This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Perry T, editor. Therapeutics Letter. Vancouver (BC): Therapeutics Initiative; 1994-.
Therapeutics Letter 123 examines biologics and biosimilars. Conclusions: Biosimilars are not identical to biologics but lie within the variability range of the originator biologic. Government regulators around the world approve biosimilars based on evidence that biosimilars are not inferior to the originator biologic product in terms of safety and effectiveness. Systematic reviews of switching from biologics to biosimilars found no meaningful differences in safety and effectiveness between them. Biosimilars are less expensive than originator biologics.
Keywords:
Biosimilar Pharmaceuticals, Drug Safety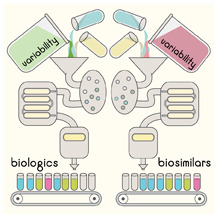
The term “biologics” can refer to a wide range of therapeutic products, including vaccines, blood products, gene and cell therapies, and bioengineered drugs. Often it is used more restrictively to refer to drugs produced through recombinant DNA technology. Health Canada notes that biologic drugs are “derived through the metabolic activity of living organisms and tend to be significantly more variable and structurally complex than chemically synthesized drugs”.1
Monoclonal antibodies (MAb) comprise one class of biologics. They are created by immune cells that are identical clones of a unique parent cell. MAb are large, complex molecules that have a binding site and may have the same primary, secondary and tertiary structure as a human protein.
According to Health Canada, a biosimilar is a “biologic drug that is highly similar to a biologic drug that was already authorized for sale. There are no expected clinically meaningful differences in efficacy and safety between a biosimilar and the biologic drug that was already authorized for sale”.2 Biosimilars have been approved for use in Canada since 2009 and were developed, similar to generic medications, to reduce the expenditure of healthcare on costly biological treatments and improve patient access to these drugs.
Biologics and variability
Manufacturing chemical drugs is a controlled, predictable chemical process, allowing identical copies (generic versions) to be replicated exactly. The manufacturing process for biologics is complex, involving several stages of cell selection, cell culture, purification, formulation, production and validation of the final product.
Every biologic manufacturer uses a unique cell line and a proprietary process. No two production batches of biologics are identical. Originator biologics also have intrinsic natural variability because batches can differ and the manufacturing process can itself change.3
During manufacturing, primary amino acid sequences can become modified through glycosylation, changing the shape of a protein because of alterations in the way it folds. These modifications are not controlled by the recombinant DNA inserted into the host cell but are affected by the cell line and the environment in which the cell line is grown.4
How are biosimilars assessed?
Regulators around the world (Health Canada, FDA, EMA) use strict, specific approval criteria to ensure that the biosimilar product lies within the variability range of the originator biologic.
Health Canada, in its submission guidance document, explains: “similarity does not signify that the quality attributes of the two products being compared are identical, but that they are highly similar with two consequences:
- that the existing knowledge of both products is sufficient to predict that any differences in quality attributes should have no adverse impact upon safety or efficacy of the biosimilar; and
- that non-clinical and clinical data previously generated with the reference biologic drug are relevant to the biosimilar”.1
Canada, US, Europe, Australia and New Zealand all follow similar scientific principles for the authorization of biosimilars.5 Health Canada accepts use of a reference product from another ICH country and information from other regulators such as the European Medicines Agency (EMA) and the US FDA regarding full biosimilar program development, including analytical similarity, non-clinical and clinical studies.2
Biosimilars can be substantially less expensive than their originator biologics. For example, the biosimilars to infliximab can save Canadians almost 50%, compared with the originator (Remicade).6
Infliximab versus biosimilars: an example of current evidence for bioequivalence
Infliximab (Remicade), a chimeric MAb derived from human and mouse DNA, is approved in Canada for treatment of rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease and ulcerative colitis, and psoriasis. Biosimilars Inflectra (CT-P13) and Renflexis have identical approvals.
We identified 12 systematic reviews of studies comparing infliximab with its biosimilars.7 Patients started treatment either as naïve to infliximab or by randomization to continue the originator therapy (Remicade) or to switch to a biosimilar.
We looked for any claim of clinically meaningful differences in rates of disease control, safety or immunogenicity between infliximab and its biosimilars. No such claims were made, so we did not examine the reviews further.
One important RCT and two of the more robust systematic reviews are summarized below. The remainder are described in a summary posted on our website.7
- Jørgensen KK et al, NOR-SWITCH RCT, 2017:8 52-week, non-inferiority RCT with 482 patients with Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis. Switching from infliximab to CT-P13 (Inflectra) “was not inferior to continued treatment with infliximab according to a pre-specified non-inferiority margin of 15%.”
- Ebada MA et al, Systematic review, 2019:9 32 studies in 3464 patients. Included RCTs, prospective, retrospective, cohort and case series studies. Patients diagnosed with IBD received CT-P13 either naïve to biological therapy or switched from infliximab. Overall, CT-P13 was “effective and well tolerated in short and long-term periods.”
- Gisbert JP et al, Systematic review, 2018:10 24 observational studies, registries, cohorts and real-world experiences studies in 1326 IBD patients evaluating safety and efficacy upon switching to CT-P13 (and to other biosimilars). Effectiveness and safety were also found to be similar between the biosimilar and infliximab.
Conclusions
- Biosimilars are not identical to biologics but lie within the variability range of the originator biologic.
- Government regulators around the world approve biosimilars based on evidence that biosimilars are not inferior to the originator biologic product in terms of safety and effectiveness.
- Systematic reviews of switching from biologics to biosimilars found no meaningful differences in safety and effectiveness between them.
- Biosimilars are less expensive than originator biologics.
References
- 1.
