This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Show detailsIntroduction
The blood-brain barrier (BBB) is a selective semi-permeable membrane between the blood and the interstitium of the brain, allowing cerebral blood vessels to regulate molecule and ion movement between the blood and the brain.[1] The BBB is composed of endothelial cells (ECs), pericytes (PCs), capillary basement membrane, and astrocyte end-feet, all of which aim to shield the brain from toxic substances, filter harmful compounds from the brain to the bloodstream, and supply brain tissue with nutrients. To do this, the BBB has physical (tight junctions) and metabolic (enzyme) barriers.[2] Central nervous system (CNS) structures are unique in structure and function and therefore require a stable environment with a composition that differs from that of the peripheral circulation. For this reason, the BBB exists to maintain a homeostatic environment in which CNS structures can function without disruption from other bodily functions.
Structure and Function
As previously mentioned, the blood-brain barrier (BBB) is composed of a capillary basement membrane and three cellular elements: endothelial cells, pericytes, and astrocyte end-feet. The BBB is responsible for creating and maintaining homeostasis for neuronal functions, defending the system against toxic insults, regulating the communication between the periphery and the CNS, and providing the brain with nutrients. This is achieved via four main mechanisms: prevention of the paracellular diffusion of hydrophilic compounds, mediation of the active transport of nutrients to the brain, activation of efflux transport of hydrophobic molecules and drugs from the brain to the blood, and regulation of the transendothelial migration of circulating blood cells and pathogens.[3]
Tight Junctions (TJs)
Located between cerebral endothelial cells, TJs form a highly-selective diffusion barrier prohibiting most blood-borne substances from entering the brain. TJs block the paracellular aqueous diffusional pathways between adjacent endothelial cells. Due to their adhesive function, they seal microvessels and impede the passive diffusion of proteins and polar solutes in and out of the CNS.[4]
Astrocyte End-Feet
Astrocyte end-feet ensheath the vessel wall and play a critical role in the induction and maintenance of the TJ barrier. Astrocyte end-feet are not believed to have a barrier function within the mammalian brain.[5]
Endothelial Cells (ECs)
The ECs of the BBB differ from those of the rest of the body. BBB ECs have no fenestrations, more extensive tight junctions, and sparse pinocytic vesicular transport. EC tight junctions limit the paracellular flux of hydrophilic molecules across the BBB.
Pericytes (PCs)
Embedded within the capillary basement membrane, PCs play a crucial role in angiogenesis, the structural integrity and differentiation of the microvessels, and the formation of endothelial tight junctions.[6]
Circumventricular Organs (CVOs)
The blood-brain barrier is present in all regions of the brain except the circumventricular organs (CVOs), located around the third and fourth ventricles.[7] Blood vessels around the CVOs have fenestrations that permit the diffusion of blood-borne molecules across the vessel wall. These unprotected areas of the brain regulate the autonomic nervous system and endocrine glands. The CVOs are:
- pineal gland
- median eminence
- subcommisural organ
- neurohypophysis
- subfornical organ
- area postrema
- organum vasculosum of the lamina terminalis
Embryology
The development and differentiation of the blood-brain barrier occur in three stages: angiogenesis, differentiation, and maturation.
Angiogenesis
The development of the blood-brain barrier begins with angiogenesis, which occurs early in gestation during neural tube development. In angiogenesis, preexisting vessels guided by vascular endothelial growth factor (VEGF) invade a developing neuroectoderm and give rise to new vessels. Downstream VEGF signaling is essential, supporting angiogenesis via endothelial cell proliferation, migration, and survival.[8] These new vessels forming in the neuroectoderm exhibit many properties of the BBB at early stages, including the expression of tight junctions and nutrient transporters that will later play a role in the selectivity of this barrier. These early vessels also contain high levels of transcytotic vesicles and exhibit increased expression of leukocyte adhesion molecules.[9]
Differentiation
The differentiation phase allows the BBB to be appropriately structured through the induction of anti-angiogenic signals and the recruitment of astrocytes and pericytes to the newly formed vessels.
Maturation
Maturation and maintenance of the BBB are accomplished by the persistence of TJ protein expression and their redistribution throughout the BBB structure. Close contact between endothelial cells, astrocytes, and pericytes sustains BBB integrity and function as a stabilized neurovascular unit.[10] The BBB is formed and completely functional by the third trimester of gestation.
