NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
What Did We Know?
In January 2022, we published an evolving rapid review,1 meta-analysis, and data visualization that compared the risk of reinfection in adults with prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection to the risk of infection in adults without a prior infection. We found that prior infection with the Alpha variant or the wild-type SARS-CoV-2 virus reduced the risk of another infection by 80–97 percent (pooled estimate 87%; 95% confidence interval [CI] 84–90%) compared with uninfected individuals in studies with a median followup of 8 months (range 4 to 13 months). Protection remained above 80 percent for at least 7 months.
Our original review, published in March 2021,2 described the antibody response after infection with the SARS-CoV-2 virus, but found little information on the duration of the response beyond 6 months or on antibody formation in asymptomatic patients or in individuals who are immunocompromised.
Questions for This Update
| Key Question | Evidence Gaps Addressed in This Update |
|---|---|
1. What is the prevalence, level, and duration of detectable antibodies to SARS-CoV-2 among adults infected with or recovered from SARS-CoV-2 infection that has been confirmed with reverse transcription-polymerase chain reaction?
| Persistence of IgG antibodies for longer than 12 months after infection. Characteristics of those who don’t seroconvert after previous infection. How the antibody response may vary in those who are immunocompromised. |
2. What is the risk of reinfection by SARS-CoV-2 among adults in the general population compared with the risk of infection in people who have never been infected?
|
Impact of Omicron and Delta variants on risk of reinfection. Relation of antibody levels to protection against reinfection. Relation of age and initial symptom status to protection against reinfection. |
3. What is the duration of protection against reinfection among adults following a primary SARS-CoV-2 infection?
| Duration of protection in the context of Omicron and Delta variants. |
| 4. What are the unintended consequences of antibody testing after SARS-CoV-2 infection? | Retired due to lack of relevance after widespread vaccination campaigns. |
IgG= Immunoglobulin G; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2
What Is New?
Updated: July 2022
Search current as of 07/08/2022
This update aims to fill the above gaps and retire this topic from continual update. It adds 30 observational studies and 1 systematic review. Our main findings are that:
- A high proportion of adults maintained detectable levels of IgG antibodies more than 12 months after SARS-CoV-2 infection confirmed by reverse transcription-polymerase chain reaction (RT-PCR) (Low Strength of Evidence [SoE]).
- Most immunocompromised adults develop IgG antibodies, but the overall proportion of those who develop antibodies is lower compared to immunocompetent adults (Moderate SoE for patients post-solid organ transplant and Low SoE for patients with cancer or HIV).
- Non-seroconversion rates were low to moderate (2–25%) and having had a mild or asymptomatic primary infection was associated with non-seroconversion (Low SoE).
- Prior infection with wild-type SARS-CoV-2 or the Alpha variant protected against reinfection by the Delta variant (80–97%) (High SoE). During the Delta wave, protection from prior infection with the wild-type virus or Alpha variant persisted for at least 13 months, and up to 20 months, in the general population, but waned after 13 months in elderly individuals (Low SoE).
- Prior infection with wild-type SARS-CoV-2, Alpha, or Delta variants provided weaker protection against symptomatic reinfection by the Omicron variant (43–56%) but was more protective against severe disease (88%), hospitalization (47%), or death (78%) (Moderate SoE).
Background
The United States Centers for Disease Control and Prevention reported that by the end of February 2022, 58 percent of the U.S. population and 75 percent of children under 12 years old have infection-induced antibodies to COVID-193, and 94.7 percent of adults have antibodies from either vaccination or previous infection.4 More than 80 COVID-19 antibody tests are available in the United States under Emergency Use Authorizations from the U.S. Food and Drug Administration (FDA). Commercial antibody tests vary considerably: some broadly detect any antibody that binds to any part of the SARS-CoV-2 virus, some only detect one specific antibody isotope (i.e., IgM, IgG, or IgA), while others only recognize antibodies that neutralize the virus. Some tests quantify antibody concentrations whereas others assess antibody function more qualitatively, while others distinguish between individuals who were infected or vaccinated (i.e., detect anti-SARS-CoV-2 nucleocapsid protein antibodies). Commercial antibody tests detect either binding antibodies (such as IgM, IgG, and IgA) or neutralizing antibodies and may be qualitative or quantitative. Some antibody tests—those that detect antibodies to the SARS-CoV-2 nucleocapsid protein—can identify individuals who have antibodies from previous infection rather than from vaccination. The FDA posts results of an ongoing, government-sponsored independent evaluation of all COVID-19 serological tests available in the United States.5
Purpose
This is the final update for a rapid, evolving, pragmatic review that was initially undertaken to inform the American College of Physicians’ Rapid Living Practice Points on the antibody response after SARS-CoV-2 infection.6 While antibody testing is a critical tool in research and public health, the value of using antibody testing at the individual patient level is unclear.
Currently, the most salient use of antibody testing is as a correlate of protection against infection. In our original review and first update, respectively, we confirmed some aspects of the antibody response and degree and duration of immunity after SARS-CoV-2 infection.1,2 These reviews also served to refine the scope of this final update by identifying gaps in the evidence on antibody testing and reinfection.
The aims of this final update are to synthesize evidence on these gaps. It is important to note that, in a rapidly changing field such as COVID-19 immunity, key questions, search strategies, and selection criteria do not remain static. In our initial protocol, we argued for a pragmatic, adaptive approach, which we described as a SWATH review.7 Our findings to date, the evolution of the pandemic, and new developments in the field have enabled us to tailor our searches, selection, and synthesis to address persistent gaps and emerging topics within the scope of the original Key Questions. For example, because of recent experiences with new variants, we refocused our surveillance for reinfection on studies that report on Delta or Omicron variants.
The gaps addressed in this final update underlie uncertainty about the value of antibody tests as a correlate of protection, that is, to predict immunity against reinfection. Since this review was conceived, the question of the role of antibody testing has changed, and narrowed, due to widespread vaccination and the wider availability of nucleic acid amplification tests (NAATs) and antigen tests to diagnose recent infections. In the setting of high vaccination rates, the question of protective immunity has become increasingly important, leading researchers to develop new methods to study immunity from previous infection and vaccination alike. While the original questions of whether there is a specific antibody isotype, antigenic target, and/or antibody level that correlates with protection remain relevant, attention has recently focused on other aspects of the immune system that may protect against reinfection, such as T-cell immunity, particularly in individuals who do not seroconvert or who are immunocompromised.
