NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2004. (Treatment Improvement Protocol (TIP) Series, No. 40.)
This publication is provided for historical reference only and the information may be out of date.

Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction.
Show detailsOverview
Office‐based treatment of opioid addiction has been unavailable in the United States since the early 1900s. Thus, most U.S. physicians today have little or no experience in the management of opioid addiction. As a consequence, physicians often treat substance‐related disorders (e.g., infectious diseases) without having the resources to treat the concurrent substance‐use disorder itself. With the introduction of buprenorphine, office‐based physicians now will have the ability to treat both the complications of opioid addiction and opioid addiction itself. (For articles on managing opioid‐dependent patients in the office setting, please see (Fiellin et al. 2001; Fiellin and O'Connor 2002; O'Connor et al. 1996, 1998)
Physicians who use buprenorphine to treat opioid addiction must consider the entire process of treatment, from induction, through stabilization, and then maintenance. At each stage of the process, many different factors must be considered if the physician is to provide comprehensive and maximally effective opioid addiction care. Physicians should conduct a comprehensive assessment to understand the nature of an individual’s addiction problem, especially with regard to the primary type of opioid abused. Before initiating buprenorphine treatment, physicians should obtain a signed release of information (see Title 42, Part 2 of the Code of Federal Regulations [42 C.F.R. Part 2]) from patients who are currently enrolled in Opioid Treatment Programs (OTPs) or other programs (42 C.F.R. Part 2 2001). (See “Confidentiality and Privacy” in chapter 6.) This chapter provides detailed protocols on the use of buprenorphine for the treatment of opioid addiction. The chapter begins with a discussion of some general issues regarding treatment with buprenorphine.
Buprenorphine Monotherapy and Combination Buprenorphine/Naloxone Treatment
The consensus panel recommends that the buprenorphine/naloxone combination be used for induction treatment (and for stabilization and maintenance) for most patients. However, pregnant women who are determined to be appropriate candidates for buprenorphine treatment should be inducted and maintained on buprenorphine monotherapy. In addition, patients who desire to change from long‐acting opioids (e.g., methadone, levo‐alpha‐acetyl‐methadol [LAAM]) to buprenorphine should be inducted using buprenorphine monotherapy.* If the buprenorphine monotherapy formulation is elected for induction treatment, it is recommended that patients who are not pregnant be switched to the buprenorphine/naloxone combination form as early in treatment as possible to minimize the possibility of diversion of Subutex® to abuse via the injection route. When the buprenorphine monotherapy formulation is used for induction, it is recommended that it be used for no more than 2 days before switching to the buprenorphine/naloxone combination formulation (for patients who are not pregnant). If buprenorphine alone is to be used for extended periods, the number of doses to be prescribed should be limited, and the use of the monotherapy formulation should be justified in the medical record.
Although controlled trials have not compared buprenorphine monotherapy to the buprenorphine/naloxone combination for induction, clinical experience in office‐based trials conducted by the National Institute on Drug Abuse (NIDA) has demonstrated that physicians were comfortable starting patients on either the monotherapy formulation or the combination formulation and did not report adverse events when patients began directly on combination treatment. Physicians will need to find their own comfort level with the induction protocols, but the consensus panel sees no contraindication to the use of the buprenorphine/naloxone combination in the initiation of buprenorphine treatment, except as noted above.
Opioid Withdrawal Syndrome With Buprenorphine Induction
Because buprenorphine (and particularly buprenorphine/naloxone) can precipitate an opioid withdrawal syndrome if administered to a patient who is opioid dependent and whose receptors are currently occupied by opioids, a patient should no longer be intoxicated or have any residual opioid effect from his or her last dose of opioid before receiving a first dose of buprenorphine.
Due to this required abstinence before initiating buprenorphine treatment, it is likely that patients will feel that they are experiencing the early stages of withdrawal when they present for buprenorphine induction treatment, unless they are on maintenance treatment with a long‐acting opioid agonist (e.g., methadone). If a patient has early symptoms of withdrawal, then the opioid receptors are unlikely to be occupied fully; precipitated withdrawal from administration of buprenorphine will be avoided, and the efficacy of buprenorphine in alleviating withdrawal symptoms can be assessed more easily.
