This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Perry T, editor. Therapeutics Letter. Vancouver (BC): Therapeutics Initiative; 1994-.
Therapeutics Letter 54 considers whether the use of benzodiazepines in British Columbia (BC), Canada is consistent with recommendations. Conclusions: The pattern of utilization of benzos in BC appears inconsistent with the recommendations of educational groups, regulators and manufacturers: Approximately 170,000 people are receiving amounts incompatible with short-term or intermittent use. The two groups most vulnerable to adverse effects, women and the elderly, are the highest users. Use of long half-life drugs (>10 hours) predominates. The overall benefits and harms from this drug exposure in BC is unknown.
Keywords:
Benzodiazepines, British Columbia, Drug-Related Side Effects and Adverse Reactions, Inappropriate PrescribingTherapeutics Letter #11,1 on the treatment of insomnia, concluded: “If indicated, prescribe benzodiazepines with short half-lives, in low doses, for short duration, and not for regular nightly use.” Therapeutics Letter #18,2 on the management of anxiety disorders, concluded: “Current evidence suggests that non-benzodiazepine treatment, particularly psychotherapy, is safer and as effective for most patients with anxiety disorders.”
This Letter describes the utilization of benzodiazepines in BC from 1996 to 2002.
Benzodiazepines may impair functional status by causing confusion, memory loss, dizziness, daytime sleepiness, falls/fractures and depression.3,4 Despite this potential for major harm and scant evidence of clinically meaningful benefit,4 the use of benzodiazepines in BC grew between 1996 and 2002 (Figure 1). This drug class is currently near the top in terms of pills dispensed, 84 million pills in 2002. This is fewer than the 124 million antidepressant pills, but exceeds the 74 million acid suppressant pills (proton pump inhibitors and H2 blockers), 72 million lipid lowering pills (statins and fibrates), 63 million non-steroidal anti-inflammatory pills (non-selective and COX-2 selective NSAIDs), and 55 million diuretic pills.
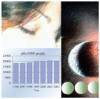
Fig. 1
Benzo use in BC.
This analysis focuses on the quantity of pills dispensed. We grouped the Z drugs, zopiclone and zaleplon, with the benzodiazepines based on the similar pharmacologic mechanism of action. In this Letter we call this larger grouping “benzos”. A recent systematic review5 by the United Kingdom National Institute for Clinical Excellence (NICE) is summarized in a British Medical Journal editorial:6 “Although initially promoted as superior to benzodiazepines in terms of daytime sedation, dependence and withdrawal, the Z drugs have not delivered on several fronts. On the quality of evidence, of the 17 randomised trials with a total of 1284 patients, all were industry funded, outcomes were poorly and often selectively reported in favour of positive findings, comparators were suboptimal, durations were very short (maximum 6 weeks), and surrogate markers (generally sleep variables) were highlighted. On the risk-benefit front, no consistent difference was found between the Z drugs and benzodiazepines for either effectiveness or safety.”6
Benzodiazepines and Z drugs used in BC in 2002 are shown in the Table. All the drugs are available in a generic form except for zaleplon. The average cost/tablet and percentages of people using the drugs are calculated from year 2002 Pharmanet data.
Table
Benzodiazepines and Z drugs (benzos) in BC.
Has prescribing of these drugs changed between 1996 and 2002?
Figure 1 shows that the quantity of pills per 1000 population is rising and peaks in 2001. Benzo use increased by 11% between 1996 and 2002; antidepressant use increased by 73% over the same time period.
Which benzos were most frequently prescribed in 1996 and 2002?
Figure 2 shows the proportions of different drugs used in 1996 and 2002. The seven most used benzos: lorazepam, clonazepam, zopiclone, oxazepam, alprazolam, diazepam and temazepam comprised 92% of the total in 1996 and 95% of the total in 2002. Five out of these seven have half-lives >10 hours (see Table). When such long half-life drugs are taken at night, daytime sedation is expected and common.

Fig. 2
Utilization of individual benzos in BC in 1996 and 2002.
What proportion of people in BC received a benzo and how does that vary by gender and age?
9.7% of the population of BC (400,000 people) received at least one prescription for a benzo in 2002. A lower percentage of males, 7.1%, than females, 12.2%, received a benzo. Figure 3 shows that this gender relationship holds for all ages and that the percentage increases steadily with age for both genders.
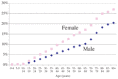
Fig. 3
Benzodiazepine prescription rates by gender.
What proportion of patients are using benzos as recommended?
We have conservatively defined patients receiving <100 pills per year as those using the drugs as recommended by educational groups 1,2,5, regulators and manufacturers, (short-term or intermittently). Patients receiving <100 pills per year comprised 5.5% of the population (230,000 people). The remaining 4.2% of the population (170,000 people) received >100 pills per year. These 43% of users received 88% of the 84 million tablets dispensed. Over 10,000 people in BC received >1000 pills per year in 2002.