This review does not address all potential uses of antibody testing in clinical practice. Most notably, this review does not address the use of antibody testing for the diagnosis of SARS-CoV-2 infection. Specifically, we did not address the use of antibody tests when there is high clinical suspicion of COVID-19 despite repeatedly negative NAATs, or in patients with prolonged symptoms that could be attributable to COVID-19 who have no documented history of infection.8,9
Methods
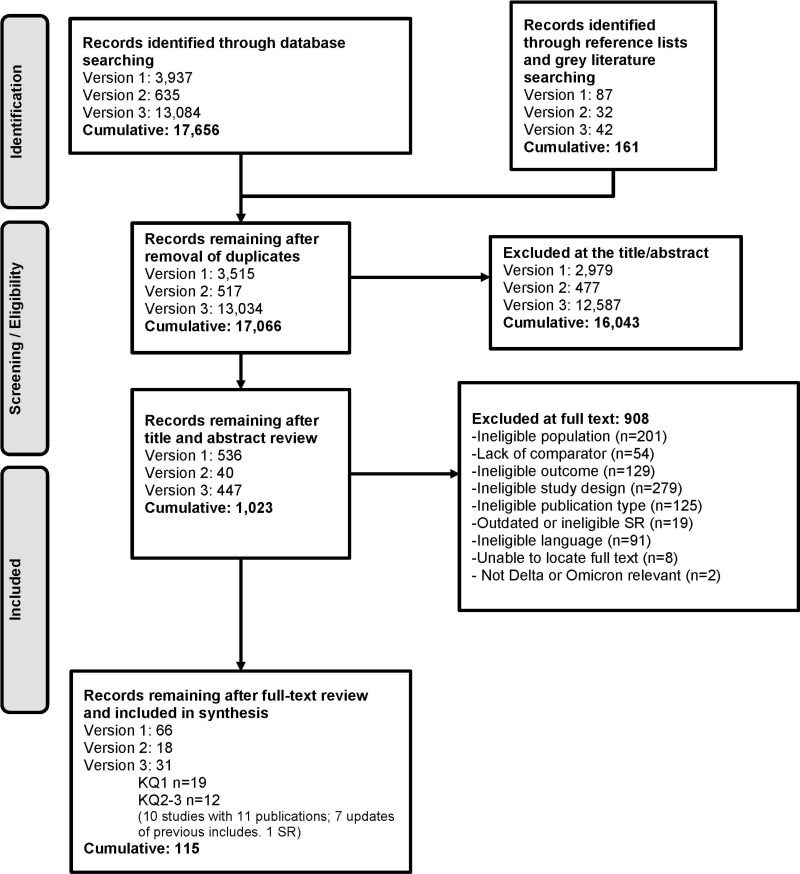
The protocol for this living rapid review was developed with the American College of Physicians, registered at PROSPERO (CRD42020207098), and posted to the Agency for Healthcare Research and Quality (AHRQ) Effective Health Care Program website.7 Methods are described in detail in our previous report and update.1,2 We stated in our original report that we would “consider modifying the scope of the review to address new developments in SARS-CoV-2 immunity research.” Appendix A describes methodological modifications relevant to this update, and Appendix B contains the PRISMA Flow Diagram (Figure B-1) and Risk of Bias assessments for included studies (Table B-2).
Results
Key Question 1. Durability of the Antibody Response
IgG duration >12 months
In our first report,2 we found that IgG may remain detectable for at least 120 days according to the study with the longest followup at the time.10 For this final update, we focused on longitudinal studies of IgG antibody status with at least 12 months of followup (i.e., studies with the longest followup currently) among participants who were infected with SARS-CoV-2 during the first year of the pandemic and who remained unvaccinated. We identified three prospective cohort studies of IgG status with median followup of at least 12 months (range 12.7 to 14 months). In all three studies, a high proportion of participants had detectable IgG over the long-term.11–13 In the largest study, conducted among 367 adults in Finland, 97 percent of participants had detectable anti-S IgG antibodies at a median followup of 12.7 months (range 11.9 to 14 months) and 88 percent had neutralizing antibodies.12 Similarly, in a study of 46 workers at a sewing company in Lithuania, 83 percent of adults who were infected in April 2020 and remained unvaccinated had detectable anti-S IgG approximately 13 months later (range 387–401 days; 1 participant was excluded because of reinfection).13 In another small study of 32 adults in Italy, 97 percent of participants had detectable anti-S-RBD IgG at 14 months.11 Across these three studies of 445 people, only one case of reinfection was reported. Additional details of the included studies are provided in Supplementary Appendix Tables B-1 and B-4.
We have limited confidence in the finding that most adults retain detectable IgG for at least 12 months following infection (Low SoE – Appendix Table B-3). While results were direct and consistent across studies, two out of three studies were small (fewer than 50 participants). Although all three studies measured IgG at different time points after initial infection and asked participants about reinfection, studies could not rule out the possibility that participants had an asymptomatic or mild reinfection accounting for persistent antibodies. Moreover, all studies were conducted in European countries early in the pandemic among adults who were mostly symptomatic during their primary infection. Results may not be generalizable to other settings or time periods or among adults with a mild or asymptomatic primary infection.
Immunocompromised Populations
In our original review, three observational studies provided insufficient evidence on antibody response in immunocompromised populations. These studies stratified antibody prevalence results according to patient comorbidities,14–16 but no study focused on the comparison of antibody response between immunocompromised and immunocompetent individuals from the same cohort.
In this update, we identified ten additional observational studies of the antibody response in immunocompromised patients compared to immunocompetent comparators: three studies in patients with cancer,17–19 one study in patients living with HIV,20 and six studies in patients who had undergone solid organ transplant (Appendix Table B-5).21–26 Consistent and direct evidence found that IgG antibodies were detected in most immunocompromised patients (≥65% at the first test after RT-PCR diagnosis for all included studies, except for a single cohort study in which the first test was conducted at just 15 days following infection, when IgG antibodies may not yet be detectable); however, IgG prevalence was consistently lower among immunocompromised patients compared to controls. The study with the most robust methods included 71 liver transplant recipients matched with 71 immunocompetent controls by propensity score, helping to ensure that the groups were comparable in terms of COVID-19 severity and baseline characteristics.22 In this study, liver transplant recipients had a lower prevalence of IgG antibodies compared to immunocompetent controls at 3 months (77% vs. 100%) and 6 months (63% vs. 90%). A study in the same cohort with 12-month follow-up found that anti-S IgG was slightly lower in transplant recipients at 3, 6, and 12 months (95%, 90%, and 88%, respectively) compared to immunocompetent controls (97%, 94%, and 100%, respectively).24 Another well-reported prospective cohort study conducted in 65 solid organ transplant recipients and 65 matched controls found that at one and nine months after SARS-CoV-2 infection, IgG prevalence decreased from 96 percent to 82 percent in immunocompromised patients and 100 percent to 96 percent in immunocompetent controls.23 Only one study in immunocompromised patients evaluated the neutralizing antibody response.21 In this study, 35 liver transplant recipients were matched with 35 immunocompetent controls and were followed for a median of 6.3 and 6.9 weeks, respectively. The prevalence of neutralizing antibodies was 83 percent in immunocompromised transplant recipients compared with 100 percent in immunocompetent controls.
