Continuing Education Activity
Exudative retinal detachment is multifactorial in origin and can develop in patients of all age groups when there is defective clearance of subretinal fluid or when there is excessive exudation in the subretinal space. Most of the cases may be successfully managed medically. If diagnosed on time and necessary medical and surgical interventions are initiated, reasonably good visual recovery could be achieved. Prompt diagnosis and identification of the cause of exudative retinal detachment can help to avoid high ocular morbidity associated with this condition. This activity reviews the evaluation and management of exudative retinal detachment and highlights the interprofessional team's role in evaluating and treating patients with this condition.
Objectives:
- Identify the etiology and epidemiology of exudative retinal detachment.
- Review the pathophysiology, histopathology, and clinical features of exudative retinal detachment.
- Describe the evaluation, management, and differential diagnoses of exudative retinal detachment.
- Summarize the prognosis, complications, and role of interprofessional collaboration in managing patients with exudative retinal detachment.
Introduction
Accumulating excessive fluid in the subretinal space between the retinal pigment epithelium (RPE) and neurosensory retina leads to retinal detachment (RD).[1] The subretinal space is the remnant of the embryonic optic vesicle.[1] Depending on the etiology which leads to subretinal fluid accumulation, retinal detachment is divided into three categories- rhegmatogenous, tractional, or exudative retinal detachment.[2][3]
There may be a combination of these categories in some cases. Rhegmatogenous retinal detachment occurs when there are retinal tears or holes leading to fluid seeping into the subretinal space.[4] Tractional retinal detachment occurs when there is traction on the retina due to fibrovascular proliferation over the retina resulting from ischemic or hypoxic stimuli or other causes.[4]
Exudative retinal detachment occurs when excessive subretinal fluid accumulates in the absence of any retinal breaks or tractional forces.[3][4] Exudative retinal detachment occurs due to disruption of the integrity of the blood-retinal barrier.[3]
There is no anatomical adhesion between the retinal pigment epithelium and the neurosensory retina.[1] The apical surface of the retinal pigment epithelial cells expresses neural cell adhesion molecules, which create adhesion between retinal pigment epithelium and photoreceptor cells.[1][5]
Any etiologic factor, whether inflammatory, idiopathic, infectious, surgical, neoplastic, vascular, or drug-induced, can induce ischemic-hypoxic stimuli, leading to the loss of integrity of the blood-retinal barrier.[6][3]
If diagnosed and treated on time, reasonably good visual acuity can be recovered in patients with exudative retinal detachment.[3] Based on the cause of exudation, exudative retinal detachment is mainly managed on medical lines. Very rarely, if all medical interventions fail, a surgical line of management with scleral buckling or vitrectomy surgery may be considered.[3]
Etiology
Exudative retinal detachment occurs when excess fluid accumulates in the subretinal space either due to hypersecretion of fluid into the subretinal space or defective transport of fluids from the subretinal space. Any inflammatory, idiopathic, infectious, surgical, neoplastic, vascular, or drug-induced etiology can lead to loss of integrity of the blood-retinal barrier, thereby leading to exudative retinal detachment.[6][3] The causes of exudative retinal detachment are as follows-
- Inflammatory[6][7][8]
- Vogt Koyanagi Harada Syndrome (VKH)[9]
- Orbital Inflammation/ orbital pseudotumor/ idiopathic orbital inflammatory disease[13]
- Posterior Scleritis[14]
- Benign reactive lymphoid hyperplasia[15]
- APMPPE (Acute posterior multifocal placoid pigment epitheliopathy)[16]
- Serpiginous Choroiditis[17]
- Behçet disease (BD)[18]
- Intermediate uveitis[19]
- Relapsing polychondritis (RPC)[20]
- Inflammatory bowel disease[21]
- Sarcoidosis[22]
- Unilateral acute idiopathic maculopathy[23]
- Neoplastic or paraneoplastic[43][44]
- Metastatic disease[45]
- Choroidal melanoma and nevus[46]
- Retinoblastoma[47]
- Choroidal hemangioma[48]
- Retinal or optic disc capillary hemangioblastoma (Von Hippel-Lindau syndrome)[49]
- Choroidal osteoma[43]
- Lymphoma[50]
- Multiple myeloma[53]
- Leukemia[54]
- Lymphomatoid granulomatosis[55]
- Carcinoma associated retinopathy[56]
- Vascular/Hematologic[57][58][59][60]
- Toxemia of pregnancy[61]
- Malignant hypertension[62]
- Disseminated intravascular coagulation[63]
- Thrombotic thrombocytopenic purpura[64]
- Wegener's granulomatosis[65]
- HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count) syndrome[60]
- Systemic lupus erythematosus [66]
- Goodpasture syndrome[67]
- Organ transplantation/hemodialysis[68]
- Kidney diseases like IGA nephropathy, chronic renal failure, type II membranoproliferative glomerulonephritis, and crescentic membranous nephropathy.[71]
- Choroidal neovascularization due to various causes, including age-related macular degeneration (AMD)[72]
- Idiopathic polypoidal choroidal vasculopathy (IPCV)[73]
- Coats' disease[74]
- Retinal vein occlusion[70]
- Carotid obstruction (venous overload choroidopathy) [75]
- Familial exudative vitreoretinopathy (FEVR)[76]
- Retinopathy of prematurity (ROP)[77]
Epidemiology
Age predilection in exudative retinal detachment is different for different diseases. Coats disease is commonly seen in the younger age group, exudative retinal detachment secondary to malignancy, exudative age-related macular degeneration is more common in the older age group, exudative retinal detachment secondary to pre-eclampsia, eclampsia, HELLP syndrome, Vogt Koyanagi Harada syndrome, central serous chorioretinopathy is more common in middle age group.[85]
Choroidal malignancies and exudative age-related macular degeneration are more common in whites.[86][87] The black population has a higher incidence of inflammatory ocular diseases.[88] Vogt Koyanagi Harada syndrome is more common in Asians.[6] Because of the multifactorial origin of exudative retinal detachment, no previous data on the frequency of the disease was available in reviewing the literature. Sex predilection in exudative retinal detachment is also different for different diseases. Central serous chorioretinopathy, Coats disease, and Uveal effusion syndrome are more common in males.[89][27][90]
Individuals with a stressful lifestyle, type A personality, and pregnancy has a higher incidence of central serous chorioretinopathy.[91]
Pathophysiology
Disruption of the blood-retinal barrier leads to the development of exudative retinal detachment.[92] The key role of the blood-retinal barrier is in managing fluid and molecular transport between retinal vasculature and retinal layers.[93][94]
The blood-retinal barrier is divided into two categories: the outer blood-retinal barrier and the inner blood-retinal barrier. The outer blood-retinal barrier is formed by the tight junction between the cells of the retinal pigment epithelium.[95]
No anatomical attachment is present between the retinal pigment epithelium and the neurosensory retina. The apical surface of the retinal pigment epithelial cells has the expression of neural cell adhesion molecules, which help in the attachment between the retinal pigment epithelium and the neurosensory retina.[95] The inner blood-retinal barrier is formed by the tight junction between the endothelial cells of the capillaries.[95]
Normal functioning of the tight junctions of the cells forming the blood-retinal barrier is responsible for maintaining the integrity of the blood-retinal barrier.[94] If the tight junction is disrupted due to any infective, inflammatory, vascular, infiltrative, neoplastic, or degenerative causes, it leads to failure of the blood-retinal barrier functioning, leading to fluid accumulation in the subretinal space.[92][94]
Other causes include loss of polarity of retinal pigment epithelium, which causes reversal of pumping and accumulation of fluid in the subretinal space.[91] There is a widening of venous channels and an increase in hydrostatic pressure due to nonperfusion of the choriocapillaris in central serous chorioretinopathy.[96][97]
Histopathology
In the acute stages of exudative retinal detachment, loss of photoreceptor outer segments is seen. In chronic disease, RPE proliferation, cysts, excessive leakage into the subretinal space, and retinoschisis are seen.[3] In the acute stages of Vogt Koyanagi Harada syndrome, eosinophilic exudation is seen in the subretinal space, which disappears in the late stages.[98][99]
Circumscribed choroidal hemangiomas are benign hamartomas that typically present in the second to fourth decade of life. They usually occur sporadically in the absence of systemic disease. Histopathology reveals that the tumor is composed of vascular channels lined with endothelium.[100]
Choroidal haemangioma shows well-demarcated "pushing" peripheral margins. It is separated from the normal choroid by a layer of choroidal lamellae and compressed melanocytes. While in some cases, the hemangiomas merge with the choroidal vasculature.[101] Diffuse choroidal hemangioma is usually associated with Sturge-Weber syndrome. Orbital pseudotumor presents as an idiopathic inflammation. It consists of pleomorphic inflammatory cellular reactions and a fibrovascular tissue complex. Histopathologic analysis shows a granulomatous inflammation with fibrosis.[102][103]
Choroidal osteoma shows dense, bony trabeculae with marrow spaces. These spaces are traversed by pathognomonic dilated thin-walled spider or feeder blood vessels. These vessels connect the choriocapillaris to the larger choroidal vessels.[104][105][106] Bilateral Diffuse Uveal Melanocytic Proliferation (BDUMP) shows predominantly spindle-shaped melanocytic cells. These cells are mixed with epithelial cells.[51] These are predominantly spindle-shaped melanocytic cells.[107][108] They are S100 positive.[109]
On histopathology, multiple myeloma exhibits round plasma cells with an eccentric nucleus and prominent nucleolus. Organized chromatin is present with a clear area next to the nucleus known as Hoffa clear zone. It represents the Golgi apparatus involved in immunoglobulin production.[110] On histopathology, lymphoma shows epidermotropism, which is characteristic of T-cell infiltrates. There is an intraepidermal cluster of lymphocytes known as Pautrier's abscess. It is pathognomic of mycosis fungoides. Angiodestruction and tissue infarction are commonly seen.[111]
History and Physical
Proper assessment of a patient with exudative retinal detachment involves:
- Careful history
- General physical examination
- Ophthalmic examination
- Anterior segment examination
- Posterior segment examination
- Locating the presence or absence of retinal break or tractional bands.
