NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Toxicological Profile for Chlordane. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2018 Feb.

Toxicological Profile for Chlordane.
Show detailsSection 104(i)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the Administrator of EPA and agencies and programs of the Public Health Service) to assess whether adequate information on the health effects of chlordane is available. Where adequate information is not available, ATSDR, in conjunction with NTP, is required to assure the initiation of a program of research designed to determine the adverse health effects (and techniques for developing methods to determine such health effects) of chlordane.
Data needs are defined as substance-specific informational needs that, if met, would reduce the uncertainties of human health risk assessment. This definition should not be interpreted to mean that all data needs discussed in this section must be filled. In the future, the identified data needs will be evaluated and prioritized, and a substance-specific research agenda will be proposed.
6.1. Information on Health Effects
Studies evaluating the health effects of inhalation, oral, and dermal exposure of humans and animals to chlordane that are discussed in Chapter 2 are summarized in Figure 6-1. The purpose of this figure is to illustrate the information concerning the health effects of chlordane. The number of human and animal studies examining each endpoint is indicated regardless of whether an effect was found and the quality of the study or studies.
Although most occupational exposure scenarios and residential exposures following application of chlordane to homes for termite control likely involved inhalation and dermal exposure, these exposure scenarios were classified as inhalation exposures and are presented as such in Figure 6-1. Studies that evaluated potential health effects in humans based on blood levels of chlordane, chlordane constituents, and/or chlordane metabolites were assumed to have involved oral exposure in cases where information regarding the likelihood of inhalation exposure was not available.
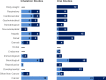
Figure 6-1
Summary of Existing Health Effects Studies on Chlordane By Route and Endpoint. Hepatic, neurological, and cancer endpoints were the most studied endpoints The majority of the studies examined oral exposure in animals (versus humans)
6.2. Identification of Data Needs
Missing information in Figure 6-1 should not be interpreted as a “data need”. A data need, as defined in ATSDR’s Decision Guide for Identifying Substance-Specific Data Needs Related to Toxicological Profiles (ATSDR 1989a), is substance-specific information necessary to conduct comprehensive public health assessments. Generally, ATSDR defines a data gap more broadly as any substance-specific information missing from the scientific literature.
Acute-Duration MRLs. Sufficient information was not available on the health effects of chlordane to derive an MRL for acute-duration inhalation exposure. Lethality was noted at the same exposure levels at which other effects were observed. Additional animal studies should be designed to evaluate nonlethal effects associated with acute-duration inhalation exposure, particularly if human populations can be identified with potential for significant exposure to chlordane.
Intermediate-Duration MRLs. The database of information regarding effects of intermediate-duration exposure of laboratory animals to chlordane was considered sufficient for inhalation and oral exposure to derive intermediate-duration inhalation and oral MRLs. It does not appear necessary to perform additional intermediate-duration inhalation or oral studies in animals.
Chronic-Duration MRLs. The database of information regarding effects of intermediate- and/or chronic-duration exposure of laboratory animals to chlordane was considered sufficient for inhalation and oral exposure to derive chronic-duration inhalation and oral MRLs. The chronic-duration inhalation MRL is based on results from an intermediate-duration inhalation study; an uncertainty factor of 10 was applied to account for extrapolation from an intermediate- to chronic-duration exposure scenario. A well-designed chronic-duration inhalation study in animals could serve as the basis for deriving a chronic-duration inhalation MRL, particularly if present human populations can be identified with potential for significant chronic-duration inhalation exposure to chlordane. Well-designed, chronic-duration oral studies in animals provide adequate information to support the chronic-duration oral MRL.
Health Effects.
- Respiratory. Respiratory effects have been associated with exposure to chlordane among humans (EPA 1980a; Menconi et al. 1988) and in inhalation studies of animals (EPA 1987f; Khasawinah et al. 1989). No signs of respiratory effects were observed in animal studies that employed oral or dermal exposure routes (EPA 1985a; Khasawinah and Grutsch 1989a, 1989b; NCI 1977; Truhaut et al. 1974, 1975). People living in areas where chlordane may be detected in air should be monitored for possible exposure-related effects on the respiratory system.