CONCLUSIONS
The pattern of utilization of benzos in BC appears inconsistent with the recommendations of educational groups, regulators and manufacturers:
- Approximately 170,000 people are receiving amounts incompatible with short-term or intermittent use.
- The two groups most vulnerable to adverse effects, women and the elderly, are the highest users.
- Use of long half-life drugs (>10 hours) predominates.
- The overall benefits and harms from this drug exposure in BC is unknown.
One reviewer’s comment
“Other drugs used as sedative/hypnotics, such as antihistamines, antidepressants and antipsychotics, may be more harmful than benzodiazepines and Z drugs.” We plan to explore this issue in a future Letter. We welcome feedback from our readers as well.
References
- 1.
- Therapeutics Initiative. To Sleep or not to Sleep: Here are your Questions. Therapeutics Letter. Nov–Dec 1995;11:1–2.
- 2.
- Therapeutics Initiative. Management of Anxiety Disorders in Primary Care. Therapeutics Letter. Feb–April 1997;18:1–4. [PubMed: 38620484]
- 3.
- Wagner AK, Zhang F, Soumerai SB, et al. Benzodiazepine use and hip fractures in the elderly. Who is at greatest risk? Arch Intern Med. 2004; 164:1567–1572. [PubMed: 15277291]
- 4.
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225–233. [PMC free article: PMC1232276] [PubMed: 10674059]
- 5.
- National Institute for Clinical Excellence. Guidance on the use of zaleplon, zolpidem, and zopiclone for the short-term management of insomnia. NICE (Technology Appraisal 77) 2004; www
.nice.org.uk/TA077 (accessed 31 Dec 04). - 6.
- Holbrook AM. Treating Insomnia: Use of drugs is rising despite evidence of harm and little meaningful benefit. Brit Med J. 2004;329:1198–1199.
- 7.
- Compendium of Pharmaceuticals and Specialties, 2004; Benzodiazepine monograph, Imovane, Starnoc: 253–256, 953–954, 1898–1901.
- This Letter contains an assessment and synthesis of publications up to December 2004. We attempt to maintain the accuracy of the information in the Therapeutics Letter by extensive literature searches and verification by both the authors and the editorial board. In addition this Therapeutics Letter was submitted for review to 60 experts and primary care physicians in order to correct any inaccuracies and to ensure that the information is concise and relevant to clinicians.The Therapeutics Letter presents critically appraised summary evidence primarily from controlled drug trials. Such evidence applies to patients similar to those involved in the trials, and may not be generalizable to every patient. We are committed to evaluate the effectiveness of our educational activities using the Pharmacare/PharmaNet databases without identifying individual physicians, pharmacies or patients. The Therapeutics Initiative is funded by the BC Ministry of Health through a 3-year grant to the University of BC. The Therapeutics Initiative provides evidence-based advice about drug therapy, and is not responsible for formulating or adjudicating provincial drug policies.
- Prescribing benzodiazepines for noninstitutionalized elderly.[Can Fam Physician. 1995]Prescribing benzodiazepines for noninstitutionalized elderly.Thomson M, Smith WA. Can Fam Physician. 1995 May; 41:792-8.
- Correlates of opioid and benzodiazepine co-prescription among people living with HIV in British Columbia, Canada: A population-level cohort study.[Int J Drug Policy. 2019]Correlates of opioid and benzodiazepine co-prescription among people living with HIV in British Columbia, Canada: A population-level cohort study.Parent S, Nolan S, Fairbairn N, Ye M, Wu A, Montaner J, Barrios R, Ti L, STOP HIV AIDS Study Group. Int J Drug Policy. 2019 May; 67:52-57. Epub 2019 Mar 18.
- Inappropriate Use of Proton Pump Inhibitor Among Elderly Patients in British Columbia: What are the Long-term Adverse Events?[Curr Drug Saf. 2024]Inappropriate Use of Proton Pump Inhibitor Among Elderly Patients in British Columbia: What are the Long-term Adverse Events?Ben-Eltriki M, Chhabra M, Cassels A, Wright JM. Curr Drug Saf. 2024; 19(2):244-247.
- Review Stimulants for ADHD in children: Revisited.[Therapeutics Letter. 1994]Review Stimulants for ADHD in children: Revisited.. Therapeutics Letter. 1994
- Review Prescription drug costs: BC versus Canada.[Therapeutics Letter. 1994]Review Prescription drug costs: BC versus Canada.. Therapeutics Letter. 1994
- Use of Benzodiazepines in BC Is it consistent with recommendations? - Therapeuti...Use of Benzodiazepines in BC Is it consistent with recommendations? - Therapeutics Letter
Your browsing activity is empty.
Activity recording is turned off.
See more...
