%.128 HCWs should be reminded that failure to remove gloves between patients or when moving between different body sites of the same patient may contribute to the transmission of organisms.73,927,930,932,941 In two reports, failure to remove gloves and gowns and to wash hands when moving between patients was associated with an increase in MRSA transmission during the SARS outbreak.942,943
Whether hand hygiene should be performed before donning non-sterile gloves is an unresolved issue and therefore this moment should not be recommended as an indication for hand hygiene. In this connection, a study found that volunteers did not contaminate the outside of their gloves significantly more often when they did not wash their hands before donning gloves, compared with the level of glove contamination that occurred when they washed their hands first.944 The study did not determine whether or not HCWs transmitted pathogens to patients more frequently when they did not wash their hands before donning gloves.
23.1.4. Appropriate and safe use of gloves
The use of gloves in situations when their use is not indicated represents a waste of resources without necessarily leading to a reduction of cross-transmission.884,930 The wide-ranging recommendations for glove use have led to very frequent and inappropriate use in general, far exceeding the frame of real indications and conditions for appropriate glove use that remain poorly understood among HCWs. Careful attention should be paid to the use of medical gloves according to indications907 for donning, but also for their removal. Moreover, numerous conditions regulate glove use and are aimed at preventing glove contamination and further consequences.
General indications for gloving and for glove removal are listed in Table I.23.1 and practical examples of care situations with indication for glove use are included in the pyramid (Figure I.23.1). It is important that HCWs are able to: 1) identify clinical situations when gloves are not indicated; 2) differentiate these from situations where gloves should be worn; and 3) correctly select the most appropriate type of gloves to be worn. Indications including indirect health-care activities, such as preparing parenteral nutrition or handling soiled waste, are also shown in the figure. In general, the moment for glove removal meets the recommendations for single use, i.e. related to a single patient and to a single care situation within the same patient.
Table I.23.1
Indications for gloving and for glove removal.

Figure I.23.1
Situations requiring and not requiring glove use. Gloves must be worn according to STANDARD and CONTACT PRECAUTIONS. The pyramid details some clinical examples in wich gloves are not indicated, and others in which examination or sterile gloves are indicated. (more...)
Conditions for glove use also imply the existence of a glove use procedure. Proper glove use requires continuous reasoning and a behavioural adjustment according to the care situation (Table I.23.2). These conditions are associated with equipment procurement and management (supply, availability, storage, and disposal) and with rigorous sequences and techniques for glove donning and removal (Figures I.23.2 and I.23.3). Conditions for glove use in health care are as crucial as the identification of indications. Indications represent a frame to limit the start and end of glove use. Importantly, gloves must be donned immediately before the contact or the activity that defines the indication and removed immediately after this contact or activity is over.945
Table I.23.2
A question-frame to capture practical conditions for appropriate and safe glove use.

Figure I.23.2
How to don and remove non-sterile gloves.

Figure I.23.3
How to don and remove sterile gloves.
Glove use does not obviate the need to comply with hand hygiene.884 1) When the hand hygiene indication occurs before a contact requiring glove use, handwashing or handrubbing must be performed before donning gloves to prevent glove contamination and possible cross-transmission in case of glove damage or improper use/efficacy. 2) Gloves must be removed to perform handwashing or handrubbing to protect a body site from the flora from another body site or skin area previously touched within the same patient. 3) Hand hygiene must be performed immediately after glove removal to prevent HCW contamination and further transmission and dissemination of microorganisms. It should be noted that handwashing with soap and water is necessary when gloves are removed because of a tear or a puncture and the HCW has had contact with blood or another body fluid; this situation is considered to be equivalent to a direct exposure to blood or another body fluid.
Further crucial conditions for appropriate glove use are their mechanical and microbiological integrity. Medical gloves should be kept in their original package or box until they are donned;945 this requires that gloves are available at the point of care as well as alcohol-based handrubs. Moreover, it is appropriate to have more than one type of gloves available, thus allowing HCWs to select the type that best suits their patient-care activities as well as their hand size. When removed, gloves should be discarded and disposed of; ideally, gloves should not be washed, decontaminated, or reprocessed for any reuse purpose.
These conditions are essential to prevent germ transmission through contaminated gloves to the patient and the HCW, and their further dissemination in the environment. When gloving is required continously because contact precautions are in place, all these conditions are difficult to integrate as part of usual care activities. Indeed, while the general indication to don gloves should remain until the contact with the patient and his/her immediate surroundings is completed, indications for glove removal, hand hygiene and, again, further indications for gloving may occur.
23.1.5. Factors potentially interfering with glove use
The use of petroleum-based hand lotions or creams may adversely affect the integrity of latex gloves.946 Following the use of powdered gloves, some alcohol-based hand rubs may interact with residual powder on HCWs’ hands, resulting in a gritty feeling on hands. In facilities where powdered gloves are commonly used, a variety of alcohol-based hand rubs should be tested following removal of powdered gloves in order to avoid selecting a product that causes this undesirable reaction.520,914 As a general policy, health-care settings should preferably select non-powdered gloves for both examination and surgical purposes.
