NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2005. (Treatment Improvement Protocol (TIP) Series, No. 43.)
This publication is provided for historical reference only and the information may be out of date.

Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs.
Show detailsIn This Chapter …
Contraindications to Opioid Pharmacotherapy
Stages of Pharmacotherapy
Medically Supervised Withdrawal
Take-Home Medications
Office-Based Opioid Therapy
This chapter describes pharmacotherapy in opioid treatment programs (OTPs), in particular the clinical use of methadone, with limited discussion of levo-alpha acetyl methadol (LAAM) and buprenorphine. More limited coverage is provided on the opioid antagonist naltrexone, which is not used widely for opioid addiction treatment in the United States. As explained in chapter 3, at this writing most OTPs have discontinued the use of LAAM for new patients, and its continued availability is uncertain. TIP 40, Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction (CSAT 2004a ), provides more detailed information about buprenorphine.
In general, the choice of medication used in medication-assisted treatment for opioid addiction (MAT) is based on safety and efficacy, patient preferences, and treatment goals. Methadone maintenance treatment has the longest successful track record in patients addicted to opioids for more than a year and has been shown to control withdrawal symptoms, stabilize physiologic processes, and improve functionality. Studies also have found that methadone maintenance treatment reduces criminality, noncompliance with HIV/AIDS therapy, seroconversion to HIV/AIDS, and mortality associated with opioid addiction (Appel et al. 2001; Ball and Ross 1991). Since 2001, LAAM, although effective in opioid pharmacotherapy, has carried a restrictive label precluding its use as the initial medication for MAT. As reviewed in chapter 3, the effectiveness of buprenorphine has been found to be similar to that of methadone and LAAM (Johnson et al. 2000). Sublingual buprenorphine formulations have been approved for use in OTPs and by physicians in office-based and other health care settings. Some patients prefer buprenorphine maintenance in an office-based opioid treatment (OBOT) setting to the daily observed dosing that is part of methadone maintenance in an OTP. However, patients who progress in MAT while in an OTP eventually may qualify for take-home medication lasting up to 30 days at a time, as detailed below, and patients desiring ongoing buprenorphine pharmacotherapy now can receive buprenorphine on a less-than-daily basis in either an OTP or OBOT setting. For some patients, these options may reduce the attendance requirements for MAT in an OTP.
For patients who do not qualify for or do not prefer opioid maintenance treatment (see “Contraindications to Opioid Pharmacotherapy” below), a primary issue during treatment is what to do about withdrawal symptoms. Naturally occurring opioid withdrawal is almost never life threatening, but it can produce discomfort severe enough to warrant urgent intervention. Treatment for withdrawal symptoms usually involves administration of a long-acting opioid medication such as methadone or buprenorphine, which can be followed by gradual tapering of the medication as withdrawal symptoms diminish.
Control of withdrawal symptoms often is insufficient treatment to prevent a relapse to opioid abuse, and detoxification alone may yield only short-term benefits. Research has shown that retention in treatment over an extended period is key to successful outcomes for opioid addiction in many patients, just as it is for other chronic diseases like hypertension, diabetes, and asthma (McLellan et al. 2000). Therefore, when detoxification from short-acting opioids is provided, the consensus panel recommends linkage to ongoing psychosocial treatment, with or without additional maintenance therapy with an opioid antagonist such as naltrexone. Comprehensive, long-term opioid agonist maintenance remains the treatment with the best track record of controlling opioid use and saving lives, although opioid partial agonist therapy is promising. Access and easy transfer to this care should remain available as part of any detoxification program.
Contraindications to Opioid Pharmacotherapy
The consensus panel believes that few psychiatric or medical diagnoses categorically should rule out admission to an OTP or access to opioid pharmacotherapy. Inclusion rather than exclusion should be the guiding principle. Types of people who possibly should not be admitted to an OTP and should receive other interventions include
- Individuals who abuse opioids but whose conditions do not meet criteria for opioid dependence outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000). If a clear history of opioid abuse or addiction exists but a person currently is not addicted, regulations allow admission to an OTP in two cases in which a person might relapse without treatment: pregnancy and release from incarceration (42 Code of Federal Regulation [CFR], 8 Part § 12(e)(3)).
- Individuals with less than 1 year of opioid addiction and no addiction treatment history, except patients receiving OBOT with buprenorphine. Detoxification might be attempted with applicants who have a shorter history of addiction. Applicants receiving buprenorphine may be admitted to an OTP for either medically supervised withdrawal or maintenance treatment.
- Applicants who cannot attend treatment sessions regularly, especially for medication dosing (unless a clinical exception can be obtained [see chapter 7]); this requirement is less of a hindrance for patients receiving OBOT with buprenorphine.
- Previous patients who have had allergic reactions to methadone, LAAM, or buprenorphine.
- For LAAM, applicants with cardiac abnormalities such as prolonged QT interval.
In addition, people who are opioid addicted and meet DSM-IV-TR criteria for alcohol or sedative dependence might be problematic candidates for opioid pharmacotherapy because the combined effects of alcohol or sedatives that depress the central nervous system (CNS) can cause serious adverse events during MAT (see discussion of drug interactions in chapter 3). Some treatment providers require detoxification from alcohol and sedatives before opioid pharmacotherapy, followed by careful monitoring such as daily Breathalyzer™ tests, ongoing drug tests, and reduction or withholding of medication if a test is positive. The consensus panel endorses this strategy, provided that adequate alcohol or sedative detoxification facilities are readily available. If not, both opioid addiction and alcohol or sedative dependence should be treated concurrently at the OTP site with a combination of psychosocial and pharmacological interventions.
Stages of Pharmacotherapy
The stages of pharmacotherapy with methadone, LAAM, or buprenorphine include induction, stabilization, and maintenance. The stages of naltrexone pharmacotherapy may differ.
Induction
Induction procedures for methadone, LAAM, and buprenorphine depend on the unique pharmacologic properties of each medication, prevailing regulatory requirements, and patient characteristics. Regardless of the medication used, safety is key during the induction stage.
General considerations
Timing. When to begin the first dose of opioid treatment medication is important. Most treatment providers begin treating new patients when there are no signs of opioid intoxication or sedation and some beginning signs of opioid withdrawal. Administration of the first dose also should await a physical assessment to rule out any acute, life-threatening condition that opioids might mask or worsen (see chapter 4 for more information on medical assessment). For naltrexone, patients should be abstinent from all short-acting opioids for at least 7 days and from long-acting opioids, such as methadone, for at least 10 days before beginning the medication to prevent potentially severe withdrawal symptoms (O'Connor and Fiellin 2000).
Other substance use. The presence of sedatives such as benzodiazepines or alcohol should be ruled out before induction to minimize the likelihood of oversedation with the first dose. OTP staff should ensure that patients known to abuse sedatives, tranquilizers, tricyclic antidepressants, benzodiazepines, alcohol, or other CNS depressants are told in clear language of the dangers of adverse effects if they take these substances while being stabilized or maintained on methadone, LAAM, or buprenorphine.
