Continuing Education Activity
Cerebral palsy is a group of permanent disorders affecting the development of movement and causing a limitation of activity. Non-progressive disturbances that manifest in the developing fetal or infant brain lead to cerebral palsy. It is the most common cause of childhood disability. The degree and type of motor impairment and functional capabilities vary depending on the etiology. Cerebral palsy may have several associated comorbidities, including epilepsy, musculoskeletal problems, intellectual disability, feeding difficulties, visual abnormalities, hearing abnormalities, and communication difficulties. Treatment of cerebral palsy should be with an interprofessional approach. This activity reviews the evaluation, treatment, and complications of cerebral palsy and underscores the importance of an interprofessional team approach to its management.
Objectives:
- Outline the various pre-, peri-, and post-natal etiologies that can result in cerebral palsy.
- Describe the physical examination process needed to evaluate a patient who presents with cerebral palsy.
- Review the interprofessional team approach that is necessary to manage cases of cerebral palsy.
- Explain the importance of interprofessional team strategies for improving care coordination and communication to aid in the diagnosis of cerebral palsy and improving outcomes in patients diagnosed with the condition.
Introduction
Cerebral palsy is a group of permanent disorders affecting the development of movement and causing a limitation of activity. Non-progressive disturbances that manifest in the developing fetal or infant brain lead to cerebral palsy.[1] It is the most common cause of childhood disability. The degree and type of motor impairment and functional capabilities vary depending on the etiology. Cerebral palsy may have several associated comorbidities, including epilepsy, musculoskeletal problems, intellectual disability, feeding difficulties, visual abnormalities, hearing abnormalities, and communication difficulties. Treatment of cerebral palsy should take an interprofessional approach.
Etiology
Abnormal development or damage to the fetal or infant's brain causes cerebral palsy. The brain insult/injury causing CP is non-progressive ("static") and can occur in the prenatal, perinatal, or postnatal periods. The etiology in an individual patient is often multifactorial.
- Congenital brain malformations
- Intrauterine infections
- Intrauterine stroke
- Chromosomal abnormalities
- Hypoxic-ischemic insults
- Central nervous system (CNS) infections
- Stroke
- Kernicterus
- Accidental and non-accidental trauma
- CNS infections
- Stroke
- Anoxic insults
Prematurity is a significant risk factor for cerebral palsy. Complications of prematurity that can cause cerebral palsy include[2][3]:
- Periventricular leukomalacia
- Intraventricular hemorrhage
- Periventricular infarcts.
Other risk factors associated with cerebral palsy are multiple gestation, intrauterine growth restriction, maternal substance abuse, preeclampsia, chorioamnionitis, abnormal placental pathology, meconium aspiration, perinatal hypoglycemia, and genetic susceptibility.[4][5]
Epidemiology
Cerebral palsy is the most common cause of childhood disability. It occurs in 1.5 to 2.5 per 1000 live births.[6] The prevalence is significantly higher in infants born prematurely than infants born at term. The risk of developing cerebral palsy increases with declining gestational age, with infants born at less than 28 weeks gestational age being at most risk.[6] The prevalence is also higher in low birth weight infants. Very low birth weight (less than 1500 grams) infants born are at greatest risk; 5% to 15% of infants born weighing less than 1500 grams develop cerebral palsy.[6] Prenatal events cause approximately 80% of cerebral palsy cases, and postnatal events cause about 10% of cases.
History and Physical
Cerebral palsy is a clinical diagnosis based mostly on information gathered from the patient’s history and physical exam. The clinical history should focus on identifying risk factors and likely etiologies of the patient’s cerebral palsy. The history should include a detailed prenatal, birth, and developmental history. Developmental history should pay particular attention to motor development. In cerebral palsy, there is a delay in motor development. A history of developmental regression is not consistent with cerebral palsy. Family history is important, as well. Multiple family members with similar delayed development or neurologic disorders as the patient should prompt consideration of a genetic etiology of cerebral palsy or a disorder that mimics CP.[7][8] Clinical history should also focus on screening for co-morbid disorders, including epilepsy, musculoskeletal abnormalities, pain, visual and hearing difficulties, feeding problems, communication disorders, and behavioral disorders.