Withdrawal symptoms can occur if either too much or too little buprenorphine is administered (i.e., spontaneous withdrawal if too little buprenorphine is given, precipitated withdrawal if buprenorphine is administered while the opioid receptors are occupied to a high degree by an opioid agonist). Therefore, physicians must be careful when timing initiation of buprenorphine induction. Each patient’s history and concerns must be considered carefully, and patient counseling about potential side effects from buprenorphine overdosing (especially in combination with benzodiazepines) or underdosing (e.g., a reemergence of opioid craving) must be emphasized. Before undertaking buprenorphine treatment of opioid addiction, physicians should be familiar with the signs, symptoms, and time course of the opioid withdrawal syndrome. (See figure 3-7.)
Method of Administration
Buprenorphine sublingual tablets should be placed under the tongue until they are dissolved. For doses requiring the use of more than two tablets, patients should either place all the tablets at once or alternatively, if they cannot fit in more than two tablets comfortably, place two tablets at a time under the tongue. Either way, the tablets should be held under the tongue until they dissolve; swallowing the tablets reduces the bioavailability of the drug. To ensure consistency in bioavailability, patients should follow the same manner of dosing with continued use of the medication. Dissolution rates vary, but, on average, the sublingual tablets should dissolve in approximately 5–10 minutes.
Treatment Approach
There are two general approaches to the medication‐assisted treatment of opioid addiction: (1) opioid maintenance treatment, and (2) medically supervised withdrawal (detoxification) with either opioid (e.g., methadone) or nonopioid (e.g., clonidine) medications. Because opioid‐assisted maintenance and medically supervised withdrawal treatments have not been available outside the OTP setting, many patients may not be aware that these forms of treatment are now available in new clinical settings. Thus, a discussion with patients of all available treatment options is essential.
For many patients, it may be inappropriate to decide arbitrarily on the length of treatment at initial evaluation. It is more likely that patients will need to be started in treatment within a flexible timeframe that responds to the progress and needs of the patient. For example, in one report of rapid‐term opioid detoxification using buprenorphine, it was noted that 25 percent of patients initially requesting detoxification subsequently switched to maintenance treatment within the 10‐day study (Vignau 1998). Thus, as treatment progresses, it may become a more appropriate time to assess the duration of various aspects of treatment, including medications, counseling therapies, and self‐help groups. Therefore, it is important to assess initially, and to reassess periodically, a patient’s motivation for treatment, as well as his or her willingness to engage in appropriate counseling and/or a structured rehabilitation program. (See “Assessment” in chapter 3.)
Maintenance Treatment With Buprenorphine
The three phases of maintenance treatment with buprenorphine for opioid addiction are (1) induction, (2) stabilization, and (3) maintenance. The following sections describe these phases.
Induction Phase
Buprenorphine induction (usual duration approximately 1 week), the first phase of treatment, involves helping a patient begin the process of switching from the opioids of abuse to buprenorphine. The goal of the induction phase is to find the minimum dose of buprenorphine at which the patient discontinues or markedly diminishes use of other opioids and experiences no withdrawal symptoms, minimal or no side effects, and no uncontrollable cravings for drugs of abuse. The physician should assess for signs and symptoms of withdrawal or inadequate dosing during induction. Patients should be advised to avoid driving or operating other machinery until they are familiar with the effects of buprenorphine and their dose is stabilized. Induction protocols differ, depending on the type of opioid to which the patient is addicted (e.g., short‐ or long‐acting) and whether or not the patient is in active withdrawal at the time of induction.
The consensus panel recommends that physicians administer initial induction doses as observed treatment (e.g., in the office); further doses may be provided via prescription thereafter. This ensures that the amount of buprenorphine located in the physician’s office is kept to a minimum. Following the initial buprenorphine dose, patients should be observed in the physician’s office for up to 2 hours. For patients who do not experience excessive opioid agonist symptoms after the initial dose, induction protocols can be followed as described below.
Induction Days 1 and 2: Who Is the Patient and What Does He or She Need?