23.1.6. Caveats regarding washing, decontaminating and reprocessing gloves
Manufacturers are not responsible for glove integrity when the principle of “single usage” is not respected. Any practice of glove washing, decontamination or reprocessing is not recommended as it may damage the material integrity and jeopardize the glove’s protective function. Although these practices are common in many health-care settings, essentially in developing countries, where glove supply is limited,947 no recommendation exists concerning the washing and reuse of gloves, nor the washing or decontamination of gloved hands followed by reuse on another patient.
In one study, washing gloved hands between patient treatments using 4% chlorhexidine and 7.5% povidone-iodine liquid soaps for 30 seconds eradicated all organisms inoculated from both glove surfaces.948 Another study describes a significant reduction of bacterial count on perforated gloves to permit their reuse for non-sterile procedures after cleansing of the gloved hand using an alcohol-based preparation with chlorhexidine.949 Although the microbial efficacy of glove washing and decontamination is demonstrated, the consequences of such processes on material integrity still remain unknown. More research on glove integrity after washing, decontaminating, and reprocessing is necessary to answer numerous unsolved issues before arriving at consistent recommendations. To this end, we call upon the manufacturers of gloves for medical application to concentrate on this issue and to conduct research to develop recyclable gloves for both examination and surgical use, and to provide also information about safe reprocessing methods for the reuse of gloves in resource-limited settings.
Cleansing gloved hands to allow for prolonged use on the same patient may result in considerable savings of disposable examination gloves. Some evidence exists that cleansing latex-gloved hands using an alcohol-based handrub solution is effective in removing micro-organisms and shows increasing contamination rates of hands only after 9–10 cycles of cleansing.950,951 However, cleansing plastic-gloved hands with an alcohol-based formulation leads to early dissolving of the plastic material. If there is an intention to proceed with the process of glove decontamination, this should be started only after performing a local study using the type of gloves and products provided at the facility. It should be noted that this process may be applied only in the framework of contact precautions implementation907 and as long as gloves are not soiled with blood and other body fluids. As a consequence, this limited context for glove decontamination probably does not represent an effective response to the serious problem of glove shortage in developing countries.
In conclusion, no evidence-based recommendation currently exists regarding glove reprocessing. While this may be an interesting option at facilities where supply is insufficient, all consequences of the reprocessing should be anticipated and measured before putting it into practice. A reprocessing method has been suggested by the Johns Hopkins Program for International Education in Reproductive Gynaecology and Obstetrics (JHPIEGO).952 This process is not standardized nor validated, and no recommendation of this or any other reprocessing process can be expressed in the absence of good quality research. This protocol firstly includes a situation analysis assessment and some criteria for opting for reprocessing gloves in order to minimize the risks and to optimize the results. Before planning or continuing the reprocessing of used gloves, every health-care facility should first undertake an assessment of factors leading to the shortage of single-use gloves, such as budget constraints or interrupted supply chains. Efforts should focus on reducing the need for gloves by avoiding wastage caused by unnecessary use and by providing a secure stock of good quality single-use surgical and examination gloves, together with a budget for regular restocking. Opting for glove reprocessing without having made these assessments would amount to contributing to the maintenance of inappropriate glove use. Health administrators are encouraged to purchase good quality disposable gloves and replenish stocks in time. In addition, clinic managers and supervisors should check that gloves are not wasted, and HCWs should be educated to appropriate use of gloves (see Figure I.23.1).
In institutions with limited resources, some authors suggest that if the necessity for the reprocessing of single-use gloves persists after a thorough evaluation, the reprocessing of previously decontaminated and thoroughly cleaned surgical gloves using sterilization (autoclaving) or high-level disinfection (steaming) can produce an acceptable product; when combined with double gloving, this may constitute a temporary tolerable practice.952,953 However, the practice could be retained only if basic criteria, such as glove quality, are satisfied and the selected processes and technologies for reprocessing are reliable and under control. A universal problem is the introduction of equipment, technology, and method with no evaluation of associated needs. In this case, their reliability and safety are not guaranteed.929 If reprocessing does take place, the institution should develop clear policies to define clinical situations where gloves are needed, when the use of reprocessed gloves can be tolerated, and when gloves should be discarded and not reprocessed (e.g. when holes are detected). Only surgical latex gloves may be reused either as surgical gloves using double gloving or as gloves for examination purposes. Some authors recommend that latex rubber surgical gloves should be discarded after three reprocessing cycles because gloves tear more easily with additional reprocessing.954,955 Examination gloves should never be reprocessed because of their particular composition properties, thinness, and inelasticity.
Systematic research is urgently needed to evaluate reprocessing methods and to develop and validate a process that leads to a product of acceptable quality. Furthermore, well-conducted cost–benefit studies are required to evaluate the potential benefits of reprocessing gloves and the general need for investing in preventive measures. Through an analysis of the financing structures of health-care delivery systems in developing countries, incentives for investment in the prevention of HCAIs from the individual, institutional, and societal perspectives can be identified.