History
The patient may present with dimness of vision, floaters, pain, visual field defects, redness of the eye, leukocoria, and metamorphopsia. The duration of presenting symptoms should be inquired. Children or rarely ignorant adults may sometimes present with squinting of the eye or leukocoria if unilateral blurring is not noticed early. The history of previous intraocular surgeries and systemic or topical medications (including) should be inquired. History of any associated systemic disease (including hypertension), excessive weight loss, and loss of appetite in suspected cases of malignancy should be thoroughly evaluated. A history of flu-like illness in the prodromal phase is seen preceding the acute uveitic phase of Vogt Koyanagi Harada syndrome.[7]
General Physical Examination
Exudative retinal detachment is multifactorial in origin. It could occur from local as well as systemic causes. A thorough physical examination is necessary to rule out the systemic causes of exudative retinal detachment like Behçet disease, relapsing polychondritis, Von Hippel-Lindau syndrome, tuberculosis, syphilis, dengue, Lyme disease, cytomegalovirus retinitis, nematode infection, fungal infection, diabetes, hypertension, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome, preeclampsia, eclampsia, hematological malignancies like acute myeloid leukemia, chronic myeloid leukemia, acute lymphoid leukemia, chronic lymphoid leukemia as well as to rule out syndromic associations with exudative retinal detachment.[3]
Ophthalmic Examination
Best-corrected visual acuity should be evaluated. Intraocular pressure should be monitored. The presence of longstanding amblyopia should be ruled out. There may be ptosis, proptosis, and pain in ocular movements in posterior scleritis and orbital inflammation.[112][113]
Anterior Segment Examination
Anterior segment examination may be unremarkable. Inflammatory etiologies are characterized by circumcorneal congestion, aqueous cells, anterior chamber flare, posterior synechiae, complicated cataracts, keratic precipitates, hypopyon, retrolental (anterior vitreous) cells on slit-lamp examination.[114] Raised intraocular pressure may be noted due to secondary angle closure in ciliary congestion and anterior rotation of the ciliary body in scleritis, after pan-retinal photocoagulation, and in VKH syndrome.[16]
Neovascularization of the iris and angle may be noted in VKH.[115] The pupillary examination should be done to check for RAPD (relative afferent pupillary defect) to assess the severity of the posterior segment involvement.[116] Features of rhegmatogenous retinal detachment, like retrolental pigments (Shafer sign) and hypotony, should be ruled out.[117]
Posterior Segment Examination
Exudative retinal detachment is characterized by the presence of shifting fluid across the retina.[118] As the patient shifts position, the subretinal fluid accumulates in the most dependent area of the retina. Previously shifting fluid was considered to be the hallmark of exudative retinal detachment.[119] However, it is found that shifting fluid is also seen with aphakic and longstanding rhegmatogenous retinal detachment and in patients in whom retinal holes were small.[120]
Other characteristic features of exudative retinal detachment include the smooth surface of retinal detachment without any retinal folds or corrugations, the convex and bullous contour of retinal detachment, and vitreous cells. Optic disc hyperemia may be seen in inflammatory etiologies causing exudative retinal detachment.[119] Choroidal neovascularization may be seen in chronic non-resolving cases of exudative retinal detachment.[121]
Choroidal metastases can present with exudative retinal detachment. The choroidal metastases are small in these cases, but the exudative detachment is relatively more.[122][45] There is an absence of associated Shaffer sign, posterior vitreous detachment, and vitreous hemorrhage in exudative retinal detachment contrary to rhegmatogenous retinal detachment.[3] The differences between exudative retinal detachment and rhegmatogenous retinal detachment are discussed in detail in the differential diagnosis section.
Locating the Presence or Absence of Retinal Break or Tractional Bands
Exudative retinal detachment is a diagnosis of exclusion. So thorough central and peripheral fundus examination is required to rule out the presence of any fibrovascular proliferation causing traction over the retina leading to tractional retinal detachment, or the presence of any retinal tear, hole, or dialysis which may lead to rhegmatogenous retinal detachment.[123][124]
Especially in cases of inferior bullous retinal detachment with shifting fluid, the presence of superior small breaks must be ruled out.[123] If proliferative vitreoretinopathy is present, then it points towards rhegmatogenous retinal detachment. This differentiation is essential as both rhegmatogenous and tractional retinal detachment need a surgical line of management, whereas exudative retinal detachment is primarily managed on medical lines.[125]
Evaluation
Diagnosis of exudative retinal detachment is mainly clinical, but to find out the exact etiology of exudative retinal detachment, further laboratory, radiographic, and other ancillary tests are required.
Laboratory Investigations
Blood pressure is one of the most important investigations for a patient with papilledema and exudative retinal detachment.
The workup should be individualized and may include a combination of complete blood cell count, differential leukocyte count, erythrocyte sedimentation rate, Mantoux test, chest X-ray, Quantiferon Gold (interferon-gamma release assay), VDRL (venereal disease research laboratory) test, and fluorescein treponema antibody (FTA-ABS) test, serum homocysteine levels, serum cortisol levels, renal function test, liver function test, Toxoplasma, Rubella, Cytomegalovirus, Herpes (TORCH) markers, prothrombin time, bleeding time, clotting time, diabetic profile including fasting blood sugar, postprandial blood sugar, glycosylated hemoglobin.[126] In patients suspected of having BDUMP, evaluation should be done for ovarian cancer, lung carcinoma, and urogenital cancer.[127][128][129]
Amsler grid is a simple method for the detection of metamorphopsia and scotoma.[130]
Ocular Imaging
- Optical coherence tomography (OCT) of the macula- OCT scanning shows the macular serous detachment of the macula with or without septae. Enhanced depth imaging OCT shows increased choroidal thickness in VKH syndrome, posterior scleritis, uveal effusion syndrome, and central serous chorioretinopathy.[131] Bacillary layer detachment can be seen in VKH, sympathetic ophthalmia, APMPPE, hematological disorders, posterior scleritis, choroidal metastases, and central serous chorioretinopathy.[132]
In VKH, a thickened choroid is seen on OCT with enhanced depth imaging. There are multiple serous retinal detachments with septa. Choroidal undulation and inward bulging are seen in the acute stage.[136][137]
In CSR on OCT, neurosensory detachment is seen. Hyperreflective dots [in Spectral Domain (SD)-OCT] are seen in the intraretinal and subretinal space.[138][139][140][141] Serous PED is seen in 50% of CSRC-affected eyes.[142][143] Elongation of photoreceptor outer segments is a frequent SD-OCT finding.
In PCV, thumb-like or sharp-peaked pigmentary epithelial detachment (PED) is seen. It signifies the polypoidal lesion at the margin of the PED. Double-layer sign is also present. It is seen as two hyperreflective lines on SD-OCT representing shallow irregular RPE elevation and Bruch's membrane separation due to an abnormal vascular network.[144][145][146]
- Fundus fluorescein angiography (FFA) -It reveals areas of hyperfluorescence and hypofluorescence, multiple window defects, pooling of the dye in the areas of serous retinal detachment, and disc staining.[147] Angiography is also helpful for detecting choroidal neovascularization, neovascularization of the disc, neovascularization elsewhere, retinochoroidal and arteriovenous anastomoses, and for detecting the peripheral ischemic zone to plan for further appropriate lines of management.[148]
In VKH, FFA shows hypofluorescent dots in the early phase, followed by multiple focal areas of leakage and subretinal fluid accumulation in the late phase.[134]
In CSR, an inkblot pattern is seen. There is pinpoint leakage that occurs in the early phase, which then concentrically enlarges in the late phase. In a smokestack pattern, the leakage starts as a pinpoint and gradually expands to form an umbrella-like (or tree-like) appearance.[91]
In Posterior scleritis, FFA shows blocked fluorescence in the initial arteriovenous phase and diffuse hyperfluorescence in the late phase with no leak. Choroidal folds are seen as alternating hyperfluorescent and hypofluorescent bands.[149][150]
In Uveal effusion syndrome, angiography shows hyperfluorescence in a leopard-spot pattern.[151]
In APMPPE, hypofluorescence is seen in the early phase due to the blockage of choroidal fluorescence. Later in the angiogram, there is staining of the lesions. As the process becomes inactive, hyperfluorescence corresponding to window defects in the mottled RPE develops.[152]
In BDUMP FFA early phase shows a reticular pattern of hypofluorescence surrounded by a background of choroidal hyperfluorescence.[153]
- Indocyanine green angiography (ICG)
In CSR, ICG shows hypocyanescence in the early phase implying choriocapillaris nonperfusion or delayed filling. In the mid-phase, hypercyanescence is seen, indicating choroidal vessel hyperpermeability. This hypercyanescence slowly fades in the late phase.[154][155][156]
In VKH, ICG shows early hyperfluorescence and leakage and hypofluorescent dark dots at the level of the choroid.[157][158]
In IPCV, ICG shows interconnecting channels. An abnormal vascular network appears within 1 min of dye injection. The polyp and feeder vessel are seen.[159][160]
In APMPPE, acute lesions show early hypofluorescence. In the late phases, these lesions become more defined in shape. These areas are more than the placoid lesions seen clinically. As the lesions heal, the hypofluorescence in the late phases becomes small and ill-defined.[161][162][163][164]
- Ultrasonography (B- scan) - Ultrasound is very useful for measuring the dimensions of tumors before and after treatment, measuring choroidal thickness, diagnosis of choroidal detachment, and T sign in posterior scleritis.[112] Ultrasonography is especially useful in patients having hazy media that does not allow posterior segment examination.
- Autofluorescence (AF)
Carcinoma Associated Retinopathy (CAR)- On AF, a parafoveal ring of hyper autofluorescence with normal autofluorescence within the ring and hypo autofluorescence outside the ring is seen.[165]
BDUMP- Ultra-widefield color fundus photography shows greater pigmentary changes and lesions in the periphery. Ultra-widefield AF shows the characteristic 'giraffe-pattern' fundus changes.[166]
CSCR- In acute cases, focal areas of hypo-autofluorescence are seen that may correspond to the leak on FFA. In chronic CSCR, hyper-autofluorescent tracks are present due to the accumulation of photoreceptor pigments.[91]
Best disease- AF shows uniform hyper auto fluorescence of vitelliform lesions, while sub-RPE fibrosis or atrophy shows hypofluorescence.[167][168] The increased autofluorescence is due to the lipofuscin content.[169][170][171]
- Electrophysiological tests
Full-field ERG in CAR shows attenuated or absent photopic and scotopic responses. In CAR, where only the cones are affected, full-field ERG could be normal, but multifocal ERG will be abnormal.[172][173]
Treatment / Management
Exudative retinal detachment is multifactorial in origin, so the management approach is individualized depending on the type, severity, and stage of presentation of the disease. Exudative retinal detachment is mainly managed on the medical lines; surgical intervention is rarely indicated if all measures to improve the exudation medically fail.[76]
Inflammatory Diseases like Vogt Koyanagi Harada syndrome and posterior scleritis should be treated with intravenous methylprednisolone pulse therapy at 1 g/day for 3-5 days, followed by a shift to oral steroids according to weight.[7] The role of inflammation and steroid in uveal effusion may be debatable.[27][174]
Along with corticosteroids, immunosuppressive agents such as methotrexate, azathioprine, cyclosporine A, mycophenolate mofetil, and alkylating agents have been used successfully to treat VKH.[175][176][177][178]
The American Uveitis Society and the International Uveitis Study Group, thus, have recommended immunosuppressive agents as mandatory in treating VKH to prevent recurrences; however, appropriate initial treatment of the disease. While high-dose corticosteroids also affect recurrences, as outlined above, so care must be taken to balance the initial treatment with the need for immunosuppressive agents later on.[7]
Oral nonsteroidal can be effective with various appropriate regimens, such as ibuprofen 600 to 800 mg 4 times daily, piroxicam 20 mg daily, and Naproxen 375 mg twice daily, to name a few. In cases unresponsive to oral NSAIDs (nonsteroidal anti-inflammatory drugs), high-dose systemic corticosteroids are often used, with typical doses of 1 mg/kg/day or approximately 60 mg of prednisone daily.[179][180][181]
These are tapered slowly over several weeks, carefully considering potential side effects such as weight gain, mood instability, blood-sugar abnormalities, etc. If the patient continues to demonstrate active disease or is unable to tolerate corticosteroids, prompt corticosteroid-sparing immunosuppression is often warranted. Anti-metabolites such as methotrexate and mycophenolate mofetil are employed, often to their maximal doses of 25 mg/week of methotrexate or 1,500 mg twice daily of mycophenolate.[182][180] These medications may take up to six months for full effect, and some patients may require a faster-acting solution. In these cases, biologics such as TNF inhibitors (e.g., adalimumab, infliximab) may be necessary.[183][184]
Malignant hypertensive retinopathy should be managed with the control of blood pressure and the cause of systemic hypertension.
