Continuing Education Activity
The term amaurosis fugax is often used interchangeably to describe transient visual loss (TVL). However, it is employed widely in medicine to refer to any cause of transient monocular visual loss. Amaurosis fugax is a harbinger of an imminent stroke. This activity describes the causes, presentation, and diagnosis of amaurosis fugax and highlights the role of the interprofessional team in the management of these patients.
Objectives:
- Review the etiology of amaurosis fugax with special attention to the diseases presenting with this symptom.
- Describe the pathophysiology behind the phenomenon of amaurosis fugax.
- Summarize the clinical evaluation and differential diagnosis for diseases presenting with amaurosis fugax.
- Explain the importance of improving care coordination among the interprofessional team to enhance the delivery of care for patients presenting with amaurosis fugax.
Introduction
The term amaurosis fugax is often used interchangeably to describe transient visual loss (TVL). However, it is employed widely in medicine to refer to any cause of transient monocular visual loss. Here, we will refer to amaurosis fugax only in the context of transient monocular visual loss associated with vascular thromboembolic events arising from the internal carotid arterial system. [1],[2],[3]
The condition is not common in children and more likely to have a benign cause. In adults, in most cases, the cause is atherosclerotic emboli from the carotid artery bifurcation.
Etiology
Amaurosis fugax is a result of an occlusion or stenosis of the internal carotid artery circulation. Thromboembolism originating from the carotid circulation, as well as hypoperfusion caused by the stenosis of this circulation, are the underlying mechanisms. The ocular ischemic syndrome results from chronic hypoperfusion due to unilateral or bilateral carotid artery occlusion. [3]
Epidemiology
Amaurosis fugax usually occurs in patients over the age of 50 who have other vascular risk factors which include hypertension, hypercholesterolemia, smoking, previous episodes of transient ischemic attacks (TIAs), and claudication. The risk of hemispheric stroke in patients with amaurosis fugax is estimated to be 2% per year and 3% per year for those presenting with retinal emboli.
Risk factors for amaurosis fugax include:
- Diabetes
- Heart disease
- Smoking
- Hypertension
- Hyperlipidemia
- Advanced age
- Use of cocaine
Pathophysiology
Amaurosis fugax may occur due to the following:
- Thromboembolism from a carotid plaque
- Hypoperfusion
- Vasospasm
- Elevated plasma viscosity, for example with leukemia, multiple myeloma
- Atherosclerotic cerebrovascular disease
In amaurosis fugax, the loss of vision is usually unilateral, painless and transient. In most cases, the vision loss may vary from a few seconds to a few minutes. The embolus in most cases is from an atherosclerotic plaque in the carotid bifurcation. Hypoperfusion from any cause can also mimic amaurosis fugax.
Histopathology
During the retinal examination, the health care provider may see the cholesterol plaque lodged within a retinal vessel. It is known as a Hollenhorst plaque, and the cholesterol particle appears refractile, yellow, and bright. [4]
History and Physical
Patients complain of sudden monocular vision loss that can last between 2 to 30 minutes. The vision loss can involve the entire visual field or can be partial. Patients often describe it as a "curtain coming down” in front of their eye or as a generalized darkening or shadow. These episodes spontaneously resolve. Patients can experience 1 or multiple episodes. In anyone over the age of 60 experiencing multiple episodes, giant cell arteritis should be suspected, and further investigations should be undertaken.
Some patients can present with signs of intraretinal emboli, such as cholesterol plaques (Hollenhorst plaques). Depending on the extent of the resulting retinal ischemia, central retinal artery occlusion or branch retinal artery occlusion can develop.
In some patients, particularly those with the ocular ischemic syndrome, exposure to bright lights can provoke the episodes, as the ipsilateral severe internal carotid occlusion causes hypoperfusion of the retinal circulation and the resulting vascular insufficiency of the retinal photoreceptors combined with increased metabolic demand leads to blurred or decreased vision in those patients. Vision usually returns to normal once the photoreceptors have completely hyperpolarized.
Patients with ocular ischemic syndrome typically present with blurred vision that can be transient. The visual acuity declines with the progression of the disease. The affected eye shows episcleral injection and, on slit-lamp examination, minimal anterior chamber inflammation is present. Intraocular pressure can be low or normal due to the decreased perfusion of the ciliary body which is responsible for aqueous production. The fundus examination usually demonstrates dilated and tortuous retinal veins with narrowed arterioles accompanied by mid-peripheral dot-and-blot retinal hemorrhages. With persistent hypoperfusion, ischemia progresses and manifests as neovascularization of the iris, anterior chamber angle, retina, and optic disc. Ocular pain is a hallmark of the disease and is usually alleviated in the supine position. The latter is a result of ischemia of the ocular and orbital branches of the trigeminal nerve. [5]
Evaluation
Assessment of vascular risk factors is most important in anyone presenting with symptoms of amaurosis fugax. Patients should be assessed for hypertension, diabetes mellitus, hyperlipidemia, cardiac disease and asked about tobacco use. A careful physical examination, including full ophthalmological examination with a dilated fundus examination, as well as auscultation of the carotid arteries and the heart, should be performed. Blood work, including a complete blood count, blood glucose level, lipid profile, prothrombin time and partial thromboplastin time, erythrocyte sedimentation rate and C-reactive protein should be ordered as well. [6],[7]
Carotid Doppler is a non-invasive test used to assess the extracranial and intracranial status of the vessels. Magnetic resonance angiography and computed tomography angiography can also be used to evaluate the patency of the internal carotid arteries.
