NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Toxicological Profile for Molybdenum. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2020 May.

Toxicological Profile for Molybdenum.
Show details3.1. TOXICOKINETICS
- A number of factors can influence the oral absorption of molybdenum; absorption can range between 40 and 100%. The amount absorbed decreased with increasing doses and was lower when the molybdenum was ingested with a meal. There is evidence for absorption of airborne molybdenum, but no data on the amount absorbed. Molybdenum is poorly absorbed (approximately 0.2%) through the skin.
- Absorbed molybdenum is widely distributed throughout the body, with the highest concentrations found in the kidneys and liver.
- Molybdenum is not metabolized; however, it can undergo oxidation and reduction.
- Molybdenum is primarily excreted in the urine, with lesser amounts excreted in feces.
3.1.1. Absorption
Inhaled molybdenum particles that deposit in the respiratory tract are subject to three general distribution processes: (1) bronchial and tracheal mucociliary transport to the gastrointestinal tract; (2) transport to thoracic lymph nodes (e.g., lung, tracheobronchial, mediastinal); or (3) absorption into blood and/or lymph and transfer to other tissues (e.g., peripheral lymph tissues, liver, kidney). The above processes apply to all forms of deposited molybdenum, although the relative contributions of each pathway and rates associated with each pathway vary with the physical characteristics (e.g., particle size, solubility). Particles having diameters >5 μm are deposited primarily in the upper airways (extrathoracic, tracheobronchial regions) and are cleared from the respiratory tract primarily by mucociliary transport to the gastrointestinal tract (Bailey et al. 2007; ICRP 1994). Smaller particles (≤5 μm) are deposited primarily in the pulmonary region (terminal bronchioles and alveoli). Particles are cleared from the pulmonary region primarily by absorption, lymph drainage, macrophage phagocytosis and migration, and upward mucociliary flow. Dissolved molybdenum is absorbed into blood. The rate of absorption will depend on solubility. ICRP (2012) assigns molybdenum sulfide, oxides, and hydroxides to a “slow” classification in their absorption, which equates to an expected terminal absorption half-time of approximately 19 years (Bailey et al. 2007; ICRP 1994). More soluble forms of molybdenum, such as molybdenum trioxide (MoVIO3), would be expected to undergo more rapid dissolution and absorption.
Quantitative estimates of absorption following inhalation exposure to molybdenum in humans or animals were not identified. Evidence for absorption of molybdenum trioxide is available from inhalation studies on molybdenum trioxide conducted in rodents (Fairhall et al. 1945; NTP 1997). Fairhall et al. (1945) showed distribution to several tissues following inhalation exposure of guinea pigs to molybdenum trioxide. In rats and mice exposed to inhaled molybdenum trioxide (6.7–67 mg molybdenum/m3, 6 hours/day, 5 days/week for 2 years), exposure-dependent increases in blood molybdenum were observed (NTP 1997). The respective blood molybdenum levels in the 0, 6.7, 20, and 67 mg molybdenum/m3 groups were 0.221, 0.800, 1.774, and 6.036 μg/g in male rats, 0.059, 0.355, 0.655, and 2.411 μg/g in female rats, 0.102, 0.208, and 0.770 μg/g in male mice (no data were available for controls), and 0.043, 0.066, 0.198, and 0.523 μg/g for female mice.
Absorption of ingested molybdenum has been studied in human adults and infants (Cantone et al. 1993, 1997; Engel et al. 1967; Giussani et al. 1998, 2006, 2007; Novotny and Turnlund 2006, 2007; Robinson et al. 1973; Sievers et al. 2001a, 2001b; Turnlund et al. 1995a, 1995b; Werner et al. 1998; Yoshida et al. 2006). These studies fall into two general categories: mass balance studies and bioavailability studies. Mass balance studies estimate the absorption fraction from measurements of the difference between the ingested dose of molybdenum and fecal excretion (the difference being net absorption). Bioavailability studies estimate the absorption fraction from measurements of the plasma concentration of molybdenum following the oral dose. These methods provide estimates of net absorption in that absorbed molybdenum that is excreted into the gastrointestinal tract (e.g., biliary excretion) may not be accurately quantified from mass balance or bioavailability estimates. However, both approaches have been facilitated by the use of stable isotopes of molybdenum (95Mo, 96Mo), which allow measurement of plasma and excretion kinetics following concurrent intravenous and oral dosing. The use of stable isotopes also allows measurement of the administered molybdenum in plasma and excreta, distinct from background sources of molybdenum derived from other sources and preexisting body stores. By incorporating elimination kinetics data into mathematical models that include parameters representing absorption and fecal excretion of absorbed molybdenum, the absorption fraction can be estimated. In most reported stable isotope studies, the exact form of molybdenum administered was not reported. However, the isotope dosing material was typically prepared from an acid dissolution of metallic molybdenum (Mo0). The resulting material dissolved in water most likely was a mixture of soluble molybdate anion (MoVIO42−) and other soluble molybdenum oxide hydrates.