Observed dosing. Observed dosing with methadone, LAAM, or buprenorphine should be part of the medical safety procedure and diversion control plan in an OTP and is recommended during induction with buprenorphine. Observed dosing is the only way to ensure that a patient ingests a given dose and to monitor a patient's response. In observed dosing, staff members who dispense medication first carefully identify patients—sometimes by requiring them to remove hats or dark glasses, for example—and then provide the medication.
To ensure that patients swallow oral doses of methadone or LAAM, they should be required to speak before and after ingesting at least 2 ounces of liquid in which an appropriate dose of medication is dissolved. For buprenorphine, a sublingual tablet should be observed to have dissolved completely under the tongue. After the first dose, patients should wait in an observation area and be checked 30 to 60 minutes later for acute adverse effects. If same-day dosing adjustments must be made, patients should wait 2 to 4 more hours after the additional dosing, for further evaluation when peak effects are achieved. The consensus panel recommends that patients be observed for several hours after the first dose of any opioid treatment medication. This observation is particularly important for patients at higher risk of overdose, including those naive to methadone, LAAM, and buprenorphine; those receiving other CNS-depressant medications or known to abuse CNS depressants; and severely medically ill, frail, or elderly patients. Naltrexone typically is prescribed without observed dosing, but poor patient compliance with ongoing naltrexone therapy has led some investigators to look at using family members to ensure that patients take their medication (Fals-Stewart and O'Farrell 2003).
Initial dosing. The first dose of any opioid treatment medication should be lower if a patient's opioid tolerance is believed to be low, the history of opioid use is uncertain, or no signs of opioid withdrawal are evident. Some former patients who have been released from incarceration or are pregnant and are being readmitted because they have a history of addiction might have lost their tolerance. Loss of tolerance should be considered for any patient who has abstained from opioids for more than 5 days. In general, the safety principle “start low and go slow” applies for early medication dosages in an outpatient OTP. The amount of opioid abuse estimated by patients usually gives only a rough idea of their tolerance and should not be used as a dosing guide for induction, nor should initial dosages be determined by previous treatment episodes or patient estimates of dollars spent per day on opioids. Patients transferred from other treatment programs should start with medication dosages identical to those prescribed at their previous OTPs.
Dosage adjustments in the first week of treatment should be based on how patients feel at the peak period for their medication (e.g., 2 to 4 hours after a dose of methadone is administered), not on how long the effects of a medication last. As stores of medication accumulate in body tissues (see below), the effects begin to last longer.
Steady state. Initial dosing should be followed by dosage increases over subsequent days until withdrawal symptoms are suppressed at the peak of action for the medication. Methadone, LAAM, and buprenorphine are stored in body tissues, including the liver, from which their slow release keeps blood levels of medication steady between doses. It is important for physicians, staff members, and patients to understand that doses of medication are eliminated more quickly from the bloodstream and medication effects wear off sooner than might be expected until sufficient levels are attained in tissues. During induction, even without dosage increases, each successive dose adds to what is present already in tissues until steady state is reached. Steady state refers to the condition in which the level of medication in a patient's blood remains fairly steady because that drug's rate of intake equals the rate of its breakdown and excretion.
Steady state is based on multiples of the elimination half-life. Approximately four to five half-life times are needed to establish a steady state for most drugs. For example, because methadone has a half-life of 24 to 36 hours, its steady state—the time at which a relatively constant blood level should remain present in the body—is achieved in 5 to 7.5 days after dosage change for most patients. However, individuals may differ significantly in how long it takes to achieve steady state.
Patients should stay on a given dosage for a reasonable period before deciding how it will “hold.” During induction, patients should be instructed to judge their doses by how they feel during the peak period (the point of maximum concentration of medication in the blood [for methadone, 2 to 4 hours after taking a dose]), rather than during the trough period (the low point of medication concentration in blood just before the next dose [for methadone, approximately 24 hours after ingestion]). Patients who wake up sick during the first few days of opioid pharmacotherapy might become convinced that they need a dose increase, when in fact they need more time for tissue stores to reach steady state. In contrast, patients who wake up sick after the first week of treatment—when tissue stores have reached steady-state levels—might indeed need higher doses.
In closely monitored settings such as inpatient programs, multiple split doses can be administered per day based on patients' symptoms at peak blood levels. Outpatient programs are limited in this approach because patients can be monitored only when they are at the OTP site. (Split dosing is discussed further below.) Because buprenorphine's safety profile makes overdose less of a concern, some providers opt to give even new patients receiving buprenorphine some take-home medication for multiple dosing during induction (CSAT 2004a ).
Induction with methadone and LAAM
Because methadone overdose deaths have occurred in the first few days of treatment (Caplehorn and Drummer 1999; Zador and Sunjic 2000), it is important to adjust methadone dosage carefully until stabilization and tolerance are established. Federal regulations require that methadone initially be given daily under observation for either 6 or 7 days per week. (A take-home dose is allowed for all patients when the OTP is closed on Sunday.) LAAM must continue to be given under observation and administered no more than every 2 to 3 days.
Initial dosing. For a patient actively abusing opioids, a typical first dose of methadone is 20 to 30 mg (Joseph et al. 2000) and is limited by regulations to no more than 30 mg. If withdrawal symptoms persist after 2 to 4 hours, the initial dose can be supplemented with another 5 to 10 mg (Joseph et al. 2000). The total first-day dose of methadone allowed by Federal regulations is 40 mg unless a program physician documents in the patient record that 40 mg was insufficient to suppress opioid withdrawal symptoms (42 CFR, Part 8 § 12(h)(3)(ii)).
Since 2001, LAAM has carried a restriction that precludes its use as an initial medication for pharmacotherapy because of concerns about its cardiovascular effects. Although direct induction with LAAM can be accomplished with an initial dose of 20 to 40 mg every 48 hours, LAAM has been used almost exclusively in cases involving transfer of patients from methadone maintenance. LAAM must never be given on 2 consecutive days because its extended duration of action can result in toxic blood levels leading to fatal overdose.
Variations in individual response and optimal dosing. Most differences in patient response to methadone can be explained by variations in individual rates of absorption, digestion, and excretion of the drug, which in turn are caused by such factors as body weight and size, other substance use, diet, co-occurring disorders and medical diseases, and genetic factors. Because variation in response to methadone is considerable, the consensus panel believes that the notion of a uniformly suitable dosage range or an upper dosage limit for all patients is unsupported scientifically. Whereas 60 mg of methadone per day may be adequate for some patients, it has been reported that some patients require much more for optimal effect. Treatment providers should avoid thinking of “high dosage” as being above a certain uniform threshold; however, there are few data on the safety of methadone doses above 120 mg/day. For example, diversion of very high doses can be associated with significant risk because the tolerance of the person taking the diverted dose may be insufficient to avoid overdose.
