Introduction
The endoplasmic reticulum (ER) is a structure found within the cytoplasm of eukaryotic cells. Its composition has two components: the smooth endoplasmic reticulum (SER) and the rough endoplasmic reticulum (RER). The SER is generally used for the creation/ storage of lipids and steroids, while the RER plays a significant role in the synthesis of various proteins. The RER is termed "rough" due ribosomal attachments to the surface compared to the SER, which does not have ribosomes.
Structure
The RER is morphologically distinguishable by its series of convoluted, flattened like membrane sheets (called cisternae) that arise near the nucleus and extend across the cytoplasm. Sections of the cisternae contain ribosomes, held together by microtubules of the cytoskeleton. Changes in the pattern of microtubule polymerization lead to a change in RER morphology. Furthermore, the ribosomes of the RER are not permanently attached to the membrane. They constantly attach and detach to the membrane as needed for protein synthesis.
Edges of the ER sheets tend to have a degree of high-curvature that require stabilization. Proteins that help with this stabilization are reticulons and DP1/Yop1p. These integral membrane proteins contribute to the curvature by forming a transmembrane hairpin that acts as a wedge.[1] This protein made wedge displaces lipids in the outer leaflet of the bilayer, which further creates the curvature of the ER membrane.[1]
Function
The RER is associated with many roles in protein synthesis, which also include post-translational modifications, folding, and sorting. Membrane-bound ribosomes in the RER translate the mature mRNA transcript into amino acids that are attached to become polypeptides. Eukaryotic ribosomes (80S) consist of two unequal subunits: the small subunit (40S) and the large subunit (60S). The (S) refers to a Svedberg unit, which is a non-metric unit of measure for the sedimentation rate. Each Svedberg unit is equal to 10^-13 seconds. After protein synthesis, the post-translational addition of some carbohydrates and some proteolytic cleavages occur in the RER. However, most post-translational modifications take place in the Golgi complex.
The first step towards proper folding requires oligosaccharyltransferases to glycosylate the newly formed peptide chain. Glycosylation of the peptide increases its solubility and protects it until chaperones can bind to the chain and begin the folding process. Major chaperones in this process include Calnexin (CNX), Calreticulin (CRT), and binding immunoglobulin protein (BiP).[2][3] The RER also consists of enzymes that catalyze the formation of disulfide bonds necessary for a protein's tertiary and quaternary structure. If the protein does not fold correctly, the molecular chaperons rebind onto the polypeptide and attempt to fold the protein into the correct shape. After multiple failed attempts, the misfolded proteins are exported to the cytosol and degraded.
After proper synthesis and folding of the protein, it goes to the edges of the RER. The vesicular coat protein complex II (COPII) mediates the formation of vesicles at the RER edges, which transport the protein product towards the Golgi apparatus for further processing.[4] Protein products that must stay within the ER move through retrograde transport from the Golgi, using vesicles formed by coat protein complex I (COPI).[4]
Tissue Preparation
Adequate cell specimens are necessary to analyze the RER organelle properly. There are different techniques used for the different types of histological sections. A light microscopy study requires specimens prepared through either the paraffin technique, frozen sections, or semithin sections. The paraffin technique is most commonly used to prepare this type of section. For electron microscope studies, the method used to make histology sections is similar to the method for semithin sections.
Histochemistry and Cytochemistry
Histochemistry
Since the outer membrane of the RER is studded with ribosomes, it will have a relatively basophilic stain in the cytoplasm when using H&E stains, due to the presence of negatively charged rRNA.
Cytochemistry
A cytochemical study revealed that BiP is found exclusively within the RER.[5] In mammalian cells specifically, ribophorin I was found to be an RER-specific protein as well.[6] Ribosomes, along with their subunits, are a characteristic of the RER and can help identify it. However, these proteins are also found free-floating throughout the cytoplasm and other parts of the cell.
Microscopy, Light
The detailed structure of the RER can't be effectively studied through light microscopy. However, there is an abundance of membrane-bound ribosomes within the RER, making those specific areas extremely basophilic and visible. Visibility of the RER increases in cells that are highly secretory such as Nissl substance in neurons and exocrine pancreatic acinar cells.
Microscopy, Electron
Depending on the electron micrograph section, it can show a detailed view of the RER, which includes cisterna, microtubules, ribosomes, and surrounding structures. Eukaryotic cisterna and tubules have a diameter of 30 to 50 nm, while ribosomes are 25 to 30 nm.[7]
Pathophysiology
Unfolded or misfolded proteins remain in the ER, translocated to the cytoplasm by ER-associated protein degradation (ERAD), and then degraded by the proteasome. The amount of unfolded proteins can accumulate to the extent where it exceeds the capacity of ERAD machinery to dispose of it all. This accumulation leads to ER stress in the cell. Three pathways that regulate the mammalian ER stress response are PERK (translational attenuation), ATF6 (enhanced expression of ER chaperones), and IRE1 (enhanced expression of ERAD components).[8] Collectively, these pathways work to reduce the amount of unfolded proteins within the ER.
Unfolded proteins have exposed hydrophobic amino-acid residues that are normally inside the protein. This arrangement can allow the formation of protein aggregates that are toxic to cells. If the three pathways can’t suppress ER stress, it induces an apoptotic pathway to help ensure the survival of the organism. The most characterized pathway of this by mechanism is the CHOP pathway, but other apoptotic pathways that can be activated as well.[8] A few of these pathways include the IRE1-TRAF2-ASK1 pathway, caspases, Bcl2 proteins, and c-Abl activation by death signals.