Alterations in the development of the BBB and the expression of TJs may potentially lead to anomalies later in life and an increased predisposition to develop metabolic diseases. Maternal obesity increases BBB permeability in offspring, leading to higher exposure to leptin and ghrelin. This increased exposure to leptin and ghrelin can contribute to metabolic defects in adult life, predisposing children to developing metabolic syndrome in childhood or early adulthood.[11]
Clinical Significance
As previously discussed, the BBB is relatively impermeable under normal physiologic conditions. However, in pathologic conditions, several chemical mediators that increase BBB permeability may be released, including glutamate, aspartate, taurine, ATP, endothelin-1, ATP, NO, MIP-2, TNF-alpha, and IL-beta, which are produced by astrocytes. Other agents that increase BBB permeability include bradykinin, 5HT, histamine, thrombin, UTP, UMP, substance P, quinolinic acid, platelet-activating factor, and free radicals.
Tumor-mediated Changes
A major driver in BBB compromise, particularly in high-grade gliomas, is tumor-secreted VEGF. High-grade gliomas have an increased metabolic rate, resulting in local hypoxia and upregulation of hypoxia-inducible factor-1, which stimulates the production of VEGF. Secreted VEGF then induces the breakdown of existing BBB architecture and the growth of structurally altered capillaries from the existing vessels. This tumoral-induced vascular endothelium displays an abnormal expression profile of transporters and receptors to accommodate the high metabolic demands of tumor cells. Unlike the brain vessels from which they originate, newly formed capillaries are structurally altered and more permeable than non-BBB peripheral capillaries. Ultimately, tumor-mediated changes in the BBB vary with tumor type, volume, stage, and anatomical location. The severity of barrier compromise ranges from serious disruption comparable to solid, non-brain neoplasm vasculature to mild compromise as found in neurodegenerative disease, stroke, diabetes, and obesity.
Hypoxic-Ischemic Encephalopathy (HIE)
HIE is a severe birth complication affecting full-term infants in which the brain does not receive adequate blood flow due to a hypoxic-ischemic event during the prenatal, intrapartum, or postnatal period. With impaired cerebral blood flow and oxygen delivery to the brain, severe disabilities can ensue, with up to 60% of affected infants dying by the age of 2 years or ending up with severe disabilities such as cognitive disability, developmental delay, cerebral palsy, and epilepsy. The exact cause may not always be known, but known precursors include uterine rupture, abruptio placenta, placenta previa, cord prolapse, maternal hypotension, shoulder dystocia, or breech presentation.[12]
The extent of injury in HIE can be determined by the biochemical cascades that trigger the apoptosis-necrosis continuum of cell death in the brain parenchyma and by the breaching of the BBB by pro-inflammatory factors. Following a neonatal hypoxic injury, raised levels of tight junction proteins in both cerebrospinal fluid (CSF) and plasma indicate BBB dysfunction. This is likely due to direct molecular damage to the endothelial TJs during a hypoxic insult. BBB breakdown releases TJ proteins, and BBB function may be assessed by measuring these proteins in the circulation.[13]
HIV
Breakdown of the BBB occurs in response to HIV or the viral proteins Tat and gp120. This disruption results in alterations in TJ protein expression, leading to enhanced paracellular compound flux across the BBB. Exposure to HIV and viral proteins also enhances monocyte migration across the BBB, leading to alterations in the expression and function of active efflux transport proteins such as P-glycoprotein. This breakdown of the BBB can lead to neurocognitive dysfunction, including decreased concentration, memory, information processing, learning, psychomotor speed, incoordination, and tremor. HIV-associated neurocognitive disorders can range in severity from asymptomatic neurocognitive impairment to full-blown HIV-associated dementia.[14]
Multiple Sclerosis
Multiple sclerosis (MS) is an autoimmune disease of the CNS associated with demyelination of axons, eventually leading to neurodegeneration. MS exhibits many of the hallmarks of an autoimmune inflammatory disorder, including the breakdown of the BBB. Among the earliest cerebrovascular abnormalities seen in the brains of patients with MS are dysregulation of the BBB and transendothelial migration of activated leukocytes.[15] Dimethyl fumarate is a drug used to treat MS. It reduces the transendothelial migration of activated leukocytes through the blood-brain barrier. It also has neuroprotective effects via the activation of antioxidant pathways.[16] The use of contrast agents (e.g., gadolinium) can identify a breakdown of the blood-brain barrier in patients with acute MS exacerbations.