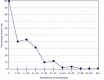
The way a person presents at the OTP is often the best indicator for determining optimal dosage. Looking for clinical signs and listening to patient-reported symptoms related to daily doses or changes in dosage can lead to adjustments and more favorable outcomes (Leavitt et al. 2000). Exhibit 5-1 illustrates the use of signs and symptoms to determine optimal methadone dosages. Generally, the disappearance of opioid withdrawal symptoms indicates adequate dosing and serum methadone levels (SMLs) within the therapeutic comfort zone.

Figure
Exhibit 5-1. Using Signs and Symptoms To Determine Optimal Methadone Levels. Adapted from Leavitt et al. (2000), modified with permission from Mount Sinai Journal of Medicine.
Research indicates that patients diagnosed with mental disorders or hepatitis C along with substance addiction may need increases of 50 percent or more in methadone dosage to achieve stabilization (Leavitt et al. 2000; Maxwell and Shinderman 2002).
Exhibit 5-2 illustrates how blood levels of methadone rise with repeated dosing until steady state is reached. It is important to understand that steady state is achieved after a dosage change. In Exhibit 5-2, because the last change (to 100 mg) occurred on day 5, steady state was not achieved until approximately day 10.

Figure
Exhibit 5-2. Induction Simulation—Moderate to High Tolerance. Adapted from Payte 2002, with permission
Induction with buprenorphine
Because buprenorphine has lower abuse potential than methadone or LAAM and is less likely to produce respiratory depression if diverted or misused, qualified practitioners can prescribe buprenorphine without the control structure of an OTP when they meet Drug Addiction Treatment Act of 2000 requirements. No stated requirement exists for observed dosing with buprenorphine, although guidelines strongly recommend dosage monitoring early in treatment (CSAT 2004a ).
Initial dosing. Awaiting signs of withdrawal before administering the first dose is especially important for buprenorphine induction because, as explained in chapter 3, buprenorphine can precipitate withdrawal in some circumstances (Johnson and Strain 1999). Precipitated withdrawal usually is more sudden and can be more severe and uncomfortable than naturally occurring withdrawal. The typical first dose of buprenorphine is 4 mg. If withdrawal symptoms persist after 2 to 4 hours, the initial dose can be supplemented with up to 4 mg for a maximum dose of 8 mg of buprenorphine on the first day (Johnson et al. 2003b ).
Three national evaluations of the buprenorphine-naloxone combination tablet found that direct induction with buprenorphine alone was effective for most people who were opioid addicted. However, buprenorphine tablets without naloxone (sometimes called monotherapy tablets) are recommended during the first 2 days of induction for patients attempting to transfer from a longer acting opioid such as sustained-release morphine or methadone (Amass et al. 2000, 2001) because most of these patients will experience withdrawal effects from the naloxone in the combination tablets. When patients' tissue levels of a full agonist are a factor and the buprenorphine-naloxone tablet is administered, it may be difficult to determine whether precipitated withdrawal is caused by the partial agonist buprenorphine or small amounts of absorbed naloxone.
For most patients who are appropriate candidates for induction with the combination tablet, the initial target dose after induction should be 12 to 16 mg of buprenorphine in a 4-to-1 ratio to naloxone (i.e., 12/3 to 16/4 mg [buprenorphine/naloxone]). Bringing patients to this target dosage may be achieved over the first 3 days of treatment by doubling the dose each successive day after initial administration. An initial dose of 4/1 mg (buprenorphine/naloxone) is recommended, followed in 2 to 4 hours with an additional 4/1 mg if indicated. The dosage should be increased on subsequent days to the target dosage (ranging from 12/3 to 16/4 mg per day). During dose induction, patients may need to visit their OTP or physician's office daily for dose adjustments and clinical monitoring. Further information and guidelines for buprenorphine induction and use can be found in TIP 40, Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction (CSAT 2004a ).
Induction with naltrexone
The standard procedure for induction to naltrexone therapy is first to make certain that there is an absence of physiological dependence on opioids. This often is done by using a Narcan challenge after a 7- to 10-day period during which opioids are not used. Then the patient is given 25 mg of naltrexone initially, followed by 50 mg the next day if no withdrawal symptoms occur after the first 25 mg dose. Thereafter, the patient is given 50 mg per day or up to 350 mg per week in three doses during the week. The first dose usually is smaller to minimize naltrexone's side effects, such as nausea and vomiting, and to ensure that patients have been abstinent from opioids for the requisite time (Stine et al. 2003).
Stabilization
The terms “steady state” and “stabilization” should be differentiated. Steady state is achieved when a treatment medication is eliminated from the blood at the exact rate that more is added. In contrast, a patient is stabilized when he or she no longer exhibits drug-seeking behavior or craving. The correct (steady-state) medication dosage contributes to a patient's stabilization, but it is only one of several factors, as discussed elsewhere in this TIP. The stabilization stage of opioid pharmacotherapy focuses on finding the right dosage for each patient. The potential for undermedication or overmedication can be avoided by a flexible approach to dosing, which sometimes requires higher dosages of treatment medication than expected, and by taking into account patient-reported symptoms (Leavitt et al. 2000).
Dosage determination
It is critical to successful patient management in MAT to determine a medication dosage that will minimize withdrawal symptoms and craving and decrease or eliminate opioid abuse. Dosage requirements for methadone, LAAM, and buprenorphine must be determined on an individual basis. There is no single recommended dosage or even a fixed range of dosages for all patients. For many patients, the therapeutic dosage range of methadone may be in the neighborhood of 80 to 120 mg per day (Joseph et al. 2000), but it can be much higher, and occasionally it is much lower.
The desired responses to medication that usually reflect optimal dosage include (Joseph et al. 2000)
- Prevention of opioid withdrawal for 24 hours or longer, including both early subjective symptoms and objective signs typical of abstinence
- Elimination of drug hunger or craving
- Blockade of euphoric effects of self-administered opioids (This is not a true blockade like that achieved by naltrexone but reflects cross-tolerance for other opioids, attenuating or eliminating desired sensations when illicit or prescription opioids are self-administered in usual “street doses.” The increasing purity of heroin and availability of highly potent prescription opioids have made it increasingly difficult to achieve complete blockade in patients through cross-tolerance; consequently, some patients require dosages considerably greater than 120 mg per day to achieve this effect.)
- Tolerance for the sedative effects of treatment medication, creating a state in which patients can function normally without impairment of perception or physical or emotional response
- Tolerance for most analgesic effects produced by treatment medication (see “Pain Management” in chapter 10).
Unfortunately, no exact way exists to determine optimal dosage for each patient. However, the consensus panel recommends that OTPs avoid exclusive reliance on drug test results and preconceived notions of correct dosage; instead, OTPs should determine dosage based primarily on patient response. Even when a medication dosage is controlled for body weight (Leavitt et al. 2000), patient responses, such as absence of withdrawal symptoms without oversedation and remission from illicit-opioid use, are the best indicators of appropriate dosage. In addition, the extent of other drug use and alcohol consumption should be considered when determining dosage adequacy. Finally, a patient's complaints (or lack thereof) are also important indicators of dosage adequacy. A patient can experience opioid craving or withdrawal but manage to abstain from illicit opioids.