- Cardiovascular. Tachycardia was among the symptoms attributed to chlordane exposure in a compilation of cases and personal reports of accidental human inhalation exposure to high concentrations of chlordane (EPA 1980b). No signs of chlordane-induced cardiovascular effects were seen in inhalation or oral animal studies that evaluated the cardiovascular system (EPA 1985a; Khasawinah and Grutsch 1989a, 1989b; NCI 1977; Truhaut et al. 1974, 1975). People living in areas where chlordane may be detected should be monitored for possible exposure-related effects on the cardiovascular system.
- Gastrointestinal. Gastrointestinal effects (cramps, diarrhea, nausea) were a consistent observation in a compilation of cases and personal reports of accidental human inhalation exposure to high concentrations of chlordane (EPA 1980a). NIOSH (1984a) also reported gastrointestinal symptoms (nausea, diarrhea) in 4 of 13 humans within 4 days of inhalation and/or dermal exposure as a result of 1% chlordane being spilled in a subterranean library room. Atrophy of gastric mucosa was reported in hamsters following single gavage dosing of chlordane technical at 1,200 mg/kg (Truhaut et al. 1974, 1975). No signs of chlordane-induced gastrointestinal effects were seen in other inhalation or oral animal studies that evaluated the gastrointestinal system (EPA 1985a; Khasawinah and Grutsch 1989a, 1989b; NCI 1977; Truhaut et al. 1974, 1975). Human populations are not likely to be exposed to chlordane levels high enough to elicit adverse gastrointestinal effects.
- Hematological. Limited information is available regarding potential chlordane-induced effects on hematological endpoints. Cases of blood dyscrasia have been observed in persons exposed to chlordane or heptachlor (Epstein and Ozonoff 1987; Infante et al. 1978). However, limitations include unquantified exposure to chlordane and confounding by exposure to other substances as well. Increased leukocytes and decreased platelets were reported in rats intermittently exposed to chlordane technical by inhalation for 90 days at 1 mg/m3 (EPA 1987f; Khasawinah et al. 1989). Increased lymphocytes were reported in mice administered trans-chlordane by gavage for 14 days at 8 mg/kg/day (Johnson et al. 1986). However, no data were located to provide support to these findings, including inhalation and oral studies of longer exposure duration. Additional animal studies could be designed to support or refute existing evidence for chlordane-induced hematological effects. People living in areas where chlordane may be detected should be monitored for possible exposure-related effects on the hematological system.
- Hepatic. Hepatic effects have been infrequently associated with chlordane exposure in humans (EPA 1980a). However, adverse liver effects were commonly observed in laboratory animals exposed by inhalation or oral routes (Ambrose et al. 1953a; Bondy et al. 2000; Den Tonkelaar and Van Esch 1974; EPA 1985a, 1987f; Khasawinah and Grutsch 1989a, 1989b; Khasawinah et al. 1989; Malarkey et al. 1995; Ogata and Izushi 1991; Truhaut et al. 1974, 1975). People living in areas where chlordane may be detected should be monitored for possible exposure-related hepatic effects.
- Endocrine. There is limited evidence of chlordane-induced effects on the thyroid in humans (Nagayama et al. 2007) and animals (EPA 1987f; Khasawinah et al. 1989). People living in areas where chlordane may be detected should be monitored for possible exposure-related thyroid effects.
- Reproductive. Data from one human case report involving a woman who was exposed to a lethal dermal dose of chlordane (Derbes et al. 1955) and a cross-sectional epidemiological investigation involving women exposed to chlordane vapors (Menconi et al. 1988) did not provide conclusive evidence that the reproductive system is a potential target organ in humans exposed to chlordane. Inhalation and oral acute-, intermediate-, or chronic-duration exposure studies in animals, in which the reproductive organs were examined histopathologically, did not identify lesions in the reproductive organs (EPA 1985a, 1987f; Khasawinah and Grutsch 1989a, 1989b; Khasawinah et al. 1989; NCI 1977; Truhaut et al. 1975). However, animal studies have identified the male reproductive system as a target of chlordane toxicity (Balash et al. 1987; Truhaut et al. 1975). Ambrose et al. (1953a) reported reduced fertility among male and female rats fed chlordane in the diet. The pharmacokinetic data in animals indicated that absorption occurs following any route of exposure, and that chlordane residues tend to accumulate in body fat; therefore, impaired reproductive performance in humans may occur following any route of exposure. Because of the tendency for chlordane residues to accumulate in body fat, multi-generation reproduction studies in animals by the inhalation and oral routes are recommended. Epidemiological investigations of reproductive effects in humans living in homes previously treated with chlordane, or those exposed during its manufacture or use as a pesticide, would also be useful if adequate populations can be identified.