The practice of autoclaving used plastic gloves in case of shortage and of autoclaving new plastic gloves meant for examination for use as surgical gloves has been described.956 The reprocessing at 125 °C leads to gloves sticking together, and separation causes tears and holes. The authors found 41% of recycled gloves with impaired integrity.956 Another potential hazard is often witnessed in developing countries: many reprocessing units use powder inside reprocessed latex gloves to prevent material sticking together and to facilitate reuse. The consequences of use of powdered latex gloves in terms of the development of latex allergies and impaired working conditions leading to sickness in HCWs are well documented.957
In general, one of the major risks of reprocessing gloves is that they could show a higher rate of non-apparent holes and tears after the reprocessing cycle than new ones. A study by Tokars et al. showed that surgeons wearing a single layer of new surgical gloves had blood contact in 14% of the procedures, and blood contact was 72% lower among surgeons who double gloved.958 Therefore, double gloving in countries with a high prevalence of HBV, HCV and HIV for long surgical procedures (>30 minutes), for procedures with contact with large amounts of blood or body fluids, for some high-risk orthopaedic procedures, or when using reprocessed gloves is considered an appropriate practice.
The illegal recovery and recycling of discarded gloves from hospital waste dumping sites, often using dubious and uncontrolled reprocessing methods, can constitute an additional health hazard and is of growing concern in countries with limited resources. Hospitals are therefore encouraged to destroy each glove before discarding.
In brief, the opinion of international experts consulted by WHO is that glove reprocessing must be strongly discouraged and avoided, mainly because at present no standardized, validated, and affordable procedure for safe glove reprocessing exists. Every possible effort should be made to prevent glove reuse in health-care settings, and financial constraints in developing countries leading to such practices should be assessed and tackled. Institutions and health-care settings should firmly avoid the reuse of gloves. In circumstances where the reprocessing of gloves has been carefully evaluated but cannot be avoided, a clear policy should be in place to limit reprocessing and reuse of gloves until a budget is allocated to ensure a secure supply of single-use gloves. Policies for exceptional reprocessing should ensure a process that follows strict procedures for collection, selection and reprocessing, including instructions for quality/integrity control and discarding of unusable gloves.
23.1.7. Conclusions
Medical glove use is an evidence-based measure to protect patients, HCWs, and the environment. The recommendations for glove use must be implemented regardless of the type of setting and the resources available. Nevertheless, glove misuse is observed regularly worldwide, irrespective of the underlying reasons. Even in institutions where gloves are widely available, HCWs often fail to remove gloves between patients or between contact with various sites on a single patient, thus facilitating the spread of microorganisms.154,744,952,959,960 Knowledge dissemination and practical training on the appropriate use of gloves are the foremost interventions leading not only to best practices, but also to resource saving. Deficient glove procurement in terms of quantity and quality causes inappropriate and unsafe practices such as glove misuse and overuse and may lead to uncontrolled reprocessing.929,947 No evidence-based recommendations for glove reuse or reprocessing exist other than those described above. Medical gloves are meant to be disposable and for single use. They are intended to complement hand hygiene and are effective as long as they are used according to the proper indications. Hand hygiene still remains the basic and most effective measure to prevent pathogen transmission and infection.
In no way does glove use modify hand hygiene indications or replace hand hygiene by washing with soap and water or handrubbing with an alcohol-based handrub.
Gloves represent a risk for pathogen transmission and infection if used inappropriately.
23.2. Importance of hand hygiene for safe blood and blood products
Providing a safe unit of blood to a patient who requires blood transfusion is a multistep process. It includes identifying safe blood donors for blood donation, safe blood collection without harming the blood donor and the donated blood, screening of donated blood for HIV, hepatitis B and C, and syphilis, processing the blood into blood products, and issue of blood or blood product to the patient, when prescribed.
Appropriate hand hygiene practice is crucial to the safety of blood and blood products at all stages in the transfusion chain during which the donated blood units are handled. The microbial contamination of blood or blood products may occur at the time of blood collection or during the processing into blood products, labelling, storage and transportation, or during administration of blood at the patient bedside. This can have fatal consequences for the recipients of the transfusion. Serious consequenses of microbial contamination can be avoided by giving particular attention to the hand hygiene of the donor care staff at the time of blood collection and by thorough cleansing of the venepuncture site on the donor arm.
Furthermore, blood collection staff frequently needs to collect blood in environments that are especially challenging. Special care must be exercised in hand hygiene while collecting blood in outdoor situations where access to running water is limited.
It is essential that all those who work in areas where blood is handled pay strict attention to hand hygiene. Standard operating procedures should be available to staff, detailing exactly how hands should be decontaminated in order to protect blood donors, patients, and the staff themselves, as well as the blood and blood products. Figure I.23.4 depicts the crucial steps during blood collection, processing, and transfusion with an associated risk for the contamination of blood or blood products attributable to poor hand hygiene of the staff involved in these processes. At each step, there are several critical procedures, including meticulous hand hygiene, which ultimately lead to the safety of blood and blood products.
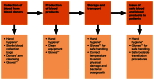
Figure I.23.4
Blood safety: crucial steps for hand hygiene action. * Hand hygiene before and after the procedure. ** Clean non-sterile gloves.