We are moderately confident that most adults who are immunocompromised due to solid organ transplant develop IgG antibodies after SARS-CoV-2 infection, but the overall proportion of those who develop antibodies is lower compared to immunocompetent controls (Moderate SoE – Appendix Table B-3). Findings from all four studies in this population were consistent and direct, although studies were small and had methodological limitations. We have low confidence that this finding is stable for cancer patients and people living with HIV (Low SoE – Appendix Table B-3) Additional details of the included studies are provided in Supplementary Appendix Table B-1.
Non-seroconversion
We identified four prospective cohort studies27–30 comparing characteristics of patients who did not seroconvert after 6 weeks following documented SARS-CoV-2 infection with those who did seroconvert, adding to the evidence from two cohort studies14, 31 identified in our first report. Across these studies, the proportion of individuals who did not develop antibodies ranged from 2 percent to 25 percent. In the largest study, conducted among a cohort of 5,230 adults in the UK with a RT-PCR cycle threshold ≤ 32 (indicating a high viral load) and confirmatory SARS-CoV-2 genomic testing, 595 (11%) of individuals did not seroconvert during median follow-up of 221 days.30 Across studies, having no or few symptoms was the most consistent factor associated with non-seroconversion (Appendix Table B-6). Higher minimum cycle thresholds with PCR testing were associated with non-seroconversion in two studies.28, 30 Additional details of the included studies are provided in Supplementary Appendix Table B-1.
Our confidence in these findings is limited, primarily due to study methodological limitations (Appendix Table B-3). In general, studies of non-seroconversion should be interpreted with caution. It is not known whether variation across studies reflects true differences in the antibody response or methodological differences due to the use of different assays. Moreover, while most studies tested participants for IgG at different time points and some studies tested participants at regular intervals (e.g., monthly), individuals who had an initial IgG response but then seroreverted could have been misclassified as individuals who never seroconverted. Finally, the clinical significance of non-seroconversion is unclear. Individuals who fail to seroconvert after infection may still have a robust humoral response with repeated exposure due to immune memory, suggesting that seronegativity cannot be interpreted as evidence of a failure to develop an immune response.32
Key Question 2. Risk of Reinfection
Protection Against the Delta Variant
The B.1.617.2 (Delta) variant originated in India and became the dominant variant in the United States in July 2021. Since then, well-conducted, controlled studies, including updates of studies from the United States, the United Kingdom, Israel, Denmark, and Qatar included in our meta-analysis in the earlier update, have shown that previous infection protected against the Delta variant as well as it had protected against wild-type and previous variants.33–37 We have high confidence in the finding that prior infection provided similar protection against reinfection by the Delta variant as was found in our previous report (80–97% reduced risk of reinfection) (High SOE – Appendix Table B-3). Additional details of the included studies are provided in Supplementary Appendix Tables B-1 and B-7.
Protection Against the Omicron Variant
The B.1.1.529 (Omicron) variant was designated a variant of concern in November 2021. From the beginning, Omicron appeared to be associated with more significant escape from immune protection from previous infection and from vaccination.38, 39 Updates from the studies in our previous meta-analysis confirmed that in unvaccinated individuals, protection from prior infection against Omicron was 43 percent to 56 percent compared to those without prior infection.33, 35, 37 In the Qatari study, for example, previous infection was 55.9 percent effective in protecting against symptomatic infection from Omicron versus unvaccinated controls who had never been infected.33
Because Omicron became dominant over a year after the initial waves of SARS-CoV-2 infection, protection against it from previous infection could be confounded by waning immunity over time, or by stepwise changes in viral epitopes that may make more remote infection less effective. Additionally, in some countries, a high rate of immunity from vaccination would make it difficult to isolate the effect of previous infection on risk of reinfection. In the Danish cohort update,37 protection against Omicron was 43.1 percent if previous infection occurred 3-6 months earlier and 22.2 percent if infection had occurred 6 or more months earlier. In the U.K. updates, prior infection was estimated to be only 44 percent (95% CI: 4, 67) protective against reinfection by Omicron.35, 39–41
While the Omicron variant is more infectious, a universal finding was that Omicron was less likely to cause serious disease than previous variants.39, 41, 42 Current estimates indicate that protection from prior infection against severe disease due to Omicron was substantial but estimates from different cohorts are inconsistent. In the Qatari cohort, previous infection was 87.8 percent effective in protecting against severe COVID-19 disease.33 In the Danish cohort, protection against COVID-19 related hospitalization was 47.1 percent compared with controls who were not previously infected.37 The most detailed analysis of severity, from the United Kingdom, found that, for unvaccinated individuals, prior infection reduced the risk of hospitalization after a positive NAAT test by 45 percent (hazard ratio [HR]=0.55; 95% CI: 0.48, 0.63) and reduced the risk of COVID-19 related death after a positive NAAT by 82 percent (HR=0.18; 95% CI: 0.06, 0.57).41 For vaccinated individuals, prior infection had no effect on the risk of hospitalization but reduced the risk of death substantially (HR=0.47; 95% CI: 0.32, 0.68).41 We have moderate confidence in the finding that prior infection provided weaker protection against reinfection by the Omicron variant than was seen in our previous report, but was similarly protective against severe disease (Moderate SOE – Appendix Table B-3). Additional details of the included studies are provided in Supplementary Appendix Table B-1 and B-7.
Asymptomatic and Mildly Symptomatic Infection
The set of cohorts we reviewed in Version 2 focused primarily on protection against reinfection in patients with documented, symptomatic initial infection. Since then, the evidence, while still sparse, suggests that protection against symptomatic reinfection may be slightly less for individuals whose initial infections were asymptomatic or mildly symptomatic.37, 39 Additional details of the included studies are provided in Supplementary Appendix Tables B-1 and B-7.
Role of Antibodies in Protection
The role of antibodies in the degree and duration of protection against reinfection remains uncertain for those infected with SARS-COV-2. Many of the studies included in our reinfection meta-analysis in the previous report defined the “previously infected” cohort as seroconversion soon after the first or second wave of COVID-19. In this situation, seroconversion was associated with significant protection against reinfection.43–50 However, antibody testing provided no additional information over the more widely used RT-PCR test. Two of the studies attempted to look at the correlation between persistence of antibodies and reinfection risk,44, 46 but because antibodies persisted in nearly all participants throughout the followup period, it was not possible to draw conclusions about loss of antibody response and risk of reinfection.
Subsequently, a systematic review and meta-analysis with broader inclusion criteria than ours sought to evaluate the prognostic value of a positive antibody test in settings other than seroconversion after the first or second waves.51 In most of the studies, the criterion for reinfection was a positive RT-PCR test after a baseline seropositive result. Overall, the relative risk of infection was 0.16 (95% CI: 0.14, 0.18) for seropositive individuals versus seronegative individuals. A limitation of this meta-analysis was that all included studies were completed before Delta and Omicron variants became prevalent.