- Developmental. Limited information is available regarding potential for chlordane-induced developmental effects in human (Fenster et al. 2006; Gladen et al. 2003; Trabert et al. 2012). Available animal data suggest that subtle behavioral and immunological effects occur in developing mice (Al-Hachim and Al-Baker 1973; Barnett et al. 1985a, 1985b, 1990a, 1990b; Chernoff and Kavlock 1982; Cranmer et al. 1984; Menna et al. 1985; Spyker-Cranmer et al. 1982; Theus et al. 1992; Usami et al. 1986). Additional developmental studies in other animal species may clarify the developmental effects that could be anticipated in humans. Particularly useful would be studies designed to locate thresholds for subtle immunological and neurological effects following both pre- and postnatal exposure. Epidemiological investigation of developmental effects in humans living in homes treated with chlordane, or those exposed during its manufacture or use as a pesticide, would also be useful if adequate populations can be identified.
- Immunotoxicity. Data from one human study suggest that chlordane may cause autoimmunity as well as impaired proliferative responses to plant mitogens following inhalation exposure to chlordane (McConnachie and Zahalsky 1992). In vitro studies with rhesus monkey peripheral blood mononuclear cells suggest that chlordane may impair cell-mediated immunity (Chuang et al. 1992). Mice exposed to chlordane exhibited leukocytosis and decreased thymus weight (Johnson et al. 1986; Khasawinah et al. 1989). Decreased myeloid cell colony forming capacity and depressed delayed type hypersensitivity occurred in the offspring of mice exposed orally to chlordane during gestation (Barnett et al. 1985a, 1985b, 1990a, 1990b; Menna et al. 1985; Spyker-Cranmer et al. 1982). Further testing of immune function in mice and other animals may provide useful information regarding chlordane-induced immunotoxicity in humans. In addition, epidemiology studies, perhaps comparing persons with high and low levels of chlordane residues in the blood, fat, or breast milk, may provide useful information. Parameters evaluated may include the frequency of allergic and autoimmune disorders, susceptibility to opportunistic infections (e.g., colds or flu), and alterations in absolute and differential leukocyte counts.
- Neurotoxicity. Neurotoxicity is a consistent and predictable finding in humans (Aldrich and Holmes 1969; Balistreri et al. 1973; Barnes 1967; Curley and Garrettson 1969; Dadey and Kammer 1953; EPA 1980a, 1986d; Harrington et al. 1978; Kilburn and Thornton 1995; Lensky and Evans 1952; Menconi et al. 1988; NIOSH 1984a; Olanoff et al. 1983) and animals (Drummond et al. 1983; Frings and O’Tousa 1950; Hrdina et al. 1974; Ingle 1952; Khasawinah et al. 1989; NCI 1977; Stohlman et al. 1950) exposed to chlordane. In the human studies, clinical signs and symptoms included migraines, convulsions, and seizures following inhalation, oral, or dermal routes of exposure. In the animal studies, convulsions and seizures were consistent findings after inhalation, oral, and dermal routes of exposure to chlordane (Ambrose et al. 1953a; EPA 1987f; Hrdina et al. 1974; Ingle 1953; Khasawinah et al. 1989; NCI 1977). Further testing should be designed to investigate subtle effects on neurobehavior and central nervous system function. Epidemiological investigation of subtle neurological and behavioral effects in humans living in homes treated with chlordane, or those exposed during its manufacture or use as a pesticide, would also be useful if adequate populations can be identified.