23.3. Jewellery
Several studies have shown that skin underneath rings is more heavily colonized than comparable areas of skin on fingers without rings.961–963 A study by Hoffman and colleagues962 found that 40% of nurses harboured Gram-negative bacilli such as E. cloacae, Klebsiella spp., and Acinetobacter spp. on skin under rings and that some nurses carried the same organism under their rings for months. In one study involving more than 60 ICU nurses, multivariable analysis revealed that rings were the only significant risk factor for carriage of Gram-negative bacilli and S. aureus and that the organism bioburden recovered correlated with the number of rings worn.964 Another study showed a stepwise increased risk of contamination with S. aureus, Gram-negative bacilli, or Candida spp. as the number of rings worn increased.153 In a Norwegian study comparing hand flora of 121 HCWs wearing a single plain ring and 113 wearing no rings, there was no significant differences in the total bacterial load or rates of carriage of S. aureus or non-fermentative Gram-negative rods on hands, but personnel wearing rings were more likely to carry Enterobacteriaceae (P=0.006).965 Among 60 volunteers from perioperative personnel and medical students, Wongworawat & Jones966 found no significant difference in bacterial counts on hands with or without rings when an alcohol product was used, but there were significantly more bacteria on ringed hands when povidone-iodine was used for handwashing (P<0.05). Furthermore, Rupp and colleagues707 reported that having longer fingernails and wearing rings were associated with increased numbers and species of organisms on hands. In addition, at least one case of irritant dermatitis under the ring has been reported as a result of wearing rings.967
A survey of knowledge and beliefs regarding nosocomial infections and jewellery showed that neonatal ICU HCWs were not aware of the relationship between bacterial hand counts and rings, and did not believe that rings increased the risk of nosocomial infections; 61% regularly wore at least one ring to work.960
Whether the wearing of rings results in greater cross-transmission of pathogens remains unknown. Two studies found that mean bacterial colony counts on hands after handwashing were similar among individuals wearing rings and those not wearing rings.963,968 One study compared the impact of wearing rings on the efficacy of several different products in 20 subjects who wore a ring on one hand and no ring on the other: an alcohol-based formulation; a waterless, alcohol-chlorhexidine lotion; and a povidone-iodine scrub. There were no significant differences in bacterial counts when the two alcohol-based formulations were used, but there were higher counts on the ringed hands (p<0.05) after povidone-iodine scrub966.
Further studies are needed to establish if wearing rings results in a greater transmission of pathogens in health-care settings. Nevertheless, it is likely that poorly maintained (dirty) rings and jewellery might harbour microorganisms that could contaminate a body site with potential pathogens. Rings with sharp surfaces may puncture gloves. Hand hygiene practices are likely to be performed in a suboptimal way if voluminous rings or rings with sharp edges or surfaces are worn. Jewellery may also be a physical danger to either patients or the HCW during direct patient care, e.g. a necklace may be caught in equipment or bracelets may cause injury during patient handling.
The consensus recommendation is to strongly discourage the wearing of rings or other jewellery during health care. If religious or cultural influences strongly condition the HCW’s attitude, the wearing of a simple wedding ring (band) during routine care may be acceptable, but in high-risk settings, such as the operating theatre, all rings or other jewellery should be removed.969 A simple and practical solution allowing effective hand hygiene is for HCWs to wear their ring(s) around their neck on a chain as a pendant.
23.4. Fingernails and artificial nails
Numerous studies have documented that subungual areas of the hand harbour high concentrations of bacteria, most frequently coagulase-negative staphylococci, Gram-negative rods (including Pseudomonas spp.), Corynebacteria, and yeasts.63,534,970 Freshly applied nail polish does not increase the number of bacteria recovered from periungual skin, but chipped nail polish may support the growth of larger numbers of organisms on fingernails.971,972 Even after careful handwashing or surgical scrubs, HCWs often harbour substantial numbers of potential pathogens in the subungual spaces.154,973,974 In particular, the presence of fingernail disease may reduce the efficacy of hand hygiene and result in the transmission of pathogens. A cluster of P. aeruginosa SSIs resulted from colonization of a cardiac surgeon’s onychomycotic nail.523
A growing body of evidence suggests that wearing artificial nails may contribute to the transmission of certain health care-associated pathogens. HCWs who wear artificial nails are more likely to harbour Gram-negative pathogens on their fingertips than those who have natural nails, both before and after handwashing154,534,974,975 or handrub with an alcohol-based gel.154 It is not clear if the length of natural or artificial nails is an important risk factor, since most bacterial growth occurs along the proximal 1 mm of the nail, adjacent to subungal skin.154,972,974 An outbreak of P. aeruginosa in a neonatal ICU was attributed to two nurses (one with long natural nails and one with long artificial nails) who carried the implicated strains of Pseudomonas spp. on their hands.976 Case patients were significantly more likely than controls to have been cared for by the two nurses during the exposure period, suggesting that colonization of long or artificial nails with Pseudomonas spp. may have played a role in causing the outbreak. HCWs wearing artificial nails have also been epidemiologically implicated in several other outbreaks of infection caused by Gram-negative bacilli or yeast.159,167,977 In a recent study, multiple logistic regression analysis showed the association of an outbreak of extended-spectrum beta-lactamase-producing K. pneumoniae in a neonatal ICU resulting from exposure to an HCW wearing artificial fingernails.155 A cluster of five cases of S. marcescens bacteraemia in haemodialysis was associated with a nurse who used an artificial fingernail to open a vial of heparin that was mixed to make a flush solution. The strains isolated from the five patients and the nurse were indistinguishable.856 Allergic contact dermatitis resulting in months of sick leave has been reported in an office worker with artificial nails.978