The physical exam should focus on identifying clinical signs of cerebral palsy. Head circumference, mental status, muscle tone and strength, posture, reflexes (primitive, postural, and deep tendon reflexes), and gait should undergo evaluation. Clinical signs and symptoms of cerebral palsy can include micro- or macrocephaly, excessive irritability or diminished interaction, hyper- or hypotonia, spasticity, dystonia, muscle weakness, the persistence of primitive reflexes, abnormal or absent postural reflexes, incoordination, and hyperreflexia.
The physical exam can also identify the cerebral palsy type. Cerebral palsy characteristically demonstrates the kind of tone abnormality and distribution of motor abnormalities. The subtypes of cerebral palsy are[9]:
- Spastic diplegic: The patient has spasticity and motor difficulties affecting the legs more than the arms
- Spastic hemiplegic: The patient has spasticity and motor difficulties affecting one side of the body; the arms are often involved more than the legs
- Spastic quadriplegic: The patient has spasticity and motor difficulties affecting all four extremities; often, there is often greater involvement of the upper extremities than the legs
- Dyskinetic/hyperkinetic (choreoathetoid): The patient has excessive, involuntary movements characterized as a combination of rapid, dance-like contractions of muscles and slow writhing movements
- Dystonic: The patient has involuntary, sustained muscle contractions causing twisting and repetitive movements
- Ataxic: The patient has unsteadiness and incoordination, they are often hypotonic
Evaluation
Clinical history and physical exam combined with neuroimaging and standardized developmental assessments are useful in making a diagnosis of cerebral palsy. Brain MRI is the preferred imaging modality for evaluating the cause of cerebral palsy. MRI has a higher diagnostic yield than CT and provides better detail of the brain’s anatomy. MRI has an 86% to 89% sensitivity for detecting abnormal neuroanatomy in the motor areas of the brain.[10] A cranial ultrasound performed in the neonatal/early infancy period can be useful in identifying intraventricular hemorrhage, ventriculomegaly, and periventricular leukomalacia.
For early detection of cerebral palsy standardized developmental assessments along with neuroimaging should be used. The General Movements Assessment (GM) is a standardized motor assessment used in children under age five months corrected age.[10] The GM observes the quality of spontaneous movements in infants while lying supine. Cramped-synchronized general movements and the absence of fidgety movements between 9 to 20 months reliably predict cerebral palsy. It has a 98% sensitivity and 89% to 93% inter-rater reliability.[10] The Hammersmith Infant Neurological Exam (HINE) is a standardized neurological assessment that is available for children between the ages of 2 and 24 months. It consists of 37 items and is subdivided into three sections: physical exam, documentation of motor development, and evaluation of the behavior state. The HINE has a 90% sensitivity for detecting cerebral palsy.[10]
An EEG is necessary for patients suspected of having seizures. Patients with stroke as a cause of their cerebral palsy should undergo thrombophilia screening. Pro-thrombotic coagulation abnormalities are present in 50% to 60% of patients with a history of stroke.[8]
The clinical signs and symptoms of cerebral palsy can occur in many other conditions. Slowly progressive disorders can be mistaken for cerebral palsy, and sometimes these disorders have a specific treatment that halts the progression, prevents complications, or treats the primary underlying pathophysiology of the condition. Therefore, it is essential to screen for disorders that mimic cerebral palsy when the clinical history, physical exam, and neuroimaging are atypical for cerebral palsy. The following historical features are concerning for an alternative diagnosis: family history of cerebral palsy or other neurologic disorders, no known risk factor for cerebral palsy, developmental regression, hypotonia associated with weakness, the rapid loss of neurologic skills, worsening during fasting or illness, oculomotor abnormalities, or sensory loss.[8][11] A metabolic work up to screen for inborn errors of metabolism is necessary for patients who have a progressive course or decompensation during periods of catabolism.[11] Genetic workup is a recommendation in those with dysmorphic features, brain malformations, family history of cerebral palsy, or if there is a history of consanguinity. Lumbar puncture should be obtained in patients with unexplained refractory seizures or movement disorders to screen for neurotransmitter disorders and glucose transporter deficiency.[8][11]
Treatment / Management
Treatment of cerebral palsy takes an interprofessional team approach. The team includes physicians (primary care, neurologists, physiatrists, orthopedists, and other specialists needed based on co-existing conditions), therapists (physical, occupational, and speech), behavioral health specialists, social workers/case managers, and educational specialists. Interventions should focus on maximizing the quality of life and decreasing the disability burden. The patient, family, and team should set functional goals that are realistic and periodically reevaluated.[10]
Oral and injectable (e.g., botulinum toxin) medications can help to treat tone abnormalities, pain, and comorbid conditions such as epilepsy, sialorrhea, gastrointestinal disturbances, and behavior disorders. Medications used for spasticity include benzodiazepines, baclofen, dantrolene, tizanidine, cyclobenzaprine, botulinum toxin, and phenol.[12] Clinicians often treat dystonia with trihexyphenidyl, gabapentin, carbidopa-levodopa, and benztropine. Sialorrhea treatment includes glycopyrrolate, atropine drops, and scopolamine patches. Anti-seizure medications are used in patients with epilepsy. Constipation is a frequent complication of cerebral palsy, requiring stool softeners and pro-motility agents. Anti-inflammatories address pain and antidepressants for depression and anxiety.