Management options for CSCR include observation, laser, PDT (photodynamic therapy), and anti-VEGF (anti-vascular endothelial growth factor) agents. The RPE leakage sites, as seen on angiography, can be treated with laser photocoagulation. Such therapy seals the leakage point and hastens the resolution of subretinal fluid.[185]
CSCR with a subfoveal leak, juxtafoveal leak, multiple leaks, and chronic cases with diffuse decompensation of RPE are better managed with PDT.[186][187] Anti-VEGF therapy has been proposed to reduce choroidal hyperpermeability. They upregulate the tight junctions between endothelial cells and the reduction of vascular fenestrations.[188][189][190] Anti
Surgical intervention is considered the last resort for exudative retinal detachment due to various causes once all efforts to manage the disease medically fail.[2] Options for surgical management include:-[191]
- Scleral buckling with external drainage of the subretinal fluid with the laser of the nonperfused retina
Management of Familial exudative vitreoretinopathy (FEVR) depends on the stage of presentation:[194]
- Stage I FEVR - Observation
- Stage II FEVR - Laser photocoagulation of the areas with abnormal vessels and ischemia (avascular zone)is recommended.[195]
- Advanced stage - In cases of retinal detachment, a surgical approach is required. Scleral buckling and/or vitrectomy, along with lasering of the avascular zone, is recommended.
- Screening of the family members is advisable.
- A Dexa scan should be done to assess bone mineral density in FEVR patients with LRP5 mutation.[76]
- The use of anti-VEGF is still a matter of debate at present. According to a study by Tagami M. et al., regression of neovascularization was noted after one intravitreal bevacizumab injection.[196]
- A study by Henry CR et al. showed favorable results when intravitreal bevacizumab was used along with laser or surgical treatment.[197]
- There are chances of worsening of tractional forces leading to the tractional retinal detachment on treatment with anti-VEGF injections, so proper guidelines are still awaited for the usage of anti-VEGF as an adjunct to laser or surgical therapy.[197]
Treatment of Coats disease also depends on the stage of the disease.
- Mild cases without exudation can be observed.[20]
- Direct laser photocoagulation of the telangiectatic vessels is useful in mild to moderate cases of exudation.[90]
- Laser therapy will not be effective in cases of massive subretinal exudation and exudative retinal detachment. In such cases, cryotherapy with a double freeze-thaw technique is done over the diseased retina.[198]
- Neovascular glaucoma could be treated with transscleral diode laser photocoagulation.[198]
- Endstage painful blind eye should be enucleated if all measures to save the eye fail.[90]
Exudative retinal detachment due to wound leak or hypotony after ocular surgery responds satisfactorily with timely wound suturing and anti-inflammatory agents coverage.[3] Exudative detachments secondary to pan-retinal photocoagulation or scleral buckling may respond to topical and systemic anti-inflammatory or steroid therapy.[40] If the scleral buckle is too tight, it has to be readjusted to release the pressure on the vascular system.[41]
Radiation therapy is commonly used in the management of malignant uveal melanoma.[46][202] After radiation therapy, especially with proton beam radiation, there are high chances of worsening exudative retinal detachment.[202] Treatment of choroidal tumors with exudative retinal detachment includes proton beam radiation or brachytherapy with plaque, trans retinal tumor biopsy for prognostication of the disease, and surgical intervention as the last measure with pars plana vitrectomy and drainage of subretinal fluid with endotamponade.[46]
Choroidal hemangioma could be treated with various treatment options, including laser photocoagulation, low-dose external beam radiation, proton beam irradiation, gamma knife radiosurgery, radiotherapy, cryotherapy, photodynamic therapy with verteporfin, transpupillary thermotherapy, oral beta-blockers, or intravitreal anti-VEGF.[203] Metastatic tumors should be managed with chemotherapy or focal radiation therapy.[204]
Infectious causes leading to exudative retinal detachment should be treated with appropriate antibiotics.[205] Tuberculous exudative detachment should be treated with antitubercular drugs along with steroids.[206] Syphilis cause needs to be treated like neurosyphilis with penicillin.[207][208][207] Viral causes warrant urgent treatment with antiviral drugs and corticosteroids.[209]
Pregnancy-induced hypertension can cause marked loss of visual acuity, but these changes are mostly reversible in the postpartum period.[62] Control of blood pressure is the most important intervention. Visual prognosis is better in pregnancy-induced hypertension as compared to malignant hypertension.[62]
Secondary exudative retinal detachment in systemic hematologic and vascular etiologies like disseminated intravascular coagulation, malignant hypertension, Goodpasture syndrome, thrombotic thrombocytopenic purpura, post organ transplantation, renal failure, SLE, Wegener's granulomatosis can resolve once the primary disease is controlled and treated.[210] [211][212]
Exudative retinal detachment in hepatitis C patients treated with interferon-a and ribavirin improves with cessation of drugs and initiation of corticosteroid treatment.[80]
Differential Diagnosis
Many conditions can cause exudative retinal detachment. Choroidal pathologies like choroidal melanoma, choroidal lymphoma, choroidal hemangioma, choroidal osteoma, choroidal metastasis, leukemia including ALL (acute lymphoid leukemia), AML (acute myeloid leukemia), CLL (chronic myeloid leukemia), CML (chronic myeloid leukemia).[3] Retinal pathologies like Coats' disease, familial exudative vitreoretinopathy (FEVR), retinal astrocytic hamartoma, vasoproliferative tumor, vitreoretinal lymphoma, retinal vein occlusion, diabetic macular edema. Wet ARMD(age-related macular degeneration), idiopathic macular telangiectasia, thrombotic thrombocytopenic purpura and hemolytic uremic syndrome, pregnancy-induced hypertension including preeclampsia, eclampsia, and HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome.
Miscellaneous causes like central serous chorioretinopathy (CSCR), mitogen-activated protein kinase (MEK) inhibitor-associated retinopathy, uveal effusion syndrome, paraneoplastic retinopathy including acute paraneoplastic polymorphous vitelliform maculopathy (AEPPVM) and bilateral diffuse uveal melanocytic proliferation (BDUMP).
The causes of exudative retinal detachment, rhegmatogenous retinal detachment (RD), and tractional RD are different. Thus exudative retinal detachment should be differentiated from rhegmatogenous retinal detachment (RD) and tractional RD.
In hemorrhagic retinal detachment, there is subretinal blood compared to an exudative retinal detachment which is serous. Causes of hemorrhagic retinal detachment include peripheral exudative hemorrhagic chorioretinopathy (thought by many authors to be a variant of IPCV), trauma, leukemia, and age-related macular degeneration, specifically in patients on blood thinners.
Prognosis
In exudative retinal detachment secondary to uveitis, the prognosis of the disease depends on the severity of inflammation and its response to treatment.[6] Exudative retinal detachment secondary to uveal effusion and VKH may respond to the high dose of systemic steroids, but the chances of the resurgence of exudation are high.[7][8] Exudative retinal detachment in patients with pre-eclampsia and eclampsia is usually self-limiting and resolves completely with time.[59]
In patients with Coats disease, the prognosis depends on the severity of macular involvement. Some baseline improvement might occur with treatment but rarely returns to normal visual acuity.[8] Chronic neurosensory detachment and chronic cystoid macular edema may permanently lose cones, leading to limited visual acuity in patients with optic disc pits.[91]
Patients with acute central serous chorioretinopathy (CSCR) may recover completely, but the chances of recurrences are very high. Once the CSCR becomes chronic, the damage caused to the macula because of recurrent detachments may lead to permanent anatomical alterations in the macular area leading to guarded visual recovery.[24]
The prognosis of exudative retinal detachment secondary to choroidal tumors depends on the severity and extent of the tumor, presence or absence of macular involvement, response to treatment, chances of recurrence and toxicity, and complications secondary to treatment (radiotherapy, chemotherapy, surgery).[46]
The exudative retinal detachment secondary to infection such as us tuberculosis, dengue, syphilis, and cat-scratch disease is treated by specific antimicrobial agents with or without steroids. The prognosis is good if the condition is diagnosed and treated early.[213]
Long-standing pathology causes permanent damage to photoreceptors and RPE, and the prognosis is poor even after complete treatment.[214]
Vascular or haematologic causes such as retinal vein occlusion, carotid obstruction (venous overload choroidopathy), familial exudative vitreoretinopathy, and retinopathy of prematurity can cause exudative retinal detachment. The prognosis is excellent if detected early. Timely laser photocoagulation of the ischaemic retina in familial exudative vitreoretinopathy and retinopathy of prematurity prevent retinal detachment with a good prognosis.[215][195]
Complications
If not treated on time, secondary complications may develop in patients with an exudative retinal detachment.[216] These include:
- Phthisis bulbi[217]
- Neovascular glaucoma,
- Subretinal fibrosis,
- Choroidal neovascularization,
- Pigmentary changes of the fundus,
- Festooned pupil,
- Vitreous hemorrhage, and
- Complicated cataract
Deterrence and Patient Education
The differential diagnosis for exudative retinal detachment is large. Differentiation of exudative, tractional, and rhegmatogenous retinal detachment is of utmost importance and is often achieved through clinical examination alone. In cases of diagnostic query, ultrasonography, fundus fluorescein angiography, indocyanine green angiography, and various laboratory investigations are required for correct diagnosis and treatment. Appropriate and timely intervention by the treating physician may thus help to improve the visual and systemic status of the patient.
Enhancing Healthcare Team Outcomes
Any patient presenting with exudative retinal detachment should not only receive treatment solely by an ophthalmologist in isolation, but it also requires a multispecialty evaluation by a chest physician, radiologist, infectious disease expert, rheumatologist, gastroenterologist, endocrinologist, and the neurologist.[3] Interprofessional communication can lead to better patient management. The patient will most often present to the primary health care provider, including mid-level practitioners, and these professionals should be aware of the condition as it is treatable. A prompt referral to an ophthalmologist is necessary.
These patients can then be followed by their primary clinicians, who should ensure correct dosing on the medication management aspect of the condition. Nursing will be the first department to come in contact with the patients on follow-up and can assess the treatment progress as well as evaluate compliance with both medication and lifestyle measures, and report any issue to the primary care physician. This collaborative, interprofessional team approach to care can ensure optimal patient outcomes.