The Chen et al review included 19 studies of unvaccinated individuals, 10 of which were included in our earlier meta-analysis.51 In seven of the other nine studies, which recruited participants between March 2020 and May 2020, seropositivity coincided with very recent infection.52–59 The two remaining studies recruited in June 2020, when new daily cases were relatively low.60, 61 In one of these, a fair-quality controlled cohort study, 3 percent of 4,812 Swiss healthcare workers were seropositive for anti-nucleocapsid SARS-CoV-2 antibodies at baseline.60 The study relied on frequent symptom surveys rather than routine surveillance with a NAAT to detect new infections. During followup (median 7.9 months), 3 of 67 (4.5%) of the seropositive group had symptomatic reinfection versus 547 of 2,645 (21%) of the seronegative cohort, corresponding to approximately 80 percent protection (RR=0.22; 95% CI: 0.07 to 0.66). Seropositivity at baseline also reduced the incidence of symptoms associated with COVID-19, such as loss of smell or limb and muscle pain. Additional details of the included studies are provided in Supplementary Appendix Table B-1.
As noted above, protection against reinfection in the subset of COVID-19 patients who do not develop an antibody response is uncertain. None of the controlled studies we reviewed, including those we excluded, provided a useful estimate of the risk of reinfection after non-seroconversion.
Key Question 3. Duration of Protection
In our previous meta-analysis, the duration of protection from reinfection was at least 6 to 8 months, and as long as 10 months.1 Recent studies suggest that, at least through the Delta wave, protection lasted longer. An update of a U.S. study from the Cleveland Clinic found that protection lasted 13 months (protection was 87.3% for all reinfections and 95% for symptomatic reinfections 390 days after initial infection).36 The SIREN study update found that, in the small subset of individuals who were never vaccinated, previous infection was more than 70 percent effective against symptomatic infection even after a year and in the setting of Delta and Omicron.35, 46
The largest and most recently published cohort study to focus on the duration of protection from previous infection reported on the entire COVID-19 experience in Sweden from March 20, 2020, until October 4, 2021.62 Three cohorts were constructed retrospectively from a national registry: unvaccinated and infected in the first wave versus never infected (Cohort 1), and infected +1 or +2 doses of vaccine versus previously infected and unvaccinated (Cohorts 2 and 3). Cohort 1 included individuals who were infected in the earliest waves, while Cohorts 2 and 3 began after vaccination became available and so had shorter followup times. For Cohort 1, at 3 months of followup, previous infection was associated with a 95 percent reduction in the risk of infection (Adjusted HR 0.05; 95% CI: 0.05, 0.05) and an 87 percent reduction in the risk of hospitalization (Adjusted HR 0.13; 95% CI: 0.11, 0.16). From 9 to 20 months of followup, during which time Delta was prevalent, protection against infection (HR=0.07; 95% CI: 0.06, 0.08) and hospitalization (HR=0.22; 95% CI: 0.15, 0.34) remained high. Protection against reinfection waned substantially for individuals older than 64 years. The study reported no data regarding the Omicron variant. We have low confidence in the finding that for the Delta variant, high levels of protection persisted for at least 13 months, and up to 20 months, in the general population (Low SOE – Appendix Table B-3).
As described above, in the Danish study, protection against Omicron depended on the time between initial infection and the Omicron wave. Before Omicron, this phenomenon was less pronounced or absent. It is worth noting that some form of immune protection persisted, as indicated by the relatively low incidence of serious infection and death. We have low confidence in the finding that for the Omicron variant, the duration of protection from reinfection waned over time (Low SOE – Appendix Table B-3). Additional details of the included studies are provided in Supplementary Appendix Tables B-1 and B-7.
Discussion
The emergence of the Omicron variant, and more recently, its subvariants, has intensified interest in immune escape. These variants have evolved and spread despite high rates of vaccination and previous infection. From a public health perspective, interest in antibodies or another correlate of protection has intensified, because vaccination or a history of infection are not, by themselves, sufficient to assume protection against reinfection.
In our review, a central question is whether an antibody test obtained in everyday practice provides useful information about the future risk of infection. A related but separate question is whether the test reliably confirms or excludes previous infection with SARS-COV-2. Another important question is whether the presence of antibodies is itself the mechanism of protection, or only a marker of protection, which could be mediated, at least in part, by other immune mechanisms.
We did not find evidence about whether an antibody test obtained in everyday practice provides information about protection when it is not known whether or when a prior infection occurred. Based on our updated results, it is highly likely that an antibody test specific for previous infection will be positive if an individual was infected in the previous year, and it is likely that, if antibodies are present, the individual will have some protection at the time of the serological test itself. While the evidence about the persistence of antibodies and the duration of protection is reassuring, particularly for severe infection, the degree of protection over time depends on whether additional variants with new immune evasion properties emerge.
The evidence base for Key Question 1 has several limitations. First, studies did not consistently measure the same antibody types and at the same time points, limiting comparability across studies. Second, reporting varied widely across studies. Longitudinal studies of the antibody response usually did not acknowledge or account for missing data. Third, most studies were small (fewer than 200 participants), leading to lack of precision in estimates. Fourth, cohort studies were limited by high potential for unmeasured confounding, and in some cases, inadequate or lack of statistical adjustment techniques to reduce bias due to confounding. Finally, most studies were conducted in 2020 and 2021, and it is possible that the nature of the antibody response to SARS-CoV-2 infection differs according to new and emerging variants.
Limitations of our review methods include single review at the abstract screening level, which could have led to missing eligible studies, and sequential review for study selection, data abstraction, and quality assessment (in contrast to dual independent review for all steps).
In conclusion, the evidence for a sustained serological response to SARS-CoV-2 infection is considerable, and previous infection provides substantial and sustained protection against future infection. However, this information has limited applicability to clinical practice because of uncertainty about whether infection with Omicron and subsequent variants will protect against reinfection. While understanding population seroprevalence has important public health implications, whether an antibody test obtained in everyday clinical practice provides useful information remains uncertain.
Appendix A. Methods
In our protocol, we argued for an evolving, adaptive, pragmatic approach, in which searches, selection of studies, and synthesis is tailored to address persistent gaps and emerging questions, which we described as a SWATH review. Our findings to date, the evolution of the pandemic, and new developments in a rapidly changing field have enabled us to tailor our searches, selection, and synthesis to address persistent gaps and emerging topics within the scope of the original key questions. As the field of living reviews on COVID-19 topics evolved, it became important to focus successive updates on selected key questions, timed to coincide with the emergence of higher quality evidence for each question. For the key questions and evidence gaps addressed in this final update, only controlled, longitudinal cohorts and case-control designs provide useful estimates of duration of the antibody response, antibody formation in asymptomatic patients or in individuals who are immunocompromised, and the protection afforded by immunity from prior infection. Additional detail is available in our published protocol and report updates. Additional notes on methods for this update are below.