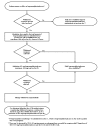
It is important to identify the opioid(s) that patients have been using, as the response to buprenorphine treatment in individuals dependent on long‐acting opioids is different than that seen with short‐acting opioids and, therefore, the appropriate induction protocol must be chosen. Most patients starting buprenorphine induction will be physically dependent on a short‐acting opioid (e.g., heroin, oxycodone, hydrocodone) and should be in the early stages of withdrawal at the time they receive their first dose of buprenorphine. (See figure 4-1 and appendix B.)
Figure
Figure 4-1. Induction Days 1–2.
Patients Dependent on Short‐Acting Opioids
Before the initial buprenorphine induction dose is administered to a patient dependent on short‐acting opioids, a minimum of 12–24 hours should have elapsed since the last use of opioids. The patient should preferably be exhibiting early signs of opioid withdrawal (e.g., sweating, yawning, rhinorrhea, lacrimation). (See figure 3-7.) Patients who are not in active withdrawal because they have not abstained from using opioids for a sufficient period should receive a careful explanation of the advantages of waiting and should be urged to wait until they begin to experience the symptoms of withdrawal.
Patients who are experiencing objective signs of opioid withdrawal and whose last use of a short‐acting opioid was more than 12–24 hours prior to the initiation of induction can receive a first dose of 4/1–8/2 mg of the buprenorphine/naloxone combination (buprenorphine monotherapy for pregnant women). (See figure 4-1.) If the initial dose of the buprenorphine/naloxone combination is 4/1 mg and opioid withdrawal symptoms subside but then return (or are still present) after 2 hours, a second dose of 4/1 mg can be administered. The total amount of buprenorphine administered in the first day should not exceed 8 mg.
Patients Dependent on Long‐Acting Opioids
Induction onto buprenorphine from long‐acting opioids (e.g., methadone, LAAM) may be complicated and is best managed by physicians experienced with this procedure. If this treatment will be conducted in an office‐based setting, the physician’s office must contact the patient’s OTP (after receiving signed consent) to determine the methadone or LAAM dosage levels and time of last dose. Such contact will ensure that the physician knows the exact quantity and time of the last methadone or LAAM dose, as well as prevent patients from receiving opioid agonist treatment (OAT) and office‐based buprenorphine treatment simultaneously. To allow this exchange of addiction treatment information per Federal confidentiality regulation 42 C.F.R. Part 2 (see “Confidentiality and Privacy” in chapter 6), the patient must provide signed consent to both the OTP and the buprenorphine‐treating physician.
For patients taking methadone, the methadone dose should be tapered to 30 mg or less per day for a minimum of 1 week before initiating buprenorphine induction treatment. Patients should not receive buprenorphine until at least 24 hours after the last dose of methadone. The first dose of buprenorphine should be 2 mg of the monotherapy formulation. (See figure 4-1.) If a patient develops signs or symptoms of withdrawal after the first dose, a second dose of 2 mg should be administered and repeated, if necessary, to a maximum of 8 mg buprenorphine on Day 1.
It should be noted that not all patients maintained on methadone may be good candidates for the switch to buprenorphine treatment at a methadone dose of 30 mg/day. As a methadone taper approaches 30 mg/day many patients become uncomfortable, develop withdrawal symptoms, and are at increased risk of relapse to opioid abuse. Such patients may request the transfer to buprenorphine at higher daily doses of methadone. The decision to transfer a patient to buprenorphine at higher daily methadone doses should be based on clinician judgment, informed by the patient’s subjective and objective findings. While there have been case reports of transferring patients to buprenorphine from methadone doses as high as 80 mg/day, there is insufficient data to formulate recommendations regarding which patients may be able to tolerate a switch at these higher doses or the best way to manage the transfer.
No clinical experience with inducting patients from LAAM to buprenorphine is documented. However, extrapolating from consensus panel members’ experience with such patients, the panel recommends that the dose of LAAM be tapered down to 40 mg or less per 48‐hour dose, and buprenorphine induction should not be undertaken until at least 48 hours after the last dose of LAAM. Induction should then proceed in the same manner and at the same dosage levels as recommended for methadone patients.
Induction Management When Withdrawal Symptoms Are Not Relieved by 8 mg Buprenorphine in the First 24 Hours
If withdrawal symptoms are still not relieved after a total of 8 mg of buprenorphine on Day 1, symptomatic relief with nonopioid medications should be provided and the patient asked to return the following day for dose management. (See “Induction Day 2 and Forward” below.)