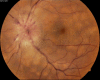
Figure
Vogt Koyanagi Harada syndrome - Fundus photograph Contributed by Unnati Shukla M.S., DNB, FVRS(FNERF), PhD Scholar (Retina)
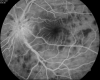
Figure
Vogt Koyanagi Harada Syndrome - Early phase FFA. Contributed by Unnati Shukla M.S., DNB, FVRS(FNERF), PhD Scholar (Retina)
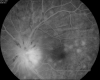
Figure
Vogt Koyanagi Harada Syndrome- Late phase FFA Contributed by Unnati Shukla M.S., DNB, FVRS(FNERF), PhD Scholar (Retina)

Figure
Familial Exudative Vitreoretinopathy - Preoperative fundus photograph Contributed by Unnati Shukla M.S., DNB, FVRS(FNERF), PhD Scholar (Retina)

Figure
Familial Exudative Vitreoretinopathy- Postoperative Fundus Photograph Contributed by Unnati Shukla M.S., DNB, FVRS(FNERF), PhD Scholar (Retina)
References
- 1.
- Kolb H. Facts and Figures Concerning the Human Retina. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System [Internet]. University of Utah Health Sciences Center; Salt Lake City (UT): May 1, 2005. [PubMed: 21413409]
- 2.
- Gariano RF, Kim CH. Evaluation and management of suspected retinal detachment. Am Fam Physician. 2004 Apr 01;69(7):1691-8. [PubMed: 15086041]
- 3.
- Amer R, Nalcı H, Yalçındağ N. Exudative retinal detachment. Surv Ophthalmol. 2017 Nov-Dec;62(6):723-769. [PubMed: 28506603]
- 4.
- Blair K, Czyz CN. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Dec 26, 2022. Retinal Detachment. [PubMed: 31855346]
- 5.
- Gundersen D, Powell SK, Rodriguez-Boulan E. Apical polarization of N-CAM in retinal pigment epithelium is dependent on contact with the neural retina. J Cell Biol. 1993 Apr;121(2):335-43. [PMC free article: PMC2200109] [PubMed: 8468350]
- 6.
- Shah DN, Al-Moujahed A, Newcomb CW, Kaçmaz RO, Daniel E, Thorne JE, Foster CS, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Sen HN, Suhler EB, Bhatt NP, Kempen JH., Systemic Immunosuppressive Therapy for Eye Diseases Research Group. Exudative Retinal Detachment in Ocular Inflammatory Diseases: Risk and Predictive Factors. Am J Ophthalmol. 2020 Oct;218:279-287. [PMC free article: PMC7529966] [PubMed: 32621891]
- 7.
- Andreoli CM, Foster CS. Vogt-Koyanagi-Harada disease. Int Ophthalmol Clin. 2006 Spring;46(2):111-22. [PubMed: 16770158]
- 8.
- Suzani M, Moore AT. Intraoperative fluorescein angiography-guided treatment in children with early Coats' disease. Ophthalmology. 2015 Jun;122(6):1195-202. [PubMed: 25824326]
- 9.
- Tripathy K, Sengupta T. Is there a link between hyperhomocysteinemia and Vogt-Koyanagi-Harada syndrome? Med Hypotheses. 2017 Jul;104:116. [PubMed: 28673567]
- 10.
- Paulbuddhe V, Addya S, Gurnani B, Singh D, Tripathy K, Chawla R. Sympathetic Ophthalmia: Where Do We Currently Stand on Treatment Strategies? Clin Ophthalmol. 2021;15:4201-4218. [PMC free article: PMC8542579] [PubMed: 34707340]
- 11.
- Chawla R, Kapoor M, Mehta A, Tripathy K, Vohra R, Venkatesh P. Sympathetic Ophthalmia: Experience from a Tertiary Care Center in Northern India. J Ophthalmic Vis Res. 2018 Oct-Dec;13(4):439-446. [PMC free article: PMC6210884] [PubMed: 30479714]
- 12.
- Tripathy K, Mittal K, Chawla R. Sympathetic ophthalmia following a conjunctival flap procedure for corneal perforation. BMJ Case Rep. 2016 Mar 14;2016 [PMC free article: PMC4800231] [PubMed: 26976837]
- 13.
- Yuen KS, Lai CH, Chan WM, Lam DS. Bilateral exudative retinal detachments as the presenting features of idiopathic orbital inflammation. Clin Exp Ophthalmol. 2005 Dec;33(6):671-4. [PubMed: 16402970]
- 14.
- Mazumdar S, Tripathy K. The Clinical Entity Called Panscleritis. J Emerg Med. 2020 Jul;59(1):e37. [PubMed: 32900464]
- 15.
- Chang TS, Byrne SF, Gass JD, Hughes JR, Johnson RN, Murray TG. Echographic findings in benign reactive lymphoid hyperplasia of the choroid. Arch Ophthalmol. 1996 Jun;114(6):669-75. [PubMed: 8639077]
- 16.
- Lee GE, Lee BW, Rao NA, Fawzi AA. Spectral domain optical coherence tomography and autofluorescence in a case of acute posterior multifocal placoid pigment epitheliopathy mimicking Vogt-Koyanagi-Harada disease: case report and review of literature. Ocul Immunol Inflamm. 2011 Feb;19(1):42-7. [PubMed: 21034311]
- 17.
- Balakrishnan D, Mathai A, Gogte P, Tibra N, Chhablani J. Serpiginous choroiditis with atypical presentation treated with intravenous methyl prednisolone. Semin Ophthalmol. 2015 Mar;30(2):157-9. [PubMed: 24117451]
- 18.
- Vrabec TR. Exudative retinal detachment in Behçet disease. Arch Ophthalmol. 2001 Sep;119(9):1383-6. [PubMed: 11545652]
- 19.
- Chauhan K, Tripathy K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Pars Planitis. [PubMed: 28613790]
- 20.
- Bhagat N, Green RL, Feldon SE, Lim JI. Exudative retinal detachment in relapsing polychondritis : case report and literature review. Ophthalmology. 2001 Jun;108(6):1156-9. [PubMed: 11382646]
- 21.
- Lee HJ, Song HJ, Jeong JH, Kim HU, Boo SJ, Na SY. Ophthalmologic manifestations in patients with inflammatory bowel disease. Intest Res. 2017 Jul;15(3):380-387. [PMC free article: PMC5478763] [PubMed: 28670235]
- 22.
- Watts PO, Mantry S, Austin M. Serous retinal detachment at the macula in sarcoidosis. Am J Ophthalmol. 2000 Feb;129(2):262-4. [PubMed: 10682988]
- 23.
- Yannuzzi LA, Jampol LM, Rabb MF, Sorenson JA, Beyrer C, Wilcox LM. Unilateral acute idiopathic maculopathy. Arch Ophthalmol. 1991 Oct;109(10):1411-6. [PubMed: 1929931]
- 24.
- Gass JD, Little H. Bilateral bullous exudative retinal detachment complicating idiopathic central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology. 1995 May;102(5):737-47. [PubMed: 7777273]
- 25.
- Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015 Dec 22;2015(12):CD011841. [PMC free article: PMC5030073] [PubMed: 26691378]
- 26.
- Venkatesh P, Chawla R, Tripathy K, Singh HI, Bypareddy R. Scleral resection in chronic central serous chorioretinopathy complicated by exudative retinal detachment. Eye Vis (Lond). 2016;3(1):23. [PMC free article: PMC5016948] [PubMed: 27617266]
- 27.
- Elagouz M, Stanescu-Segall D, Jackson TL. Uveal effusion syndrome. Surv Ophthalmol. 2010 Mar-Apr;55(2):134-45. [PubMed: 20159229]
- 28.
- Song JH, Koreishi AF, Goldstein DA. Tuberculous Uveitis Presenting with a Bullous Exudative Retinal Detachment: A Case Report and Systematic Literature Review. Ocul Immunol Inflamm. 2019;27(6):998-1009. [PubMed: 29969330]
- 29.
- Kim RY, Loewenstein JI. Systemic diseases manifesting as exudative retinal detachment. Int Ophthalmol Clin. 1998 Winter;38(1):177-95. [PubMed: 9532481]
- 30.
- Chan DP, Teoh SC, Tan CS, Nah GK, Rajagopalan R, Prabhakaragupta MK, Chee CK, Lim TH, Goh KY., Eye Institute Dengue-Related Ophthalmic Complications Workgroup. Ophthalmic complications of dengue. Emerg Infect Dis. 2006 Feb;12(2):285-9. [PMC free article: PMC3373088] [PubMed: 16494756]
- 31.
- Aguilar-González M, Fernández-Santodomingo AS, Marín-Payá E, Rahhal-Ortuño M, Udaondo P. Bilateral exudative retinal detachment in undiagnosed ocular syphilis after treatment with corticosteroids. Eur J Ophthalmol. 2021 Mar;31(2):NP86-NP90. [PubMed: 31746221]
- 32.
- Matsuo T, Kato M. Submacular exudates with serous retinal detachment caused by cat scratch disease. Ocul Immunol Inflamm. 2002 Jun;10(2):147-50. [PubMed: 12778351]
- 33.
- Yen M, Chen J, Ausayakhun S, Kunavisarut P, Vichitvejpaisal P, Ausayakhun S, Jirawison C, Shantha J, Holland GN, Heiden D, Margolis TP, Keenan JD. Retinal detachment associated with AIDS-related cytomegalovirus retinitis: risk factors in a resource-limited setting. Am J Ophthalmol. 2015 Jan;159(1):185-92. [PMC free article: PMC4262697] [PubMed: 25448999]
- 34.
- Grinager HS, Krason DA, Olsen TW. Lyme disease: resolution of a serous retinal detachment and chorioretinal folds after antibiotic therapy. Retin Cases Brief Rep. 2012 Summer;6(3):232-4. [PubMed: 25389719]
- 35.
- Sawant SD, Biswas J. Fungal scleritis with exudative retinal detachment. Ocul Immunol Inflamm. 2010 Dec;18(6):457-8. [PubMed: 20846054]
- 36.
- Poppert S, Heideking M, Agostini H, Fritzenwanker M, Wüppenhorst N, Muntau B, Henneke P, Kern W, Krücken J, Junker B, Hufnagel M. Diffuse Unilateral Subacute Neuroretinitis Caused by Ancylostoma Hookworm. Emerg Infect Dis. 2017 Feb;23(2):343-344. [PMC free article: PMC5324813] [PubMed: 28098549]
- 37.
- Goodart RA, Riekhof FT, Beaver PC. Subretinal nematode. An unusual etiology for uveitis and retinal detachment. Retina. 1985 Spring-Summer;5(2):87-90. [PubMed: 4048662]
- 38.
- Al-Zahrani YA, Al-Dhibi HA, Al-Abdullah AA. Atypical Presentation of Ocular Toxoplasmosis: A Case Report of Exudative Retinal Detachment and Choroidal Ischemia. Middle East Afr J Ophthalmol. 2016 Jan-Mar;23(1):150-2. [PMC free article: PMC4759896] [PubMed: 26957857]
- 39.
- Le TD, Weisbrod D, Mandelcorn ED. Chorioretinitis with exudative retinal detachment secondary to varicella zoster virus. Can J Ophthalmol. 2015 Oct;50(5):e91-3. [PubMed: 26455991]
- 40.
- Reddy SV, Husain D. Panretinal Photocoagulation: A Review of Complications. Semin Ophthalmol. 2018;33(1):83-88. [PubMed: 29172937]
- 41.
- Matsuo T, Eguchi K, Matsuo N. Unusual exudative retinal detachment 9 months after scleral buckling surgery. Ophthalmologica. 1990;201(2):79-82. [PubMed: 2234819]
- 42.