Long, sharp fingernails, either natural or artificial, can puncture gloves easily.123 They may also limit HCWs’ performance in hand hygiene practices. In a recent survey among neonatal ICU HCWs, 8% wore artificial fingernails at work, and knowledge among them about the relationship between Gram-negative bacterial hand contamination and long or artificial fingernails was limited.960
Jeanes & Green979 reviewed other forms of nail art and technology in the context of hand hygiene in health care, including: applying artificial material to the nails for extensions; nail sculpturing; protecting nails by covering them with a protective layer of artificial material; and nail jewellery, where decorations such as stones may be applied to the nails or the nails are pierced. In addition to possible limitations of care practice, there may be many potential health problems, including local infection for individuals who have undergone some form of nail technology.979
Each health-care facility should develop policies on the wearing of jewellery, artificial fingernails or nail polish by HCWs. These policies should take into account the risks of transmission of infection to patients and HCWs, rather than cultural preferences.
Consensus recommendations are that HCWs do not wear artificial fingernails or extenders when having direct contact with patients and natural nails should be kept short (≤ 0.5 cm long or approximately ¼ inch long).
23.5. Infrastructure required for optimal hand hygiene
Compliance with hand hygiene is only possible if the healthcare setting ensures the adequate infrastructure and a reliable supply of hand hygiene products at the right time and at the right location in alignment with the concept of “My five moments for hand hygiene” (Part I, Section 21.4).1 An important cause of poor compliance may be the lack of user-friendliness of hand hygiene equipment, as well as poor logistics leading to limited procurement and replenishment of consumables. The latter is one of the most commonly cited obstacles to hand hygiene improvement in developing countries (reports of workshops hosted by the WHO Regional Offices for Africa (AFRO) and South-East Asia (SEARO) in 2007, see http://www.who.int/gpsc/in/). As an example, very low overall hand hygiene compliance (8%) was shown in a university hospital in Mali where, at the same time, a survey on infrastructure for hand hygiene demonstrated that no alcohol-based handrub was available. Only 14.3% of patient rooms were equipped with sinks, and soap and towels were available at only 47.4% of sinks.980 In developed countries, Suresh & Cahill981 described several deficiencies in the structural layout of hand hygiene resources that hinder their usage: poor visibility, difficulty of access, placement at undesirable height, and wide spatial separation of resources that are used sequentially.
Other parts of these Guidelines have already described the need for clean water for handwashing and have elaborated on the advantages of handrubs over handwashing, namely, the freedom from the requirement of sinks and the possibility to clean hands at the point of care. While describing the overall infrastructure necessary, this section is particularly focused on soap and handrub dispensers.
23.5.1. General guidelines
All health-care settings should have written guidelines describing the appropriate placement of sinks and soap and handrub dispensers. Furthermore, the delegated responsibility with regards to supply of hand hygiene products, replenishment of consumables, and maintenance of the dispensers should be clearly described and communicated.
23.5.2. Sinks
While not all settings have a continuous water supply, tap water (ideally drinkable, is preferable for handwashing (see Part I, Section 11.1). In settings where this is not possible, water “flowing” from a pre-filled container with a tap is preferable to still-standing water in a basin. Where running water is available, the possibility of accessing it without the need to touch the tap with soiled hands is preferable. This may be achieved by taps that are opened by using an elbow or foot. In settings without budget restrictions, sensor-activated taps may be used for handwashing, although it must be noted that the system reliability is paramount since its failure completely prevents any access to handwashing facilities. In summary, manual or elbow-or foot-activated taps could be considered the optimal standard within health-care settings. Their availability is not considered among the highest priorities, however, particularly in settings with limited resources. Of note, recommendations for their use are not based on evidence.
To avoid water splashes, the water stream should not be directed straight into the drain, and taps should be fitted with an aerator screen. The mesh of the aerator screen should be sufficiently wide to ensure that no water remains on top of the aerator screen, as this may lead to bacterial contamination and consequent spread of microbes.982
23.5.3. Dispensers
In most health-care facilities, alcohol-based handrub dispensers have historically been located close to the sink, often adjacent to the wall-mounted liquid soap. Part of their function was to dispense pre-set amounts of handrub (mostly 1. 5 ml, half of what was needed according to older guidelines). Frequently, these dispensers were designed to allow the user to apply handrub without using their contaminated hands to touch the dispenser (elbow-activated). While wall-mounted dispensers at the sink seemed a logical place to start promoting hand antisepsis with rubs over handwashing, the main advantage of handrubs is the fact that they can (and should) be used at the point of care, for example at the end of the bed. Placement of handrubs exclusively at the sink therefore disregards one of their unique features and is not aligned with promoting hand hygiene at the five moments when it is required in health care.