An electrocardiogram, as well as Holter monitoring, and, in some cases, transesophageal echocardiography, should be obtained to rule out any possible source of rhythmic disturbance or cardiac emboli.
Neuroimaging studies are almost always done to rule out other causes of vision loss. In addition, the CT scan can help rule out a hemorrhagic lesion in the brain.
Treatment / Management
Treatment is first aimed at controlling and treating the underlying vascular risk factors, such as hypertension, diabetes, and hyperlipidemia.
Two large studies, the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST) found that endarterectomy in symptomatic patients who have carotid stenosis greater than 70% reduces future risks of stroke. However, these studies did not address ocular TIAs separately. The NASCET further compared patients with hemispheric TIAs and ocular TIAs demonstrating that patients with hemispheric TIAs had a 2-year stroke incidence of about 44% compared to about 17% in ocular TIAs. The patients with ocular TIAs did not experience a major stroke episode, which was defined as a functional deficit persisting for more than 90 days. Both groups also had similar grades of carotid stenosis (about 83%). [2],[8],[9]
Therefore, the clinician needs to address if the risks of death and preoperative stroke in patients undergoing endarterectomy outweigh the low risk of observing these specific patients and treating them with anticoagulants alone, such as aspirin, warfarin or clopidogrel.
It is important to note that both NASCET and ECST evaluated open endarterectomy but, since then, carotid stenting has become commonly used. Carotid stenting is comparable to endarterectomy, regarding treatment, and has similar low risks of death, stroke and myocardial infarction in patients with asymptomatic severe carotid artery stenosis. [10]
Treatment of ocular ischemic syndrome involves reducing the oxygen drive to the eye to decrease the neovascularization. This involves panretinal photocoagulation or intravitreal injections of anti-vascular endothelial growth factor (VEGF).
Differential Diagnosis
The differential diagnosis of unilateral vision loss includes the following:
- Central retinal artery/vein occlusion
- Giant cell arteritis
- Multiple sclerosis
- Papilledema
- Epilepsy
- Sickle cell anemia
Prognosis
If amaurosis fugax is not diagnosed or treated, the patient risks a major stroke in the future. Most untreated patients with significant carotid artery plaques will develop a major stroke within 12 months. Those who undergo carotid endarterectomy have a good prognosis but the risk of adverse cardiac events still remains. Patients who do suffer a stroke have a guarded prognosis.
Complications
The main complications of amaurosis fugax include:
- Stroke
- Death
- Adverse cardiac events
Enhancing Healthcare Team Outcomes
Amaurosis fugax is a harbinger of an imminent stroke. The first study of choice is an ultrasound to determine the presence of carotid artery stenosis. When an individual has been diagnosed with amaurosis fugax, it is vital to refer the patient first to a neurologist to confirm the diagnosis and examine for any other neurological deficits. Since many of these patients are smokers and diabetics and may have hypertension, they need a thorough presurgical workup to ensure that they can undergo general or local anesthesia. The time to undergo carotid endarterectomy is variable but expert opinion states that it should be within 4 to 8 weeks. [11],[12] (Level C)
While awaiting surgery, the nurse should educate the patient and the family on the dangers of smoking and the importance of blood pressure control. The pharmacist should make sure that the patient is on aspirin and compliant with his blood pressure and diabetes medications. Before surgery, the patient should be advised to stop all blood thinners.
An interdisciplinary team approach to patient care and education will lead to better outcomes. [Level 5]
Outcomes
There is a large amount of evidence to show that treating patients with amaurosis fugax can prevent a full-blown stroke. However, the treatment of carotid stenosis is in a state of flux. The condition can also be managed with angioplasty and stenting. For patients who are unfit for surgery or those who are asymptomatic, the endovascular approach may be viable, but symptomatic patients need an open procedure. There still remains debate as to which procedure is safer and the data is mixed and confusing because of the heterogeneity of the patients. The most important thing is to ensure that a patient with amaurosis fugax gets the appropriate referral to a neurologist first to confirm the diagnosis. The choice between a vascular or an endovascular procedure depends on personal preference. Both are safe procedures, and both have a role in the treatment of this serious disorder. [13],[14] (Level III) Whatever choice one makes, the person doing the procedure must be experienced, and have the support of an interprofessional team to achieve optimal outcomes. [Level 5]
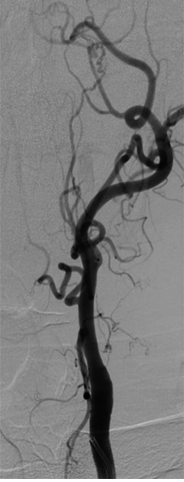
Figure
Occlusion of the Internal Carotid Artery (ICA). Occlusion of the ICA results in collateral formation via the external carotid artery (ECA). Contributed by Scott Dulebohn, MD
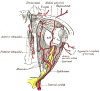
Figure
Arteries of the Eye, Ophthalmic Artery; Internal Carotid Artery Henry Vandyke Carter, Public Domain, via Wikimedia Commons
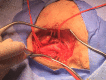
Figure
Exposure and Isolation of External and Internal Carotid Artery Contributed by Erion Qaja, Wyckoff Heights Medical Center
Figure
amaurosis fugax Image courtesy S bhimji MD
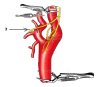
Figure
Carotid artery and nerves Image courtesy S Bhimji MD
References
- 1.