- Solberg T, Ytrehus T, Ringvold A. Hypotony and retinal detachment. Acta Ophthalmol (Copenh). 1986 Feb;64(1):26-32. [PubMed: 3962615]
- 43.
- Padhy SK, Mandal S, Gagrani M. Bone inside eye: choroidal osteoma presenting as exudative retinal detachment: a challenge to diagnosis. BMJ Case Rep. 2018 Jun 17;2018 [PMC free article: PMC6011542] [PubMed: 29914906]
- 44.
- Stewart MW, Gitter KA, Cohen G. Acute leukemia presenting as a unilateral exudative retinal detachment. Retina. 1989;9(2):110-4. [PubMed: 2772418]
- 45.
- Vicini G, Nicolosi C, Pieretti G, Mazzini C. Large choroidal metastasis with exudative retinal detachment as presenting manifestation of small cell lung cancer: A case report. Respir Med Case Rep. 2020;30:101074. [PMC free article: PMC7218149] [PubMed: 32420018]
- 46.
- Gibran SK, Kapoor KG. Management of exudative retinal detachment in choroidal melanoma. Clin Exp Ophthalmol. 2009 Sep;37(7):654-9. [PubMed: 19788660]
- 47.
- Rowlands MA, Mondesire-Crump I, Levin A, Mauguen A, Francis JH, Dunkel IJ, Brodie SE, Gobin YP, Abramson DH. Total retinal detachments due to retinoblastoma: Outcomes following intra-arterial chemotherapy/ophthalmic artery chemosurgery. PLoS One. 2018;13(4):e0195395. [PMC free article: PMC5919618] [PubMed: 29698399]
- 48.
- Anaya-Pava EJ, Saenz-Bocanegra CH, Flores-Trejo A, Castro-Santana NA. Diffuse choroidal hemangioma associated with exudative retinal detachment in a Sturge-Weber syndrome case: photodynamic therapy and intravitreous bevacizumab. Photodiagnosis Photodyn Ther. 2015 Mar;12(1):136-9. [PubMed: 25560419]
- 49.
- Ruppert MD, Gavin M, Mitchell KT, Peiris AN. Ocular Manifestations of von Hippel-Lindau Disease. Cureus. 2019 Aug 04;11(8):e5319. [PMC free article: PMC6776162] [PubMed: 31588386]
- 50.
- Kormann BA, Holzgreve H, Wolff-Kormann PG, Riedel KG. Systemic malignant lymphoma presenting as bilateral exudative retinal detachment. Klin Wochenschr. 1990 Oct 17;68(20):1027-31. [PubMed: 2283792]
- 51.
- Klemp K, Kiilgaard JF, Heegaard S, Nørgaard T, Andersen MK, Prause JU. Bilateral diffuse uveal melanocytic proliferation: Case report and literature review. Acta Ophthalmol. 2017 Aug;95(5):439-445. [PubMed: 28636126]
- 52.
- Breazzano MP, Bacci T, Wang H, Francis JH, Yannuzzi LA. Bacillary Layer Detachment in Bilateral Diffuse Uveal Melanocytic Proliferation Masquerading as Neovascular AMD. Ophthalmic Surg Lasers Imaging Retina. 2020 Jul 01;51(7):413-417. [PubMed: 32706900]
- 53.
- Brody JM, Butrus SI, Ashraf MF, Rabinowitz AI, Whitmore PV. Multiple myeloma presenting with bilateral exudative macular detachments. Acta Ophthalmol Scand. 1995 Feb;73(1):81-2. [PubMed: 7627765]
- 54.
- Yoshida K, Hasegawa D, Takusagawa A, Kato I, Ogawa C, Echizen N, Ohkoshi K, Yamaguchi T, Hosoya R, Manabe A. Bullous exudative retinal detachment due to infiltration of leukemic cells in a child with acute lymphoblastic leukemia. Int J Hematol. 2010 Oct;92(3):535-7. [PubMed: 20838956]
- 55.
- Cameron JR, Cackett P. Lymphomatoid granulomatosis associated with bilateral exudative retinal detachments. Arch Ophthalmol. 2007 May;125(5):712-3. [PubMed: 17502519]
- 56.
- Zarzecki M, Saeed E, Mariak Z, Konopińska J. Recurrent monocular exudative retinal detachment as the first manifestation of squamous cell lung cancer: A case report. Medicine (Baltimore). 2021 Mar 19;100(11):e25189. [PMC free article: PMC7982169] [PubMed: 33726010]
- 57.
- Sánchez Zamora P, Alina Mejía Arnaud R, Saz Castro R, Gómez Del Pulgar Vázquez B, José Correa Barrera J. Bilateral serous retinal detachment in a patient with atypical presentation of preeclampsia due to HELLP syndrome. Rev Esp Anestesiol Reanim (Engl Ed). 2021 Jun 17; [PubMed: 34148693]
- 58.
- Çelik G, Eser A, Günay M, Yenerel NM. Bilateral Vision Loss after Delivery in Two Cases: Severe Preeclampsia and HELLP Syndrome. Turk J Ophthalmol. 2015 Dec;45(6):271-273. [PMC free article: PMC5082267] [PubMed: 27800247]
- 59.
- Vigil-De Gracia P, Ortega-Paz L. Retinal detachment in association with pre-eclampsia, eclampsia, and HELLP syndrome. Int J Gynaecol Obstet. 2011 Sep;114(3):223-5. [PubMed: 21719013]
- 60.
- Schönfeld CL. Bilateral Exudative Retinal Detachment in HELLP Syndrome. Case Rep Ophthalmol. 2012 Jan;3(1):35-7. [PMC free article: PMC3357143] [PubMed: 22615699]
- 61.
- Singalavanija A, Dangosintr N, Namatra C. Retinal detachment in toxemia of pregnancy. J Med Assoc Thai. 1989 Oct;72(10):597-600. [PubMed: 2584907]
- 62.
- Lee CS, Choi EY, Lee M, Kim H, Chung H. Serous retinal detachment in preeclampsia and malignant hypertension. Eye (Lond). 2019 Nov;33(11):1707-1714. [PMC free article: PMC7002678] [PubMed: 31089238]
- 63.
- Hoines J, Buettner H. Ocular complications of disseminated intravascular coagulation (DIC) in abruptio placentae. Retina. 1989;9(2):105-9. [PubMed: 2672208]
- 64.
- Sampo M, Yin GH, Hoffart L, Denis D, Soler V, Matonti F. Exudative Retinal Detachment Treatment in a Patient with Thrombotic Thrombocytopenic Purpura. Case Rep Ophthalmol. 2016 Jan-Apr;7(1):90-5. [PMC free article: PMC4899654] [PubMed: 27293407]
- 65.
- Jaben SL, Norton EW. Exudative retinal detachment in Wegener's granulomatosis: case report. Ann Ophthalmol. 1982 Aug;14(8):717-20. [PubMed: 7125466]
- 66.
- Hannouche D, Korobelnik JF, Cochereau I, Hayem G, Beaudreuil J, Meyer O, Hoang-Xuan T. Systemic lupus erythematosus with choroidopathy and serous retinal detachment. Int Ophthalmol. 1995;19(2):125-7. [PubMed: 8586496]
- 67.
- Hoscheit AM, Austin JK, Jones WL. Nonrhegmatogenous retinal detachment in Goodpasture's syndrome: a case report and discussion of the clinicopathologic entity. J Am Optom Assoc. 1993 Aug;64(8):563-7. [PubMed: 8409194]
- 68.
- Otuka OAI, Eweputanna LI, Okoronkwo NC, Kalu A. Bilateral Exudative Retinal Detachment in a Young Patient with Chronic Renal Failure. Int Med Case Rep J. 2021;14:139-144. [PMC free article: PMC7947333] [PubMed: 33716512]
- 69.
- Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015 Apr 15;6(3):489-99. [PMC free article: PMC4398904] [PubMed: 25897358]
- 70.
- Ferencz JR, Rosen E, Tam G, Gilady G, Rubowich A, Assia EI, Korzets Z. Treatment of total exudative retinal detachment due to central retinal vein occlusion by intravitreal bevacizumab in a patient with p-ANCA vasculitis. Clin Ophthalmol. 2007 Sep;1(3):347-51. [PMC free article: PMC2701120] [PubMed: 19668494]
- 71.
- Chang YS, Weng SF, Chang C, Wang JJ, Chen HI, Ko SY, Tu IT, Chien CC, Wang JJ, Wang CM, Jan RL. Risk of serous retinal detachment in patients with end-stage renal disease on dialysis. PLoS One. 2017;12(6):e0180133. [PMC free article: PMC5489197] [PubMed: 28658289]
- 72.
- AlAli A, Bourgault S, Clark I, Lam WC. EXUDATIVE RETINAL DETACHMENT CAUSED BY A CHOROIDAL NEOVASCULAR MEMBRANE IN HALLERMANN-STREIFF SYNDROME. 2018 WinterRetin Cases Brief Rep. 12(1):45-47. [PubMed: 27648586]
- 73.
- Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10(1):1-8. [PubMed: 1693009]
- 74.
- Khairil-Ridzwan KK, Azian A, Hanizasurana H, Shatriah I. Exudative Retinal Detachment due to Coats Disease in a Teenager with Senior-Loken Syndrome: Case Report and Review of Literature. Cureus. 2019 Apr 15;11(4):e4460. [PMC free article: PMC6561513] [PubMed: 31205846]
- 75.
- Spaide RF, Gemmy Cheung CM, Matsumoto H, Kishi S, Boon CJF, van Dijk EHC, Mauget-Faysse M, Behar-Cohen F, Hartnett ME, Sivaprasad S, Iida T, Brown DM, Chhablani J, Maloca PM. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res. 2022 Jan;86:100973. [PubMed: 34029721]
- 76.
- Tauqeer Z, Yonekawa Y. Familial Exudative Vitreoretinopathy: Pathophysiology, Diagnosis, and Management. Asia Pac J Ophthalmol (Phila). 2018 May-Jun;7(3):176-182. [PubMed: 29633588]
- 77.
- Moshfeghi DM, Silva RA, Berrocal AM. Exudative retinal detachment following photocoagulation in older premature infants for retinopathy of prematurity: description and management. Retina. 2014 Jan;34(1):83-6. [PubMed: 23881225]
- 78.
- Takahashi K, Kishi S. Serous macular detachment associated with retinal arterial macroaneurysm. Jpn J Ophthalmol. 2006 Sep-Oct;50(5):460-464. [PubMed: 17013700]
- 79.
- Singh D, Tripathy K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Retinal Macroaneurysm. [PubMed: 35015432]
- 80.
- Modorati G, Matteo DF, Miserocchi E, Colucci A, Bandello F. Serous Retinal Detachments Complicating Interferon-α and Ribavirin Treatment in Patients with Hepatitis C. Case Rep Ophthalmol. 2011 Jan;2(1):105-10. [PMC free article: PMC3219447] [PubMed: 22110438]
- 81.
- Chan CK, Gass JD, Lin SG. Acute exudative polymorphous vitelliform maculopathy syndrome. Retina. 2003 Aug;23(4):453-62. [PubMed: 12972754]
- 82.