Balance and bioavailability studies conducted in humans have shown that the fraction of ingested molybdenum that is absorbed depends on numerous factors, including molybdenum dose level, fasting, diet, and nutritional status. Absorption was estimated to be 80–100% in replete fasted adults who ingested molybdenum dissolved in water or in a beverage (Giussani et al. 2006; Novotny and Turnlund 2006, 2007; Turnlund et al. 1995a). Absorption was 80–100% following a single dose of 20–40 μg molybdenum/kg dissolved in water and decreased with increasing dose level; absorption was 60% after a dose of 60 μg molybdenum /kg (Giussani et al. 2006). Absorption was lower when molybdenum was ingested with a meal (40–60%), when dissolved in black tea (20%), or when incorporated into vegetables cultivated with 96Mo (30–60%), compared to when ingested without a meal (80–100%) (Giussani et al. 2006; Werner et al. 1998). Absorption was lower when molybdenum was incorporated into the diet (83%) compared to when it was administered in a beverage (90–94%) (Novotny and Turnlund 2007). Absorption appears to be affected by dietary molybdenum intake and molybdenum nutritional status. The absorption fraction was 90% in adults fed a diet containing 22 μg/day (approximately 0.3 μg molybdenum/kg/day), compared to 94% when fed a diet containing 467 μg molybdenum/day (approximately 7 μg molybdenum/kg/day) (Novotny and Turnlund 2007). Absorption in infants (gestational age 30–39 weeks) was 96–99% when a stable isotope of molybdenum was mixed with breast milk or infant formula (Sievers et al. 2001a, 2001b).
Long-term diet mass balance studies, without the aid of stable isotopes, have been conducted in adults and children (Engel et al. 1967; Robinson et al. 1973; Tipton et al. 1966). Because these studies cannot distinguish between the ingested dose of molybdenum and molybdenum excreted from body stores, these studies will underestimate the absorption fraction. A 50-week balance study conducted in two adult males (age 23 and 25 years) found absorption to range from 60 to 80% (Tipton et al. 1966). A 3-week balance study conducted in women (age 19–21 years) found absorption to range from 40 to 70% (Robinson et al. 1973). An 8-day balance study conducted in women (age 18–23 years) found absorption to range from 72 to 84% (Yoshida et al. 2006). Balance studies (18–30 days) conducted in female children (age 6–10 years) estimated the absorption fraction from diet to range from 67 to 85% (Engel et al. 1967).
Measurements of the time course of plasma molybdenum following oral doses of molybdenum indicate that absorption is relatively rapid, with peak concentrations in plasma attained within 100 minutes of dosing (Giusanni et al. 2006; Novotny and Turnlund 2007).
Studies of absorption and elimination kinetics conducted in swine provide estimates of absorption of ingested molybdenum. Based on cumulative urinary and fecal excretion measurements in swine dosed with a stable isotope of molybdenum, absorption was estimated to be between 80 and 90% (Bell et al. 1964). Studies conducted in rats have shown that tetrathiomolybdate (MoVIS42−) is more poorly absorbed when ingested in the diet; approximately 21% was absorbed when the copper content of the diet was 8 ppm (Mills et al. 1981b).
Roper (2009) evaluated the in vitro percutaneous absorption of sodium molybdate through human skin. Following an 8-hour application of 3.97 or 19.83 mg molybdenum/mL, the potentially absorbable doses were 0.21 and 0.16%, respectively.
Mechanisms that participate in absorptive transport of molybdenum in the gastrointestinal tract have not been characterized. Molybdate (MoO42−) and sulfate (SO42−) show mutually competitive inhibition for absorptive transport in rat small intestine, suggesting involvement of a common transporter for both anions (Cardin and Mason 1975, 1976). This transporter may be the Na+/SO42− symporter (NaS1 or SLC13A1) expressed in rodent small intestine and renal proximal tubule (Markovich and Aronson 2007; Murer et al. 1994). In humans, NaS1 is expressed in kidney but not small intestine, suggesting that mechanisms of absorptive transport in humans may be different from that of rodents (Lee at al. 2000).