- Cancer. Many available epidemiology studies have found no convincing evidence for the carcinogenicity of chlordane in humans (Brown 1992; Cantor et al. 2003; Demers et al. 2000; Falck et al. 1992; Gammon et al. 2002; MacMahon et al. 1988; Shindell and Ulrich 1986; Wang and MacMahon 1979a; Ward et al. 2000; Weiderpass et al. 2000; Wolff et al. 2000). Other studies reported slight (statistically significant) associations between serum or adipose tissue levels of selected chlordane components and/or metabolites and risk of cancer of the male reproductive system (Cook et al. 2011; Hardell et al. 2003, 2006a; McGlynn et al. 2008), non-Hodgkin’s lymphoma (Hardell et al. 1996; Quintana et al. 2004; Spinelli et al. 2007), and pancreatic cancer (Hardell et al. 2007). Other population-based studies reported significant associations between self-reported chlordane use and risk of breast cancer (Mills and Yang 2005), non-Hodgkin’s lymphoma (Cantor et al. 1992; Colt et al. 2006), and rectal cancer (Purdue et al. 2006), except for a weak association with leukemia and neuroblastoma (Epstein and Ozonoff 1987; Infante et al. 1978). However, epidemiological studies are typically limited by lack of quantitative exposure data and the likelihood of significant exposure to other potentially toxic substances as well.Oral studies in animals have confirmed that chlordane induces liver tumors in mice, but not rats, exposed to high levels (Becker and Sell 1979; EPA 1985a; Epstein 1976; IRDC 1973; Khasawinah and Grutsch 1989a, 1989b; NCI 1977; Williams and Numoto 1984). Chronic-duration inhalation and dermal studies were not located, but it seems likely that the carcinogenicity of chlordane in mice is not route-dependent, because the pharmacokinetic data in animals indicate that absorption occurs following any route of exposure, and because the liver is a target organ for noncancer effects regardless of route of exposure. Most genotoxicity tests with chlordane yielded negative results (Arnold et al. 1977; Ashby and Tennant 1988; Blevins and Sholes 1978; Brandt et al. 1972; Maslansky and Williams 1981) (see Section 2.20), suggesting an epigenetic mechanism of carcinogenicity. In support of this theory, chlordane inhibited gap junction intercellular communication (Ruth et al. 1990; Tong and Williams 1988). These results suggest that chlordane acts as a tumor promoter, depressing intercellular communication that checks uncontrolled proliferation of transformed or neoplastic cells. Further mechanistic studies may provide useful information regarding the potential carcinogenicity to humans chronically exposed to low levels. These studies are important because humans may be chronically exposed by living in previously treated homes or near hazardous waste sites.
- Genotoxicity. Studies of genotoxic effects in humans are limited to an in vitro study of chlordane-induced sister chromatid exchange in lymphoid cells and a positive response was obtained (Sobti et al. 1983). In vivo mouse studies provided mixed results; chlordane did not induce dominant lethal mutations (Arnold et al. 1977; Epstein et al. 1972) or DNA adducts (Whysner et al. 1998) in mice, but did induce micronucleus formation (Schop et al. 1990) and chromosomal aberrations (Sarkar et al. 1993) in mouse bone marrow cells and nuclei aberrations in hair follicles (Schop et al. 1990). The most prevalent metabolite of the chlordane isomers, oxychlordane, although an epoxide, appears to be relatively inert (Khasawinah 1989; Sasaki et al. 1992), and probably does not bind strongly to tissue macromolecules. Free radicals formed as a result of reductive dehalogenation, however, may bind to DNA and other macromolecules (Brimfield and Street 1981; Kawano et al. 1989), inducing genetic defects or interfering with DNA repair. Additional in vivo mutation and chromosomal aberration tests in animals may clarify the ability of chlordane to induce genotoxicity in humans.
Epidemiology and Human Dosimetry Studies. Several epidemiological studies have investigated the cancer and noncancer effects of chlordane in humans exposed in their homes or occupationally in the manufacture of chlordane or in its application as a pesticide (Alvarez and Hyman 1953; Brown 1992; Cantor et al. 1992; Ditraglia et al. 1981; Kawano and Tatsukawa 1982; MacMahon et al. 1988; Menconi et al. 1988; Ogata and Izushi 1991; Wang and MacMahon 1979a, 1979b; Woods and Polissar 1989). Limitations of these studies include unquantified exposure levels of chlordane, exposure to a mixture of chemicals, and failure to investigate subtle neurological, behavioral, and hepatic effects in the exposed persons. Additional multi-endpoint epidemiological studies should be designed to study these subtle effects, as well as hematological effects such as blood dyscrasia and leukemia, in the exposed populations mentioned above. Further, case-control and longitudinal epidemiological studies would help to establish a cause/effect relationship among a better defined population.