- Health Canada, Health Products and Foods Branch. Guidance Document: Information and Submission Requirements for Biosimilar Biologic Drugs, adopted 2010/03/05 Revised Date 2016/11/14. https://www
.canada.ca /content/dam/hc-sc/migration /hc-sc/dhp-mps /alt_formats/pdf/brgtherap /applic-demande /guides/seb-pbu/seb-pbu-2016-eng.pdf [Accessed 26 Sep 2019] - 2.
- Health Canada. Biosimilar biologic drugs in Canada: Fact Sheet. 2019-08-23. https://www
.canada.ca /content/dam/hc-sc/migration /hc-sc/dhp-mps /alt_formats/pdf/brgtherap /applic-demande /guides/Fact-Sheet-EN-2019-08-23.pdf [Accessed 26 Sep 2019] - 3.
- Windisch J. Biosimilars versus original biologics. Similarities and differences from development to approval. Z Rheumatol 2015; 74(8): 672–81. DOI:10.1007/s00393-014-1486-9 [PubMed: 26323591] [CrossRef]
- 4.
- Ventola CL. Biosimilars: part 1: proposed regulatory criteria for FDA approval. P T. 2013;38(5):270–287. PMC3737980 [PMC free article: PMC3737980] [PubMed: 23946620]
- 5.
- Rannanheimo P, Richardson M, Perras C, Mai H, Hodgson A. Biosimilars — regulatory, health technology assessment, reimbursement trends, and market outlook. CADTH Environmental Scan; no.68; 2018. https://cadth
.ca/sites /default/files/pdf/ES0317_biosimilars .pdf [Accessed 26 Sep 2019] - 6.
- Patented Medicines Prices Review Board. Potential Savings from Biosimilars in Canada. Date modified: 2018-02-05 https://www
.pmprb-cepmb .gc.ca/CMFiles/NPDUIS /2017_Conference_Posters /post_6_biosim.pdf [Accessed 26 Sep 2019] - 7.
- Therapeutics Initiative. Summary of infliximab originator vs. biosimilar randomized controlled trial and systematic reviews. September 2019. https://ti
.ubc.ca/letter123-appendix [Accessed 26 Sep 2019] - 8.
- Jørgensen KK, Olsen IC, Goll GL et al., on behalf of the NOR-SWITCH study group. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017; 389:2304–2316. DOI:10.1016/S0140-6736(17)30068-5 [PubMed: 28502609] [CrossRef]
- 9.
- Ebada MA, Elmatboly AM, Ali AS et al. An updated systematic review and meta-analysis about the safety and efficacy of infliximab biosimilar, CT-P13, for patients with inflammatory bowel disease. International Journal of Colorectal Disease. 2019; 34(10):1633–52. DOI:10.1007/s00384-019-03354-7 [PubMed: 31492986] [CrossRef]
- 10.
- Gisbert JP, Chaparro M. Switching from an originator anti-TNF to a biosimilar in patients with inflammatory bowel disease: Can it be recommended? A systematic review. Gastroenterología y Hepatología (English Edition). 2018; 41(6): 389–405. DOI:10.1016/j.gastrohep.2018.04.005 [PubMed: 29753532] [CrossRef]
- The draft of this Therapeutics Letter was submitted for review to 130 experts and primary care physicians in order to correct any inaccuracies and to ensure that the information is concise and relevant to clinicians.The Therapeutics Initiative is funded by the BC Ministry of Health through a grant to the University of BC. The Therapeutics Initiative provides evidence-based advice about drug therapy, and is not responsible for formulating or adjudicating provincial drug policies.
- Review Biosimilars: impact of biologic product life cycle and European experience on the regulatory trajectory in the United States.[Clin Ther. 2012]Review Biosimilars: impact of biologic product life cycle and European experience on the regulatory trajectory in the United States.Ahmed I, Kaspar B, Sharma U. Clin Ther. 2012 Feb; 34(2):400-19. Epub 2012 Jan 13.
- Review Efficacy and Safety Outcomes for Originator TNF Inhibitors and Biosimilars in Rheumatoid Arthritis and Psoriasis Trials: A Systematic Literature Review.[BioDrugs. 2018]Review Efficacy and Safety Outcomes for Originator TNF Inhibitors and Biosimilars in Rheumatoid Arthritis and Psoriasis Trials: A Systematic Literature Review.Moots RJ, Curiale C, Petersel D, Rolland C, Jones H, Mysler E. BioDrugs. 2018 Jun; 32(3):193-199.
- Review Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology.[Rheumatol Int. 2016]Review Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology.Mysler E, Pineda C, Horiuchi T, Singh E, Mahgoub E, Coindreau J, Jacobs I. Rheumatol Int. 2016 May; 36(5):613-25. Epub 2016 Feb 27.
- Switching from Biologic to Biosimilar Products: Insight from an Integrated Health Care System.[BioDrugs. 2022]Switching from Biologic to Biosimilar Products: Insight from an Integrated Health Care System.Bhardwaja B, Klocke S, Ekinci E, Jackson A, Kono S, Olson KL. BioDrugs. 2022 Jan; 36(1):1-11. Epub 2021 Nov 24.
- Safety of Marketed Cancer Supportive Care Biosimilars in the US: A Disproportionality Analysis Using the Food and Drug Administration Adverse Event Reporting System (FAERS) Database.[BioDrugs. 2021]Safety of Marketed Cancer Supportive Care Biosimilars in the US: A Disproportionality Analysis Using the Food and Drug Administration Adverse Event Reporting System (FAERS) Database.Tanni KA, Truong CB, Almahasis S, Qian J. BioDrugs. 2021 Mar; 35(2):239-254. Epub 2021 Jan 13.
- Biosimilars or Biologics: What’s the difference? - Therapeutics LetterBiosimilars or Biologics: What’s the difference? - Therapeutics Letter
Your browsing activity is empty.
Activity recording is turned off.
See more...