Patients Not Physically Dependent on Opioids
Patients who are not physically dependent on opioids but who have a known history of opioid addiction, have failed other treatment modalities, and have a demonstrated need to cease the use of opioids, may be candidates for buprenorphine treatment. Patients in this category will be the exception rather than the rule, however. Other patients in this category would be those recently released from a controlled environment who have a known history of opioid addiction and a high potential for relapse.
Patients who are not physically dependent on opioids should receive the lowest possible dose (2/0.5 mg) of buprenorphine/naloxone for induction treatment.
Induction Day 2 and Forward
If buprenorphine monotherapy was administered on Day 1, switch to buprenorphine/naloxone on Day 2 (for a patient who is not pregnant).
For patients who do not experience any difficulties with the first day of buprenorphine dosing, and who are not experiencing withdrawal symptoms on Day 2, the induction schedule shown in figure 4-2 can be followed. The daily buprenorphine/naloxone dose is established as equivalent to the total amount of buprenorphine/naloxone (or buprenorphine) that was administered on Day 1. Doses may be subsequently increased in 2/0.5 to 4/1 mg increments each day, if needed for symptomatic relief, with a target dose of 12/3 to 16/4 mg per day to be achieved within the first week, unless side effects occur. If side effects occur, the dose of buprenorphine should be maintained or lowered until these side effects disappear.
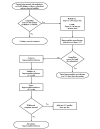
Stabilization Phase
The induction phase is completed and the stabilization phase (usual duration approximately 1 to 2 months) is begun when the patient is experiencing no withdrawal symptoms, is experiencing minimal or no side effects, and no longer has uncontrollable cravings for opioid agonists. (See figure 4-3.) As with any pharmacotherapy, the goal of buprenorphine treatment is to treat with the minimum dose of medication needed to address target signs, symptoms, desired benefits, and laboratory indices while minimizing side effects. Elimination of objective evidence of opioid use (negative toxicology) represents the key target sign for which to strive. The goal is to reduce self‐reported cravings and self‐reported use of illicit opioids. One benefit worth achieving is a self‐reported increase in opioid blockade such that self‐administered illicit opioids induce little or no euphoria. A reduction in opioid‐positive toxicology specimens confirms a successful direction in treatment.
Maintenance Phase
The longest period that a patient is on buprenorphine is the period of maintenance. This period may be indefinite. It is easy for physicians to lessen their vigilance during this period, but significant considerations still must be addressed. Attention must be maintained to the psychosocial and family issues that have been identified during the course of treatment. Other issues that will need continual monitoring are related to cravings for opioids and to preventing relapse. Some other issues related to opioid abuse that need to be addressed during maintenance treatment include, but are not limited to, the following:
- Psychiatric comorbidity
- Somatic consequences of drug use
- Family and support issues
- Structuring of time in prosocial activities
- Employment and financial issues
- Legal consequences of drug use
- Other drug and alcohol abuse
The frequent presence of some or all of these problems underscores the importance of providing nonpharmacological services to address comprehensively the needs of patients and to maximize the chances of the best possible outcomes.
Long‐Term Medication Management
The design of long‐term treatment depends in part on the patient’s personal treatment goals and in part on objective signs of treatment success. Maintenance can be relatively short‐term (e.g., <12 months) or a lifetime process. Treatment success depends on the achievement of specific goals that are agreed on by both the patient and the physician. Following successful stabilization, decisions to decrease or discontinue buprenorphine should be based on a patient’s desires and commitment to becoming medication‐free, and on the physician’s confidence that tapering would be successful. Factors to be considered when determining suitability for long‐term medication‐free status include stable housing and income, adequate psychosocial support, and the absence of legal problems. For patients who have not achieved these indices of stabilization, a longer period of maintenance, during which they work through any barriers that exist, may be appropriate. Data suggest that longer duration of medication treatment is associated with less illicit drug use and fewer complications.