Biomarkers of Exposure and Effect. Biomarkers of exposure include various components of commercial chlordane (principally cis- and trans-chlordane, cis- and trans-nonachlor) and its metabolites (principally oxychlordane). These substances are specific for exposure to chlordane. Detection of heptachlor and its metabolite, heptachlor epoxide, could reflect exposure to chlordane, because heptachlor is a component of commercial chlordane, or exposure to heptachlor, which is an insecticide in its own right.
Detectable levels in urine would probably reflect recent or ongoing exposure, because urinary excretion is not prominent for chlordane (Aldrich and Holmes 1969; Barnett and Dorough 1974; Ewing et al. 1985; Ohno et al. 1986; Tashiro and Matsumura 1977). Higher levels of chlordane residues occur in the feces of acutely poisoned humans (Aldrich and Holmes 1969). Generally, levels of chlordane residues in blood are below those in liver and fat (Mussalo-Rauhamaa 1991); blood levels may be reasonable indicators of recent or ongoing exposure (Ogam and Izushi 1991).
Some studies evaluated the use of levels of chlordane residues in skin surface lipids as a biomarker of exposure to avoid the invasive techniques necessary to obtain blood or body fat (Sasaki et al. 1991b; Wariishi and Nishiyama 1989). In monkeys, levels of trans-chlordane and oxychlordane in surface skin lipids correlated fairly well with levels in subcutaneous fat. Further refinement of this technique could increase the utility of chlordane residues in skin surface lipids as biomarkers of exposure.
Known biomarkers of effect are limited to slight alterations in serum chemistry. Evidence of liver effects (elevated serum triglycerides, CPK, and LDH) were observed in pesticide workers (Ogata and Izushi 1991). The elevated CPK was considered somewhat specific for chlordane exposure. Carefully performed epidemiological studies might provide data that clarify the relation between exposure to chlordane and optic neuritis or other disease states. Such studies may also identify alterations in blood chemistry indices or other clinicopathological endpoints that are useful for identifying the presence or pathogenesis of disease states associated with chlordane exposure.
Absorption, Distribution, Metabolism, and Excretion. There are no quantitative absorption data regarding human exposure by any route. However, that chlordane is absorbed by humans is indicated from measurement of blood and tissue levels of chlordane in persons exposed via inhalation from homes treated for termite control (Kawano and Tatsukawa 1982; Saito et al. 1986; Taguchi and Yakushiji 1988; Takamiya 1987), in case reports of accidental ingestion (Aldrich and Holmes 1969; Curley and Garrettson 1969; Kutz et al. 1983; Olanoff et al. 1983), and from systemic toxicity after dermal exposure (Barnes 1967). One human study also attempted to correlate skin chlordane levels with blood chlordane levels in 248 male and 227 female outpatients (Hirai and Tomokuni 1993). Although chlordane was potentially absorbed by the dermal route, the data from this study did not demonstrate any strong correlations between skin chlordane levels and blood chlordane levels. The rat data for inhalation absorption are limited to an intratracheal study, which is inadequate for estimating absorption via the respiratory tract (Nye and Dorough 1976). Oral data in rats and mice indicate that gastrointestinal absorption occurs readily (Ewing et al. 1985; Ohno et al. 1986). Quantitative dermal absorption data are lacking, but absorption is indicated by lethality in rats and rabbits exposed dermally (Gaines 1960; Ingle 1965). Quantitative inhalation and dermal absorption data would be useful, because these routes are toxicologically significant to humans.