The advantages and disadvantages of the different dispenser systems are discussed below and summarized in Table I.23.3. Although the same wall-mounted dispensers are used frequently for handrubs and liquid soaps, this section will focus on handrub dispersion. It is obvious that economic constraints as well as local logistics have a major influence on the choice of dispensing system. Furthermore, in many settings, the different forms of dispensers, such as wall-mounted and those for use at the point of care, should be used in combination to achieve maximum compliance. Some of the prerequisites for all dispensers and their placement are given in Table I.23.4. Some examples of dispensers for use at the point of care are shown in Figure I.23.5.
Table I.23.3
Advantages and disadvantages of different dispensing methods.
Table I.23.4
Characteristics to be considered as a prerequisite for all dispensers and their placement.

Figure I.23.5
Different types of dispensers at the point of care.
23.5.3.1. Wall-mounted systems
Wall-mounted soap dispensing systems are recommended to be located at every sink in patient and examination rooms, when affordable. Wall-mounted handrub dispensers should be positioned in locations that facilitate hand hygiene at the point of care, in accordance with the concept of the “My five moments for hand hygiene”. Careful consideration should be given to the placement of these dispensers in areas with patients who are likely to ingest the product, such as disoriented elderly patients, psychiatric patients, young children, or patients with alcohol dependence. In patient areas where beds are geographically in very close proximity, common in developing countries, wall-mounted, alcohol-based handrubs can be placed in the space between beds to facilitate hand hygiene at the point of care. Some institutions have customized dispensers to fit on carts or intravenous-pools to ensure use during care delivery.
Splashes on the floor from wall-mounted dispensers have been reported as a potential problem, as this may lead to the discolouration of certain floor surfaces or even result in the floor surface becoming slippery. Some manufacturers in developed countries offer dispensers with a splash-guard intended to catch splashes and droplets to avoid these problems.
Dispensers should be mounted on the wall in a manner that allows unrestricted, easy access (i.e. not in corners or under hanging cupboards). They should be used preferably with disposable, transparent containers of a standardized size, thus allowing the use of products from different suppliers (e.g. Euro-dispenser for standardized 500 ml and 1000 ml bottles). The product should be placed in the dispenser in such a way that the label and content is visible to ensure timely replacement of empty containers by housekeeping or maintenance staff. Dispersion of the handrub should be possible in a “nontouch” fashion to avoid any touching of the dispenser with contaminated hands, e.g. “elbow-dispensers” or pumps that can be used with the wrist.58 Despite the fact that ease of access may lead to increased use, as shown by Larson and colleagues654 when comparing the frequency of handrub use of manually operated and touch-free dispensers in a paediatric ICU, robust mechanical systems are preferable over electronic “non-touch systems” that are more susceptible to malfunction, more costly, and frequently only usable with the supplier’s own hand hygiene formulation. In general, the design and function of the dispensers that will ultimately be installed in a healthcare setting should be evaluated, because some systems were shown to malfunction continuously, despite efforts to rectify the problem.983
23.5.3.2. Table-top dispensers (pumps)
A variation of wall-mounted dispensers are holders and frames that allow placement of a container that is equipped with a pump. The pump is screwed onto the container in place of the lid. It is likely that this dispensing system is associated with the lowest cost. Containers with a pump can also be placed easily on any horizontal surface, e.g. cart/trolley or night stand/bedside table. Several manufacturers have produced dispenser holders that allow positioning of the handrub onto a bed frame, thus enabling access to the handrub at the point of care. A disadvantage of these “loose” systems is the fact that the bottles can be moved around easily and may be misplaced, resulting in decreased reliability. Where possible, the combination of fixed (wall-mounted) and loose dispensers should be used.
23.5.3.3. Pocket or clip-on dispensers
Studies that compared the use of personal alcohol-based handrub dispensing systems with the traditional wall-mounted dispenser and sinks were unable to show a sustained effect on hand hygiene compliance,709 possibly because the increased availability of hand hygiene products is only a single intervention within a broad multimodal approach. Individual, portable dispensers are ideal if combined with wall-mounted dispensing systems, to increase point-of-care access and enable use in units where wall-mounted dispensers should be avoided or cannot be installed. Also, wall-mounted systems can be used for back-up, as many of the pocket bottles or clip-ons are frequently not transparent and may be found to be empty when required. In some of these systems, the amount of handrub may be so small (10–20 ml) that several containers per HCW are needed each day. Costs and dependency on a single manufacturer and its products may be a problem especially with the clip-on system. Because many of these systems are used as disposables, environmental considerations should also be taken into account. In some situations, concern has been expressed about the potential contamination of the external surface of the bottle. However, this is considered to be almost theoretical and negligible because of the excess spillage of the disinfectant and the overall short time until replacement.