Opioid Detoxification With Buprenorphine
This section discusses the use of buprenorphine for the medically supervised withdrawal (detoxification) from short‐acting opioids and from OAT with methadone or LAAM. The goal of medically supervised withdrawal from opioids is to provide a smooth transition from a physically dependent to a physically nondependent state. A patient can then engage in further rehabilitation with or without the use of opioid antagonist treatment to assist in relapse prevention. Before considering the use of buprenorphine for withdrawal from illicit opioids or to discontinue OAT, a patient’s appropriateness as a candidate for withdrawal or cessation must be determined at the time of assessment. Withdrawal treatment must be followed by long‐term drug‐free, or naltrexone, treatment in order to minimize the risk of relapse to opioid abuse. It should be noted, however, that absent a compelling need for the complete avoidance of all opioids, long‐term maintenance treatment with buprenorphine is to be preferred in most instances to any form of detoxification or withdrawal treatment.
Buprenorphine for Detoxification From Short‐Acting Opioids
Detoxification in patients addicted to short‐acting opioids is only a part of the overall approach to treatment. The purpose of using buprenorphine for detoxification from short‐acting opioids is to provide a transition from the state of physical dependence on opioids to an opioid‐free state, while minimizing withdrawal symptoms (and avoiding side effects of buprenorphine).
Induction Phase
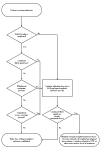
The consensus panel recommends that patients dependent on short‐acting opioids be inducted directly onto buprenorphine/naloxone tablets. Before initiating buprenorphine induction, patients should have discontinued the use of illicit opioids and should be exhibiting the early symptoms of withdrawal. An initial 4/1 mg dose of buprenorphine/naloxone is recommended. This dose can be followed in 2–4 hours with a second dose of 4/1 mg, if indicated. Over the next 2 days, the dose of buprenorphine/naloxone should be increased to 12/3–16/4 mg per day. The objectives of induction should be to stabilize the patient as rapidly as possible, to minimize any withdrawal symptoms, and to eliminate further use of illicit opioids. Only after a patient has completely discontinued use of illicit opioids should the dose‐reduction phase begin. Unless a patient is in a controlled environment (e.g., a hospital or residential setting), cessation of opioid use should be documented with a negative toxicology test for illicit opioids. If a patient is unable to discontinue illicit opioid use, as documented by negative toxicology results, a further period of stabilization or maintenance should be considered. (See figure 4-4.)
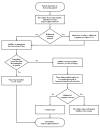
Figure
Figure 4-4. Detoxification From Short‐Acting Opioids.
Dose Reduction Phase
Long‐Period Reduction. The literature suggests that the use of buprenorphine for gradual detoxification over long periods is probably more effective than its use for rapid detoxification over short or moderate periods; however, little research has been conducted on this use of buprenorphine. Patients who are unwilling or unable to engage actively in rehabilitation services without agonist support may not be appropriate candidates for short‐term detoxification; however, such patients may benefit from long‐term detoxification (or, even more so, from maintenance treatment).
Moderate‐Period Reduction. Patients without a compelling need to undergo short‐term detoxification, but with a desire to become opioid free and to engage in rehabilitation aimed at an opioid‐free lifestyle, can be detoxified over a 10‐ to 14‐day (or longer) period by gradually decreasing the initial stabilization dose of buprenorphine (usually 8–16 mg per day) by 2 mg every 2–3 days. It is extremely important that patients engage in rehabilitation programs during the detoxification period and that they remain engaged in such programs after the conclusion of the detoxification protocol.
Short‐Period Reduction. Patients with a compelling reason to achieve an opioid‐free state quickly (e.g., impending incarceration, foreign travel, job requirement) may have their buprenorphine dose reduced over 3 days and then discontinued. When compared to clonidine for the treatment of short‐term opioid withdrawal, buprenorphine is better accepted by patients and more effective in relieving withdrawal symptoms (Cheskin et al. 1994). Relapse rates and long‐term outcomes from such rapid opioid withdrawal using buprenorphine have not been reported, however. Studies of other withdrawal modalities have shown that such brief withdrawal periods are (1) unlikely to result in long‐term abstinence and (2) produce minimal, if any, long‐term benefits in the treatment of patients dependent on opioids.