Key Question 1 Methods Notes
KQ 1: What is the prevalence, level, and duration of detectable antibodies to SARS-CoV-2 among adults infected with or recovered from SARS-CoV-2 infection that has been confirmed with reverse transcription-polymerase chain reaction (RT-PCR)?
- Do the level and duration of detectable SARS-CoV-2 antibodies vary by patient characteristics (e.g., age, gender, race/ethnicity, and comorbidities), COVID-19 disease severity, presence of symptoms, time from symptom onset, or the characteristics of the immunoassay (i.e., sensitivity/specificity)?
Data Sources and Searches
A research librarian searched for English language articles in the following databases: Ovid MEDLINE ALL, Elsevier Embase, Cochrane Central Register of Controlled Trials, CINAHL, ClinicalTrials.gov, the World Health Organization Global Literature Database, and COVID19reviews.org. The original database search was from 1 January to 15 December 2020 and the search for this final update was from 16 December 2020 to 8 July 2022. See previous reports for complete search strategy information.
Study Selection
We included studies of adults (aged ≥18 years) with SARS-CoV-2 infection diagnosed via RT-PCR who had serologic testing when the study addressed at least one of the following evidence gaps: (1) IgG antibody duration after SARS-CoV-2 infection at timepoints greater than 12 months among unvaccinated adults, (2) predictors of non-seroconversion at least six weeks after SARS-CoV-2 infection, and (3) whether the antibody response among immunocompromised populations differs from that of immunocompetent populations. Using a sequential process, one reviewer screened abstracts for inclusion and reviewed full texts and a second reviewer verified decisions. Disagreements were resolved through consensus.
Quality Assessment
Two reviewers sequentially assessed methodologic study quality using criteria from the Joanna Briggs Institute Checklist for Cohort Studies (Appendix Table B-2).63
Data Synthesis and Analysis
We performed a qualitative synthesis of the studies using a “best evidence” approach, meaning that we focused on the studies most germane to our outcomes of interest and of the highest methodological quality.64
Grading the Strength of the Body of Evidence
Following the approach outlined in the AHRQ Methods Guide for Comparative Effectiveness Reviews, two reviewers rated the overall Strength of Evidence (SoE) for each outcome to describe our confidence in effect estimates as high, moderate, low, or insufficient evidence. The assessment used criteria that assessed study methodologic quality, how directly studies evaluated the outcomes and populations of interest, precision of effect estimates, consistency of findings across studies, and, when applicable, plausible confounding and strength of association (Appendix Table B-3).65
Key Question 2 and 3 Methods Notes
KQ 2. What is the risk of reinfection by SARS-CoV-2 among adults in the general population compared with the risk of infection in people who have never been infected?
- Does the risk of reinfection vary by factors such as initial antibody levels, patient characteristics, presence of symptoms, or severity of disease?
KQ 3. What is the duration of protection against reinfection among adults following a primary SARS-CoV-2 infection?
- Does the duration of protection vary by factors such as initial antibody levels, patient characteristics, case identification method (e.g., surveillance, symptomatic testing only), presence of symptoms, or COVID-19 severity?
Data Sources and Study Selection
Our previous update, on reinfection, focused on 18 large, controlled studies that estimated protection against reinfection relative to the risk of infection in a concurrent control group of previously uninfected individuals. As it would be difficult to mount new cohort studies of this kind, we anticipated that additional publications from these studies would provide the best evidence regarding protection against reinfection in the Delta and Omicron variant era. We also searched for publications of additional studies through December 31, 2021, using the original search strategies for KQ2, supplemented by searches of articles citing the 18 original studies through July 8, 2022. We excluded studies that did not provide data on the Omicron or Delta variant.66, 67 We also excluded studies that compared vaccinated individuals to previous infection alone if they did not provide a protection estimate for prior infection alone.68
Quality Assessment
As described in our previous update, we used the Joanna Briggs Institute (JBI) cohort study checklist to screen for methodological limitations and excluded studies likely to have serious methodological limitations that would invalidate results. For included studies, we identified potential biases in the following areas: (1) sampling, (2) cohort assignment, (3) case definition, and (4) ascertainment of cases during follow-up, and evaluated the impact on risk of reinfection estimates. We empirically determined that variation in the four areas did not account for the variation in our protection against reinfection estimate.
Data Synthesis and Analysis
In previous update, we conducted a meta-analysis of controlled studies of protection against reinfection, but synthesized evidence on related questions qualitatively. More recent publications from the controlled studies concern protection in the setting of new variants and additional follow-up time. As this makes the estimates from the new studies qualitatively different from those in the original reports, we decided not to add these results to the meta-analysis, but rather summarize all results qualitatively.
Grading the Strength of the Body of Evidence
Two reviewers rated the overall strength of evidence (SoE) for each outcome to describe our confidence in effect estimates as high, moderate, low, or insufficient evidence. The assessment used criteria that assessed study methodologic quality, how directly studies evaluated the outcomes and populations of interest, precision of effect estimates, consistency of findings across studies, and, when applicable, plausible confounding and strength of association (Appendix Table B-3).65
Appendix B. Results
See supplemental Excel files located on the report page: Table B-1. Observational studies examining serology and reinfection for individuals with SARS-CoV-2.