Methadone. Strong evidence supports the use of daily methadone doses in the range of 80 mg or more for most patients (Strain et al. 1999), but considerable variability exists in patient responses. Some do well on dosages below 80 to 120 mg per day, and others require significantly higher dosages (Joseph et al. 2000). OTPs should exercise additional caution with higher dosages, guarding against diversion of take-home methadone to individuals who are opioid intolerant because higher dosages can be lethal for such individuals.
Buprenorphine. Buprenorphine dosage should be determined in a manner similar to that used for methadone or LAAM. The recommended dosage of buprenorphine to begin stabilization is 12 to 16 mg per day for most patients, with increases provided thereafter as applicable (Johnson et al. 2003b ). As reviewed by Johnson and colleagues (2003b), if patients continue to show evidence of opioid abuse or withdrawal, the dosage should be increased using the same types of guidelines as for methadone. For example, if the goal is to suppress opioid withdrawal symptoms, then dose increases can be less frequent (e.g., weekly or biweekly) because the desired therapeutic response likely will become detectable more slowly.
Most patients are likely to remain stable on 12 to 24 mg per day, although some might need dosages of up to 32 mg per day. Increasing the buprenorphine dosage to 24 mg per day or higher has been shown to prolong the duration of its effects and usually is necessary if patients are to be dosed every other day, which is an option with buprenorphine; however, such an increase usually does not increase buprenorphine's opioid agonist effects to the same degree because of its partial agonist properties (Johnson et al. 2003b ). Because buprenorphine is a partial agonist, patients who continue to abuse opioids after sufficient exposure to buprenorphine treatment and ancillary psychosocial services or who experience continued symptoms of withdrawal at optimal daily doses of buprenorphine (12 to 32 mg) should be considered for therapy with methadone or LAAM (CSAT 2004a ; Johnson et al. 2003b ).
As with all medications used for MAT, when buprenorphine dosage changes are contemplated, the intensity and frequency of other available psychosocial services (see chapter 8) affect patients' ability to refrain from opioid abuse (Bickel et al. 1997) and should be considered.
LAAM. Most patients who begin LAAM are being transferred from methadone and should have been screened for cardiac risk. Equivalency dosing tables for methadone and LAAM are available in the ORLAAM® package insert (Roxane Laboratories, Inc., 2001), and transfer can be done easily. Because of the long-acting nature of LAAM, a patient's reaction should be monitored closely during the first 2 weeks of treatment and adjustments in dosage made accordingly.
Patients may request transfer from methadone to LAAM for various reasons: (1) to avoid the hardship of methadone's daily observed dosing, (2) to provide negative drug test results at work (LAAM is less likely to show up on screening tests), (3) because they are not doing well on methadone (Borg et al. 2002), (4) because LAAM can be less sedating, and (5) because the patients are rapid metabolizers of methadone and would benefit from LAAM because it is longer acting.
LAAM can be given every other day if an OTP is open all week or three times per week (i.e., two 48-hour doses and one 72-hour dose) if that is more convenient. Although some patients take the same dose on Monday, Wednesday, and Friday, most benefit from an increase on Friday (i.e., 10 to 40 percent more than the Monday and Wednesday doses) with or without an additional small dose of methadone to be taken home and used on Sunday. For stable patients, the best option is a regular LAAM dose on Friday and a full methadone dose (80 percent of the LAAM dose) as a take-home dose for Sunday. The efficacy of LAAM dosing is determined clinically and by patient history and examination; an affordable means to determine blood levels of LAAM and its metabolites is unavailable at this writing.
Naltrexone. Naltrexone can be administered either daily (usually at a dosage of 50 mg per day) or thrice weekly. For the latter, the usual practice is to give 100 mg on Monday and Wednesday and 150 mg on Friday (Stine et al. 2003).
Studies of the importance of dosing
Much evidence shows a positive correlation between medication dosage during MAT and treatment response (e.g., Strain et al. 1999). Higher dosages in some studies probably produced greater cross-tolerance. Cross-tolerance occurs when medication diminishes or prevents the euphoric effects of heroin or other short-acting opioids so that patients who continue to abuse opioids no longer feel “high.” The medication dosage needed for this result depends on how long and how recently a patient has abused heroin or other opioids and how much he or she has used, along with individual differences in the level of brain receptor adaptation induced by chronic opioid use.
An Australian study connected the importance of dosage with patient retention in MAT (Caplehorn and Bell 1991). The importance of retention for successful treatment outcomes is discussed further in chapter 8. In addition to the benefits of eliminating illicit opioids (see below), reductions in the threats of HIV and hepatitis B and C make adequate dosing and treatment retention high priorities and justify additional studies on the safety and efficacy of methadone doses exceeding 120 mg.
In their classic study, Ball and Ross (1991) clearly demonstrated an inverse relationship between frequency of recent heroin use and methadone dosage. The data in Exhibit 5-3 are based on their study of 407 patients who received methadone maintenance treatment. These data support the premise that lower methadone dosages are less effective than higher or adequate dosages in facilitating abstinence from heroin among patients in MAT. The low end of the effective range has been accepted widely as about 60 mg for most patients (reviewed in Faggiano et al. 2003).
Another study (Maxwell and Shinderman 2002) monitored 144 patients who were not doing well at 100 mg of methadone per day and reported excellent results after raising dosages based on clinical signs and symptoms. Patients receiving more than 200 mg per day (mean 284.9 mg per day) had improved responses with no apparent increase in adverse events. However, additional controlled research is needed to determine the safety of very high doses of methadone or other medications used in MAT.
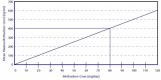
With the increased availability of blood testing in OTPs, measurements of blood concentrations of methadone at peak and trough are used more commonly as aids to determine individual methadone dosage requirements. A study in England (Wolff et al. 1991) showed a positive correlation between methadone dosages and concentrations in serum (Exhibit 5-4). Moreover, mean SMLs near or above 400 ng/mL are gaining increasing consensus as ideal levels for treatment effectiveness (Payte et al. 2003). Although mean SMLs of 400 ng/mL generally are considered to be sufficient to block the effects of illicit opioids and prevent withdrawal symptoms, some patients may require higher SMLs for stabilization. More research is needed to understand better the relationship between methadone blood levels and cessation of opioid abuse. SML results should continue to be considered along with patient symptoms. For example, a patient with an SML below 400 ng/mL with no symptoms of discomfort would not require a dosage increase, whereas a patient with an SML of 600 ng/mL but with persisting withdrawal symptoms would.
Figure
Exhibit 5-4. Methadone Dose/Mean Plasma Levels. Adapted from Wolff et al. (1991) by permission of the AACC.