Buprenorphine for Discontinuation of OAT
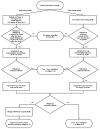
The use of buprenorphine (either as buprenorphine monotherapy or as buprenorphine/naloxone combination treatment) to taper off OAT with methadone or LAAM should be considered only for those patients who have evidence of sustained medical and psychosocial stability. Requests to provide pharmacological withdrawal with buprenorphine or buprenorphine/naloxone should be entertained with caution. Only a small proportion of patients who have achieved stability with OAT are likely to maintain abstinence without medication. Ideally, this decision would be made in conjunction, and in coordination, with a patient’s OTP. The option of continued maintenance with buprenorphine/naloxone if withdrawal proves unsuccessful should be discussed.
The guidelines in figure 4-5 describe both short‐period (3‐day) and moderate‐period (2‐week) discontinuation of OAT with buprenorphine. Short‐period discontinuation is not recommended unless there is a compelling need for rapid discontinuation.
Methadone Discontinuation
In general, patients who are clinically stable and are being slowly tapered off methadone maintenance treatment experience little difficulty until the daily methadone dose reaches 30 mg or less. As the daily dose drops below 30 mg, opioid withdrawal symptoms often emerge between methadone doses. Additionally, the euphoria‐blocking and anticraving effects of methadone are much diminished at this low dose level.
LAAM Discontinuation
Cessation of OAT with LAAM follows a protocol similar to that for methadone cessation. Patients previously stabilized on LAAM may be candidates for buprenorphine once the LAAM dose is tapered to 40 mg or less per 48 hour dose. At this point, buprenorphine monotherapy can be instituted similarly to procedures for methadone discontinuation, although LAAM’s pharmacology must be taken into account. (See figure 4-5.) When the patient has been stabilized on buprenorphine monotherapy, the physician should employ the same decision process described above for methadone discontinuation. If there is a compelling reason for OAT discontinuation, short‐term discontinuation with buprenorphine monotherapy can be achieved with a 3‐day protocol as described above. In the absence of a compelling reason, the patient should be switched to buprenorphine/naloxone combination treatment, which can be reduced subsequently and eventually discontinued if the patient remains clinically stable without evidence of illicit opioid use. Physicians should remember that patients are most likely to relapse during or after discontinuation. Therefore, patients should be monitored closely for relapse to illicit opioid use, and the dose of buprenorphine should be increased in response to cravings or withdrawal symptoms.
Discontinuation of Buprenorphine/Naloxone
When the decision is made to discontinue buprenorphine/naloxone combination treatment, the daily dose should be decreased gradually over a predetermined period or at a rate negotiated by the patient and the physician together. Withdrawal symptoms may emerge as the buprenorphine/naloxone dose is decreased. In this event, the taper may be temporarily suspended.
As with the protocols described above, discontinuation of buprenorphine/naloxone combination treatment may be performed over short periods (e.g., 3 days), but this approach should be used only in the presence of a compelling urgency to discontinue buprenorphine/naloxone in this manner; discontinuation over a longer period is the preferred manner.
Patient Management
Psychosocial Treatment Modalities and Adjuncts
Pharmacotherapy alone is rarely sufficient treatment for drug addiction (McLellan et al. 1993). Treatment outcomes demonstrate a dose‐response effect based on the level or amount of psychosocial treatment services that are provided. Therefore, physicians have an additional level of responsibility to patients with opioid addiction problems; this responsibility goes beyond prescribing and/or administering buprenorphine. For most patients, drug abuse counseling—individual or group—and participation in self‐help programs (e.g., Alcoholics Anonymous [AA]; Narcotics Anonymous [NA]; Methadone Anonymous, a 12‐Step group that supports recovery concurrent with OAT; Self Management and Recovery Training [SMART] Recovery; or Moderation Management) are considered necessary. Self‐help groups may be beneficial for some patients and should be considered as one adjunctive form of psychosocial treatment. It should be kept in mind, however, that the acceptance of patients who are maintained on medication for opioid treatment is often challenged by many 12‐Step groups. Furthermore, many patients have better treatment outcomes with formal therapy in either individual or group settings.
