NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Knowles CH, Booth L, Brown SR, et al. Non-drug therapies for the management of chronic constipation in adults: the CapaCiTY research programme including three RCTs. Southampton (UK): NIHR Journals Library; 2021 Nov. (Programme Grants for Applied Research, No. 9.14.)

Non-drug therapies for the management of chronic constipation in adults: the CapaCiTY research programme including three RCTs.
Show detailsSpecific background and rationale
Transanal irrigation, for which there are a variety of commercially available devices, has been rapidly disseminated internationally since about 2000, first in CC patients with neurological injury112,113 and subsequently in other groups CC groups.114,115 Despite a lack of published data other than from small selected case series, TAI is now available on drug tariff (at a cost of ≈ £2500 per patient per annum) and is generally considered to be the next step for patients failing other nurse-led interventions. TAI has permeated the UK market without robust efficacy data and with ongoing concerns regarding longevity of treatment and complications.112,116 Retrospective clinical audit data and review116,117 by the applicants suggest a continued response rate after 1 year of ≈ 50%, with patients thus avoiding or delaying surgical intervention, but an accurate assessment of response rate and acceptability of this intervention required confirmation in a trial. In addition, two alternative systems for delivery of TAI exist: low-volume systems delivering ≈ 70 ml per TAI, and high-volume systems delivering up to 2 l of TAI (although typically only 0.5–1.5 l is required per TAI). The theoretical benefit of higher-volume TAI is greater efficacy – simply put, more washout. However, the low-volume system is cheaper, costing ≈£750 per annum, based on alternate-day use, compared with a cost of £1400–1900 per annum for high-volume TAI, and it may also be less traumatic and more acceptable to patients.
This underpinned our first specific research question:
- In patients with CC who have failed conservative treatment (HT or HTBF), what is the impact on disease-specific QoL of TAI initiated with a low-volume and a high-volume system measured by PAC-QoL score at 3 months’ follow-up?
Transanal irrigation is an invasive therapy that requires, every day or every couple of days, insertion of the device into the anus followed by a period spent filling the rectum with fluid and then evacuating it. It is reasonable to suppose that patients would discontinue therapy that they felt was ineffective or unacceptable, or switch between low-volume and high-volume systems (permissible in the trial design).
This underpinned our second trial-specific research question:
- What is the survival rate of therapy of TAI initiated with a low-volume and a high-volume system and do patients prefer one system to another?
Both research questions were embedded in a single, pragmatic, two-group parallel design (Figure 4) and required a total sample size of 300 patients (150 per group). Patients used one system only (plus defined ‘rescue therapies’) for a minimum of 3 months. After this time point they could switch to the other system if their initial therapy was ineffective/unsatisfactory. This allowed identification of response rates to each system in the short term (3 months) and, thereafter, a comparison between treatment strategies (TAI initiated with low-volume therapy or high-volume therapy) rather than a pure comparison of the two techniques. This was a patient-centred study design aiming to limit the time that patients spent using ineffective therapy without being allowed to try an alternative. Reasons for switching were captured qualitatively.
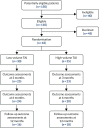
FIGURE 4
The CapaCiTY trial 2 CONSORT flow diagram.
For a full description of this trial, including interventions, trial-specific design procedures and all results and analyses, see Appendix 1. Only the main results and conclusions are summarised in this report.
Results
High-volume compared with low-volume transanal irrigation
First recruitment and intervention took place on 11 November 2015; recruitment ended 30 June 2018. A total of 65 patients (target 300 patients) were randomised from 150 screened (21.7%) from seven sites. Three sites opened but failed to recruit; the remainder randomised between 1 and 33 patients, with approximately half of the patients recruited from secondary care and half recruited from tertiary care. A total of 30 patients were randomised to low-volume TAI and 35 to high-volume TAI (see Figure 4). The primary outcome (PAC-QoL score) was available at baseline and 3 months for only 43 patients.
At 3 months there was a modest reduction in mean PAC-QoL score from 2.4 points to 2.2 points (SD –0.2 points) in the low-volume TAI group and a larger reduction of –0.5 points in the high-volume TAI group (Table 3). Although this difference was not large there was consistency of findings across some of the other outcome measures. For example, global satisfaction score, global improvement score and EQ-VAS score showed greater improvement in the high-volume TAI group. These results were based on an ITT analysis even though two patients crossed over (from low-volume to high-volume TAI) before the 3-month outcome (possibly diluting the difference in effect).