Table B-2Joanna Briggs Institute cohort study checklist assessments
| Outcome | Author, Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG duration >12 months | Dehgani-Mobaraki, 2021 | Y | Y | Y | NA | NA | U | Y | Y | Y | NA | NA |
| Haveri, 2021 | Y | Y | Y | NA | NA | U | Y | Y | Y | NA | NA | |
| Kučinskaitė-Kodzė, 2021 | Y | Y | Y | NA | NA | U | Y | Y | Y | NA | NA | |
| Immuno-compromised populations | Agarwal, 2021 | Y | Y | Y | Y | U | U | Y | Y | N | N | Y |
| Becchetti, 2021 | Y | Y | Y | Y | Y | U | Y | Y | Y | NA | Y | |
| Cattaneo, 2021 | Y | Y | Y | Y | N | U | Y | Y | N | N | Y | |
| Marra, 2020 | U | Y | Y | Y | U | U | Y | Y | Y | NA | Y | |
| Liu, 2021 | Y | Y | Y | Y | Y | U | Y | Y | N | N | Y | |
| Caballero-Marcos, 2021 | Y | Y | Y | Y | Y | U | Y | Y | U | NA | Y | |
| Caballero-Marcos, 2022 | Y | Y | Y | Y | Y | U | Y | Y | U | NA | Y | |
| Fava, 2021 | Y | Y | Y | Y | Y | U | Y | Y | N | Y | Y | |
| Fava, 2022 | Y | Y | Y | Y | Y | U | Y | Y | Y | NA | Y | |
| Softeland, 2021 | N | Y | Y | Y | Y | U | Y | Y | N | Y | Y | |
| Non-seroconversion | Johannesen, 2021 | Y | Y | Y | Y | Y | U | Y | Y | N | N | Y |
| Masia, 2021 | Y | Y | Y | Y | Y | U | Y | Y | Y | NA | Y | |
| Petersen, 2021 | Y | Y | U | Y | Y | U | Y | Y | Y | NA | Y | |
| Staines, 2021 | Y | Y | Y | Y | Y | U | Y | U | Y | NA | Y | |
| Thiruvengadam, 2021 | Y | Y | Y | Y | Y | U | Y | Y | Y | NA | Y | |
| Wei, 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | |
| Risk of reinfection | Altarawneh, 2022* | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y |
| Gazit, 2022* | Y | Y | Y | Y | Y | U | Y | Y | NA | Y | Y | |
| Hall, 2022* | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Kim, 2021* | Y | N | Y | Y | Y | Y | Y | Y | Y | U | Y | |
| Kohler, 2021 | Y | Y | Y | Y | Y | Y | Y | Y | N | NA | Y | |
| Krutikov, 2021* | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Leidi, 2022* | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Michlmayr, 2022* | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Nordstrom, 2022 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Nyberg, 2022 | Y | Y | Y | Y | Y | U | Y | Y | NA | Y | Y |
Abbreviations: IgG = Immunoglobulin G; N= No; NA= Not Applicable; U= Unclear; Y= Yes
- *
New studies that were updates of longitudinal studies included in Version 2.
Criteria (From the Joanna Briggs Institute Checklist for Cohort Studies):
- Were the two groups similar and recruited from the same population?
- Were the exposures measured similarly to assign people to both exposed and unexposed groups?
- Was the exposure measured in a valid and reliable way?
- Were confounding factors identified?
- Were strategies to deal with confounding factors stated?
- Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)?
- Were the outcomes measured in a valid and reliable way?
- Was the follow up time reported and sufficient to be long enough for outcomes to occur?
- Was follow up complete, and if not, were the reasons to loss to follow up described and explored?
- Were strategies to address incomplete follow up utilized?
- Was appropriate statistical analysis used?
Table B-3Strength of evidence ratings
| Finding | N Studies, N total Cohort | Study Limitations | Directness | Precision | Consistency | Plausible Confounding | Strength of Association | Strength of Evidence |
|---|---|---|---|---|---|---|---|---|
| A high proportion of adults maintained detectable levels of IgG antibodies more than 12 months after SARS-CoV-2 infection confirmed by RT-PCR |
N= 445 | Moderate | Direct | Imprecise | Consistent | N/A | N/A | Low |
| Most immunocompromised adults post-solid organ transplant develop IgG antibodies, but the overall proportion of those who develop antibodies is lower compared to immunocompetent adults |
N= 618 | Moderate | Direct | Imprecise | Consistent | Present | N/A | Moderate |
| Most immunocompromised cancer patients develop IgG antibodies, but the overall proportion of those who develop antibodies is lower compared to immunocompetent adults. |
N= 464 | Moderate | Direct | Imprecise | Inconsistent | Present | N/A | Low |
| Most immunocompromised adults living with HIV develop IgG antibodies, but the overall proportion of those who develop antibodies is lower compared to immunocompetent adults. |
1 Study20 N=203 | Moderate | Direct | Imprecise | Consistency unknown (single study) | Present | N/A | Low |
| Having had a mild or asymptomatic primary infection was associated with non-seroconversion. |
N= 11,721 | Moderate | Direct | Imprecise | Inconsistent | Present | Weak | Low |
| Prior infection with the wild-type SARS-CoV-2 or the Alpha variant protected against reinfection by the Delta variant (80-97%). |
N=4,038,444 | Low | Direct | Precise | Consistent | Present | Strong | High |
| Prior infection with the wild-type SARS-CoV-2, Alpha, or Delta variants provided weaker protection against symptomatic reinfection with the Omicron variant (43-56%) but was highly protective against severe disease. |
N=3,453,218 | Low | Direct | Imprecise | Consistent | Present | Strong | Moderate |
| For the Delta variant, high levels of protection persisted for at least 13 months, and up to 20 months, in the general population. |
N=6,316,985 | Moderate | Direct | Precise | Inconsistent | Present | Strong | Low |
| For Omicron, the duration of protection conferred from earlier variants of concern waned over time (51% protection if first infection was within the past 3-6 months and 19% protection if first infection was greater than 12 months earlier) |
N=3,436,672 | Moderate | Direct | Precise | Inconsistent | Present | Strong | Low |
Table B-4Persistence of IgG antibodies for longer than 12 months after infection
| Author, Year | N | Time Since SARS-CoV-2 PCR Positive | Antibody Measured | Number Antibody Positive/Number Tested (%) |
|---|---|---|---|---|
| Dehgani-Mobaraki, 2021 | 32 | 14 months | Anti-S-RBD IgG | 31/32 (97) |
| Haveri, 2021 | 367 | 13 months | Anti-S IgG | 356/367 (97) |
| Kučinskaitė-Kodzė, 2021 | 46 | 13 months | Anti-S IgG | 38/46 (83) |
Table B-5Prevalence of antibodies in different immunocompromised populations