Distribution data available for humans are limited; levels of chlordane metabolites in several tissues after acute poisoning, and in blood, liver, fat, and breast milk after chronic-duration exposure were reported for the oral route (Aldrich and Holmes 1969; Curley and Garrettson 1969; Dearth and Hites 1991a; Hirai and Tomokuni 1991a, 1991b; Kutz 1983; Kutz et al. 1976, 1983; Mussalo-Rauhamaa 1991; Ogata and Izushi 1991; Olanoff et al. 1983; Sasaki et al. 1991a; Wariishi and Nishiyama 1989). Other human data include reports of chlordane residues in blood, adipose tissue, and cord blood (Brock et al. 1998; Glynn et al. 2000; Kang et al. 2008; Rhainds et al. 1999; Tanabe et al. 1993), and adipose tissue and brain and liver autopsy samples (Dewailly et al. 1999). Rat studies regarding inhalation, oral, and parenteral exposure demonstrate that initial distribution is to the liver and kidney, followed by redistribution to body fat (Ambrose et al. 1953b; Balba and Saha 1978; Barnett and Dorough 1974; Dearth and Hites 1991b; Ewing et al. 1985; Khasawinah 1989; Nye and Dorough 1976; Ohno et al. 1986; Poonawalla and Korte 1971; Sasaki et al. 1992; Street and Blau 1972; Takeda et al. 1984). One oral study in mice demonstrated that chlordane is initially distributed to the muscles and that the cis isomer accumulates in tissues to a greater extent than the trans isomer (Satoh and Kikawa 1992). Additional dermal exposure studies would be useful for elucidating patterns of distribution by this route. Of particular interest would be studies of distribution to the central nervous system, since neurological effects are a consistent part of the clinical picture in humans exposed by any route. The ability of chlordane to cross the placenta and its presence in milk should also be investigated, because data show that prenatally exposed mice are more sensitive than adults to the immunological effects of chlordane (Barnett et al. 1985a, 1985b; Menna et al. 1985; Spyker-Cranmer et al. 1982). Determination of apparent volume of distribution and the extent of binding to tissue proteins may provide data that would be useful in the management of clinical cases of poisoning.
Human metabolism data are limited to in vitro studies (Kutz et al. 1976, 1979; Tashiro and Matsumura 1978). In vivo and in vitro animal studies, however, are sufficient to propose probable metabolic pathways (Balba and Saha 1978; Barnett and Dorough 1974; Brimfield et al. 1978; Nomeir and Hajjar 1987; Poonawalla and Korte 1964; Sasaki et al. 1992; Tashiro and Matsumura 1978). Further studies could be designed to estimate metabolic rate constants and to determine the levels at which saturation of specific pathways occurs with different routes of exposure.
Data from environmentally exposed humans suggest that substantial excretion occurs via lactation (Barnett et al. 1979; Strassman and Kutz 1977; Taguchi and Yakushiji 1988; WHO 1984). Data regarding acute poisoning in humans after acute oral exposure to chlordane indicate that most chlordane-derived material is excreted in the feces (Aldrich and Holmes 1969; Curley and Garrettson 1969; Olanoff et al. 1983). Studies involving intratracheal dosing of rats and acute, oral dosing of rats, mice, and rabbits confirm that fecal, probably biliary, excretion is more important than renal excretion (Barnett and Dorough 1974; Ewing et al. 1985; Tashiro and Matsumura 1977; Nye and Dorough 1976; Ohno et al. 1986). Additional animal studies might elucidate the relative importance of various routes of excretion following inhalation and dermal exposure, and might provide useful information regarding the rate and extent of excretion via lactation.
Comparative Toxicokinetics. Data in rats, mice, and rabbits following oral exposure to chlordane indicate that there are some species differences in absorption, distribution, and excretion (Balba and Saha 1978; Barnett and Dorough 1974; Ewing et al. 1985; Ohno et al. 1986; Poonawalla and Korte 1971; Satoh and Kikawa 1992). The available data on metabolites in human tissues and in vitro studies both indicate qualitatively that metabolism of chlordane in rats and humans is similar (Tashiro and Matsumura 1978). Analysis of the urine of humans with known exposure to chlordane (e.g., those living in previously treated houses) could provide a means of further studying the differences and similarities between animal species and humans and of monitoring humans for exposure. Additional studies could be designed to further evaluate the adequacy of using rats and/or mice as models for toxicokinetics of chlordane in humans.
Children’s Susceptibility. Evidence in mice indicates that the fetus may be particularly susceptible to compromised immunocompetence due to altered stem cell populations of key immunoactive cells (Barnett et al. 1990a, 1990b). Infants may be unusually susceptible to a chronic seizure disorder following exposure to chlordane, particularly if they have a hereditary predisposition, such as a positive familial history of febrile convulsions (Bernad 1989). Since chlordane is no longer used as a pesticide, the general population is not likely to be exposed to the chemical. However, if a population is identified with documented exposure to chlordane, it should be monitored for potential effects on the young.