TABLE 3
The CapaCiTY trial 2 primary outcome: PAC-QoL score, low-volume TAI vs. high-volume TAI
Some further evidence of the greater benefit of high-volume TAI could be inferred from the fact that, over the follow-up period (with the majority after 3 months), 18 patients switched from low-volume to high-volume TAI but only six patients switched from high-volume to low-volume TAI, and two of these six switched back again to high-volume TAI.
Despite under-recruitment, patient-level data from the trial still provided the most robust evidence available to inform the pathway of care. Despite differences in initial purchase prices of the basic devices, initiating high-volume TAI did not increase study cost but resulted in higher patient QoL, suggesting a dominant interventional strategy (cost-effective at a WTP threshold of £30,000 per QALY; p = 0.99).
The interventions were generally well tolerated considering the invasive nature of the procedure. A total of 16 out of the 65 patients (25%) reported AEs, with a total of 68 AEs. Of these, 40 AEs (59%) were mild and 20 (29%) were moderate. There were six SAEs, of which three were expected. All resolved with no sequelae.
Survival rate of transanal irrigation therapy
In the absence of a sham control (impossible to devise), and with the inability to compare with standard therapy, it was reasonable to use treatment continuation as a marker of efficacy. The treatment is burdensome, and it can be argued that patients who are not receiving benefit would not continue with it. There was no encouragement from research or clinical staff for patients to continue to use ineffective therapy. The 1-year survival rate of the treatment of 76% (Figure 5) implied significant continued effect. A comparison of survival rate plots of low-volume and high-volume TAI that allows for crossover has not been presented at the time of publication. This analysis is planned but was outside the SAP.118
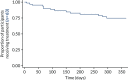
FIGURE 5
The CapaCiTY trial 2 survival rate of treatment as a surrogate of ongoing benefit: Kaplan–Meier plot for time to cessation of treatment.
Conclusions
Taking together the results from clinical, cost-effectiveness and patient experience data, the following conclusions may be drawn while accepting the major caveat of under-recruitment:
- The population studied represented a typical hospital-derived cohort of patients with severe CC that had not responded to conservative therapies.
- We did not carry out statistical significance tests owing to under-recruitment. Despite this, there is preliminary evidence that high-volume TAI may be more effective than low-volume TAI. Although effect sizes were small for both primary and secondary outcome measures (especially global improvement scores), dominant crossover from low-volume to high-volume TAI and health economic analysis point in the same direction of favouring high-volume TAI.
- Survival rate data were suggestive of a persisting benefit in the majority of patients, with three-quarters of patients still using TAI at 12 months. Overall, there were fewer patients on low-volume TAI at 12 months than on high-volume TAI. A survival rate analysis allowing for crossover is yet to be undertaken.
- Some AEs were reported, but, of six SAEs, none was related or life threatening. The most common AEs were rectal bleeding and anal pain (48 events in 16 patients).
- Despite under-recruitment, patient-level data from the trial still provided the most robust evidence currently available to inform the pathway of care. Initiating high-volume TAI did not increase overall cost (despite slightly higher basic device pricing) and resulted in higher patient QoL, suggesting a dominant interventional strategy (cost-effective at a WTP threshold of £30,000 per QALY; p = 0.99).
- Although cost-effectiveness base-case findings were robust to the complete-case and sensitivity analyses conducted, the findings should be treated with caution. Small absolute numbers of patients and high levels of missing data as follow-up progressed placed great reliance on the imputation process and output. As with CapaCiTY trial 1, although substantial efforts were made to deliver a robust imputation process, it not possible to prove that a MAR process has been attained. Actual level of TAI use (the predominant cost) was not directly recorded and was instead approximated from available variables.
- A final consideration in support of high-volume TAI concerns convergence. Health-care costs are similar comparing the two groups, suggesting that differences in health-care costs beyond 12 months are small. However, QoL diverges between groups over 12 months, suggesting that QALY gains may continue to accrue for some time beyond 12 months. Although speculative, any modelling of future cost and benefits would further increase the cost-effectiveness of high-volume TAI.