| Population | Author, Year | Ab Type(s) | Immunocompromised: Number Antibody Positive/Number Tested – Timing* | Immunocompetent Comparator: Number Antibody Positive/Number Tested – Timing* |
|---|---|---|---|---|
| Hematology-Oncology | Agarwal, 2021 | IgG anti-N |
65/80 (81%) — 1 month 39/41 (95%) — 3 months 35/37 (95%) — 6 months | 96/100 (96%) HCWs — 92 days+ |
| Cattaneo, 2021 | IgG anti-S |
32/45 (71%) — 1 month^ 27/41 (66%) — 3 months^ 21/31 (68%) — 6 months^ |
16/18 (89%) — 1 month^ 17/18 (94%) — 3 months^ 16/17 (94%) — 6 months^ | |
| IgG anti-N |
36/45 (80%) — 1 month^ 32/41 (78%) — 3 months^ 19/31 (61%) — 6 months^ |
18/18 (100%) — 1 month^ NR/NR (89%) — 3 months^ NR/NR (65%) — 6 months^ | ||
| Marra, 2020 | IgG | 29/33 (88%) — 17 days# (26) | 33/41 (80%) — 17 days# (26) | |
| HIV | Liu, 2021 | IgM | 6/18 (33%) — 15 days | 96/185 (52%) — 15 days |
| IgG |
10/18 (56%) — 15 days NR/NR (12%) — 300 days |
163/185 (88%) — 15 days NR/NR (33%) — 300 days | ||
| Solid Organ Transplant | Becchetti, 2021 | IgG anti-N | 28/35 (80%) — 6.3 weeks (5.6–9.35)~ | 35/35 (100%) — 6.9 (5.35–7.55)~ |
| IgG anti-S | 34/35 (97%) — 6.3 weeks (5.6–9.35)~ | 35/35 (100%) — 6.9 (5.35–7.55)~ | ||
| Neutralizing Ab | 29/35 (83%) — 6.3 weeks (5.6–9.35)~ | 29/29 (100%) — 6.9 (5.35–7.55)~ | ||
| Caballero-Marcos, 2021 | IgG anti-N |
48/62 (77%) — 3 months 45/71 (63%) — 6 months |
62/62 (100%) — 3 months 64/71 (90%) — 6 months | |
| Caballero-Marcos, 2022 | IgG anti-S |
NR/NR (95%) — 3 months NR/NR (90%) — 6 months 30/34 (88%) — 12 months |
NR/NR (97%) — 3 months NR/NR (94%) — 6 months 50/50 (100%) — 12 months | |
| Fava, 2021 | IgG |
20/26 (77%) — 7 days (5-12)# 22/22 (100%) — 23 (20–28)# 22/22 (100%) — 40 (36–44)# |
16/16 (100%) — 6 (4-10)# 12/12 (100%) — 24 (20-26)# 15/15 (100%) — 41 (38–44)# | |
| Fava, 2022 | IgG anti-S | 43/53 (81%) — ≥6 months | 38/48 (79%) — ≥6 months | |
| IgG anti-N | 38/53 (72%) — ≥6 months | 40/48 (83%) — ≥6 months | ||
| Softeland, 2021 | IgG anti-N |
15/22 (68%) — 1 month 22/42 (52%) — 3 months 14/41 (34%) — 6 months 6/20 (30%) — 9 months |
20/23 (87%) — 1 month 48/55 (87%) — 3 months 17/29 (59%) — 6 months 20/41 (49%) — 9 months | |
| IgG anti-S |
21/22 (96%) — 1 month 37/46 (80%) — 3 months 38/45 (84%) — 6 months 23/28 (82%) — 9 months |
21/21 (100%) — 1 month 54/55 (98%) — 3 months 26/27 (96%) — 6 months 21/22 (96%) — 9 months |
- *
Test timing in days post RT-PCR diagnosis
- +
Median days followup
- ^
Post-PCR-negative nasal swab
- #
Median (IQR) days
- ~
Median (IQR) weeks
Table B-6Factors associated with non-seroconversion
| Author, Year | N | Time Since PCR+ or Symptom Onset | Antibody Type Tested: N Seronegative/N Tested (%) | Factors Tested for Association With Non-seroconversion | Statistically Significant Predictors of Non-seroconversion in Multivariable Analysis |
|---|---|---|---|---|---|
| Johannesen, 2021* | 886 | Median 131 days | Total antibody: 21/886 (2%) | BMI (<25, 25-30, >30), presence of symptoms (no or mild symptoms compared to clinically symptomatic and bedridden at home or at hospital) | No or mild symptoms: adjusted HR 6.6 (95% CI, 2.6-17), p<0.001 |
| Masia, 2021* | 132 | Median 6 days and up to 63 days | Total antibody, anti-N IgG, and anti-S IgG: 33/132 (25%) | Age, sex, Charlson comorbidity Index, clinical status (SOFA score, SpO2/FI02 on admission, and bilateral lung infiltrates on x-ray), microbiologic data, biomarkers, outcomes, anti-COVID-19 therapy |
Higher Ct of RT-PCR (indicating low viral load): adjusted OR 1.87 (95% CI, 1.09–3.21; p = .023) Higher Charlson comorbidity index: adjusted OR 1.35 (95% CI, 1.04–1.76; p = .027) Higher SpO2/FIO2 adjusted OR: 1.014 (95% CI, 1.00–1.02; p = .036) Higher neutrophil count: adjusted OR 1.38 (95% CI, 0.96–1.97; p = .081) Lower fibrinogen levels: adjusted OR 0.99 (95% CI, 0.99–1; p = .032) |
| Petersen, 2021 | 2547 | Mean 63.6 days (± 16.0) | Anti-S IgG: 160/2547 (6%) | Age group, sex, race/ethnicity, days since symptom onset, weight (under/normal, overweight, obesity, severe obesity), immunosuppressed, immunosuppressing therapies or medications, sought medical care, hospitalized, number of symptoms |
Immunosuppressive medications (31.9% lacking antibodies vs 6.2% for persons not taking such medications) Non-Hispanic White and Hispanic race/ethnicity (6.4% and 8.6%, respectively, lacking antibodies vs 2.7% for non-Hispanic Black race/ethnicity) Under/normal weight status (9.4% lacking antibodies vs 5.4% for obesity) Fewer symptoms (persons with 0-2 symptoms had higher risk of lacking antibodies compared with persons with 6-9 symptoms) |
| Staines, 2021 | 177 | Median 6 days (3-9) | Anti-S/N IgG:15/177 (8%) | Age (<70 or >70 years), sex, presence of respiratory symptoms, peak CRP levels, co-morbidities | No significant results in multivariable analysis |
| Thiruvengadam, 2021* | 743 | 6-10 weeks | Anti-RBD or anti-N IgG: 170/743 (23%) | Age (30-60 years and >60 years compared to 0-30 years), sex, comorbidities, presence or absence of symptoms at presentation |
Predictors of seroconversion: Older age: adjusted OR 1.03 (95% CI 1.02–1.05) Presence of symptoms at presentation: adjusted OR 3.23 (95% CI 2.01–5.17) |
| Wei, 2021* | 5230** | Median 221 days (14-251) | Anti-S IgG: 595/5230 (11%) | Age, sex, ethnicity, presence of long-term health conditions, working in patient-facing healthcare, Ct value, symptoms, have ≥PCR+ swab in the infection episode, days between first and last positive |
Higher minimum Ct: adjusted OR 1.33 (95% CI 1.31-1.36, p <0.001) Other symptoms compared to no symptoms: Adjusted OR 0.23, 95% CI 0.19-0.29, p<0.001 and adjusted OR classic symptoms compared to no symptoms 0.07, 95% 0.06-0.09, <0.001) Older age (OR NR) Not working in patient-facing healthcare: adjusted: OR working in patient-facing healthcare vs not 0.39, 95% CI 0.18-0.83, p< 0.01) |
- *
New study for this update
- **
Cohort with strong evidence for true-positive PCR: Ct ≤ 32 and ≥2 genes detected
Table B-7Risk of reinfection and duration of protection
| Author, Year | Country | Length of Followup | SARS-CoV-2 Variants | Total N Included in Analysis | Risk of Reinfection, % (95% CI) | Duration of Protection, % (95% CI) |