- Feroze KB, Wang J. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 18, 2022. Interferon-Induced Retinopathy. [PubMed: 28722892]
- 83.
- Mahendradas P, Parab S, Sasikumar R, Kawali A, Shetty BK. Topiramate-induced acute angle closure with severe panuveitis: A challenging case report. Indian J Ophthalmol. 2018 Sep;66(9):1342-1344. [PMC free article: PMC6113830] [PubMed: 30127167]
- 84.
- Mazumdar S, Tripathy K, Sarma B, Agarwal N. Acquired myopia followed by acquired hyperopia due to serous neurosensory retinal detachment following topiramate intake. Eur J Ophthalmol. 2019 Jan;29(1):NP21-NP24. [PubMed: 30175623]
- 85.
- Chee SP, Jap A, Bacsal K. Prognostic factors of Vogt-Koyanagi-Harada disease in Singapore. Am J Ophthalmol. 2009 Jan;147(1):154-161.e1. [PubMed: 18834575]
- 86.
- Shields CL, Manalac J, Das C, Ferguson K, Shields JA. Choroidal melanoma: clinical features, classification, and top 10 pseudomelanomas. Curr Opin Ophthalmol. 2014 May;25(3):177-85. [PubMed: 24614143]
- 87.
- Stahl A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch Arztebl Int. 2020 Jul 20;117(29-30):513-520. [PMC free article: PMC7588619] [PubMed: 33087239]
- 88.
- Egwuagu CE, Sun L, Kim SH, Dambuza IM. Ocular Inflammatory Diseases: Molecular Pathogenesis and Immunotherapy. Curr Mol Med. 2015;15(6):517-28. [PubMed: 26238372]
- 89.
- Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008 Mar;86(2):126-45. [PubMed: 17662099]
- 90.
- Shapiro MJ, Chow CC, Karth PA, Kiernan DF, Blair MP. Effects of green diode laser in the treatment of pediatric Coats disease. Am J Ophthalmol. 2011 Apr;151(4):725-731.e2. [PubMed: 21257148]
- 91.
- Gupta A, Tripathy K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Central Serous Chorioretinopathy. [PubMed: 32644399]
- 92.
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995 Oct;269(4 Pt 1):G467-75. [PubMed: 7485497]
- 93.
- Marmor MF. Control of subretinal fluid: experimental and clinical studies. Eye (Lond). 1990;4 ( Pt 2):340-4. [PubMed: 2199242]
- 94.
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261-98. [PubMed: 16487793]
- 95.
- Cunha-Vaz JG. The blood-retinal barriers. Doc Ophthalmol. 1976 Oct 15;41(2):287-327. [PubMed: 1009819]
- 96.
- Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010 Mar;149(3):361-363. [PubMed: 20172062]
- 97.
- Okushiba U, Takeda M. [Study of choroidal vascular lesions in central serous chorioretinopathy using indocyanine green angiography]. Nippon Ganka Gakkai Zasshi. 1997 Jan;101(1):74-82. [PubMed: 9028111]
- 98.
- Rao NA. Pathology of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2007 Apr-Jun;27(2-3):81-5. [PubMed: 17435969]
- 99.
- Lavezzo MM, Sakata VM, Morita C, Rodriguez EE, Abdallah SF, da Silva FT, Hirata CE, Yamamoto JH. Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis. 2016 Mar 24;11:29. [PMC free article: PMC4806431] [PubMed: 27008848]
- 100.
- Sen M, Honavar SG. Circumscribed choroidal hemangioma: An overview of clinical manifestation, diagnosis and management. Indian J Ophthalmol. 2019 Dec;67(12):1965-1973. [PMC free article: PMC6896540] [PubMed: 31755430]
- 101.
- Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976 May-Jun;20(6):415-31. [PubMed: 820013]
- 102.
- Mombaerts I, Schlingemann RO, Goldschmeding R, Koornneef L. Idiopathic granulomatous orbital inflammation. Ophthalmology. 1996 Dec;103(12):2135-41. [PubMed: 9003349]
- 103.
- Mombaerts I, Goldschmeding R, Schlingemann RO, Koornneef L. What is orbital pseudotumor? Surv Ophthalmol. 1996 Jul-Aug;41(1):66-78. [PubMed: 8827931]
- 104.
- Gass JD, Guerry RK, Jack RL, Harris G. Choroidal Osteoma. Arch Ophthalmol. 1978 Mar;96(3):428-35. [PubMed: 629679]
- 105.
- Williams AT, Font RL, Van Dyk HJ, Riekhof FT. Osseous choristoma of the choroid simulating a choroidal melanoma. Association with a positive 32P test. Arch Ophthalmol. 1978 Oct;96(10):1874-7. [PubMed: 697626]
- 106.
- Chen J, Lee L, Gass JD. Choroidal osteoma: evidence of progression and decalcification over 20 years. Clin Exp Optom. 2006 Mar;89(2):90-4. [PubMed: 16494612]
- 107.
- Duong HV, McLean IW, Beahm DE. Bilateral diffuse melanocytic proliferation associated with ovarian carcinoma and metastatic malignant amelanotic melanoma. Am J Ophthalmol. 2006 Oct;142(4):693-5. [PubMed: 17011873]
- 108.
- Pulido JS, Flotte TJ, Raja H, Miles S, Winters JL, Niles R, Jaben EA, Markovic SN, Davies J, Kalli KR, Vile RG, Garcia JJ, Salomao DR. Dermal and conjunctival melanocytic proliferations in diffuse uveal melanocytic proliferation. Eye (Lond). 2013 Sep;27(9):1058-62. [PMC free article: PMC3772365] [PubMed: 23788206]
- 109.
- Chen C, Jing W, Gulati P, Vargas H, French SW. Melanocytic differentiation in a solid pseudopapillary tumor of the pancreas. J Gastroenterol. 2004 Jun;39(6):579-83. [PubMed: 15235877]
- 110.
- Brigle K, Rogers B. Pathobiology and Diagnosis of Multiple Myeloma. Semin Oncol Nurs. 2017 Aug;33(3):225-236. [PubMed: 28688533]
- 111.
- Slater D. Histopathological aspects of cutaneous lymphoma. J R Soc Med. 2001 Jul;94(7):337-40. [PMC free article: PMC1281597] [PubMed: 11418703]
- 112.
- Vermeirsch S, Testi I, Pavesio C. Choroidal involvement in non-infectious posterior scleritis. J Ophthalmic Inflamm Infect. 2021 Oct 27;11(1):41. [PMC free article: PMC8554953] [PubMed: 34705127]
- 113.
- Yeşiltaş YS, Gündüz AK. Idiopathic Orbital Inflammation: Review of Literature and New Advances. Middle East Afr J Ophthalmol. 2018 Apr-Jun;25(2):71-80. [PMC free article: PMC6071347] [PubMed: 30122852]
- 114.
- Zhu M, Tang A, Amatya N, Qiu L. Exudative retinal detachment. Neth J Med. 2011 Nov-Dec;69(11):527, 530. [PubMed: 22173366]
- 115.
- Magliyah MS, Al-Fakhri AS, Al-Dhibi HA. Proliferative retinopathy as a feature of Vogt Koyanagi Harada Disease: a report of two cases. BMC Ophthalmol. 2020 Dec 01;20(1):470. [PMC free article: PMC7706217] [PubMed: 33261580]
- 116.
- Simakurthy S, Tripathy K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Marcus Gunn Pupil. [PubMed: 32491607]
- 117.
- García-Arumí J, Martínez-Castillo V, Boixadera A, Blasco H, Marticorena J, Zapata MÁ, Macià C, Badal J, Distéfano L, Rafart JM, Berrocal M, Zambrano A, Ruíz-Moreno JM, Figueroa MS. Rhegmatogenous retinal detachment treatment guidelines. Arch Soc Esp Oftalmol. 2013 Jan;88(1):11-35. [PubMed: 23414946]
- 118.
- Obuchowska I, Mariak Z. [Choroidal detachment--pathogenesis, etiology and clinical features]. Klin Oczna. 2005;107(7-9):529-32. [PubMed: 16417015]
- 119.
- Spaide RF, Goldbaum M, Wong DW, Tang KC, Iida T. Serous detachment of the retina. Retina. 2003 Dec;23(6):820-46; quiz 895-6. [PubMed: 14707834]
- 120.
- Kirkby GR, Chignell AH. Shifting subretinal fluid in rhegmatogenous retinal detachment. Br J Ophthalmol. 1985 Sep;69(9):654-5. [PMC free article: PMC1040705] [PubMed: 4041411]
- 121.
- Sartini F, Menchini M, Posarelli C, Casini G, Figus M. Bullous Central Serous Chorioretinopathy: A Rare and Atypical Form of Central Serous Chorioretinopathy. A Systematic Review. Pharmaceuticals (Basel). 2020 Aug 28;13(9) [PMC free article: PMC7559580] [PubMed: 32872388]
- 122.
- Lin SH, Xu YG, Zhao JH, Cui H, Jin H, Jia YJ, Zhao J, Li YJ. Choroidal metastasis with retinal detachment: A case report. Medicine (Baltimore). 2021 Dec 23;100(51):e28009. [PMC free article: PMC8702016] [PubMed: 34941041]
- 123.
- Feltgen N, Walter P. Rhegmatogenous retinal detachment--an ophthalmologic emergency. Dtsch Arztebl Int. 2014 Jan 06;111(1-2):12-21; quiz 22. [PMC free article: PMC3948016] [PubMed: 24565273]
- 124.
- Stewart MW, Browning DJ, Landers MB. Current management of diabetic tractional retinal detachments. Indian J Ophthalmol. 2018 Dec;66(12):1751-1762. [PMC free article: PMC6256889] [PubMed: 30451175]
- 125.
- Liao L, Zhu XH. Advances in the treatment of rhegmatogenous retinal detachment. Int J Ophthalmol. 2019;12(4):660-667. [PMC free article: PMC6469565] [PubMed: 31024823]
- 126.
- Kirchhof B. [The diseased vitreous body: Malformations, developmental disorders and opacities]. Ophthalmologe. 2015 Jul;112(7):559-63. [PubMed: 26149492]
- 127.
- Miles SL, Niles RM, Pittock S, Vile R, Davies J, Winters JL, Abu-Yaghi NE, Grothey A, Siddiqui M, Kaur J, Hartmann L, Kalli KR, Pease L, Kravitz D, Markovic S, Pulido JS. A factor found in the IgG fraction of serum of patients with paraneoplastic bilateral diffuse uveal melanocytic proliferation causes proliferation of cultured human melanocytes. Retina. 2012 Oct;32(9):1959-66. [PubMed: 22791177]
- 128.
- Jampol LM, Leskov I, Lyon AT. Diffuse Uveal Melanocytic Proliferation With Primary Vitreoretinal Lymphoma-Reply. JAMA Ophthalmol. 2019 Dec 01;137(12):1466-1467. [PubMed: 31647494]
- 129.
- Pefkianaki M, Agrawal R, Desai P, Pavesio C, Sagoo MS. Bilateral Diffuse Uveal Melanocytic Proliferation (BDUMP) associated with B-cell lymphoma: report of a rare case. BMC Cancer. 2015 Jan 30;15:23. [PMC free article: PMC4320603] [PubMed: 25633015]
- 130.
