Continuing Education Activity
A melanoma is a tumor produced by the malignant transformation of melanocytes. Melanocytes are derived from the neural crest; consequently, melanomas, although they usually occur on the skin, can arise in other locations where neural crest cells migrate, such as the gastrointestinal tract and brain. The five-year relative survival rate for patients with stage 0 melanoma is 97%, compared with about 10% for those with stage IV disease. This activity reviews the evaluation and treatment of melanoma and the role of the interprofessional team in evaluating, treating, and education required of patients to decrease the incidence of this condition.
Objectives:
- Describe the pathophysiology of malignant melanoma.
- Review why malignant melanoma may appear in multiple regions of the body.
- Summarize the survival statistics in malignant melanoma based on the time of diagnosis.
- Outline the role of the interprofessional team in managing a patient with malignant melanoma.
Introduction
A melanoma is a tumor produced by the malignant transformation of melanocytes. Melanocytes are derived from the neural crest; consequently, melanomas, although they usually occur on the skin, can arise in other locations where neural crest cells migrate, such as the gastrointestinal tract and brain.[1][2] The five-year relative survival rate for patients with stage 0 melanoma is 97%, compared with about 10% for those with stage IV disease.
Etiology
The causes may be related to:
- Family history - Positive family history in 5% to 10% of patients; a 2.2-fold higher risk with at least one affected relative
- Personal characteristics - Blue eyes, fair and/or red hair, pale complexion; skin reaction to sunlight (easily sunburned); freckling; benign and/or dysplastic melanocytic nevi (the number shows a stronger correlation than size); immunosuppressive states (transplantation patients, hematologic malignancies)
- Sun exposure over a lifetime - High UVB and UVA radiation exposure (Recent evidence has shown that the risk of melanoma is higher in people who use sunscreen. Because sunscreen mostly blocks UVB, people using sunscreen may be exposed to UVA more than the general public, provided those people are exposed to the sun more than the public at large); low latitude; the number of blistering sunburns; use of tanning beds.
- Atypical mole syndrome (formerly termed B-K mole syndrome, dysplastic nevus syndrome, familial atypical multiple mole melanoma) - Over ten years, 10.7% risk of melanoma (vs. 0.62% of controls); higher risk of melanoma depending on the number of family members affected (nearly 100% risk if two or more relatives have dysplastic nevi and melanoma)
- Socioeconomic status - Lower socioeconomic status may be linked to more advanced disease at the time of detection. One survey of newly-diagnosed patients found that low-SES individuals have decreased melanoma risk perception and knowledge of the disease.
Epidemiology
The incidence of malignant melanoma is rapidly increasing worldwide, and this increase is occurring at a faster rate than that of any other cancer except lung cancer in women. Melanoma is more common in Whites than in Blacks and Asians. Overall, melanoma is the fifth most common malignancy in men and the seventh most common malignancy in women, accounting for 5% and 4% of all new cancer cases, respectively. The average age at diagnosis is 57 years, and up to 75% of patients are younger than 70 years of age.[3][4]Melanoma is notorious for affecting young and middle-aged people, unlike other solid tumors, mainly affecting older adults. It is commonly found in patients younger than 55 years, and it accounts for the third-highest number of lives lost across all cancers.
Pathophysiology
Melanomas may develop in or near a previously existing precursor lesion or in healthy-appearing skin. A malignant melanoma developing in healthy skin is said to arise de novo, without evidence of a precursor lesion. Solar irradiation induces many of these melanomas. Melanoma also may occur in unexposed areas of the skin, including the palms, soles, and perineum.
Certain lesions are considered to be precursor lesions of melanoma. These include the following nevi:
- Common acquired nevus
- Dysplastic nevus
- Congenital nevus
- Cellular blue nevus
Melanomas have 2 growth phases, radial and vertical. During the radial growth phase, malignant cells grow in a radial fashion in the epidermis. With time, most melanomas progress to the vertical growth phase, in which the malignant cells invade the dermis and develop the ability to metastasize.
Clinically, lesions are classified according to their depth, as follows:
- Thin - 1 mm or less
- Moderate - 1 mm to 4 mm
- Thick- greater than 4 mm
The 4 major types of melanoma, classified according to growth pattern, are as follows:
- Superficial spreading melanoma constitutes approximately 70% of melanomas, usually flat but may become irregular and elevated in later stages; the lesions average 2 cm in diameter, with variegated colors, as well as peripheral notches, indentations, or both.