3.1.2. Distribution
Very little information on the distribution on molybdenum following inhalation exposure is available. Following exposure of guinea pigs to inhaled molybdenum trioxide (150–300 mg/m3, 1 hour/day, 5 days/week for 5 weeks), molybdenum was distributed to the lungs, liver, kidneys, and bone (Fairhall et al. 1945). Tissue levels decreased by approximately 20% in the 2-week postexposure period.
Absorbed molybdenum distributes to various tissues. Human autopsy studies have found that the kidney and liver have the highest amounts of molybdenum (Iyengar et al. 1978; Schroeder et al. 1970; Sorensen and Archambault 1963; Sumino et al. 1975; Tipton and Cook 1963; Tipton et al. 1965; Yoo et al. 2002; Zeisler et al. 1988). Based on a review of these data, Giussani (2008) estimated liver and kidney molybdenum concentrations to be approximately 0.5–1.5 μg molybdenum/g wet in liver (700–2,700 μg) and 0.2–0.4 μg molybdenum/g wet in kidney (55–120 μg). Coughtrey and Thorne (1983) reported relatively high concentrations (56 μg molybdenum/g) in bone, based on their recalculation of measurements of molybdenum in bone ash reported in Nusbaum et al. (1965) and Iyengar et al. (1978). However, these results are not supported by other studies (previously cited) and have been attributed to overestimation of tissue concentrations measured by arc emission spectrometry in the Nusbaum et al. (1965) and Iyengar et al. (1978) studies (Giussani 2008).
Results of studies in rats and guinea pigs exposed to oral molybdenum show that molybdenum is widely distributed (Bibr et al. 1977; Howell et al. 1993; Murray et al. 2014b; Pandey et al. 2002). Generally, the highest molybdenum tissue concentration is observed in the kidney. Molybdenum also is distributed to liver, spleen, brain, lungs, heart, bone, muscles, testes, epididymides, seminal vesicles, prostate, blood cells, and plasma.
Maternal-Fetal Transfer. Results of studies in humans and animals show that molybdenum is distributed to the fetus. In humans, maternal and fetal cord blood levels obtained from 33 maternal-fetal pairs at parturition show similar molybdenum levels (maternal: 1.44±0.75 μg/L, mean±standard deviation [SD]; fetal: 1.44±0.89 μg/L) (Bougle et al. 1989). Molybdenum concentrations in venous cord blood (flowing from the placenta to the fetus; 0.7±0.2 μg/L, mean±SD) were slightly higher than in arterial cord blood (flowing from the fetus to the placenta; 0.6±0.2 μg/L), indicating fetal retention of molybdenum (Krachler et al. 1999); the study did not evaluate whether there was a statistical difference between the molybdenum concentrations in venous and arterial blood.
Gestational exposure of rats to ammonium molybdate and thiomolybdate shows distribution of molybdenum to fetal liver, kidney, bone, and brain (Howell et al. 1993). Levels in liver, kidney, and bone were similar, with lower levels in brain. In rats, dose-dependent increases in placental and maternal liver content of molybdenum were observed following gestational exposure to molybdenum (sodium molybdate) in drinking water (5–100 mg molybdenum/L; equivalent to approximately 0.76–15 mg/kg/day, based on intermediate exposure to nonpregnant female rats) over the full dose range (Fungwe et al. 1989). However, neonatal whole-body levels of molybdenum reached a plateau at drinking water concentrations ≥50 mg/L (Fungwe et al. 1989). Results suggest that molybdenum levels in the fetus reach steady state more rapidly than in dams.
Maternal-Infant Transfer. Several studies have measured molybdenum in breast milk (Anderson 1992; Aquilio et al. 1996; Biego et al. 1998; Bougle et al. 1988; Casey and Neville 1987; Dang et al. 1984; Friel et al. 1999a; Krachler et al. 1998; Wappelhorst et al. 2002); the mean concentrations ranged from 0.02 to 24 μg/L. Breast milk concentrations are highest at the start of breast feeding and then decline (EFSA 2013). In the only study comparing maternal intake to breast milk levels, Wappelhorst et al. (2002) did not find a correlation between breast milk concentrations of molybdenum and maternal molybdenum intake. The mean concentration in breast milk was 72 μg/L and the mean maternal intake was 132 μg/day.