Okruhlica and colleagues (2002) investigated 69 patients receiving methadone dosages of 10 to 270 mg per day and found a significant positive relationship between dosage and mean SMLs, although, at each dosage level, patients' resulting SMLs differed widely. Some had relatively low (subtherapeutic) SMLs, even at daily doses considerably above 100 mg, which would be expected to affect treatment negatively (Leavitt et al. 2000). Given these and similar data, it is incorrect to conclude that a particular methadone dosage causes a specific SML; many other factors are likely to affect SMLs for individual patients. However, measuring SMLs can be useful to determine why a relatively high methadone dosage does not appear to benefit a patient. In such cases, a blood test may show that a patient's SML remains low and that he or she requires a higher dose.
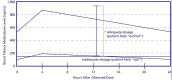
In their review, Leavitt and colleagues (2000) noted a broad range of SMLs among patients in MAT. They suggested that individual differences in metabolic enzyme activity and other factors may lead to higher or lower serum levels of the (R)-methadone enantiomer, explaining some of the variation in dosage ranges needed for clinical effectiveness. In one study of the clinical uses of methadone blood level measurements, it was suggested that the peak level should be no more than twice the trough level and that, if it is more, the patient should be considered a “fast metabolizer” and be administered split dosing. When split dosing is used, patients receive two or three doses per day to achieve the targeted peak-to-trough ratio in blood level measurements and to avoid withdrawal symptoms for 24 hours (Payte et al. 2003). Exhibit 5-5 shows 24-hour SML curves at both inadequate and adequate dosages. These curves include peak SMLs at roughly 4 hours after dose ingestion (0 hour) and trough SMLs at 24 hours after ingestion. Data were derived by averaging a series by Inturrisi and Verebely (1972) and another one by Kreek (1973). Exhibit 5-6 shows an example of plasma levels in a fast metabolizer, illustrating that when a day's dose is split into two (lower curve), the peak SML achieved after each of the two split doses is lower than the peak achieved after a single daily dose (upper curve), and the trough SML reached just before the next split dose is higher than the trough level reached just before the next single dose.
Figure
Exhibit 5-5. Blood Plasma Levels Over 4 and 24 Hours With an Adequate and Inadequate Methadone Dose.

Figure
Exhibit 5-6. SMLs After Single and Split Methadone Dosing in a Fast Metabolizer.
The consensus panel recommends that a maintenance dosage of methadone not be predetermined or limited by policy if that policy does not allow adjustments for individual patients.
Other common dosing issues
Signs and symptoms associated with lesser degrees of withdrawal and acute opioid overdose are well known, but patient changes associated with overmedicating and undermedicating are less dramatic and often more subjective.
Certain medical factors may cause a patient's dosage requirements to change, including (but not limited to) starting, stopping, or changing the dosage of other prescription medications; onset and progression of pregnancy; onset of menopause; progression of liver disease; significant increase or decrease in weight; or aging (elderly patients are sometimes more sensitive to drugs such as opioids). Patient complaints of opioid craving, withdrawal symptoms, medication side effects, or intoxication always should be investigated and never should be dismissed.
Overmedication . Mildly to moderately overmedicated patients might show “nodding” and closing of the eyes or might fall asleep at inappropriate times. These patients might scratch their faces continuously, especially their noses. In some cases, sedation might occur but be unapparent, and some overmedicated patients might feel mildly stimulated. Nausea also can occur, particularly in newer patients. Patients should be told when overmedication is suspected, and their dosage should be reduced. Patients also might report feeling high or “loaded” and ask for a reduced dosage. Such a reduction can be helpful for patients committed to abstinence rather than ongoing medication maintenance because they may find physical reminders of intoxication discouraging, frightening, or relapse triggering.
Vomited doses. Patients who report that they have vomited their medication pose special problems. The consensus panel recommends that only doses lost to witnessed emesis be replaced. Emesis 30 minutes after dosing can be handled by reassuring patients that the full dose has been absorbed. Emesis at 15 to 30 minutes after dosing can be handled by replacing half the dose, and the whole dose should be replaced if emesis occurs within 15 minutes of dosing. If vomiting persists, it is important to remember that only a portion of the gut is emptied with forceful emesis; therefore, the risk of accumulated toxicity increases with repeated dose replacements. Causes of emesis—including pregnancy—should be explored. Ingestion of smaller amounts of medication over a few minutes can be helpful and prudent, as can the occasional use of antiemetic medicines.
“Triggered” withdrawal. Environmental cues, including people, places, things, and feelings associated with drug taking, can be associated strongly with opioid craving and withdrawal. Such reactions may be identical to opioid withdrawal symptoms and can stimulate drug craving and relapse long after opioid use has stopped and physical dependence has been controlled (Self and Nestler 1998). Environmental changes and other stressors can cause patients to perceive that a dose on which they were stabilized is no longer adequate and to experience increased drug craving. Events that increase the availability of substances of abuse, such as another person who uses drugs moving into a patient's home or new sources of illicit drugs, can intensify craving. When their discomfort resumes after a period of abstinence, patients might feel that they are weak willed. They need reassurance that this reaction is a condition of their brain chemistry, not a weakness of will. In animal models, withdrawal symptoms have been conditioned to appear with environmental cues after months of abstinence from opioids (Self and Nestler 1998). The consensus panel believes that increased medication dosages are appropriate in such cases, although efforts also should focus on resolving the troublesome situations such as developing ways to avoid people, places, and things that trigger opioid craving or relapse. Conversely, diminished triggers and reduced drug availability can diminish drug craving and might indicate the possibility of decreasing medication dosage if a patient prefers.
Contingent use of dosage. The consensus panel believes that any manipulation of dosage as either a positive or a negative consequence of behavior is inappropriate and has no place in MAT. The only type of contingency contracting related to medication that should be supported in MAT is that associated with take-home medication. Take-home medication is controlled by Federal regulations, and access is based on several factors, including drug abstinence, OTP attendance, length of time in treatment, and overall functioning. An increase in medication dosage should not be a reward for positive behavior change, although not everyone in the MAT field shares this viewpoint. For example, extensive work has demonstrated the effectiveness of using increased dosage (as well as extra take-home doses) as an incentive to decrease substance abuse and increase treatment program attendance (e.g., Stitzer et al. 1986, 1993; see also Petry 2000). Although the consensus panel acknowledges important behavioral aspects of addiction and the value of contingency management as an aid to behavioral change, using medication dosage as a reward or punishment is considered inappropriate.
Maintenance Pharmacotherapy
The maintenance stage of opioid pharmacotherapy begins when a patient is responding optimally to medication treatment and routine dosage adjustments are no longer needed. Patients at this stage have stopped abusing opioids and other substances and have resumed productive lifestyles away from the people, places, and things associated with their addictions. These patients typically receive scheduled take-home medication privileges. Patients who continue to abuse substances, do not seek employment, or remain connected to their drug-using social networks have not reached this stage. Along with continued observed medication treatment, these latter patients are candidates for intensified counseling and other services to help them reach the maintenance stage.