- Pula JH, Yuen CA. Eyes and stroke: the visual aspects of cerebrovascular disease. Stroke Vasc Neurol. 2017 Dec;2(4):210-220. [PMC free article: PMC5829892] [PubMed: 29507782]
- 2.
- Qaja E, Theetha Kariyanna P. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 24, 2023. Carotid Artery Surgery. [PubMed: 28722976]
- 3.
- Makhkamova DК. [Etiopathogenesis of ocular ischemic syndrome]. Vestn Oftalmol. 2017;133(2):120-124. [PubMed: 28524151]
- 4.
- McCullough HK, Reinert CG, Hynan LS, Albiston CL, Inman MH, Boyd PI, Welborn MB, Clagett GP, Modrall JG. Ocular findings as predictors of carotid artery occlusive disease: is carotid imaging justified? J Vasc Surg. 2004 Aug;40(2):279-86. [PubMed: 15297821]
- 5.
- Kvickström P, Lindblom B, Bergström G, Zetterberg M. Amaurosis fugax: risk factors and prevalence of significant carotid stenosis. Clin Ophthalmol. 2016;10:2165-2170. [PMC free article: PMC5096748] [PubMed: 27826182]
- 6.
- Hagiwara Y, Shimizu T, Hasegawa Y. Contrast-enhanced transoral carotid ultrasonography for the diagnosis and follow-up of extracranial internal carotid artery dissection: A case report. J Clin Ultrasound. 2018 Jun;46(5):368-371. [PubMed: 28990690]
- 7.
- Pula JH, Kwan K, Yuen CA, Kattah JC. Update on the evaluation of transient vision loss. Clin Ophthalmol. 2016;10:297-303. [PMC free article: PMC4755435] [PubMed: 26929593]
- 8.
- Hayreh SS, Zimmerman MB. OCULAR ARTERIAL OCCLUSIVE DISORDERS AND CAROTID ARTERY DISEASE. Ophthalmol Retina. 2017 Jan-Feb;1(1):12-18. [PMC free article: PMC5439962] [PubMed: 28547004]
- 9.
- McBride R, Porter J, Al-Khaffaf H. The modified operative technique of partial eversion carotid endarterectomy. J Vasc Surg. 2017 Jan;65(1):263-266. [PubMed: 28010865]
- 10.
- Pribán V, Fiedler J, Chlouba V. [Ocular symptoms as an indication for carotid endarterectomy]. Cesk Slov Oftalmol. 2006 Sep;62(5):354-9. [PubMed: 17039923]
- 11.
- Reid TD, Finney LJ, Hedges AR. The National Stroke Strategy - is it achievable? Ann R Coll Surg Engl. 2009 Nov;91(8):641-4. [PMC free article: PMC2966237] [PubMed: 19686616]
- 12.
- Meyer D, Karreman E, Kopriva D. Factors associated with delay in carotid endarterectomy for patients with symptomatic severe internal carotid artery stenosis: a case-control study. CMAJ Open. 2018 May 15;6(2):E211-E217. [PMC free article: PMC7869657] [PubMed: 29769180]
- 13.
- Pothof AB, Zwanenburg ES, Deery SE, O'Donnell TFX, de Borst GJ, Schermerhorn ML. An update on the incidence of perioperative outcomes after carotid endarterectomy, stratified by type of preprocedural neurologic symptom. J Vasc Surg. 2018 Mar;67(3):785-792. [PMC free article: PMC5828919] [PubMed: 29074118]
- 14.
- Cheng I, Vyas KS, Velaga S, Davenport DL, Saha SP. Outcomes of Carotid Endarterectomy with Primary Closure. Int J Angiol. 2017 Jun;26(2):83-88. [PMC free article: PMC5446247] [PubMed: 28566933]
Disclosure: Prasanna Tadi declares no relevant financial relationships with ineligible companies.
Disclosure: Kinda Najem declares no relevant financial relationships with ineligible companies.
Disclosure: Edward Margolin declares no relevant financial relationships with ineligible companies.
Publication Details
Author Information and Affiliations
Authors
Prasanna Tadi1; Kinda Najem; Edward Margolin2.Affiliations
Publication History
Last Update: August 7, 2023.
Copyright
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Publisher
StatPearls Publishing, Treasure Island (FL)
NLM Citation
Tadi P, Najem K, Margolin E. Amaurosis Fugax. [Updated 2023 Aug 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.