Alzheimer Disease
BBB dysfunction is closely associated with the pathogenesis of Alzheimer disease (AD). BBB dysfunction results in the failure of A-beta transport from the brain to the peripheral circulation across the BBB, leading to the accumulation of A-beta in the brain. Changes and dysfunction of the BBB structural components, including pericytes, astrocytes, vascular endothelial cells, and TJs between endothelial cells, are associated with an increased risk of AD. BBB dysfunction triggers neuroinflammation and oxidative stress, enhances the activity of beta-secretase and gamma-secretase, and ultimately promotes A-beta generation. A vicious cycle between A-beta pathology and BBB damage is well-established and will continue to destroy the neurons and glial cells and damage the neural networks. Thus, the progressive accumulation of A-beta in the brain and BBB dysfunction can become a damaging feedback loop that gives rise to cognitive impairment and the onset of dementia.[17]
Vasogenic Edema
Vasogenic edema is the extracellular accumulation of fluid resulting from the disruption of the BBB and extravasations of serum proteins, such as albumin, into the cerebral parenchyma. The extravasated fluid accumulates outside the cells, and the excessive extracellular fluid accumulation increases brain volume and intracranial pressure (ICP).[18] According to the Monro-Kellie doctrine, the sum of brain tissue, CSF, and intracranial blood volumes is constant. Any increase in one of these three components will always be at the expense of the other two. In the case of vasogenic edema, volume shifts into the extracellular compartments of the brain will cause increased ICP, resulting in compression of brain structures and vasculature.[19]
Hepatic Encephalopathy
Typically, ammonia enters the portal circulation and is converted by the liver to urea. However, when a hepatocellular disease is present, such as hepatic encephalopathy, ammonia does not get converted to urea. Instead, it is shunted through the portosystemic collateral vessels into the systemic circulation, traversing the BBB and inducing neuronal edema and resultant hepatic encephalopathy. ATP-binding cassette (ABC) transporters prevent the brain from accumulating toxins by pumping them out of the brain. Accumulation of toxins such as ammonia during hepatic encephalopathy or other cases of severe liver disease supports the conclusion that liver diseases alter the expression and function of ABC transporters at the BBB. There is growing evidence to suggest that altered ABC transporter expression is implicated in the development of hepatic encephalopathy.[20]
Neuromyelitis Optica (Devic Disease)
Neuromyelitis optica is a disease involving the synchronous or near-synchronous development of bilateral optic neuritis and spinal cord demyelination. It results in elevated serum levels of aquaporin-4 antibodies, a protein found in astrocytic foot processes surrounding blood vessels that are involved in maintaining the blood-brain barrier.[21]
Intraventricular Hemorrhage (IVH)
IVH in premature infants is caused by dysfunction of the TJ seal and immaturity of the BBB in the germinal matrix. A lack of TJs or pericytes, coupled with incomplete coverage of blood vessels by astrocyte endfeet, may explain the fragility of blood vessels in the germinal matrix of premature infants.[22]
Other Issues
Current Strategies for Delivery of Drugs Across the BBB
As it is the tightest endothelium in the body, the BBB represents the main impediment to drug delivery to the brain. It has been estimated that greater than 90% of all small-molecule drugs and close to 100% of all larger therapeutics cannot overcome the BBB.[23]
Generally, only lipophilic (lipid soluble), positively-charged molecules with a low molecular weight (less than 400 to 600 Da) can cross the BBB. Several transport routes have been established to allow for the movement of molecules across the BBB.
- Diffusion - allows for movement of water and small ions (Na, K, Cl)
- Membrane transport - allows for movement of:
- Small lipophilic molecules (O2, CO2)
- Anesthetics, barbiturates
- Ethanol
- Nicotine
- Caffeine
- Efflux pumps - facilitate movement of P-glycoprotein, BRCP, and MRP 1,2,4, and 5
- Receptor-mediated transcytosis - facilitates the movement of insulin, leptin, IgG, transferrin, and TNF-alpha
- Adsorptive-mediated transcytosis - allows for the movement of albumin and histones
- Carrier-mediated transport - allows for movement of glucose, creatine, lactate, pyruvate, and large neutral amino acids
Ongoing research focuses on developing new strategies and techniques for drugs to effectively cross the BBB and deliver therapeutic products in the CNS. These techniques can be classified into invasive and non-invasive or miscellaneous techniques.