Surgical management options include placement of a baclofen pump, selective dorsal rhizotomy, tendon releases, hip derotation/rotation surgery, spinal fusion, strabismus repair, and deep brain stimulation.[12][13][12]
Differential Diagnosis
Conditions that can mimic cerebral palsy include neurodegenerative disorders, inborn errors of metabolism, developmental abnormalities of the spinal cord, neuromuscular disorders, movement disorders, and neoplasms. Below is a list of differential considerations based on the predominant clinical feature.[3][7][11]
Spasticity
- Hereditary spastic paraplegia
- Tethered cord
- Spinal cord tumor
- Adrenoleukodystrophy
- Arginase deficiency
- Pyruvate dehydrogenase deficiency
- Rett syndrome
- Lesch-Nyhan syndrome
- Pelizaeus-Merzbacher
- Glut 1 transporter deficiency
Dystonia
- Dopa-responsive dystonia
- Glutaric aciduria type 1
- Pyruvate dehydrogenase deficiency
- Lesch-Nyhan syndrome
- Leigh's disease
- Niemann-Pick type C
- Glut 1 transporter deficiency
Hypotonia
- Holocarboxylase synthetase deficiency
- Zellweger syndrome
- Infantile Refsum disease
- Pontocerebellar hypoplasias
- Metachromatic leukodystrophy
Ataxia
- Ataxia-telangiectasia
- X-linked spinocerebellar ataxia
- Angelman's syndrome
- Glut 1 transporter deficiency
- Leigh disease
- Joubert syndrome
Choreoathetosis
- Pelizaeus-Merzbacher
- Lesch-Nyhan syndrome
Weakness
- Muscular dystrophies
- Metachromatic leukodystrophy
- Pontocerebellar hypoplasias
Prognosis
Most children with cerebral palsy will survive into adulthood.[14] Severely affected patients have a reduced life expectancy. The most common cause of early death is a respiratory disease, usually aspiration pneumonia.