- Tripathy K, Salini B. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Amsler Grid. [PubMed: 30844168]
- 131.
- Laviers H, Zambarakji H. Enhanced depth imaging-OCT of the choroid: a review of the current literature. Graefes Arch Clin Exp Ophthalmol. 2014 Dec;252(12):1871-83. [PubMed: 25363655]
- 132.
- Benson WE. Posterior scleritis. Surv Ophthalmol. 1988 Mar-Apr;32(5):297-316. [PubMed: 3043740]
- 133.
- Joye A, Suhler E. Vogt-Koyanagi-Harada disease. Curr Opin Ophthalmol. 2021 Nov 01;32(6):574-582. [PubMed: 34545845]
- 134.
- Wong IY, Koizumi H, Lai WW. Enhanced depth imaging optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011 Jul;42 Suppl:S75-84. [PubMed: 21790115]
- 135.
- Vasconcelos-Santos DV, Sohn EH, Sadda S, Rao NA. Retinal pigment epithelial changes in chronic Vogt-Koyanagi-Harada disease: fundus autofluorescence and spectral domain-optical coherence tomography findings. Retina. 2010 Jan;30(1):33-41. [PMC free article: PMC2903055] [PubMed: 20010321]
- 136.
- Heussen FM, Vasconcelos-Santos DV, Pappuru RR, Walsh AC, Rao NA, Sadda SR. Ultra-wide-field green-light (532-nm) autofluorescence imaging in chronic Vogt-Koyanagi-Harada disease. Ophthalmic Surg Lasers Imaging. 2011 Jul-Aug;42(4):272-7. [PubMed: 21553702]
- 137.
- Kon Y, Iida T, Maruko I, Saito M. The optical coherence tomography-ophthalmoscope for examination of central serous chorioretinopathy with precipitates. Retina. 2008 Jun;28(6):864-9. [PubMed: 18536604]
- 138.
- Yalcinbayir O, Gelisken O, Akova-Budak B, Ozkaya G, Gorkem Cevik S, Yucel AA. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina. 2014 Apr;34(4):705-12. [PubMed: 24100708]
- 139.
- Iacono P, Battaglia PM, Papayannis A, La Spina C, Varano M, Bandello F. Acute central serous chorioretinopathy: a correlation study between fundus autofluorescence and spectral-domain OCT. Graefes Arch Clin Exp Ophthalmol. 2015 Nov;253(11):1889-97. [PubMed: 25563727]
- 140.
- Landa G, Barnett JA, Garcia PM, Tai KW, Rosen RB. Quantitative and qualitative spectral domain optical coherence tomography analysis of subretinal deposits in patients with acute central serous retinopathy. Ophthalmologica. 2013;230(2):62-8. [PubMed: 23774198]
- 141.
- Ahlers C, Geitzenauer W, Stock G, Golbaz I, Schmidt-Erfurth U, Prünte C. Alterations of intraretinal layers in acute central serous chorioretinopathy. Acta Ophthalmol. 2009 Aug;87(5):511-6. [PubMed: 19508461]
- 142.
- Yang L, Jonas JB, Wei W. Optical coherence tomography-assisted enhanced depth imaging of central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2013 Jul 12;54(7):4659-65. [PubMed: 23737472]
- 143.
- Alshahrani ST, Al Shamsi HN, Kahtani ES, Ghazi NG. Spectral-domain optical coherence tomography findings in polypoidal choroidal vasculopathy suggest a type 1 neovascular growth pattern. Clin Ophthalmol. 2014;8:1689-95. [PMC free article: PMC4159396] [PubMed: 25214762]
- 144.
- De Salvo G, Vaz-Pereira S, Keane PA, Tufail A, Liew G. Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2014 Dec;158(6):1228-1238.e1. [PubMed: 25152500]
- 145.
- Liu R, Li J, Li Z, Yu S, Yang Y, Yan H, Zeng J, Tang S, Ding X. DISTINGUISHING POLYPOIDAL CHOROIDAL VASCULOPATHY FROM TYPICAL NEOVASCULAR AGE-RELATED MACULAR DEGENERATION BASED ON SPECTRAL DOMAIN OPTICAL COHERENCE TOMOGRAPHY. Retina. 2016 Apr;36(4):778-86. [PubMed: 26428604]
- 146.
- Ruia S, Tripathy K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Fluorescein Angiography. [PubMed: 35015403]
- 147.
- Cavallerano AA. Ophthalmic fluorescein angiography. Optom Clin. 1996;5(1):1-23. [PubMed: 8963072]
- 148.
- Norton EW. A characteristic fluorescein angiographic pattern in choriodal folds. Proc R Soc Med. 1969 Feb;62(2):119-28. [PMC free article: PMC1810726] [PubMed: 5775225]
- 149.
- Biswas J, Mittal S, Ganesh SK, Shetty NS, Gopal L. Posterior scleritis: clinical profile and imaging characteristics. Indian J Ophthalmol. 1998 Dec;46(4):195-202. [PubMed: 10218301]
- 150.
- Friedel S, Polak A. [Leopard-spot pattern in fluorescein angiography]. Ophthalmologe. 2013 Apr;110(4):360-4. [PubMed: 23338531]
- 151.
- Gass JD. Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol. 1968 Aug;80(2):177-85. [PubMed: 5661882]
- 152.
- Chen TY, Bhagat S, Bhagat N. Melanocytic Lesions in Buccal Mucosa in BDUMP. Ophthalmology. 2020 Aug;127(8):1063. [PubMed: 32703386]
- 153.
- Pierce KK, Lane RG. Central serous chorioretinopathy associated with the use of ephedra. Retin Cases Brief Rep. 2009 Fall;3(4):376-8. [PubMed: 25389852]
- 154.
- Park DW, Schatz H, Gaffney MM, McDonald HR, Johnson RN, Schaeffer D. Central serous chorioretinopathy in two families. Eur J Ophthalmol. 1998 Jan-Mar;8(1):42-7. [PubMed: 9590595]
- 155.
- Muraleedharan S, Tripathy K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Indocyanine Green (ICG) Angiography. [PubMed: 35593804]
- 156.
- Herbort CP, Mantovani A, Bouchenaki N. Indocyanine green angiography in Vogt-Koyanagi-Harada disease: angiographic signs and utility in patient follow-up. Int Ophthalmol. 2007 Apr-Jun;27(2-3):173-82. [PubMed: 17457515]
- 157.
- Miyanaga M, Kawaguchi T, Miyata K, Horie S, Mochizuki M, Herbort CP. Indocyanine green angiography findings in initial acute pretreatment Vogt-Koyanagi-Harada disease in Japanese patients. Jpn J Ophthalmol. 2010 Sep;54(5):377-82. [PubMed: 21052896]
- 158.
- Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012 Sep;32(8):1453-64. [PubMed: 22426346]
- 159.
- Japanese Study Group of Polypoidal Choroidal Vasculopathy. [Criteria for diagnosis of polypoidal choroidal vasculopathy]. Nippon Ganka Gakkai Zasshi. 2005 Jul;109(7):417-27. [PubMed: 16050460]
- 160.
- Schneider U, Inhoffen W, Gelisken F. Indocyanine green angiography in a case of unilateral recurrent posterior acute multifocal placoid pigment epitheliopathy. Acta Ophthalmol Scand. 2003 Feb;81(1):72-5. [PubMed: 12631024]
- 161.
- Yuzawa M, Kawamura A, Matsui M. Indocyanine green video angiographic findings in acute posterior multifocal placoid pigment epitheliopathy. Acta Ophthalmol (Copenh). 1994 Feb;72(1):128-33. [PubMed: 8017187]
- 162.
- Montero JA, Ruiz-Moreno JM, Fernandez-Munoz M. Spectral domain optical coherence tomography findings in acute posterior multifocal placoid pigment epitheliopathy. Ocul Immunol Inflamm. 2011 Feb;19(1):48-50. [PubMed: 21250924]
- 163.
- Cheung CM, Yeo IY, Koh A. Photoreceptor changes in acute and resolved acute posterior multifocal placoid pigment epitheliopathy documented by spectral-domain optical coherence tomography. Arch Ophthalmol. 2010 May;128(5):644-6. [PubMed: 20457992]
- 164.
- Lima LH, Greenberg JP, Greenstein VC, Smith RT, Sallum JM, Thirkill C, Yannuzzi LA, Tsang SH. Hyperautofluorescent ring in autoimmune retinopathy. Retina. 2012 Jul;32(7):1385-94. [PMC free article: PMC4377132] [PubMed: 22218149]
- 165.
- Ramtohul P, Engelbert M, Malclès A, Gigon E, Miserocchi E, Modorati G, Cunha de Souza E, Besirli CG, Curcio CA, Freund KB. BACILLARY LAYER DETACHMENT: MULTIMODAL IMAGING AND HISTOLOGIC EVIDENCE OF A NOVEL OPTICAL COHERENCE TOMOGRAPHY TERMINOLOGY: Literature Review and Proposed Theory. Retina. 2021 Nov 01;41(11):2193-2207. [PubMed: 34029276]
- 166.
- Naysan J, Pang CE, Klein RW, Freund KB. Multimodal imaging of bilateral diffuse uveal melanocytic proliferation associated with an iris mass lesion. Int J Retina Vitreous. 2016;2:13. [PMC free article: PMC5088479] [PubMed: 27847631]
- 167.
- von Rückmann A, Fitzke FW, Bird AC. In vivo fundus autofluorescence in macular dystrophies. Arch Ophthalmol. 1997 May;115(5):609-15. [PubMed: 9152128]
- 168.
- Boon CJ, Theelen T, Hoefsloot EH, van Schooneveld MJ, Keunen JE, Cremers FP, Klevering BJ, Hoyng CB. Clinical and molecular genetic analysis of best vitelliform macular dystrophy. Retina. 2009 Jun;29(6):835-47. [PubMed: 19357557]
- 169.
- O'Gorman S, Flaherty WA, Fishman GA, Berson EL. Histopathologic findings in Best's vitelliform macular dystrophy. Arch Ophthalmol. 1988 Sep;106(9):1261-8. [PubMed: 3415551]
- 170.
- Weingeist TA, Kobrin JL, Watzke RC. Histopathology of Best's macular dystrophy. Arch Ophthalmol. 1982 Jul;100(7):1108-14. [PubMed: 7092654]
- 171.
- Bakall B, Radu RA, Stanton JB, Burke JM, McKay BS, Wadelius C, Mullins RF, Stone EM, Travis GH, Marmorstein AD. Enhanced accumulation of A2E in individuals homozygous or heterozygous for mutations in BEST1 (VMD2). Exp Eye Res. 2007 Jul;85(1):34-43. [PubMed: 17477921]
- 172.
- Dutta Majumder P, Marchese A, Pichi F, Garg I, Agarwal A. An update on autoimmune retinopathy. Indian J Ophthalmol. 2020 Sep;68(9):1829-1837. [PMC free article: PMC7690499] [PubMed: 32823399]
- 173.
- Mohamed Q, Harper CA. Acute optical coherence tomographic findings in cancer-associated retinopathy. Arch Ophthalmol. 2007 Aug;125(8):1132-3. [PubMed: 17698766]
- 174.