23.5.3.4. Automated wall-mounted dispensers
These types of systems have emerged from the non-medical setting, are aesthetically appealing, and are presently being marketed in many health-care settings. Such systems are truly non-touch and easy to use. Barrau and colleagues984 compared a wall-mounted, hand-activated sprayer system with “bottles on a table”, suggesting a possible benefit of the sprayer system. The study had several flaws, among them the low volume of product dispensed, which may be associated with lower efficacy.985 On average, less than 0.8 ml was supplied for a one-time handrub, an amount less than three times than that currently recommended. In addition to the costs of the dispensers and the problem of their maintenance, many of these systems have to be filled with the manufacturer’s own handrub, which is generally more expensive than other products distributed in 500 ml and 1000 ml standardized containers. In general, the maintenance is more complicated and the chance of malfunction is higher in automated systems.
23.5.3.5. Indicators/surveillance
Within the health-care setting, simple structure and performance indicators may be used to evaluate:
- the number of dispensers filled compared with the total number of dispensers in a unit;
- the number of dispensers in working order compared with the total number of dispensers in a unit;
- the proportion of patient and treatment rooms with dispensers present at the point of care;
- the number of sinks in patient and treatment rooms and sink/bed ratio;
- the proportion of sinks equipped with soap and single-use towels.
Recently, special dispensers with electronic surveillance systems have been made commercially available. While measures of use are not validated in observational studies and do not allow conclusions about individual HCW adherence to hand hygiene indications, particularly the five moments, these electronic devices, in combination with other measures, may help to collect information about soap and handrub use, including the effect of quality improvement and educational initiatives.986
23.6. Safety issues related to alcohol-based preparations
23.6.1. Fire hazard issues
Alcohols are flammable. Flashpoints of alcohol-based handrubs range from 17.5°C to 24.5°C, depending on the type and concentration of alcohol present.484,540 Therefore, risk assessment and minimization is crucial and alcohol-based handrubs should be stored away from high temperatures or flames in accordance with National Fire Protection Agency recommendations in the USA.
Although alcohol-based hand rubs are flammable, the risk of fires associated with such products is very low. For example, none of 798 health-care facilities surveyed in the USA reported a fire related to an alcohol-based handrub dispenser. A total of 766 facilities had accrued an estimated 1430 hospital-years of alcohol-based handrub use without a fire attributed to a handrub dispenser.987
In Europe, where alcohol-based handrubs have been used extensively for many years, the incidence of fires related to such products has been extremely low.484 A recent study988 conducted in German hospitals found that handrub usage represented an estimated total of 25 038 hospital-years. The median volume usage was between 31 litres/month (smallest hospitals) and 450 litres/month (largest hospitals), resulting in an overall usage of 35 million litres for all hospitals. A total of seven non-severe fire incidents was reported (0.9% of hospitals). This is equal to an annual incidence per hospital of 0.0000475%. No reports of fire caused by static electricity or other factors were received, nor any related to storage areas. Indeed, most reported incidents were associated with deliberate exposure to a naked flame, e.g. lighting a cigarette.
One recent report from the USA described a flash fire that occurred as a result of an unusual series of events, which consisted of an HCW applying an alcohol gel to her hands then immediately removing a polyester isolation gown and touching a metal door before the alcohol had evaporated.989 Removing the polyester gown created a large amount of static electricity that generated an audible static spark when she touched the metal door, igniting the unevaporated alcohol on her hands.989 This incident underscores the fact that, following the application of alcohol-based handrubs, hands should be rubbed together until all the alcohol has evaporated.
In the USA, shortly after publication of the 2002 CDC/HICPAC hand hygiene guideline, fire marshals in a number of states prohibited the placement of alcohol-based handrub dispensers in egress corridors because of a concern that they may represent a fire hazard. On 25 March 2005, the Center for Medicare and Medicaid Services adopted a revised version of the USA National Fire Protection Agency’s Life Safety Code that allows such dispensers to be placed in egress corridors. The International Fire Code recently agreed to accept alcohol-based handrubs in corridors. In addition, the CMS 3145-IFC (Fire Safety Requirement for Certain Health Care Facilities, Alcohol-Based Hand Sanitizer and Smoke Detector Amendment) was published in March 2005, addressing this issue.990
23.6.2. Other safety-related issues
Accidental and intentional ingestion and dermal absorption of alcohol-based preparations used for hand hygiene have been reported.599,778–780 Acute, severe alcohol intoxication resulting from accidental ingestion of an unknown quantity of alcohol-based handrub was recently reported in the United Kingdom, resulting in the unconsciousness of an adult male patient (Glasgow Coma Scale 3).778,781 This unusual complication of hand hygiene may become more common in the future, and security measures are needed. These may involve: placing the preparation in secure wall dispensers; labelling dispensers to make the alcohol content less clear at a casual glance and adding a warning against consumption; and the inclusion of an additive in the product formula to reduce its palatability. In the meantime, medical and nursing staff should be aware of this potential risk.