- Nodular melanoma accounts for approximately 15% to 30% of melanoma diagnoses; the tumors typically are blue-black but may lack pigment in some circumstances.
- Lentigo maligna melanoma represents 4% to 10% of melanomas; the tumors are often larger than 3 cm, flat, and tan, with marked notching of the borders; they begin as small, freckle-like lesions
- Acral lentiginous melanoma constitutes 2% to 8% of melanomas in Whites and 35% to 60% of them in dark-skinned people; may appear on the palms and soles as flat, tan, or brown stains with irregular borders; subungual lesions can be brown or black, with ulcerations in later stages.
History and Physical
Most commonly, the history includes either changing characteristics in an existing mole or the identification of a new mole.
The characteristics of melanoma are commonly known by the acronym ABCDE and include the following:
- A - Asymmetry
- B - Irregular border
- C - Color variations, especially red, white, and blue tones in a brown or black lesion
- D - Diameter greater than 6 mm
- E - Elevated surface
Also, melanomas may itch, bleed, ulcerate, or develop satellites. Patients who present with metastatic disease or with primary sites other than the skin have signs and symptoms related to the affected organ system(s).
It is also important to examine all lymph node groups.
Evaluation
Perform excisional biopsy on suggestive lesions so that a pathologist can confirm the diagnosis. Shave biopsies and electrodesiccation are inadequate; a full thickness of the skin is essential for proper histologic diagnosis and classification.[5][6][7] The most important prognostic indicator for stage I and II tumors is thickness; obtain a full-thickness biopsy specimen for adequate pathologic interpretation. Biopsy results ultimately determine the margins of resection and which patients are candidates for sentinel lymph node biopsy and other adjuvant treatment.
The following laboratory studies are indicated:
- Complete blood count
- Complete chemistry panel (including alkaline phosphatase, hepatic transaminases, total protein, and albumin)
- Lactate dehydrogenase
The following imaging modalities may be considered:
- Chest radiography
- MRI of the brain
- Ultrasonography (possibly the best imaging study for diagnosing lymph node involvement)
- CT of the chest, abdomen, or pelvis
- Positron emission tomography (PET; PET-CT may be the best imaging study for identifying other sites of metastasis)
Treatment / Management
Surgery such as wide local excision with sentinel lymph node biopsy, elective node dissection, or both is the definitive treatment for early-stage melanoma. When performing the wide local excision, first consider the surgical margins. If the primary closure is not feasible, skin grafting or tissue transfers may be needed.[8][9][10][11] Medical management is reserved for adjuvant therapy of patients with advanced melanoma.
Agents that may be used in adjuvant therapy include the following:
- Interferon alfa
- Pegylated interferon
- Granulocyte-macrophage colony-stimulating factor (GM-CSF)
- Ipilimumab
Agents that merit consideration for the treatment of advanced-stage (stage IV) melanoma include the following:
- Dacarbazine
- Temozolomide
- Interleukin-2
- Cisplatin, vinblastine, and dacarbazine (CVD)
- Cisplatin, dacarbazine, carmustine, and tamoxifen
- Carboplatin and paclitaxel
- Ipilimumab
- Pembrolizumab
- Trametinib
- Vemurafenib (BRAF Positive)
- Dabrafenib (BRAF Positive)
- Peginterferon alfa-2b
- Nivolumab
Differential Diagnosis
- Atypical fibroxanthoma
- Basal cell carcinoma
- Blue nevus
- Epithelioid tumor
- Halo nevus
- Histiocytoid hemangioma
- Lentigo malignant melanoma
- Mycosis fungoides
- Pigmented spindle cell tumor
- Sebaceous carcinoma
Prognosis
Poor prognostic factors include the following:
- Tumor thickness (worse prognosis in thicker lesions)
- Evidence of tumor in regional lymph nodes (stage III disease)
- A higher number of positive lymph nodes
- Presence of distant metastasis (stage IV disease)
- Anatomic site (trunk and/or face lesions have worse prognoses than extremity lesions)
- Presence of ulceration
- Presence of regression on histologic examination (controversial)
- Male sex
Prognosis depends on the disease stage at diagnosis, as follows:
- Patients with stage I disease - 5-year survival rate of greater than 90%
- Patients with stage II disease - 5-year survival rate ranging from 45% to 77%
- Patients with stage III disease - 5-year survival rate ranging from 27% to 70%
Patients with metastatic disease have a grim prognosis, with a 5-year survival rate of less than 20%.