- Transanal irrigation often caused initial anxiety, was not always easy to learn and was time-consuming. Ongoing staff support was much appreciated, especially as results were not always immediate. Some found it of no benefit, but many persevered and found that, although not necessarily a pleasant experience, it had an impact (sometimes large) on their symptoms and QoL. Staff were possibly more enthusiastic than patients initially, and this enthusiasm was picked up by patients in some cases and helped them to persevere.
Reflections on work programme 3
Poor recruitment (65/300; 22%) was a disappointment and reduced the inferential quality of the study. As well as the usual difficulties as discussed for CapaCiTY trial 1, we failed to recognise an important constituent: that the patients offered TAI had (1) a severe burden of illness and (2) already tried all the usual conservative measures (which had failed). They were resorting to TAI often in a state of desperation. The difficulty, then, was that recruitment to the trial resulted in a delay in treatment while baseline assessments and investigations were undertaken. At the same time, patients were aware that they could access the treatment immediately if they remained outside the trial. With hindsight, it should have been clear that this would lead to difficulties. To overcome this would have required a waiting list for non-trial treatment or a pragmatic approach in which investigations and baseline diaries were dispensed with (as they are dispensed with in emergency medicine trials). Further issues, such as the failure to obtain funding for the therapy in several UK regions, proved beyond our control despite extensive discussion with relevant CRNs and at a national level. Ironically, we received letters stating that funding would not be provided because the therapy lacked evidence from a randomised trial.
Despite gross under-recruitment, we believe that the trial provided some meaningful evidence that high-volume TAI may be more effective than low-volume TAI for the majority of patients. The CI for the effect size appeared to rule out a clinically important difference of 0.4 in favour of low-volume TAI and not in favour of high-volume TAI. This finding promotes a care pathway in which high-volume TAI might be initiated as a default for patients when there is not a compelling reason or preference for low-volume TAI. This is a useful conclusion, but it lacks identification of a more definite means of stratifying patients to one treatment or the other based on baseline phenotype. Ideally, we would have liked to study the efficacy of TAI per se, but there was no way of designing a sham control. A waiting list comparison was considered but rejected because waiting times were not long enough to provide meaningful data. A cohort design was also proposed, but the grant review panel encouraged a RCT. The appeal of considering low-volume and high-volume TAI was the ability to relate responders in the different treatment cohorts to symptoms (urge vs. no urge) and selected physiology results (notably, presence or absence of blunted rectal sensation). We could potentially have learned something about the physiology and mechanism of action of these treatments by doing this. However, with very low recruitment numbers, these additional analyses (covariates of response to each or both therapies) could not be undertaken.
It was interesting to note from the staff interviews that specialist nurses providing the treatment already had strong views that high-volume TAI was a better treatment. It is possible that some bias might have been affected by these opinions.
Research recommendations
It does not seem necessary to recommend repeating the trial on the basis of inadequate recruitment because any future trial will face many of the same challenges, even with the changes proposed here. Furthermore, a more basic question still remains: what is the value of any type of anal irrigation? There have been a significant number of retrospective observational studies and occasional prospective ones. The difficulty would be in establishing a control, as already stated here. It may be possible to develop a prospective cohort study of patients with CC that, while being monitored prospectively, might adopt TAI as an alternative to usual care. A trials within cohorts (TwiCs) design could be considered if patients would consider secondary randomisation against continuing usual care.
The qualitative evidence has been very illuminating and further analysis of this will be undertaken to understand drivers for continuing and discontinuing therapy (to guide patient selection and encourage compliance). As new equipment is developed (including devices activated by mobile telephones) there may be a desire to assess equivalence and safety. In addition, there is a need to understand the merits of contraindications such as mild colitis and pregnancy.
- Work programme 3: trial 2 – pragmatic randomised trial of low-volume compared wi...Work programme 3: trial 2 – pragmatic randomised trial of low-volume compared with high-volume initiated transanal irrigation therapy in adults with chronic constipation - Non-drug therapies for the management of chronic constipation in adults: the CapaCiTY research programme including three RCTs
- unclassified Paramyxoviridae polymerase gene, partial cds.unclassified Paramyxoviridae polymerase gene, partial cds.PopSet: 2243706996PopSet
- OR2M7 olfactory receptor family 2 subfamily M member 7 [Homo sapiens]OR2M7 olfactory receptor family 2 subfamily M member 7 [Homo sapiens]Gene ID:391196Gene
- 391196[uid] AND (alive[prop]) (1)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...