Clinical Significance
Unfolded and misfolded protein aggregates formed are toxic for a variety of reasons. Studies suggest that small aggregates can impair the ubiquitin-proteasome pathway and even sequester transcription factors like CREB-binding protein and TATA-binding protein.[9][10][11] Furthermore, other reports suggest that ER stress caused by protein aggregates is involved in some diseases. PKR and caspase-4 are proteins that both play a role in ER-stress induced apoptosis and are also involved with the onset of Alzheimer’s dementia.[12][13]
Concerning ALS, mutant superoxide dismutase-1 forms aggregates in the ER, which leads to an increase in BiP expression and activates caspase-12, leading to neuronal cell death. This mechanism provides support of how ER stress is inducable and how it contributes to the selective motor neuronal degeneration in ALS.[14] A strong correlation is suggested between ER stress and GM1 gangliosidosis since GM1 gangliosides induce expression of BiP and CHOP, which activates JNK2 and caspase-12 to cause neuronal apoptosis.[15]
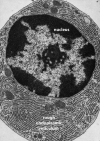
Figure
Transmission Electron Micrograph of Rough Endoplasmic Reticulum Contributed by Don W. Fawcett
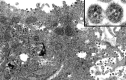
Figure
Microscopy of Rough Endoplasmic Reticulum Contributed from Cynthia S. Goldsmith, Inger K. Damon, Sherif R. Zaki, USCDCP (Public Domain https://creativecommons.org/licenses/publicdomain/)
References
- 1.
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006 Feb 10;124(3):573-86. [PubMed: 16469703]
- 2.
- Tannous A, Pisoni GB, Hebert DN, Molinari M. N-linked sugar-regulated protein folding and quality control in the ER. Semin Cell Dev Biol. 2015 May;41:79-89. [PMC free article: PMC4474783] [PubMed: 25534658]
- 3.
- Lewy TG, Grabowski JM, Bloom ME. BiP: Master Regulator of the Unfolded Protein Response and Crucial Factor in Flavivirus Biology . Yale J Biol Med. 2017 Jun;90(2):291-300. [PMC free article: PMC5482305] [PubMed: 28656015]
- 4.
- García IA, Martinez HE, Alvarez C. Rab1b regulates COPI and COPII dynamics in mammalian cells. Cell Logist. 2011 Jul;1(4):159-163. [PMC free article: PMC3265928] [PubMed: 22279615]
- 5.
- Bole DG, Dowin R, Doriaux M, Jamieson JD. Immunocytochemical localization of BiP to the rough endoplasmic reticulum: evidence for protein sorting by selective retention. J Histochem Cytochem. 1989 Dec;37(12):1817-23. [PubMed: 2685110]
- 6.
- Sanderson CM, Crowe JS, Meyer DI. Protein retention in yeast rough endoplasmic reticulum: expression and assembly of human ribophorin I. J Cell Biol. 1990 Dec;111(6 Pt 2):2861-70. [PMC free article: PMC2116400] [PubMed: 2269658]
- 7.
- Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016 Jan;73(1):79-94. [PMC free article: PMC4700099] [PubMed: 26433683]
- 8.
- Yoshida H. ER stress and diseases. FEBS J. 2007 Feb;274(3):630-58. [PubMed: 17288551]
- 9.
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001 May 25;292(5521):1552-5. [PubMed: 11375494]
- 10.
- Schaffar G, Breuer P, Boteva R, Behrends C, Tzvetkov N, Strippel N, Sakahira H, Siegers K, Hayer-Hartl M, Hartl FU. Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol Cell. 2004 Jul 02;15(1):95-105. [PubMed: 15225551]
- 11.
- Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease. Trends Genet. 2003 May;19(5):233-8. [PubMed: 12711212]
- 12.
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004 May 10;165(3):347-56. [PMC free article: PMC2172196] [PubMed: 15123740]
- 13.
- Onuki R, Bando Y, Suyama E, Katayama T, Kawasaki H, Baba T, Tohyama M, Taira K. An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer's disease. EMBO J. 2004 Feb 25;23(4):959-68. [PMC free article: PMC380987] [PubMed: 14765129]
- 14.
- Kikuchi H, Almer G, Yamashita S, Guégan C, Nagai M, Xu Z, Sosunov AA, McKhann GM, Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci U S A. 2006 Apr 11;103(15):6025-30. [PMC free article: PMC1458691] [PubMed: 16595634]
- 15.
- Tessitore A, del P Martin M, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, d'Azzo A. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell. 2004 Sep 10;15(5):753-66. [PubMed: 15350219]
Disclosure: Terrence Sanvictores declares no relevant financial relationships with ineligible companies.
Disclosure: Donald Davis declares no relevant financial relationships with ineligible companies.
Publication Details
Author Information and Affiliations
Authors
Terrence Sanvictores1; Donald D. Davis2.Affiliations
Publication History
Last Update: August 8, 2023.
Copyright
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Publisher
StatPearls Publishing, Treasure Island (FL)
NLM Citation
Sanvictores T, Davis DD. Histology, Rough Endoplasmic Reticulum. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