Bacteria and eukaryotes express cell membrane molybdate transporters, one of which (MoT2) also appears to be expressed in humans (Tejada-Jimenez et al. 2007, 2011). In eukaryotes, this transporter participates in the delivery of molybdate into cells for incorporation into molybdopterin-cofactor (Moco), the biologically active prosthetic group in molybdenum-dependent enzymes (Schwarz et al. 2009). MoT2 transport of molybdate is inhibited by sulfate, suggesting a common carrier for molybdate and sulfate. A sulfate-insensitive oxalate-sensitive molybdate transporter has been described in mammalian MEK-293T cells grown in culture (Nakanishi et al. 2013). Uptake of molybdate into human red blood cells involves participation of the Cl−1/HCO3−1 anion exchanger (Gimenez et al. 1993).
3.1.3. Metabolism
Molybdenum exists in several valence states and may undergo oxidation and reduction. The primary form of molybdenum that interacts with enzyme systems is MoVI, as the molybdate anion (MoVIO22−) (Nakanishi et al. 2013). After molybdate is taken into a cell, it is incorporated into a molybdopterin to form molybdenum cofactor (Moco). Moco is a sulfur-molybdate complex that forms the prosthetic group in molybdenum-dependent enzymes (Mendel and Kruse 2012; Schwarz et al. 2009). Given that Moco is extremely sensitive to oxidation, it is believed that it is bound to a Moco-binding protein in the cell (Mendel and Kruse 2012). This stored Moco would be readily available to meet the cell’s demand for molybdenum enzymes. Molybdate forms complexes with copper and binds to plasma proteins as a copper-molybdenum-sulfur (Cu-Mo-S) complex (Nederbragt 1980, 1982).
3.1.4. Excretion
Studies investigating the elimination and excretion of molybdenum following inhalation exposure were not identified.
Absorbed molybdenum is excreted in urine and feces in humans. Urine is the dominant excretion route, accounting for the excretion of approximately 75–90% of the absorbed dose (Giussani 2008; Novotny and Turnlund 2007). The fraction excreted in urine increases with increasing dietary intake (Novotny and Turnlund 2007). Urine also is the dominant excretory route for absorbed molybdenum in swine. Following an oral dose, approximately 90% of the dose was excreted in urine (Bell et al. 1964). To measure urinary and fecal excretion of molybdenum, Turnlund et al. (1995a, 1995b) exposed four healthy adult males to various doses of a radioactive isotope of molybdenum (24–1,378 μg 100Mo/day) and administered intravenous doses of stable isotope of molybdenum (33 μg 97Mo). Six days after exposure to 100Mo in the diet, 17.8% of the 100Mo label was excreted in the urine at the lowest dose tested (total molybdenum dose of 24 μg/day). As the molybdenum dose increased, the amount excreted in the urine also increased; at the highest dose (1488 μg/day), 82.1% of the 100Mo was excreted in the urine. A similar pattern of urinary excretion was found when 97Mo was measured: 32.7% of the label at 24 μg/day and 86.7% at 1,488 μg/day. The percentage of the molybdenum dose excreted in the feces decreased with increasing doses. At the lowest dose tested, 9.4% of the 100Mo dose was excreted in the feces; at the highest dose, 7.5% of the 100Mo dose was excreted in the feces. In contrast, no consistent pattern of fecal 97Mo excretion was found. When total molybdenum excretion was measured, the study found that 41% was excreted in feces and 59% was excreted in urine at the lowest dose tested and 6% was excreted in feces and 94% was excreted in urine at the highest dose tested. Fecal excretion of absorbed molybdenum is thought to result from biliary secretion. Studies conducted in bile-duct cannulated rats have shown that, following an intravenous dose of Mo[V] or Mo[VI], approximately 1% of the molybdenum dose was secreted into bile in a period of 4 hours (Lener and Bibr 1979).
The rate of elimination of molybdenum from plasma has been studied in human clinical studies (Cantone et al. 1997; Rosoff and Spencer 1964; Thompson et al. 1996; Werner et al. 2000). Elimination is approximately biphasic, with mean half-times of 30 minutes and 6.6 hours (Giussani 2008).