During the maintenance stage, many patients remain on the same dosage of treatment medication for many months, whereas others require frequent or occasional adjustments. Periods of increased stress, strenuous physical labor, negative environmental factors, greater drug availability, pregnancy, or increased drug hunger can reawaken the need for increased dosages over short or extended periods. Serious emotional crises may require long-term or temporary dosage adjustments. Although the counseling relationship and patient interview are paramount, drug test reports and medication blood levels are useful for dosage determination and adjustment during and after transition from stabilization to the maintenance stage.
Medically Supervised Withdrawal
When stable patients in the maintenance stage ask for dosage reductions, it is important to explore their reasons. They might believe that they can get by on less medication, or they might be responding to external pressures. Patients often perceive that those on lower dosages are “better patients.” These situations require physicians or other staff members to educate patients and their significant others about the importance of adequate dosage and how individual differences in absorption, body weight, metabolism, and tolerance can affect the dosage necessary to achieve stability (Leavitt et al. 2000).
Voluntary Tapering and Dosage Reduction
For various reasons, some patients attempt reduction or cessation of maintenance medication. Some studies indicate high relapse rates, often 80 percent or more, for this group, including patients judged to be rehabilitated before tapering (e.g., Magura and Rosenblum 2001). However, the likelihood of successful dose tapering also depends on individual factors such as motivation and family support. The possibility of relapse should be explained to patients who want to dose taper, especially those who are not stable on their current dosage, as part of the informed-consent process. Patients who choose tapering should be monitored closely and taught relapse prevention strategies. They and their families should be aware of risk factors for relapse during and after tapering. If relapse occurs or is likely, additional therapeutic measures can be taken, including rapid resumption of MAT when appropriate (American Society of Addiction Medicine 1997).
Ideally, withdrawal should be attempted when it is desired strongly by a stable patient who has a record of abstinence and has adjusted positively on MAT. However, sometimes dose tapering is necessary for administrative reasons, such as a response to extreme antisocial behavior, noncompliance with minimal program standards, or a move to a location where MAT is unavailable. In such cases, providers should refer patients to other programs that are more reasonable and practical in terms of the patients' overall situation (e.g., motivation, resource availability, ability to pay).
In a review of research on withdrawal from MAT, Magura and Rosenblum (2001) noted that many treatment providers lacked effective ways to improve outcomes for patients who undertook planned withdrawal and that opioid craving remained prevalent in this group, even after successful physiological withdrawal. They concluded, therefore, that planned withdrawal from opioid pharmacotherapy should be undertaken conservatively.
Relapse prevention techniques should be incorporated into counseling and other support services both before and during dosage reduction. Such structured techniques can be useful safeguards in preventing and preparing for relapse. Use of mutual-help techniques (see chapter 8) is recommended highly, especially during dosage reduction.
Although most data about outcomes after tapering from opioid medication come from studies of methadone maintenance, the consensus panel believes that success rates are likely to be similar for patients who taper from buprenorphine or LAAM, and similar cautions and monitoring processes should be in place.
Methadone dosage reduction
The techniques and rates of graded methadone reduction vary widely among patients. One common practice is to reduce daily doses in roughly 5- to 10-percent increments with 1 to 2 weeks between reductions, adjusting as needed for patient conditions. Because reductions become smaller but intervals remain about the same, many months may be spent in such graded reductions. The rate of withdrawal can be increased or decreased based on individual patient response. A slow withdrawal gives patients and staff time to stop the tapering or resume maintenance if tapering is not working and relapse seems likely.
Regardless of the rate of withdrawal from methadone, a point usually is reached at which steady-state occupancy of opiate receptors is no longer complete and discomfort, often with drug hunger and craving, emerges. This point may occur at any dosage but is more common with methadone when the dosage is below 40 mg per day. Highly motivated patients with good support systems can continue withdrawal despite these symptoms. Some patients appear to have specific thresholds at which further dosage reductions become difficult.
Physicians and other staff members should be alert to the possibility of patients attempting dose tapering by substituting other psychoactive substances, such as alcohol, cocaine, sedatives-hypnotics, or other nonopioid substances for their maintenance medication.
Some patients might request blind dosage reduction, that is, withdrawal from medication without their awareness of dose reductions at each step. Blind dosage reduction is appropriate only if requested by a patient. It should be discussed and agreed on before it is implemented. It is inappropriate, clinically and ethically, to withdraw a patient from maintenance medication without his or her knowledge and consent. The consensus panel recommends that OTP staff always disclose dosing information unless patients have given specific informed consent and have requested that providers not tell them their exact dosages.
Withdrawal and termination from LAAM maintenance
Few studies have addressed medically supervised withdrawal from LAAM. Because LAAM is longer acting than methadone, withdrawal should be expected to have a delayed onset and protracted course, although symptoms might be less intense than with other opioids. Patients tend to dislike longer periods of withdrawal, regardless of symptom intensity. Special counseling might be needed to address this aspect of withdrawal from LAAM.
For patients on LAAM who wish to be medication free, dosage can be reduced gradually at a rate determined by their response. Patients who prefer less protracted withdrawal can be converted to and then tapered from methadone. As with tapering from methadone (Moolchan and Hoffman 1994), tapering from LAAM should take into account a patient's level of stability, past functioning without medication, and fear of withdrawal.
Medically Supervised Withdrawal After Detoxification
For patients who neither qualify for nor desire opioid maintenance treatment, methadone or buprenorphine may be used to control withdrawal from illicit opioids or from abuse of prescription opioids (detoxification) and then can be tapered gradually (medically supervised withdrawal). Regulations specify two kinds of detoxification with methadone: short-term treatment of less than 30 days and long-term treatment of 30 to 180 days. These regulations specify that patients who fail two detoxification attempts in 12 months must be evaluated for a different treatment (42 CFR, Part 8 § 12(e)(4)).
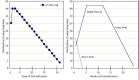
Dosing decisions in medically supervised withdrawal are related to the intended steepness of tapering. Patients undergoing short-term withdrawal may never achieve steady state, and tapering from methadone may be too steep if it begins at a dose greater than about 40 mg. In long-term withdrawal, stabilization of dosage at a therapeutic range is followed by more gradual reduction (see Exhibit 5-7).
Figure
Exhibit 5-7. Types of Detoxification From Illicit Opioids.
Involuntary Tapering or Dosage Reduction
When patients violate program rules or no longer meet treatment criteria, involuntary tapering might be indicated although it should be avoided if possible (see chapter 8). For example, if many days of dosing are missed and repeated attempts to help a patient comply with daily dosing requirements have failed, maintenance pharmacotherapy no longer may be possible. Treatment decisions should be made in the patient's best interest. If patient progress is unsatisfactory at a particular level of care, the physician should explore the possibility of increasing that patient's care while maintaining him or her on methadone. Involuntary tapering and discontinuation of maintenance medication may be necessary if a patient is unwilling to comply with treatment or tapering or discontinuation of medication appears to be in the patient's best interest.