- Patil YB, Garg R, Rajguru JP, Sirsalmath M, Bevinakatti VA, Kumar M, Sharma S. Vogt-Koyanagi-Harada (VKH) syndrome: A new perspective for healthcare professionals. J Family Med Prim Care. 2020 Jan;9(1):31-35. [PMC free article: PMC7014871] [PubMed: 32110561]
- 175.
- Baltatzis S, Tufail F, Yu EN, Vredeveld CM, Foster CS. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology. 2003 May;110(5):1061-5. [PubMed: 12750115]
- 176.
- Agarwal M, Ganesh SK, Biswas J. Triple agent immunosuppressive therapy in Vogt-Koyanagi-Harada syndrome. Ocul Immunol Inflamm. 2006 Dec;14(6):333-9. [PubMed: 17162603]
- 177.
- Bykhovskaya I, Thorne JE, Kempen JH, Dunn JP, Jabs DA. Vogt-Koyanagi-Harada disease: clinical outcomes. Am J Ophthalmol. 2005 Oct;140(4):674-8. [PubMed: 16226518]
- 178.
- Jap A, Luu CD, Yeo I, Chee SP. Correlation between peripapillary atrophy and corticosteroid therapy in patients with Vogt-Koyanagi-Harada disease. Eye (Lond). 2008 Feb;22(2):240-5. [PubMed: 16980924]
- 179.
- Abdel-Aty A, Gupta A, Del Priore L, Kombo N. Management of noninfectious scleritis. Ther Adv Ophthalmol. 2022 Jan-Dec;14:25158414211070879. [PMC free article: PMC8785299] [PubMed: 35083421]
- 180.
- Stem MS, Todorich B, Faia LJ. Ocular Pharmacology for Scleritis: Review of Treatment and a Practical Perspective. J Ocul Pharmacol Ther. 2017 May;33(4):240-246. [PubMed: 28355124]
- 181.
- Lavric A, Gonzalez-Lopez JJ, Majumder PD, Bansal N, Biswas J, Pavesio C, Agrawal R. Posterior Scleritis: Analysis of Epidemiology, Clinical Factors, and Risk of Recurrence in a Cohort of 114 Patients. Ocul Immunol Inflamm. 2016;24(1):6-15. [PubMed: 26134101]
- 182.
- Dutta Majumder P, Agrawal R, McCluskey P, Biswas J. Current Approach for the Diagnosis and Management of Noninfective Scleritis. Asia Pac J Ophthalmol (Phila). 2020 Dec 07;10(2):212-223. [PubMed: 33290287]
- 183.
- Sota J, Girolamo MM, Frediani B, Tosi GM, Cantarini L, Fabiani C. Biologic Therapies and Small Molecules for the Management of Non-Infectious Scleritis: A Narrative Review. Ophthalmol Ther. 2021 Dec;10(4):777-813. [PMC free article: PMC8589879] [PubMed: 34476773]
- 184.
- Wakefield D, Di Girolamo N, Thurau S, Wildner G, McCluskey P. Scleritis: Immunopathogenesis and molecular basis for therapy. Prog Retin Eye Res. 2013 Jul;35:44-62. [PubMed: 23454614]
- 185.
- Lim JW, Kang SW, Kim YT, Chung SE, Lee SW. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. Br J Ophthalmol. 2011 Apr;95(4):514-7. [PubMed: 20644214]
- 186.
- Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008 Oct;115(10):1756-65. [PubMed: 18538401]
- 187.
- Schmidt-Erfurth U, Laqua H, Schlötzer-Schrehard U, Viestenz A, Naumann GO. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002 Jun;120(6):835-44. [PubMed: 12049594]
- 188.
- Witkin AJ, Brown GC. Update on nonsurgical therapy for diabetic macular edema. Curr Opin Ophthalmol. 2011 May;22(3):185-9. [PubMed: 21427573]
- 189.
- Chiang A, Regillo CD. Preferred therapies for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2011 May;22(3):199-204. [PubMed: 21427571]
- 190.
- London NJ, Brown G. Update and review of central retinal vein occlusion. Curr Opin Ophthalmol. 2011 May;22(3):159-65. [PubMed: 21460724]
- 191.
- Kusaka S. Surgical Management of Coats Disease. Asia Pac J Ophthalmol (Phila). 2018 May-Jun;7(3):156-159. [PubMed: 29664241]
- 192.
- Sen M, Shields CL, Honavar SG, Shields JA. Coats disease: An overview of classification, management and outcomes. Indian J Ophthalmol. 2019 Jun;67(6):763-771. [PMC free article: PMC6552590] [PubMed: 31124484]
- 193.
- Rugwizangoga B, Mwabili T, Scanlan T, Meyer P, Kitinya J. Coats' disease in Tanzania: first case report and literature review. Afr Health Sci. 2014 Sep;14(3):763-8. [PMC free article: PMC4209640] [PubMed: 25352900]
- 194.
- Sızmaz S, Yonekawa Y, T Trese M. Familial Exudative Vitreoretinopathy. Turk J Ophthalmol. 2015 Aug;45(4):164-168. [PMC free article: PMC5082275] [PubMed: 27800225]
- 195.
- Gilmour DF. Familial exudative vitreoretinopathy and related retinopathies. Eye (Lond). 2015 Jan;29(1):1-14. [PMC free article: PMC4289842] [PubMed: 25323851]
- 196.
- Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Rapid regression of retinal hemorrhage and neovascularization in a case of familial exudative vitreoretinopathy treated with intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008 Dec;246(12):1787-9. [PubMed: 18795314]
- 197.
- Henry CR, Sisk RA, Tzu JH, Albini TA, Davis JL, Murray TG, Berrocal AM. Long-term follow-up of intravitreal bevacizumab for the treatment of pediatric retinal and choroidal diseases. J AAPOS. 2015 Dec;19(6):541-8. [PubMed: 26691034]
- 198.
- Schefler AC, Berrocal AM, Murray TG. Advanced Coats' disease. Management with repetitive aggressive laser ablation therapy. Retina. 2008 Mar;28(3 Suppl):S38-41. [PubMed: 18317343]
- 199.
- Entezari M, Ramezani A, Safavizadeh L, Bassirnia N. Resolution of macular edema in Coats' disease with intravitreal bevacizumab. Indian J Ophthalmol. 2010 Jan-Feb;58(1):80-2. [PMC free article: PMC2841384] [PubMed: 20029156]
- 200.
- Lei S, Lam WC. Efficacy and safety of dexamethasone intravitreal implant for refractory macular edema in children. Can J Ophthalmol. 2015 Jun;50(3):236-41. [PubMed: 26040225]
- 201.
- Spitznas M, Joussen F, Wessing A. Treatment of Coats' disease with photocoagulation. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976 Apr 01;199(1):31-7. [PubMed: 1083682]
- 202.
- Finger PT. Radiation therapy for exudative choroidal hemangioma. Indian J Ophthalmol. 2019 May;67(5):579-581. [PMC free article: PMC6498941] [PubMed: 31007211]
- 203.
- Singh AD, Kaiser PK, Sears JE. Choroidal hemangioma. Ophthalmol Clin North Am. 2005 Mar;18(1):151-61, ix. [PubMed: 15763200]
- 204.
- Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian J Ophthalmol. 2015 Feb;63(2):122-7. [PMC free article: PMC4399120] [PubMed: 25827542]
- 205.
- Song W, Du C, Zhang Y. A case report of exudative retinal detachment derived from orbital cellulitis in mainland China. BMC Ophthalmol. 2020 Aug 17;20(1):332. [PMC free article: PMC7433059] [PubMed: 32807110]
- 206.
- Testi I, Agrawal R, Mehta S, Basu S, Nguyen Q, Pavesio C, Gupta V. Ocular tuberculosis: Where are we today? Indian J Ophthalmol. 2020 Sep;68(9):1808-1817. [PMC free article: PMC7690544] [PubMed: 32823397]
- 207.
- Schulz DC, Orr SMA, Johnstone R, Devlin MK, Sheidow TG, Bursztyn LLCD. The many faces of ocular syphilis: case-based update on recognition, diagnosis, and treatment. Can J Ophthalmol. 2021 Oct;56(5):283-293. [PubMed: 33549544]
- 208.
- Koundanya VV, Tripathy K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2023. Syphilis Ocular Manifestations. [PubMed: 32644383]
- 209.
- Schoenberger SD, Kim SJ, Thorne JE, Mruthyunjaya P, Yeh S, Bakri SJ, Ehlers JP. Diagnosis and Treatment of Acute Retinal Necrosis: A Report by the American Academy of Ophthalmology. Ophthalmology. 2017 Mar;124(3):382-392. [PubMed: 28094044]
- 210.
- Jampol LM, Lahov M, Albert DM, Craft J. Ocular clinical findings and basement membrane changes in Goodpasture's syndrome. Am J Ophthalmol. 1975 Mar;79(3):452-63. [PubMed: 1092172]
- 211.
- Diddie KR, Aronson AJ, Ernest JT. Chorioretinopathy in a case of systemic lupus erythematosus. Trans Am Ophthalmol Soc. 1977;75:122-31. [PMC free article: PMC1311545] [PubMed: 349823]
- 212.
- Paris GL, Macoul KL. Reversible bullous retinal detachment in chronic renal disease. Am J Ophthalmol. 1969 Feb;67(2):249-51. [PubMed: 4885012]
- 213.
- Shakarchi FI. Ocular tuberculosis: current perspectives. Clin Ophthalmol. 2015;9:2223-7. [PMC free article: PMC4664543] [PubMed: 26648690]
- 214.
- Dutta Majumder P, Chen EJ, Shah J, Ching Wen Ho D, Biswas J, See Yin L, Gupta V, Pavesio C, Agrawal R. Ocular Syphilis: An Update. Ocul Immunol Inflamm. 2019;27(1):117-125. [PubMed: 29020491]
- 215.
- Daruich A, Bremond-Gignac D, Behar-Cohen F, Kermorvant E. [Retinopathy of prematurity: from prevention to treatment]. Med Sci (Paris). 2020 Oct;36(10):900-907. [PubMed: 33026333]
- 216.
- Shields JA, Shields CL. Review: coats disease: the 2001 LuEsther T. Mertz lecture. Retina. 2002 Feb;22(1):80-91. [PubMed: 11884883]
- 217.
- Tripathy K, Chawla R, Temkar S, Sagar P, Kashyap S, Pushker N, Sharma YR. Phthisis Bulbi-a Clinicopathological Perspective. Semin Ophthalmol. 2018;33(6):788-803. [PubMed: 29902388]
Disclosure: Unnati Shukla declares no relevant financial relationships with ineligible companies.
Disclosure: Abhishek Gupta declares no relevant financial relationships with ineligible companies.
Disclosure: Koushik Tripathy declares no relevant financial relationships with ineligible companies.
Publication Details
Author Information and Affiliations
Authors
Unnati V. Shukla1; Abhishek Gupta2; Koushik Tripathy3.Affiliations
Publication History
Last Update: February 22, 2023.
Copyright
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Publisher
StatPearls Publishing, Treasure Island (FL)
NLM Citation
Shukla UV, Gupta A, Tripathy K. Exudative Retinal Detachment. [Updated 2023 Feb 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.