The whole-body elimination rate in rats is dose-dependent (Bibr and Lener 1973). Following oral administration of Mo[VI] at doses <3 μg molybdenum/kg, elimination was mono-phasic with a half-time of approximately 47 hours. Following doses >3 μg molybdenum/kg, the rate of elimination increased, with an increasing proportion of elimination contributed by a fast phase having a half-time of 6 hours.
Mechanisms that participate in the renal excretion of molybdenum have not been characterized. In sheep, reabsorption of filtered molybdate (MoO42−) is saturable, which results in an increase in the fraction of filtered molybdate excreted as the plasma molybdate concentration increases and approaches or exceeds the tubular maximum (Ryan et al. 1987). In sheep and rat kidney, sodium-dependent reabsorptive transport of molybdate (MoO42−) and sulfate (SO4−2) exhibit mutual inhibition (Ryan et al. 1987). This is consistent with participation of the Na+/SO42− symporter (NaS1 or SLC13A1) in the reabsorption of molybdate. This symporter is also expressed in the human renal proximal tubule (Markovich and Aronson 2007; Murer et al. 1994).
3.1.5. Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models
PBPK models use mathematical descriptions of the uptake and disposition of chemical substances to quantitatively describe the relationships among critical biological processes (Krishnan et al. 1994). PBPK models are also called biologically based tissue dosimetry models. PBPK models are increasingly used in risk assessments, primarily to predict the concentration of potentially toxic moieties of a chemical that will be delivered to any given target tissue following various combinations of route, dose level, and test species (Clewell and Andersen 1985). Physiologically based pharmacodynamic (PBPD) models use mathematical descriptions of the dose-response function to quantitatively describe the relationship between target tissue dose and toxic endpoints.
Several multi-compartmental models of the kinetics of molybdenum in humans have been developed (Giussani 2008; Giussani et al. 1998, 2000; Novotny and Turnlund 2007; Thompson et al. 1996). The latest of these are the Giussani (2008) and Novotny and Turnlund (2007) models. Both models yield similar predictions when applied to the same dosing scenarios (Giusanni 2008). The Giussani (2008) model has been adopted for use by the International Commission on Radiological Protection (ICRP) and is described in this section.
Giussani (2008) Model
Giussani (2008) developed a model of molybdenum kinetics in humans. The structure of the model is shown in Figure 3-1 and parameter values are presented in Table 3-1. Data used to derive and evaluate the model are described in Giusanni (2008) and included human clinical studies in which subjects were administered intravenous or oral doses of stable isotopes of molybdenum (Giusanni et al. 2006, 2007; Novotny and Turnlund 2006, 2007; Turnlund et al. 1995a; Werner et al. 1998, 2000). The Giussani (2008) model has been adopted for use by the ICRP and is described in this section.
The model consists of two central compartments representing extracellular fluids (ECF) and compartments representing liver, kidney (two subcompartments), and a lumped compartment representing all other tissues. All transfers of molybdenum between compartments are first order and governed by first-order rate coefficients (day−1). The two ECF compartments represent fast and slow transfer pathways out of the ECF and were based on studies conducted in humans, which provide evidence for multi-phasic clearance of molybdenum from plasma (Giussani et al. 2007; Werner et al. 2000). The half-times for the two ECF compartments are approximately 30 minutes for ECF1 and 280 minutes for ECF2. Transfers from the fast compartment (ECF1) are to liver, kidney, and urine. Transfers from the slow compartment (ECF2) are to urine, kidney, and other tissues; the slow compartment also receives molybdenum from the liver. Retention half-times in tissues are 41 days for liver, 14.5 days for kidney, and 21.5 days for the other tissue compartment. Excretion of absorbed molybdenum occurs in urine (88%) and transfer from liver to the gastrointestinal tract (12%).
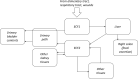
Figure 3-1
The Proposed Systemic Model for Molybdenum Radionuclides. ECF = extracellular fluid
Table 3-1
Transfer Rates (Day-1) for the Molybdenum Model.
The model can simulate absorption from the gastrointestinal tract and respiratory tract. The absorption fraction for the gastrointestinal pathway uses an absorption fraction of 0.9 for molybdenum ingested in liquids and 0.6 for molybdenum ingested in the diet. The model predicts a steady state for constant dietary intake of molybdenum in adults, in which approximately 52% of the molybdenum body burden is in liver, 3% is in kidney, 45% is in other tissues, 53% of the daily dose is excreted in urine, and 47% of the daily dose is excreted in feces (Giussani 2008). The model is constructed to be able to interface with output from the ICRP Human Respiratory Tract Model (HRTM) (Bailey et al. 2007; ICRP 1994). The inputs to the Giussani (2008) model from the HRTM would be simulated transfers of molybdenum to the gastrointestinal tract (mucociliary transfer) and to blood (absorption from the respiratory tract), depending on the particle size and solubility of the inhaled molybdenum and other physiological factors (e.g., age, activity).