If a patient is intoxicated repeatedly with alcohol or sedative drugs, the addition of an opioid medication is unsafe, and any dose should be withheld, reduced, or tapered. Disruptive or violent behavior or threats to staff and other patients might be reasons for dismissal without tapering or for immediate transfer to another facility where a patient may be treated under safer conditions.
Administrative tapering for nonpayment of fees may be part of the structure to which patients agree on admission. It should be noted that, in addiction treatment, a patient's sudden lack of funds is a marker of possible relapse.
LAAM
When involuntary withdrawal from LAAM is unavoidable, patients can be transferred to methadone before withdrawal because clinical experience with methadone withdrawal is more extensive.
Incarceration
When patients know that they must serve time in jail or prison, planned withdrawal is the best course of action. At this writing, few correctional institutions offer methadone maintenance to nonpregnant inmates (National Drug Court Institute 2002). Many jails do not provide methadone for detoxification. When a patient in MAT is arrested, program staff should make every effort to communicate with the criminal justice authorities involved and to recommend that the patient be withdrawn gradually from medication. Regardless of which opioid medication is used, maintenance or medically supervised withdrawal is preferable to sudden discontinuation of the medication. The consensus panel recommends that opioid pharmacotherapy be made available during incarceration for patients who are already in MAT when incarcerated.
Take-Home Medications
Take-home medication refers to unsupervised doses. Any OTP patient may receive a single take-home dose for a day when the OTP is closed for business, including Sundays and State and Federal holidays. Beyond this, decisions on dispensing take-home medication are determined by the medical director in accordance with eight criteria for take-home medication specified in Federal regulations (42 CFR, Part 8 § 12(i)):
- Absence of recent drug and alcohol abuse
- Regular OTP attendance
- Absence of behavioral problems at the OTP
- Absence of recent criminal activity
- Stable home environment and social relationships
- Acceptable length of time in comprehensive maintenance treatment
- Assurance of safe storage of take-home medication
- Determination that rehabilitative benefits of decreased OTP attendance outweigh the potential risk of diversion.
Once these clinical criteria are met, maximum take-home doses must be further restricted based on length of time in treatment as follows:
- First 90 days (months 1 through 3): one take-home dose per week
- Second 90 days (months 4 through 6): two take-home doses per week
- Third 90 days (months 7 through 9): three take-home doses per week
- Fourth 90 days (months 10 through 12): 6 days' supply of take-home doses per week
- After 1 year of continuous treatment: 2 weeks' supply of take-home medication
- After 2 years of continuous treatment: 1 month's supply of take-home medication, but monthly OTP visits are still required.
Additional restrictions are imposed in some States. No take-home doses are permitted for patients in short-term detoxification or interim maintenance treatment.
Specific Clinical Considerations in Take-Home Status
Demands of a concurrent medical disorder
The existence and severity of a concurrent medical disorder (see chapter 10) are additional considerations in determining whether take-home medication is appropriate. For patients with concurrent diseases causing impaired ambulation, reduced OTP attendance might be required to aid recovery and prevent complications. In these cases, OTPs should consider seeking medical exceptions for patients who would not otherwise be permitted to receive take-home doses of medication. These patient exceptions should be requested on Substance Abuse and Mental Health Services Administration (SAMHSA) form SMA-168, Exception Request and Record of Justification. Form SMA-168 is available at http://dpt.samhsa.gov/webintro.htm.
When a new medication treatment—such as rifampin, highly active antiretroviral therapy (HAART), or phenytoin—that is known to interact with an opioid treatment medication is introduced, a MAT patient might need a dosage adjustment (see chapter 3 for further discussion of medications that interact with opioid treatment medications). Take-home medication should be avoided until a patient is stable on these new medications and the risks of an undesirable outcome have diminished. In these instances, more frequent observations are important to monitor concurrent disease, to avoid methadone-related complications of a concurrent medical disorder, and to ensure that the pharmacological benefits of administering methadone are maintained during the course and treatment of the concurrent disease.
Enhancement of rehabilitative potential
Another important issue in take-home medication involves reviewing whether it is likely to help rehabilitate a patient. Take-home medication may enable patients to engage in employment, education, childcare, or other important endeavors.
Emergency circumstances
During emergency situations or unforeseen circumstances such as personal or family crises; bereavement; or medical, family, or employment hardships, the need may arise for unscheduled take-home medication. An OTP can facilitate emergency or hardship access to medication for a patient by submitting SAMHSA form SMA-168. The OTP's policies should explain who can request exceptions and how it is done. Courtesy dosing at a distant OTP usually can be arranged if unstabilized patients are traveling.
Positive drug tests, diversion control, and take-home medications
The consensus panel believes that take-home medications are inadvisable for patients who continue to abuse illicit drugs or misuse prescription medications, as evidenced by drug testing or other assessment information, and for those whose drug tests do not reflect medication ingestion. Under the disinhibiting effects of other substances, patients might be unable to safeguard or adequately store their take-home doses. They should be encouraged to keep their medication in a locked cabinet away from food or other medicines and out of the reach of children. Some programs require patients to bring a locked container to the OTP when they pick up their take-home medication to hold it while in transit. This policy should be considered carefully because most such containers are large and visible, which might serve more to advertise that a patient is carrying medication than to promote safety.
Methadone is stable and does not need refrigeration when in diskette or tablet form. However, when methadone diskettes are reconstituted or liquid methadone oral concentrate is used and diluted with juice or some other sugar-based liquid, the mixture may not remain stable beyond a few days without refrigeration. Manufacturer instructions call for adding a minimum of 30 mL or 1 fluid ounce of liquid per dose when reconstituting methadone.
Although methadone has a significant street value, a National Institutes of Health consensus statement refers to it as “a medication that is not often diverted to individuals for recreational or casual use but rather to individuals with opiate dependence who lack access to [methadone maintenance treatment] programs” (National Institutes of Health 1997b , p. 20). Nevertheless, reported deaths attributed to methadone have increased significantly in some States. According to data from the Drug Abuse Warning Network, more than 10,000 emergency room visits related to methadone were reported in 2001 compared with more than 5,000 in 1999 (Crane 2003). This increase has occurred in the context of overall increases in abuse of prescription opioids, in particular hydrocodone and oxycodone. Local reports indicate that most diverted methadone comes from medical prescriptions because it has gained acceptance as an excellent chronic pain treatment (Belluck 2003). Although the slow onset of methadone makes it less attractive than prescription opioids to potential abusers, it also makes methadone more dangerous because respiratory depression can become significant hours after ingestion. To guard against the possibility of methadone-related respiratory depression, the consensus panel recommends the following diversion control policies for take-home medication:
- Require patients to return all empty dose bottles on their next OTP visit after take-home dosing. Staff members who accept these bottles should inspect them to ensure that they are coming from the indicated patient during the appropriate period.