Alcohol toxicity usually occurs after ingestion. It is primarily metabolized by an alcohol dehydrogenase in the liver to acetone. Symptoms and signs of alcohol intoxication include headache, dizziness, lack of coordination, hypoglycaemia, abdominal pain, nausea, vomiting, and haematemesis. Signs of severe toxicity include respiratory depression, hypotension, and coma. Among alcohols, isopropyl alcohol appears to be more toxic than ethanol, but less so than methanol. Blood isopropyl alcohol levels of 50 mg/dl are associated with mild intoxication and 150 mg/dl with deep coma. Apparently, isopropyl alcohol has no adverse effects on reproduction and is not genotoxic, teratogenic, or carcinogenic.991
In addition to accidental ingestion, alcohols can be absorbed by inhalation and through intact skin, although the latter route (dermal uptake) is very low. Any absorption exceeding certain levels may result in toxicity and chronic disease in animals992 and humans.780 Recently, the Health Council of the Netherlands993 suggested to classify ethanol as carcinogenic and to include it in skin notation because of the fear of an increased risk of breast and colorectal cancer in persons with an occupational exposure to ethanol. While the Dutch Social and Economic Council advised the Ministry of Social Affairs and Employment to consider an exception for the use of alcohol-based handrubs in health-care settings, the Ministry of Social Affairs and Employment rejected such an exception and set the maximum amount of occupational absorbed ethanol at such a low level that the decision could possibly lead to a ban of ethanol-containing handrubs in the Netherlands if upheld. Obviously, such a decision would be disastrous for health-care settings and could induce other countries to consider similar measures. Indeed, while there are no data to show that the use of alcohol-based handrub may be harmful – and studies evaluating the absorption into blood show that it is not – reduced compliance with hand hygiene will lead to preventable HCAIs.
Data used by the Dutch Heath Council estimated the absorption level after spraying of the total body under occlusive circumstances and after exposure times of up to 24 hours, although this is obviously not relevant for the application of handrubs. Furthermore, they estimated a worst case dermal uptake of 30 mg ethanol after a single application to hands and forearms, and a daily uptake of 600 mg/day after 20 applications per day, an estimate that has been proven wrong by several new studies.782,784,994,995
In practice, absorption of ethanol from a handrub would be by a combination of dermal absorption and inhalation. In a study using a solution of 44% ethanol sprayed on the skin and left for 15 minutes, there was no positive identification of ethanol in any of the blood samples taken (limit of detection was 9 mg/litre).994 Turner and colleagues evaluated the dermal absorption through HCW’s intact skin599:3 ml of an isopropyl alcohol-containing handrub (52.6% (w/w) isopropyl alcohol) were applied to HCWs’ hands every 10 minutes over a 4-hour period. A blood sample was taken 5 minutes after the final application of handrub and blood isopropyl alcohol levels were measured. In 9 out of 10 participants, a rise in the blood isopropyl alcohol level was noted at very low levels (the highest observed level was 0.18 mg/dl), much less than the levels achieved with mild intoxication (50 mg/dl).
More recently, Miller and colleagues conducted two studies in which large amounts of an ethanol-based handrub were used very frequently over periods of several hours; they found that blood alcohol levels at the end of the trial periods were below the level of detection.782,995 Brown and colleagues exposed HCWs to intensive use (30 times/hour) of ethanol-and isopropanol-based handrub solutions and found only extremely low concentrations of ethanol in the blood (far too low to cause symptoms) and that blood isopropanol levels were undetectable.783 Similarly, insignificant levels of ethanol were detected in the breath of a few study participants and no trace of isopropanol. Kramer and colleagues studied the intensive use of handrub solutions containing 55–95% ethanol and found that blood ethanol concentrations were far below levels that would result in any noticeable symptoms. For example, the highest median blood ethanol concentration after intensive use of a 95% ethanol hand rub was 20.95 mg/litre, whereas levels of 200– 500 mg/litre are needed to impair fine motor coordination, and levels of 500–1000 mg/litre are needed to impair judgement.784
The presence of ethanol in the blood of human beings can also have other origins. Ethanol can be found in ripe fruit with concentrations of 0.6% or higher as a product of fermentation by natural yeasts.996 A very small amount of ethanol is present as an endogenous substance in the blood, probably resulting from microbial production in the gastrointestinal tract. Studies have shown concentrations ranging from 0 mg/litre to1.6 mg/litre.997,998 In rare instances, much higher endogenous concentrations have been reported (> 800 mg/litre) in Japanese subjects with serious yeast infections; endogenous ethanol appears to have been produced after they had eaten carbohydrate-rich foods.997
Studies to measure both alcohol and acetone levels in subjects chronically exposed to topical alcohols are required to investigate further this issue. Based on work emerging from the United Kingdom, Table I.23.5 lists the risks and recommended mitigation measures.999,1000
Table I.23.5
Summary of risks and mitigation measures concerning the use of alcohol-based hand hygiene preparations.
- Practical issues and potential barriers to optimal hand hygiene practices - WHO ...Practical issues and potential barriers to optimal hand hygiene practices - WHO Guidelines on Hand Hygiene in Health Care
Your browsing activity is empty.
Activity recording is turned off.
See more...