Novotny and Turnlund (2007) Model
The major difference between the structures of the Giussani (2008) and Novotny and Turnlund (2007) models is that the Novotny and Turnlund (2007) model has a single lumped compartment representing all tissues outside of the vasculature. The Novotny and Turnlund (2007) model illustrated in Figure 3-2 has two configurations: an intravenous configuration, which has two plasma compartments, representing fast and slower clearance, and an oral configuration, which has a single plasma compartment. Molybdenum exchanges between plasma and a lumped tissue compartment. Urinary excretion is represented as a direct transfer from plasma. Absorbed molybdenum is also transferred to the gastrointestinal tract.
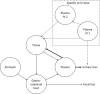
Figure 3-2
Diagram of the Compartment Molybdenum Model.
Novotny and Turnlund (2006, 2007) conducted mass balance studies with subjects who ingested stable isotopes of molybdenum in the context of varying dietary intakes of molybdenum (22–1,490 μg molybdenum/day) and found that certain model parameters were dependent on dietary intake. These included, in association with increasing dietary intake, increases in the first-order rate coefficients for gastrointestinal absorption and urinary excretion and a decrease in the rate coefficients for transfer from plasma to tissues. The largest adjustments were needed to simulate molybdenum kinetics in subjects who consumed >121 μg molybdenum/day and included a 70% decrease in the coefficient for transfer of molybdenum from plasma to tissues and a 660% increase in the rate coefficient for transfer from plasma to urine. These results suggest that high molybdenum intakes (>121 μg molybdenum/day) result in physiological adaptations that increase excretion of absorbed molybdenum (Novotny and Turnlund 2007).
3.1.6. Animal-to-Human Extrapolations
There are limited data to evaluate potential differences in the toxicity of molybdenum between laboratory animals and humans. Most of the available oral exposure studies were conducted in rats, and human data are mostly limited to a small number of cross-sectional studies. Within laboratory animal species, some differences have been observed between rats and rabbits, with rabbits appearing to be more sensitive than rats. However, the studies are not directly comparable due to differences in the copper content and other dietary constituents. In the absence of data to the contrary, it is assumed that the toxicity of molybdenum will be similar across species (excluding ruminants).
3.2. CHILDREN AND OTHER POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE
This section discusses potential health effects from exposures during the period from conception to maturity at 18 years of age in humans. Potential effects on offspring resulting from exposures of parental germ cells are considered, as well as any indirect effects on the fetus and neonate resulting from maternal exposure during gestation and lactation. Children may be more or less susceptible than adults to health effects from exposure to hazardous substances and the relationship may change with developmental age.
This section also discusses unusually susceptible populations. A susceptible population may exhibit different or enhanced responses to certain chemicals than most persons exposed to the same level of these chemicals in the environment. Factors involved with increased susceptibility may include genetic makeup, age, health and nutritional status, and exposure to other toxic substances (e.g., cigarette smoke). These parameters can reduce detoxification or excretion or compromise organ function.
Populations at greater exposure risk to unusually high exposure levels to molybdenum are discussed in Section 5.7, Populations with Potentially High Exposures.
There are limited data on the toxicity of molybdenum in children. In studies in rat pups maintained on a caries-promoting diet, administration of 50 mg molybdenum/kg/day as sodium molybdate resulted in an increase in buccal enamel lesions (Hunt and Navia 1975), but exposure to 8 mg molybdenum/kg/day did not result in increases in dental caries (Van Reen et al. 1962). Arrington and Davis (1953) exposed young (6 weeks of age at the start of the study) and mature rabbits to sodium molybdate in the diet for 30–84 days. Marked muscular/skeletal effects were observed in the young rabbits, but not in the mature rabbits. Since the investigators did not provide information on dietary intake, it is difficult to make direct comparisons across the studies.