The ability to provide counseling and education within the context of office‐based practice may vary considerably, depending on the type and structure of the practice. Psychiatrists, for example, may include components of cognitive‐behavioral therapy or motivational enhancement therapy during psychotherapy sessions. Some medical clinics may offer patient education, which generally is provided by allied health professionals (e.g., nurses, nurse practitioners, physician assistants). A drug abuse treatment program typically includes counseling and prevention education as an integral part of the clinic program. In a stand‐alone general or family practice, the opportunities for education/counseling may be more limited. As part of their training in opioid addiction treatment, physicians should obtain, at a minimum, some knowledge of the basic principles of brief intervention in case of relapse. (See appendix E.) Physicians may want to consider providing to office staff some training in brief treatment interventions and motivational interviewing; this information could also enhance the effectiveness of treatment for other medical problems. A list of trainers may be found at http://www.motivationalinterview.org.
Many physicians already have the capability to assess and link substance abuse patients to ancillary services for substance abuse. Physicians considering making buprenorphine available to their patients should ensure that they are capable of providing psychosocial services, either in their own practices or through referrals to reputable behavioral health practitioners in their communities. In fact, the Drug Addiction Treatment Act of 2000 (DATA 2000) stipulates that, when physicians submit notification to the Substance Abuse and Mental Health Services Administration (SAMHSA) to obtain the required waiver to practice opioid addiction therapy outside the OTP setting, they must attest to their capacity to refer such patients for appropriate counseling and other nonpharmacological therapies.
It is incumbent on practitioners of buprenorphine treatment to be aware of the options and services that are available in their communities and to be able to make appropriate referrals. Physicians should be able to determine the intensity of services needed by individual patients and when those needs exceed what the practitioner can offer. Contingency plans should be established for patients who do not follow through with referrals to psychosocial treatments. Physicians should work with qualified behavioral health practitioners to determine the intensity of services needed beyond the medical services.
Treatment Monitoring
Treatment Plan
Patients and their physicians together need to reach agreement on the goals of treatment through a treatment plan that is based on assessment of the patient. Treatment plans should include both treatment goals and the conditions under which treatment is to be discontinued. The initial plan should contain contingencies for treatment failure, such as referral to a more structured treatment modality (e.g., an OTP). For polysubstance users, it is also important for patients to set a goal of abstinence from all illicit drugs, provided that counseling to address other drug use is also available. (Abstinence from all illegal or inappropriate substances of abuse should be the goal of all patients, whether single or polysubstance users.) Treatment contracts are often employed to make explicit what is expected of patients in terms of their cooperation and involvement in addiction treatment. Physicians may find the sample contract (or an adapted version) in appendix H a useful tool in working with patients in an office‐based setting.
After obtaining signed patient consent (according to 42 C.F.R. Part 2), physicians should clarify assessment and treatment goals with family members. Whenever possible, significant others should be engaged in the treatment process, as their involvement is likely to have a positive effect on outcomes. Conversely, when patients refuse to involve their significant others, or when the latter refuse to become involved, positive outcomes are less likely.
Frequency of Visits
During the stabilization phase, patients receiving maintenance treatment should be seen on at least a weekly basis. Part of the purpose of the ongoing assessment is to determine whether patients are adhering to the dosing regimen and handling their medications responsibly (e.g., storing it safely, taking it as prescribed, not losing it). Once a stable buprenorphine dose is reached and toxicological samples are free of illicit opioids, the physician may determine that less frequent visits (biweekly or longer, up to 30 days) are acceptable. Visits on a monthly basis are considered a reasonable frequency for patients on stable buprenorphine doses who are making appropriate progress toward treatment objectives and in whom toxicology shows no evidence of illicit drugs. However, physicians should be sensitive to treatment barriers, such as geographical issues, travel distance to treatment, domestic issues such as child care and work obligations, as well as the cost of care.
Patients’ progress in achieving treatment goals should be reviewed periodically. Various goal‐attainment scales, which can be administered by a nurse or case manager, can assist in monitoring and documenting patients’ progress. Measures used to evaluate maintenance treatment with buprenorphine are similar to those used for other areas of addiction treatment:
- No illicit opioid drug use occurs and no other ongoing drug use (including problematic alcohol use) is found that might compromise patient safety (e.g., ongoing abuse of alcohol and/or benzodiazepines).
- Toxicity is absent.
- Medical adverse effects are absent.
- Behavioral adverse effects are absent.
- Patient is handling the medication responsibly.
- Patient is adhering to all elements of the treatment plan (e.g., seeing a psychotherapist or attending groups as scheduled, participating in recovery‐oriented activities).