Invasive Techniques
- Intra-cerebro-ventricular and intrathecal infusions or injections of therapeutic proteins directly into the CSF
- Transient disruption of the BBB via focused ultrasound, noxious agents, or hyperosmotic solutions to shrink the endothelial cells of the brain by breaking down TJs, allowing various molecules to pass into the cerebral tissue. Limitations of these methods include patient dissatisfaction and compromise to the integrity and physiologic functions of the BBB, leading to the accumulation of unwanted blood products and neurotoxic agents, ultimately causing injury to the CNS
Non-Invasive or Miscellaneous Techniques
- Enhanced transcellular transport
- Intranasal delivery facilitates direct transport into the CSF, permitted by the presence of neural pathways connecting the nasal mucosa and the brain
- Use of transport/carrier systems
- Nanoparticle-based technologies, including lipid- and polymer-based nanoparticles that allow a controlled release of their cargo by protecting loaded drugs from being metabolized[24]
- Chemically transforming water-soluble molecules into lipid-soluble molecules capable of crossing the BBB by adding lipid or functional groups to the polar ends of drug molecules[25]
- Monoclonal antibody fusion proteins re-engineer the biologic drug as an IgG fusion protein, making brain penetration possible[26]
- Inhibition of efflux transporters that impede drug delivery
- Chimeric peptides covalently couple an otherwise non-transportable drug to a BBB-transportable peptide vector (e.g., cationized albumin, insulin, transferrin, etc.) by a disulfide bond, allowing for endocytosis of the chimeric peptide by the capillary endothelial cells through receptor-mediated transcytosis
- Genetically engineered proteins cross the BBB via endogenous receptor-mediated transport processes (the Trojan-horse approach)[27]
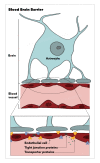
Figure
Blood Brain Barrier. The illustration depicts the astrocyte, endothelial cell, tight junction proteins, transporter proteins, and blood vessels. Illustration by E Gregory
References
- 1.
- Gawdi R, Shumway KR, Emmady PD. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Mar 17, 2023. Physiology, Blood Brain Barrier. [PubMed: 32491653]
- 2.
- Correale J, Villa A. Cellular elements of the blood-brain barrier. Neurochem Res. 2009 Dec;34(12):2067-77. [PubMed: 19856206]
- 3.
- Bellettato CM, Scarpa M. Possible strategies to cross the blood-brain barrier. Ital J Pediatr. 2018 Nov 16;44(Suppl 2):131. [PMC free article: PMC6238258] [PubMed: 30442184]
- 4.
- Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012 Nov 09;9(1):23. [PMC free article: PMC3542074] [PubMed: 23140302]
- 5.
- El-Khoury N, Braun A, Hu F, Pandey M, Nedergaard M, Lagamma EF, Ballabh P. Astrocyte end-feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2006 May;59(5):673-9. [PubMed: 16627880]
- 6.
- Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front Cell Neurosci. 2019;13:282. [PMC free article: PMC6611154] [PubMed: 31316352]
- 7.
- Kaur C, Ling EA. The circumventricular organs. Histol Histopathol. 2017 Sep;32(9):879-892. [PubMed: 28177105]
- 8.
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006 May;7(5):359-71. [PubMed: 16633338]
- 9.
- Chapouly C, Tadesse Argaw A, Horng S, Castro K, Zhang J, Asp L, Loo H, Laitman BM, Mariani JN, Straus Farber R, Zaslavsky E, Nudelman G, Raine CS, John GR. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain. 2015 Jun;138(Pt 6):1548-67. [PMC free article: PMC4614128] [PubMed: 25805644]
- 10.
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015 Nov 19;163(5):1064-1078. [PMC free article: PMC4655822] [PubMed: 26590417]
- 11.
- Kim DW, Glendining KA, Grattan DR, Jasoni CL. Maternal Obesity in the Mouse Compromises the Blood-Brain Barrier in the Arcuate Nucleus of Offspring. Endocrinology. 2016 Jun;157(6):2229-42. [PubMed: 27054554]
- 12.
- Allen KA, Brandon DH. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs Rev. 2011 Sep 01;11(3):125-133. [PMC free article: PMC3171747] [PubMed: 21927583]
- 13.
- Andersson EA, Mallard C, Ek CJ. Circulating tight-junction proteins are potential biomarkers for blood-brain barrier function in a model of neonatal hypoxic/ischemic brain injury. Fluids Barriers CNS. 2021 Feb 10;18(1):7. [PMC free article: PMC7877092] [PubMed: 33568200]
- 14.
- McRae M. HIV and viral protein effects on the blood brain barrier. Tissue Barriers. 2016 Jan-Mar;4(1):e1143543. [PMC free article: PMC4836474] [PubMed: 27141423]
- 15.