An observational study did not find an association between maternal urinary molybdenum levels and newborn body weight or infant mental development (Shirai et al. 2010). However, another study did find an association between third-trimester maternal urinary molybdenum levels and infant psychomotor development indices (Vazquez-Salas et al. 2014). Two rat studies in which the copper content of the diet was adequate did not find significant alterations in fetal growth, survival, or malformations at maternal doses of 40 mg molybdenum/kg/day (Murray et al. 2014b, 2019). However, a third study reported decreases in growth and number of live fetuses in the offspring of male rats administered 14 mg molybdenum/kg as sodium molybdate 5 days/week for 60 days prior to mating with unexposed females (Pandey and Singh 2002).
Studies in laboratory animals have found that maintenance on a copper-deficient diet enhances the toxicity of molybdenum (Brinkman and Miller 1961; Franke and Moxon 1937; Johnson and Miller 1961; Sasmal et al. 1968; Valli et al. 1969; Van Reen 1959; Widjajakuma et al. 1973). Administration of additional copper results in a reversal of the adverse effect (Arrington and Davis 1953). Thus, individuals with low copper intakes may be unusually susceptible to the toxicity of molybdenum. Additionally, individuals with high dietary molybdenum intake, including individuals taking supplements containing high levels of molybdenum, may be at an increased risk from exposure to high levels of molybdenum in the environment.
Studies in rats suggest that the toxicity of molybdenum may be increased in animals maintained on a low protein diet. The magnitudes of the decrease in body weight gain (Bandyopadhyay et al. 1981; Cox et al. 1960) and decreases in femur breaking strength (Fejery et al. 1983) were greater in rats exposed to a low protein diet, as compared to those maintained on a diet with sufficient protein.
3.3. BIOMARKERS OF EXPOSURE AND EFFECT
Biomarkers are broadly defined as indicators signaling events in biologic systems or samples. They have been classified as biomarkers of exposure, biomarkers of effect, and biomarkers of susceptibility (NAS/NRC 1989).
A biomarker of exposure is a xenobiotic substance or its metabolite(s) or the product of an interaction between a xenobiotic agent and some target molecule(s) or cell(s) that is measured within a compartment of an organism (NAS/NRC 1989). The preferred biomarkers of exposure are generally the substance itself, substance-specific metabolites in readily obtainable body fluid(s), or excreta. Biomarkers of exposure to molybdenum are discussed in Section 3.3.1. The National Report on Human Exposure to Environmental Chemicals provides an ongoing assessment of the exposure of a generalizable sample of the U.S. population to environmental chemicals using biomonitoring (see http://www.cdc.gov/exposurereport/). If available, biomonitoring data for molybdenum from this report are discussed in Section 5.6, General Population Exposure.
Biomarkers of effect are defined as any measurable biochemical, physiologic, or other alteration within an organism that (depending on magnitude) can be recognized as an established or potential health impairment or disease (NAS/NRC 1989). This definition encompasses biochemical or cellular signals of tissue dysfunction (e.g., increased liver enzyme activity or pathologic changes in female genital epithelial cells), as well as physiologic signs of dysfunction such as increased blood pressure or decreased lung capacity. Note that these markers are not often substance specific. They also may not be directly adverse, but can indicate potential health impairment (e.g., DNA adducts). Biomarkers of effect caused by molybdenum are discussed in Section 3.3.2.
A biomarker of susceptibility is an indicator of an inherent or acquired limitation of an organism’s ability to respond to the challenge of exposure to a specific xenobiotic substance. It can be an intrinsic genetic or other characteristic or a preexisting disease that results in an increase in absorbed dose, a decrease in the biologically effective dose, or a target tissue response. If biomarkers of susceptibility exist, they are discussed in Section 3.2, Children and Other Populations that are Unusually Susceptible.
3.3.1. Biomarkers of Exposure
Molybdenum levels can readily be measured in tissues, body fluids, and excreta. Dose-related increases in serum molybdenum levels were observed in rats and mice exposed via inhalation to molybdenum trioxide for 2 years (NTP 1997). In a study examining the relationship between plasma molybdenum levels and dietary intake, Turnland and Keyes (2004) reported a baseline plasma molybdenum level of 8.2±0.5 nmol/L; 25 days after the subjects were maintained on a low molybdenum diet (22 μg/day), the plasma molybdenum level was 5.1±0.5 nmol/L. Although a significant correlation between plasma molybdenum and dietary molybdenum was observed, comparison between plasma molybdenum levels at different dietary intakes showed that a significant increase in plasma molybdenum was not observed until the dietary intake exceeded 460 μg/day (6.6 mg/kg/day) and that tripling the intake resulted in a doubling of the plasma molybdenum levels. Urinary molybdenum levels were also significantly correlated to dietary intakes (Turnland and Keyes 2004) and appeared to be more responsive to changes in dietary intake. At all dietary concentrations, the urinary molybdenum levels were slightly lower than the dietary intakes (Turnland and Keyes 2004). The investigators concluded that plasma molybdenum levels are an indicator of dietary intake, but urinary levels had a more direct relationship with dietary intake.