Unstable Patients
Given these evaluations, physicians need to decide when they cannot appropriately provide further management for particular patients. For example, if a patient is abusing other drugs that a physician does not feel competent to manage, or if toxicology tests are still not free of illicit drugs after 8 weeks, then the physician may want to assess (1) whether to continue to treat that patient without additional evidence of ongoing counseling or (2) whether to refer the patient to specialists or to a more intensive treatment environment. Decisions should be based on the treatment plan to which the patient previously agreed.
Toxicology Testing for Drugs of Abuse
During opioid addiction treatment with buprenorphine, toxicology tests for all relevant illicit drugs should be administered at least monthly. Urine screening is the most common testing method, although testing can be performed on a number of other bodily fluids and tissues—including blood, saliva, sweat, and hair. A comprehensive discussion of urine drug testing in the primary care setting can be found in Urine Drug Testing in Primary Care: Dispelling the Myths & Designing Strategies (Gourlay et al. 2002).
Methadone and heroin metabolites are each detected by commercially available urine‐testing kits. Buprenorphine does not cross‐react with the detection procedures for methadone or other opioids; therefore, it will not be detected in a routine urine drug screen. Both physicians and patients should be aware of this fact.
Buprenorphine and its metabolites are excreted in urine. Urine testing for buprenorphine can be performed at a medical laboratory, but at the time of this document’s publication, there are no CLIA‐waived, in‐office buprenorphine urine test kits commercially available.
There are two primary reasons to consider testing for buprenorphine: (1) in new patients to confirm that they do not already have buprenorphine in their system, (2) to assist with evaluating adherence in patients on buprenorphine treatment. (Refer to chapter 3 for additional information on drug‐testing methodologies.) As new testing procedures and protocols are recommended for use in addiction treatment with buprenorphine, SAMHSA will be making additional information available through the Division of Pharmacologic Therapies (DPT) Web site at http://www.dpt.samhsa.gov/.
Discontinuation of Medication
Under ideal conditions, discontinuation of medication should occur when a patient has achieved the maximum benefit from treatment and no longer requires continued treatment to maintain a drug‐free lifestyle. Once this goal is achieved, buprenorphine should be tapered slowly and appropriately while psychosocial services continue to be provided. Patients should be assessed for continued stability in maintaining their drug‐free lifestyle. Patients should then be followed with psychosocial services and/or the reintroduction of medication, if needed, for continued progress.
Certain situations undoubtedly will arise, however, in which a physician may feel that a patient is not progressing satisfactorily. For example, a patient may not be in compliance with the treatment plan or with office procedures (e.g., timely payment). Under some conditions, physicians may consider involuntary termination of treatment, but must be careful to not abandon patients. Physicians can and should take a variety of actions to prevent this situation. Physicians should have written policies in place regarding patient behavior, office procedures, and adherence to treatment. These policies should be discussed with patients before initiating buprenorphine treatment, and patients should agree to comply with these policies.
Physicians should develop practices for dealing with minor infractions of rules or policies and with minor nonadherence to treatment plans. Clearly defined points should be identified at which patients will be notified that they are not adhering to treatment plans, and they should be given the opportunity to improve in this regard. In the event of involuntary termination of treatment, it is necessary for physicians to make appropriate referrals—to OTPs, to other physicians who are willing to prescribe buprenorphine, or to other appropriate treatment facilities. If a patient will not be receiving OAT in another treatment setting, the physician must manage the appropriate withdrawal of buprenorphine so as to minimize withdrawal discomfort. A patient may or may not be willing to accept referrals made on his or her behalf, but physicians must make good faith efforts to ensure that their patients have an appropriate level of care available after their own therapeutic involvement is ended.
For more information about treatment management issues, see the forthcoming TIP Medication‐Assisted Treatment for Opioid Addiction (CSAT in development). The treatment management principles addressed in that TIP will also be applicable to office‐based buprenorphine treatment.
Footnotes
Due to a number of factors, including the association of LAAM with cardiac arrhythmias in some patients, as of January 1, 2004, the sole manufacturer has ceased production of the drug.
- 4 Treatment Protocols - Clinical Guidelines for the Use of Buprenorphine in the ...4 Treatment Protocols - Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction
Your browsing activity is empty.
Activity recording is turned off.
See more...