The prognosis of motor abilities depends on the cerebral palsy subtype, the rate of motor development, ascertainment of developmental reflexes, and cognitive abilities. Children who walk independently typically achieve this milestone by three years of age. Those who walk with support may take up to age nine years to reach this milestone.[15] A child that is not walking by age nine years is unlikely to walk even with support. Children with hemiplegic, choreoathetoid, and ataxic cerebral palsy are likely to achieve walking. Good prognostic indicators for independent walking are sitting by age 24 months and crawling by 30 months.[15] Poor prognostic indicators for walking include not having achieved head balance by 20 months, primitive reflexes retained, or no postural reflexes by age 24 months, and not crawling by age five years.[15]
Complications
A variety of complications can accompany cerebral palsy, including[9]:
- Pain- occurs in 50% to 75%
- Intellectual disability occurs in 50%
- Epilepsy occurs in 25% to 45%
- Orthopedic disorders (hip subluxation/dislocation (30%), foot deformities, and scoliosis)
- Speech impairment occurs in 40% to 50%
- Hearing impairment occurs in 10% to 20%
- Blindness occurs in 10%
- Strabismus occurs in 50%
- Neurobehavioral disorders occur in 25%
- Growth failure
- Pulmonary disease
- Osteopenia occurs in 77% of those moderate-severely affected
- Urologic conditions (incontinence, neurogenic bladder): occurs in 30 to 60%
- Sleep disturbances occur in 23%
- Dental abnormalities
Deterrence and Patient Education
Cerebral palsy is a term used to describe a group of disorders that are caused by a non-progressive brain abnormality, which results in difficulty with movement, tone, and/or posture. There are several factors during pregnancy, around the time of birth, and after birth that play a role in the development of cerebral palsy. The significant risk factors for cerebral palsy are prematurity and low birth weight. Other causes of cerebral palsy include stroke, lack of oxygen to the brain, infections of the brain, and abnormal development of the brain. Cerebral palsy is the most common cause of childhood disability. Cerebral palsy is a clinical diagnosis, made by obtaining a detailed prenatal and birth history, physical exam, and neuroimaging. Treatment focuses on achieving the best functional outcomes and takes an interprofessional team approach. Routine prenatal care and measures to reduce preterm birth lower the risk of cerebral palsy.
Enhancing Healthcare Team Outcomes
Clinicians should aim to recognize the signs of cerebral palsy and make the diagnosis of cerebral palsy or "high risk of cerebral palsy" as early as possible. Historically the diagnosis of cerebral palsy was made between the ages of 12 to 24 months. There is now good evidence to support that the diagnosis can be made or accurately predicted before age six months corrected age.[10] Early diagnosis can improve functional outcomes and reduce disease burden because starting interventions early can optimize neuroplasticity. Delays in diagnosis can be harmful to parent's and caregiver's well-being. A diagnosis allows parents to receive psychological support and resources. Patients with known risk factors for cerebral palsy should receive a referral for further diagnostic testing, including neuroimaging and standardized developmental assessments.[10] [Level 1] Despite the best treatment, there is a significant reduction in the lifespan of most individuals with cerebral palsy. [16] [Level 2]
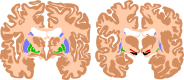
Figure
The basal ganglia are instrumental in motor function. Damage to these areas results in athetoid/ dyskinetic cerebral palsy (ADCP). Contributed by Wikimedia Commons, Mikael Häggström and Andrew Gillies (Public Domain)
References
- 1.
- The Definition and Classification of Cerebral Palsy. Dev Med Child Neurol. 2007 Feb;49(s109):1-44. [PubMed: 17371509]
- 2.
- Nelson KB. Causative factors in cerebral palsy. Clin Obstet Gynecol. 2008 Dec;51(4):749-62. [PubMed: 18981800]
- 3.
- MacLennan AH, Thompson SC, Gecz J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. 2015 Dec;213(6):779-88. [PubMed: 26003063]
- 4.
- McMichael G, Bainbridge MN, Haan E, Corbett M, Gardner A, Thompson S, van Bon BW, van Eyk CL, Broadbent J, Reynolds C, O'Callaghan ME, Nguyen LS, Adelson DL, Russo R, Jhangiani S, Doddapaneni H, Muzny DM, Gibbs RA, Gecz J, MacLennan AH. Whole-exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015 Feb;20(2):176-82. [PubMed: 25666757]
- 5.
- van Eyk CL, Corbett MA, Maclennan AH. The emerging genetic landscape of cerebral palsy. Handb Clin Neurol. 2018;147:331-342. [PubMed: 29325622]
- 6.
- Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013 Jun;55(6):509-19. [PubMed: 23346889]
- 7.
- Morgan C, Fahey M, Roy B, Novak I. Diagnosing cerebral palsy in full-term infants. J Paediatr Child Health. 2018 Oct;54(10):1159-1164. [PubMed: 30294991]
- 8.
- Whelan MA. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004 Nov 23;63(10):1985-6; author reply 1985-6. [PubMed: 15568275]
- 9.
- Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, Becher JG, Gaebler-Spira D, Colver A, Reddihough DS, Crompton KE, Lieber RL. Cerebral palsy. Nat Rev Dis Primers. 2016 Jan 07;2:15082. [PMC free article: PMC9619297] [PubMed: 27188686]
- 10.
- Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, Cioni G, Damiano D, Darrah J, Eliasson AC, de Vries LS, Einspieler C, Fahey M, Fehlings D, Ferriero DM, Fetters L, Fiori S, Forssberg H, Gordon AM, Greaves S, Guzzetta A, Hadders-Algra M, Harbourne R, Kakooza-Mwesige A, Karlsson P, Krumlinde-Sundholm L, Latal B, Loughran-Fowlds A, Maitre N, McIntyre S, Noritz G, Pennington L, Romeo DM, Shepherd R, Spittle AJ, Thornton M, Valentine J, Walker K, White R, Badawi N. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017 Sep 01;171(9):897-907. [PMC free article: PMC9641643] [PubMed: 28715518]
- 11.
- Leach EL, Shevell M, Bowden K, Stockler-Ipsiroglu S, van Karnebeek CD. Treatable inborn errors of metabolism presenting as cerebral palsy mimics: systematic literature review. Orphanet J Rare Dis. 2014 Nov 30;9:197. [PMC free article: PMC4273454] [PubMed: 25433678]
- 12.
- Nahm NJ, Graham HK, Gormley ME, Georgiadis AG. Management of hypertonia in cerebral palsy. Curr Opin Pediatr. 2018 Feb;30(1):57-64. [PubMed: 29135566]
- 13.
- Park TS, Dobbs MB, Cho J. Evidence Supporting Selective Dorsal Rhizotomy for Treatment of Spastic Cerebral Palsy. Cureus. 2018 Oct 19;10(10):e3466. [PMC free article: PMC6300384] [PubMed: 30585282]
- 14.
- Strauss D, Brooks J, Rosenbloom L, Shavelle R. Life expectancy in cerebral palsy: an update. Dev Med Child Neurol. 2008 Jul;50(7):487-93. [PubMed: 18611196]
- 15.
- da Paz Júnior AC, Burnett SM, Braga LW. Walking prognosis in cerebral palsy: a 22-year retrospective analysis. Dev Med Child Neurol. 1994 Feb;36(2):130-4. [PubMed: 8132123]
- 16.
- Morgan P, McGinley JL. Cerebral palsy. Handb Clin Neurol. 2018;159:323-336. [PubMed: 30482324]
- 17.
- Verschuren O, Peterson MD, Balemans AC, Hurvitz EA. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016 Aug;58(8):798-808. [PMC free article: PMC4942358] [PubMed: 26853808]
- 18.
- Moreau NG, Bodkin AW, Bjornson K, Hobbs A, Soileau M, Lahasky K. Effectiveness of Rehabilitation Interventions to Improve Gait Speed in Children With Cerebral Palsy: Systematic Review and Meta-analysis. Phys Ther. 2016 Dec;96(12):1938-1954. [PMC free article: PMC5131187] [PubMed: 27313240]
- 19.
- Pin T, Dyke P, Chan M. The effectiveness of passive stretching in children with cerebral palsy. Dev Med Child Neurol. 2006 Oct;48(10):855-62. [PubMed: 16978468]
- 20.
- McCoy SW, Palisano R, Avery L, Jeffries L, Laforme Fiss A, Chiarello L, Hanna S. Physical, occupational, and speech therapy for children with cerebral palsy. Dev Med Child Neurol. 2020 Jan;62(1):140-146. [PubMed: 31353456]
Disclosure: Jamika Hallman-Cooper declares no relevant financial relationships with ineligible companies.
Disclosure: Franklyn Rocha Cabrero declares no relevant financial relationships with ineligible companies.
Publication Details
Author Information and Affiliations
Authors
Jamika L. Hallman-Cooper1; Franklyn Rocha Cabrero2.Affiliations
Publication History
Last Update: October 10, 2022.
Copyright
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Publisher
StatPearls Publishing, Treasure Island (FL)
NLM Citation
Hallman-Cooper JL, Rocha Cabrero F. Cerebral Palsy. [Updated 2022 Oct 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