Physical and Chemical Properties. The physical and chemical properties of chlordane listed in Table 4-2 are not complete. These properties are difficult to specify since the technical product is a mixture of over 140 compounds. The composition of technical chlordane varies according to conditions during its manufacture. In addition, properties of a mixture differ from the properties of the components. Even minor components can affect physical and chemical properties. Some of the properties reported in Table 4-2 are those of particular components such as cis- and trans-chlordane. The value for log Kow listed in Table 4-2 is a particularly crude estimate for technical chlordane since this value was estimated for the chlordane structure. In addition, the values for water solubility of chlordane vary widely. This is probably due to differences in composition of the chlordane studied. Suntio et al. (1988) and Weil et al. (1974) did not indicate whether the chlordane used in their studies was technical grade or a mixture of chlordane isomers. Despite the limitations discussed above, the physical-chemical properties available for chlordane are adequate to estimate its partitioning in the environment. More significantly, there are numerous monitoring studies that have been performed over several decades that give us this information concerning chlordane’s fate in the environment.
Production, Import/Export, Use, Release, and Disposal. Although the use of chlordane in the United States has been banned since April 1988, chlordane may be manufactured for export. Since chlordane is extremely persistent in the environment, knowledge of the former use pattern for this compound is useful in making estimations concerning potential for human exposure and sources of release. Environmental burdens of chlordane can be roughly estimated by relying on production and use data and by using a few basic assumptions. Production methods for chlordane are documented in the literature; however, recent production volumes are not available. A breakdown of the former uses of chlordane and its use pattern is available, which indicates that chlordane was widely used in the home, on food and nonfood crops, and on lawns and gardens. Disposal information is useful in determining environmental burden and potential concentrations where environmental exposures may be high. This type of information may be obtained by polling the manufacturer or other commercial organizations to determine methods of chlordane disposal. For example, asking exterminators how they disposed of contaminated materials or unused product may provide disposal information for much of the chlordane not directly applied to soils or dwellings. Current disposal practices are not known. Chlordane has been designated as a hazardous waste, and regulations guide the disposal of such waste. Chlordane is also regulated in effluent by provisions of the Clean Water Act.
Environmental Fate. Data are available to characterize the partitioning and transport of chlordane (Atallah et al. 1979; Atlas and Giam 1988; Beeman and Matsumura 1981; Bennett et al. 1974; Bidleman and Foreman 1987; Bidleman et al. 1986, 1987; Cotham and Bidleman 1991; Foreman and Bidleman 1987; Glotfelty and Schomburg 1989; Glotfelty et al. 1984; Gustafson 1989; Jury et al. 1987a; Lau et al. 1989; Oliver and Niimi 1985; Oloffs et al. 1972, 1973; Pacynba and Oehme 1988; Singh et al. 1991; Zaroogian et al. 1985). Monitoring studies demonstrate that chlordane is very persistent in soil, lasting over 20 years in some soils (Beeman and Matsumura 1981; Bennett et al. 1974; Harris and Sans 1976; Lichtenstein and Schulz 1959; Mullins et al. 1971; Nash and Woolson 1967; Stewart and Chisholm 1971; Stewart and Fox 1971; Szeto and Price 1991; Tafuri et al. 1977; Wiese and Basson 1966). However, chlordane’s degradation products in soil have not been reported. More information would be useful on the transformation of chlordane in the environment. There are data needs regarding biodegradation and photolysis of chlordane in water systems and oxidation of this compound in air. Natural water grab sample biodegradation studies carried out under both aerobic and anaerobic conditions would be useful in establishing the biodegradation half-life of chlordane. A number of studies have been carried out that show chlordane is photolyzed in air; however, there is a lack of data pertaining to photolysis of chlordane that is adsorbed to particulate matter in air or in water and its significance for the removal of chlordane from these media. A photolysis study carried out under conditions simulating those found in the environment would be useful in establishing the significance of this reaction in air and natural waters. One of the dominant removal mechanisms for vapor phase chlordane in air is expected to be reaction with photochemically generated hydroxyl radicals; however, no experimental data are available concerning the kinetics of this reaction, the reaction pathway, or the products of these types of reactions. Photolysis, photooxidation, and biodegradation of particulate-bound chlordane is unknown. These types of data would be useful in understanding the fate of this compound in the environment.