- Institute procedures for responding to patients who frequently fail to return or have unverified reasons for failing to return empty take-home bottles. Staff should consider discontinuing take-home medication for these patients.
- Stay open 7 days a week for dispensing. In this way, take-home doses can be provided only to stable patients with a record of adherence to treatment, rather than to all patients regardless of their status with the program.
Behavior, social stability, and take-home medications
Patients appearing intoxicated; demonstrating aggressive, seriously impaired, or disordered behavior; or engaging in ongoing criminal behavior are poor candidates for take-home medication. Their home environments also are keys to the safety and storage of medication. Where social relationships are unstable, a significant risk exists that methadone take-home doses will be secured inadequately from diversion or accidental use (e.g., by children). If patients with take-home privileges develop altered mental competency, such as in dementia, frequent loss of consciousness, or delusional states, then take-home privileges should be reevaluated.
Monitoring Patients Who Receive Take-Home Medications
Monitoring should ensure that patients with take-home medication privileges are free of illicit drug use and consume their medication as directed. This goal can be met through random drug testing and periodic interdisciplinary assessment of continuing eligibility. OTPs should consider carefully whether to use pill counts or callbacks of dispensed take-home doses to verify adherence to program rules. In a pill count or callback, the patient receives an unannounced phone call and must show up at the OTP within a reasonable period (e.g., 24 to 36 hours) with all MAT medications. The number of pills remaining must correspond to the number expected based on prescribed ingestion. A physician should review periodically the status of every patient provided with take-home medication. When these strategies are followed, programs should state their policies clearly to patients. Callbacks should be used selectively, not be applied across the board, and focus on high-risk patients who have given OTP staff members reason to be concerned.
Issues for review
The rationale for providing take-home medication should be reviewed regularly and documented to determine whether initial justifications continue to apply. For example, if employment was a reason for take-home medication, the patient's continued employment should be verified. If a concurrent medical disorder was the basis, a medical reassessment is necessary to determine whether the clinical status of the concurrent medical disease still warrants reduced OTP attendance.
Reviewing the original rationale for take-home medication is a necessary but insufficient condition for increased patient monitoring. The monitoring process also should include an assessment of whether medical, psychological, or social reasons exist to rescind these privileges.
Treatment interruptions
Many circumstances, such as work-related travel, illness, funerals, planned vacations, and emergencies, might require patients to miss OTP visits. Some unstable patients might miss days because of chaotic social situations. OTPs should have policies to address treatment interruptions.
Disability or illness. When disability or illness prevents patients from coming to the OTP, authorized staff may use home delivery and observed-dosing procedures to ensure treatment continuity. OTPs should evaluate the need for continuity of other support services, as well as medication, in these circumstances.
Hospitalization. OTPs are responsible for ensuring continuity of treatment when patients are hospitalized for medical or psychiatric problems (see chapters 10 and 12). The best practice is for OTP staff to educate and stay in touch with a patient's hospital clinicians about MAT. For example, hospital staff might be unaware that certain drugs, such as partial agonists or mixed agonists and antagonists for pain management, should be avoided for patients receiving LAAM or methadone for opioid addiction (pain management is discussed in chapter 10). It usually is helpful to provide psychiatric consultation to medical or surgical staff members, especially for patients with co-occurring disorders. Written patient consent is necessary for this kind of program-to-hospital communication; however, if a medical emergency poses a threat to a patient's health, the OTP should use the medical emergency exception for treatment when it lacks patient consent. A publication by the Center for Substance Abuse Treatment (CSAT 2004b ) provides a description of the confidentiality regulations' medical emergency exception.
Hospitalization, particularly of unconscious patients, raises the issue of using identification (ID) cards. Patients can get OTP-specific Medic Alert ID Cards from Advocates for Recovery Through Medicine (www.methadonetoday.org/armhelp.htm; telephone 615-354-1320), which can include a patient's name, OTP contact information, and a list of contraindicated medications. Some large urban OTPs provide patients with a photographic ID card to gain admittance to the OTP. Their experience has been that some patients use their OTP cards as generic photographic IDs in lieu of a driver's license; for example, they use them to cash checks, despite the fact that the cards identify them as being in treatment. Smart cards containing a complete medical history are already in use in the United States, Israel, and the Netherlands and may be useful in OTPs. These cards contain electronically encoded information needed to identify and monitor a patient without outwardly identifying the cardholder as a patient.
Missed doses. When doses are missed, it is critical to evaluate patients' presenting condition. Concerns should include whether a patient has been using illicit drugs or taking other medications, has lost tolerance for previous doses (i.e., whether a previously tolerated dosage is still safe to administer), or is intoxicated.
One dose missed. For patients who miss one scheduled dose and come to the OTP the next day—for example, 3 to 4 days after the last LAAM or 2 days after the last methadone dose—the dosage can remain unchanged, and dosing should resume on schedule. For patients on LAAM who miss a dose and come to the OTP 2 days later (i.e., 4 to 5 days after their last LAAM dose), the scheduled dose still is usually well tolerated.
More than 5 days missed. For patients who are out of treatment for a significant time and might have lost tolerance, dosage reduction or reinduction is advisable. Thereafter, increases of 5 to 10 mg per dose up to the previous level can be ordered because it is unlikely that the dosage needed to maintain stability will change in 1 week. Patients might have to be reminded about steady state and that they may not feel back to normal until tissue stores have built up as well.
Office-Based Opioid Therapy
OTPs should consider assisting with transfer arrangements for long-term methadone-maintained patients who prefer to use a physician in the community for ongoing care. Various forms of this treatment have been studied in the United States and found to be safe and efficacious (King et al. 2002; Schwartz et al. 1999).
Patient selection for this treatment option should focus on a history of negative drug tests, a required length of stability in treatment (at least 1 year), social stability, and minimal need for psychosocial services. Methadone can be ordered by private physicians, through an affiliation or other arrangement with an OTP, and patients can obtain their medication at specially registered pharmacies under a SAMHSA-approved protocol. Under this arrangement, patients on extended take-home-dosing schedules (up to 1 month) no longer must ingest their doses under observation. Outcomes have been uniformly positive, with few relapses and little or no diversion reported (King et al. 2002; Schwartz et al. 1999). Patient satisfaction has been found to be significantly better compared with OTP dosing (Fiellin et al. 2001) but not significantly different from a comparable OTP-based monthly medical maintenance and take-home schedule (King et al. 2002).
- Chapter 5. Clinical Pharmacotherapy - Medication-Assisted Treatment for Opioid A...Chapter 5. Clinical Pharmacotherapy - Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs
- Mus musculus pyroglutamyl-peptidase I (Pgpep1), mRNAMus musculus pyroglutamyl-peptidase I (Pgpep1), mRNAgi|31560232|ref|NM_023217.2|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...