Complications
Most severe complications occur in cases of delayed diagnosis and treatment. Complications can include:
- Secondary infection - resulting from disruption of the normal skin barrier.
- Scarring - these can result from the lesion itself or treatments.
- Lymphedema - most commonly occurs secondary to the removal of lymph nodes but can result from cancer alone.
- Local recurrence - especially in cases that were more advanced before diagnosis.
- Metastases - more common with advanced cases, and melanomas and squamous cell carcinomas
- Depression and anxiety - because of cosmesis issues.
Deterrence and Patient Education
Patients need to receive counsel to engage in preventative activities, especially once they have been treated for melanoma. These actions include:
- Avoid midday sun
- Use sunscreen at all times of the year
- Don protective clothing to cover skin
- Avoid tanning beds
- Be familiar with their skin so they can promptly spot changes - this includes areas that may not receive much sun exposure
Enhancing Healthcare Team Outcomes
Skin cancers are frequently seen by primary care providers, nurse practitioners, internists, and pharmacists; this is why an interprofessional team approach is needed. While many skin lesions are benign, it is important always to consider melanoma- as it is potentially deadly if the diagnosis gets missed. If there is suspicion of melanoma, the patient should obtain a referral to the dermatologist/oncologist and pathologist for further workup, irrespective of which of the other healthcare providers first became suspicious. Surgery includes wide local excision with sentinel lymph node biopsy, elective node dissection, or both. These surgical procedures are the definitive treatment for early-stage melanoma.
When performing the wide local excision, first consider the surgical margins. If the primary closure is not feasible, skin grafting or tissue transfers may be needed. Medical management is reserved for adjuvant therapy of patients with advanced melanoma; here again, the pharmacist can monitor medications and consult with the dermatologist. Dermatology nursing staff will assist at all stages of case management, and provide patient counsel and monitor the condition, reporting to the treating clinician as necessary. For localized lesions, the prognosis is with surgery, but advanced melanoma has a grim prognosis, but the interprofessional team approach to care will optimize the patient's prospects for a better outcome.[12][13][14] [Level 5]
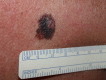
Figure
Melanoma DermNet New Zealand
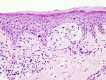
Figure
Malignant Melanoma Contributed by Scott Jones, MD

Figure
Superficial Melanoma. Histopathology shows an atypical proliferation of melanocytes in the dermis, in a diffuse pattern. The architecture is altered by epidermal invasion and erosion. H/E 4x Contributed by Fabiola Farci, MD

Figure
Nodular Melanoma. Nodular melanoma with vertical growth phase and deep dermal invasion. H/E 4x Contributed by Fabiola Farci, MD

Figure
Dermal Invasion by Melanoma. Malignant melanocytic proliferation enhanced by MART1 / MELAN-A immunohistochemical stain with deep dermal invasion. 4x Contributed by Fabiola Farci, MD
References
- 1.
- Ott PA. Intralesional Cancer Immunotherapies. Hematol Oncol Clin North Am. 2019 Apr;33(2):249-260. [PubMed: 30832998]
- 2.
- Tarhini A, Atzinger C, Gupte-Singh K, Johnson C, Macahilig C, Rao S. Treatment patterns and outcomes for patients with unresectable stage III and metastatic melanoma in the USA. J Comp Eff Res. 2019 May;8(7):461-473. [PubMed: 30832505]
- 3.
- Ghazawi FM, Darwich R, Le M, Rahme E, Zubarev A, Moreau L, Burnier JV, Sasseville D, Burnier MN, Litvinov IV. Uveal melanoma incidence trends in Canada: a national comprehensive population-based study. Br J Ophthalmol. 2019 Dec;103(12):1872-1876. [PubMed: 30819691]
- 4.
- Donley GM, Liu WT, Pfeiffer RM, McDonald EC, Peters KO, Tucker MA, Cahoon EK. Reproductive factors, exogenous hormone use and incidence of melanoma among women in the United States. Br J Cancer. 2019 Apr;120(7):754-760. [PMC free article: PMC6461881] [PubMed: 30814688]
- 5.
- Hayek SA, Munoz A, Dove JT, Hunsinger M, Arora T, Wild J, Shabahang M, Blansfield J. Hospital-Based Study of Compliance with NCCN Guidelines and Predictive Factors of Sentinel Lymph Node Biopsy in the Setting of Thin Melanoma Using the National Cancer Database. Am Surg. 2018 May 01;84(5):672-679. [PubMed: 29966567]
- 6.