Bioavailability from Environmental Media. Data are available that correlate length of exposure to atmospheric chlordane and levels of chlordane and/or metabolites in human blood and milk (Kawano and Tatsukawa 1982; Saito et al. 1986; Taguchi and Yakushiji 1988; Takamiya 1987). Similar data are not available for human exposure to chlordane in water or soil. A deficiency in the inhalation studies is that exposure concentrations have not been quantified. Therefore, it is not possible to correlate levels of chlordane and/or metabolites in human biosamples with specific concentrations in air, soil, or water. Studies in animals may provide valuable information regarding bioavailability and bioaccumulation of chlordane residues from air, water, and soil.
Food Chain Bioaccumulation. Chlordane has been found to bioconcentrate in marine and freshwater fish and shellfish, and biomagnify in animals that prey upon these fish. Available data indicate that organisms that have bioaccumulated chlordane are geographically distributed across the United States. This type of information is useful in determining how levels in the environment affect the food chain and potentially impact on human exposure levels.
Exposure Levels in Environmental Media. In general, the monitoring database for chlordane is not very broadly based or recent. Data for soils are especially out of date. Chlordane levels in soil and sediment are particularly important, as these are the repositories for chlordane, and broadly-based monitoring data in these media are the best way of assessing environmental trends. Reliable monitoring data for the levels of chlordane in contaminated media at hazardous waste sites are needed. Such information could be used in combination with the known body burden of chlordane to assess the potential risk of adverse health effects in populations living in the vicinity of hazardous waste sites.
Exposure Levels in Humans. Estimates have been made for the human intake of chlordane from food, air, and water. Chlordane has been measured in adipose tissue, blood, serum, sebum, and seminal fluid. This information is necessary for assessing the need to conduct health studies on these populations.
Exposures of Children. Studies examining potential exposure sources for children would be useful because limited human and animal data provide suggestive evidence that the fetus and young child may be at increased susceptibility to chlordane toxicity (see Section 3.2).
Analytical Methods. Levels of various components of technical chlordane (principally cis- and trans-chlordane, cis- and trans-nonachlor) and its metabolites (principally oxychlordane) in body tissue and fluids are elevated in individuals exposed to chlordane. While analytical methods are available (Aldrich and Holmes 1969; Barquet et al. 1981; EPA 1977; Griffith and Blanke 1974; LeBel and Williams 1986; Mussalo-Rauhamaa 1991; Saito et al. 1985; Tojo et al. 1986; Wariishi et al. 1986) for the quantification of chlordane compounds and their metabolites in biological matrices, there are no data correlating these levels with environmental chlordane concentrations to which a person was exposed.
The levels of chlordane in different environmental media can be used to indicate exposure of humans to mixture compounds through the inhalation of air, ingestion of drinking water and foods, and exposure to soils containing chlordane. If a correlation with human tissue or body fluid levels is available, the intake levels from different environmental sources can be used to estimate the body burden of the chemical in humans. Although the products of biotic and abiotic processes of chlordane in the environment are known, few systematic studies are available in which the concentrations of its reaction products were measured in the environment. In instances where products of an environmental reaction are more toxic than the parent compound, it is important that the level of the degradation products in the environment be known. Analytical methods for the determination of chlordane compounds and their degradation products in air, water, soil, sediment, and food are available, and these methods have good sensitivity and specificity. The methods for determining degradation products of chlordane compounds are similar to those for the parent compounds.
6.3. Ongoing Studies
A search of the National Institutes of Health (NIH) RePORTER (2017) revealed the following ongoing studies.
Dr. George E. Howell, in the Schools of Veterinary Medicine at Mississippi State University, is evaluating the effects of exposure to organochlorine pesticides (including chlordane) on hepatic lipid metabolism in Type 2 diabetes. The study is sponsored by the National Institute of Environmental Health Sciences.
Dr. Victoria W. Persky, in the Schools of Public Health at the University of Illinois at Chicago, is studying possible relationships between persistent organic pollutants (presumably including chlordane), endogenous hormones, and diabetes in Latinos. The study is sponsored by the National Institute of Environmental Health Sciences.
- ADEQUACY OF THE DATABASE - Toxicological Profile for ChlordaneADEQUACY OF THE DATABASE - Toxicological Profile for Chlordane
Your browsing activity is empty.
Activity recording is turned off.
See more...