- Ortiz GG, Pacheco-Moisés FP, Macías-Islas MÁ, Flores-Alvarado LJ, Mireles-Ramírez MA, González-Renovato ED, Hernández-Navarro VE, Sánchez-López AL, Alatorre-Jiménez MA. Role of the blood-brain barrier in multiple sclerosis. Arch Med Res. 2014 Nov;45(8):687-97. [PubMed: 25431839]
- 16.
- Krämer T, Grob T, Menzel L, Hirnet T, Griemert E, Radyushkin K, Thal SC, Methner A, Schaefer MKE. Dimethyl fumarate treatment after traumatic brain injury prevents depletion of antioxidative brain glutathione and confers neuroprotection. J Neurochem. 2017 Dec;143(5):523-533. [PubMed: 28921587]
- 17.
- Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q. Role of Blood-Brain Barrier in Alzheimer's Disease. J Alzheimers Dis. 2018;63(4):1223-1234. [PubMed: 29782323]
- 18.
- Michinaga S, Koyama Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci. 2015 Apr 30;16(5):9949-75. [PMC free article: PMC4463627] [PubMed: 25941935]
- 19.
- Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001 Jun 26;56(12):1746-8. [PubMed: 11425944]
- 20.
- Fan Y, Liu X. Alterations in Expression and Function of ABC Family Transporters at Blood-Brain Barrier under Liver Failure and Their Clinical Significances. Pharmaceutics. 2018 Jul 23;10(3) [PMC free article: PMC6161250] [PubMed: 30041501]
- 21.
- Yick LW, Ma OK, Ng RC, Kwan JS, Chan KH. Aquaporin-4 Autoantibodies From Neuromyelitis Optica Spectrum Disorder Patients Induce Complement-Independent Immunopathologies in Mice. Front Immunol. 2018;9:1438. [PMC free article: PMC6026644] [PubMed: 29988553]
- 22.
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004 Jun;16(1):1-13. [PubMed: 15207256]
- 23.
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005 Jan;2(1):3-14. [PMC free article: PMC539316] [PubMed: 15717053]
- 24.
- Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics. 2018 Dec 11;10(4) [PMC free article: PMC6321434] [PubMed: 30544966]
- 25.
- Lu CT, Zhao YZ, Wong HL, Cai J, Peng L, Tian XQ. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int J Nanomedicine. 2014;9:2241-57. [PMC free article: PMC4026551] [PubMed: 24872687]
- 26.
- Pardridge WM. Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin Drug Deliv. 2015 Feb;12(2):207-22. [PubMed: 25138991]
- 27.
- Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Discov Med. 2006 Aug;6(34):139-43. [PubMed: 17234133]
Disclosure: Ary Dotiwala declares no relevant financial relationships with ineligible companies.
Disclosure: Cassidy McCausland declares no relevant financial relationships with ineligible companies.
Disclosure: Navdeep Samra declares no relevant financial relationships with ineligible companies.
- Review Cellular elements of the blood-brain barrier.[Neurochem Res. 2009]Review Cellular elements of the blood-brain barrier.Correale J, Villa A. Neurochem Res. 2009 Dec; 34(12):2067-77. Epub 2009 Oct 25.
- Physiology, Blood Brain Barrier.[StatPearls. 2024]Physiology, Blood Brain Barrier.Gawdi R, Shumway KR, Emmady PD. StatPearls. 2024 Jan
- Review The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction.[Semin Immunopathol. 2009]Review The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction.Engelhardt B, Sorokin L. Semin Immunopathol. 2009 Nov; 31(4):497-511. Epub 2009 Sep 25.
- Pericytes regulate the blood-brain barrier.[Nature. 2010]Pericytes regulate the blood-brain barrier.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Nature. 2010 Nov 25; 468(7323):557-61. Epub 2010 Oct 13.
- Review Blood-brain barrier: structural components and function under physiologic and pathologic conditions.[J Neuroimmune Pharmacol. 2006]Review Blood-brain barrier: structural components and function under physiologic and pathologic conditions.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. J Neuroimmune Pharmacol. 2006 Sep; 1(3):223-36. Epub 2006 Jul 6.
- Anatomy, Head and Neck: Blood Brain Barrier - StatPearlsAnatomy, Head and Neck: Blood Brain Barrier - StatPearls
- Poikilocytosis - StatPearlsPoikilocytosis - StatPearls
- Anatomy, Bony Pelvis and Lower Limb, Foot Fascia - StatPearlsAnatomy, Bony Pelvis and Lower Limb, Foot Fascia - StatPearls
- Papuloerythroderma of Ofuji - StatPearlsPapuloerythroderma of Ofuji - StatPearls
- Ozone Toxicity - StatPearlsOzone Toxicity - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...