- Janz TA, Neskey DM, Nguyen SA, Lentsch EJ. Is imaging of the brain necessary at diagnosis for cutaneous head and neck melanomas? Am J Otolaryngol. 2018 Sep-Oct;39(5):631-635. [PubMed: 29929862]
- 7.
- Barker CA, Salama AK. New NCCN Guidelines for Uveal Melanoma and Treatment of Recurrent or Progressive Distant Metastatic Melanoma. J Natl Compr Canc Netw. 2018 May;16(5S):646-650. [PubMed: 29784747]
- 8.
- Reserva J, Janeczek M, Joyce C, Goslawski A, Hong H, Yuan FN, Balasubramanian N, Winterfield L, Swan J, Tung R. A Retrospective Analysis of Surveillance Adherence of Patients after Treatment of Primary Cutaneous Melanoma. J Clin Aesthet Dermatol. 2017 Dec;10(12):44-48. [PMC free article: PMC5774903] [PubMed: 29399266]
- 9.
- Blakely AM, Comissiong DS, Vezeridis MP, Miner TJ. Suboptimal Compliance With National Comprehensive Cancer Network Melanoma Guidelines: Who Is at Risk? Am J Clin Oncol. 2018 Aug;41(8):754-759. [PubMed: 28121641]
- 10.
- Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE, Daniels GA, DiMaio D, Fields RC, Fleming MD, Gastman B, Gonzalez R, Guild V, Johnson D, Joseph RW, Lange JR, Martini MC, Materin MA, Olszanski AJ, Ott P, Gupta AP, Ross MI, Salama AK, Skitzki J, Swetter SM, Tanabe KK, Torres-Roca JF, Trisal V, Urist MM, McMillian N, Engh A. NCCN Guidelines Insights: Melanoma, Version 3.2016. J Natl Compr Canc Netw. 2016 Aug;14(8):945-58. [PubMed: 27496110]
- 11.
- Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE, Daniels GA, DiMaio D, Ernstoff M, Fields RC, Fleming MD, Gonzalez R, Guild V, Halpern AC, Hodi FS, Joseph RW, Lange JR, Martini MC, Materin MA, Olszanski AJ, Ross MI, Salama AK, Skitzki J, Sosman J, Swetter SM, Tanabe KK, Torres-Roca JF, Trisal V, Urist MM, McMillian N, Engh A. Melanoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016 Apr;14(4):450-73. [PubMed: 27059193]
- 12.
- Ferguson SD, Bindal S, Bassett RL, Haydu LE, McCutcheon IE, Heimberger AB, Li J, O'Brien BJ, Guha-Thakurta N, Tetzlaff MT, Tawbi H, Davies MA, Glitza IC. Predictors of survival in metastatic melanoma patients with leptomeningeal disease (LMD). J Neurooncol. 2019 May;142(3):499-509. [PubMed: 30847840]
- 13.
- Cho SI, Lee J, Jo G, Kim SW, Minn KW, Hong KY, Jo SJ, Cho KH, Kim BJ, Mun JH. Local recurrence and metastasis in patients with malignant melanomas after surgery: A single-center analysis of 202 patients in South Korea. PLoS One. 2019;14(3):e0213475. [PMC free article: PMC6405088] [PubMed: 30845184]
- 14.
- Nyakas M, Aamdal E, Jacobsen KD, Guren TK, Aamdal S, Hagene KT, Brunsvig P, Yndestad A, Halvorsen B, Tasken KA, Aukrust P, Maelandsmo GM, Ueland T. Prognostic biomarkers for immunotherapy with ipilimumab in metastatic melanoma. Clin Exp Immunol. 2019 Jul;197(1):74-82. [PMC free article: PMC6591141] [PubMed: 30821848]
Disclosure: Jonathan Heistein declares no relevant financial relationships with ineligible companies.
Disclosure: Utkarsh Acharya declares no relevant financial relationships with ineligible companies.
Disclosure: Shiva Kumar Mukkamalla declares no relevant financial relationships with ineligible companies.
Publication Details
Author Information and Affiliations
Authors
Jonathan B. Heistein; Utkarsh Acharya1; Shiva Kumar R. Mukkamalla2.Affiliations
Publication History
Last Update: May 22, 2023.
Copyright
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Publisher
StatPearls Publishing, Treasure Island (FL)
NLM Citation
Heistein JB, Acharya U, Mukkamalla SKR. Malignant Melanoma. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.