Molybdenum levels were measured in urine samples collected during the NHANES study. The geometric mean urinary molybdenum levels in the United States in 2011–2012 was 37.1 μg/L and the creatinine-corrected value was 42.0 μg/g creatinine (CDC 2015); see Section 5.6 for additional information.
Although several studies have reported molybdenum levels in hair samples (DiPietro et al. 1989; Nagra et al. 1992; Paschal et al. 1989), no relationship between molybdenum exposure and hair levels has been established. Furthermore, Miekeley et al. (1998) demonstrated large interlaboratory differences in the molybdenum levels measured in hair.
3.3.2. Biomarkers of Effect
No biomarkers to characterize effects caused by molybdenum have been identified.
3.4. INTERACTIONS WITH OTHER CHEMICALS
The interaction between copper and molybdenum has been well-established in animals. The levels of copper in the diet have been shown to influence the toxicity of molybdenum. Marked toxicity has been reported in studies in which the copper content of the diet was inadequate. Observed effects included mortality in rabbits (Valli et al. 1969; Widjajakuma et al. 1973), marked decreases in body weight gain and weight loss in rats and rabbits (Brinkman and Miller 1961; Johnson and Miller 1961; Sasmal et al. 1968; Valli et al. 1969; Van Reen 1959), and anemia in rats and rabbits (Brinkman and Miller 1961; Franke and Moxon 1937; Gray and Daniel 1954; Johnson et al. 1969; Valli et al. 1969). In general, these effects (or the severity of the effects) have not been observed when the diet contains an adequate level of copper (Mills et al. 1958; Murray et al. 2014a; Pandey and Singh 2002; Peredo et al. 2013). Exposure to high levels of copper has been shown to reduce the toxicity of molybdenum. Administration of high doses of copper to moribund rabbits resulted in a return to normal body weight gain and increases in hemoglobin levels within 2–3 weeks (Arrington and Davis 1953). Lyubimov et al. (2004) showed that administration of a high dose of copper prevented the molybdenum-induced testicular toxicity observed in rats fed a copper-adequate diet. Similarly, in an environmental exposure study of men at infertility clinics, Meeker et al. (2008) found a greater decline in sperm concentration in men with high molybdenum blood levels and copper blood levels below the median, as compared to when the men were not stratified by blood copper levels.
Kinetic studies have demonstrated differences in plasma, liver, and kidney copper and molybdenum concentrations in rats fed copper-deficient, copper-adequate, and copper-excessive diets (Nederbragt 1980). Administration of molybdenum results in increases in plasma, liver, and kidney copper levels in rats fed a copper-adequate diet (Nederbragt 1980); the increases in copper appear to be molybdenum-dose-related. Most of the rise in plasma copper levels was in the tightly-bound fraction, which is likely to be poorly available for copper metabolism. Excess copper in the diet resulted in a smaller increase in copper concentrations in plasma, liver, and kidneys and molybdenum concentrations in the liver and kidney, as compared to levels in rats fed a copper-adequate diet. Similarly, lower rises in liver copper and molybdenum and kidney molybdenum levels were observed in rats fed a copper-deficient and high-molybdenum diet, as compared to the copper-adequate diet. At the lowest molybdenum dose, kidney molybdenum levels were higher in the copper-deficient groups. In another study (Nederbragt 1982), kidney levels of copper and molybdenum were 5 and 3 times higher, respectively, in the copper-adequate groups as compared to the copper-deficient group. Two human studies have also evaluated the effect of molybdenum on copper levels. In one study, increases in serum and urine copper levels were found following a 10-day exposure to 0.022 mg molybdenum/kg/day (Deosthale and Gopalan 1974). Another study found no significant alterations in serum copper levels in humans exposed to 0.0003–0.02 mg molybdenum/kg/day for 24 days (Turnlund and Keys 2000).
- TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS - Tox...TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS - Toxicological Profile for Molybdenum
Your browsing activity is empty.
Activity recording is turned off.
See more...