|---|---|---|---|---|---|---|
| Altarawneh, 2022 | Qatar |
Alpha, Beta, and Delta: ~8 months Omicron: 10 days | Alpha, Beta, Delta, Omicron |
Total: 16,546 Positive cohort: 3,508 Negative cohort: 13,038 |
Effectiveness of prior infection against symptomatic reinfection Alpha: 95.3 (66.0-99.3) Beta: 85.4 (72.4-92.2) Delta: 90.2 (81.9-4.6) Omicron: 61.9 (48.2-72.0) Effectiveness of past infection against severe, critical, or fatal COVID-19 Alpha: 69.4 (–143.6-96.2) Beta: 88.0 (50.7-97.1) Delta: 100 (43.3-100) Omicron: 87.8 (47.5-97.1) |
Effectiveness of prior infection against reinfection: Delta 3-8 months: 93.4 (87.6-96.5) 9-14 months: 91.1 (83.3-95.3) ≥ 15 months: 87.1 (59.4-95.9) Omicron 3-8 months: 64.0 (54.7-71.4) 9-14 months: 47.2 (37.5-55.4) ≥ 15 months: 59.6 (50.7-67.0) |
| Gazit, 2022 | Israel |
Average follow-up duration Unvaccinated: 107 days (3.5 months) Unvaccinated: 164 days (5.5 days) |
Delta (99.93% of all infections during the Delta predominance); Alpha (0.071%) | 107,413 |
Risk of reinfection Previously infected vaccinated adults compared with unvaccinated previously infected adults: HR=0.18 (95% CI 0.15, 0.20) Risk of symptomatic disease Previously infected vaccinated adults compared to unvaccinated previously infected adults: HR=0.24 (95% CI 0.20, 0.29) | NR |
| Hall, 2022 | UK | 12.5 months |
Delta *Unvaccinated previously infected cohort “were predominantly infected in the spring of 2020 and were followed in the period before emergence of the Delta variant” | 6,169 (unvaccinated only) |
All data for unvaccinated only: Crude incidence rate, n reinfections/10,000 person-days at risk F/u ≤1 year after primary infection: 2.25 F/u >1 year after primary infection: 2.40 |
Effectiveness of past infection against reinfection (adjusted for time since primary infection, sex, and race/ethnicity), % (95% CI) ≤1 year after primary infection: 0.86 (0.81-0.89) >1 year after primary infection: 0.69 (0.38- 0.84) Infection acquired immunity waned after 1 year in unvaccinated participants but remained consistently higher than 90% in those who were subsequently vaccinated, even in persons infected more than 18 months previously (data NR) |
| Kim, 2021 | U.S. | Up to 13 months | Delta |
Delta analysis: 325,157 Long-term effectiveness analysis: 152,656 Total: 477,813 |
Delta analysis: Overall protection against reinfection: 85.4% (95% CI, 80.0, 89.3) Protection against symptomatic infection: 88.2% (95% CI, 82.9, 91.9) Protection against asymptomatic infection: 66.6% (95% CI, 40.6, 81.2) Protection by age 0-64 years: 87.9% 65+: 75.1% Hospitalization Previously infected: 14/40 (35.0%) Not previously infected: 587/1494 (39.3%) ICU admission Previously infected: (3/14 [21.4%) Not previously infected: 94/587 (16.0%) Mechanical ventilation Previously infected: 2/14 [14.3%] Not previously infected: 38/587 (6.5%) Death Previously infected: 1/14 (7.1%) Not previously infected: 25/587(4.3%) |
Long-term effectiveness analysis: Overall protection against reinfection: 85.7% (95% CI, 82.2, 88.5) Protection against symptomatic infection: 92.0% (95% CI, 89.1, 94.2) Protection against asymptomatic infection: 52.2% (95% CI, 35.3, 64.7) After 5 months, protection against reinfection exceeded 90% for up to 13 months from initial infection |
| Kohler, 2021 | Switzerland | Median (IQR) 7.9 months (6.7-8.2) | NR | 4812 |
Among 2712 HCW with ≥ 1 SARS-CoV-2 test during follow-up: Seropositive individuals with positive result: 3/67 (4.5%) (1 asymptomatic) Seronegative individuals with positive result: 547/2645 (20.7%) (12 asymptomatic) RR=0.22; 95% CI 0.07, 0.66 | Approximately 80% protection against symptomatic reinfection for 8 months Reinfections all occurred at similar time (January 2021) |
| Krutikov, 2022 | U.K. | 8 months | NR | 2280 | The risk of PCR-positive infection was higher for residents who were antibody-negative at baseline than residents who were antibody-positive at baseline (adjusted HR [a HR] 0·15, 95% CI 0·05–0·44, p=0·0006), and the risk of a PCR-positive infection was also higher for staff who were antibody-negative at baseline compared with staff who were antibody-positive at baseline (aHR 0·39, 0·19–0·82; p=0·012). 1 | Up to 10 months |
| Leidi, 2022 | Switzerland | ~6.75 months (27 weeks) | NR | 10,457 |
Positive SARS-CoV-2 test in seropositive essential workers compared with seronegative essential workers: HR=0.07 (95% CI 0.03, 0.17) Between-group differences, stratified by occupational group: Occupations requiring physical proximity: HR=0.07 (95% CI 0.02, 0.29) Occupations with regular customer contact: HR=0.05 (95% CI 0.01, 0.33) Other essential occupations: HR=0.09 (95% CI 0.02, 0.40) P interaction=0.85 | At least 6 months |
| Michlmayr, 2022 | Denmark | ~17 months | Alpha, Delta, Omicron | 3,430,503 |
Estimated protection against reinfection: 83.5% (95%CI 82.2, 84.6) Protection stratified by age: Any age: 82.9% (95% CI 80.7, 84.9) 65+ years: 72.0% (95% CI 56.1, 82.2) Delta: Protection was strongest among those with a recent primary infection and ranged from 93.3% (95% CI 89.7, 92.7) among cases with a first infection 3-6 months earlier to 71.3% (95% CI 66.8, 75.2) among cases with a first infection over a year earlier Prior infection was highly protective against hospitalization with the Delta variant (estimated protection: 91.3%; 95% CI 83.8, 95.4) with no noticeable evidence among the relatively few hospitalizations of waning over time (p=0.415) Omicron: Individuals with a primary infection in the 3-6 months before the introduction of the Omicron variant were 43.1% (95% CI 41.6, 44.4) less likely to become infected with Omicron than those previously uninfected Protective effect appeared to decline rapidly with time since the primary infection, to 22.2% (19.6, 24.8) or less after 6 months. In the Omicron period, prior infection was also less protective against hospitalization (47.1%; 95%CI 33.1, 58.2) than in the Delta variant period |
Delta: > 1 year Omicron: 6 months |
| Nordstrom, 2022 | Sweden |
Up to 20 months Mean follow-up, days, (SD) Cohort 1: 164 (100) Cohort 2: 52 (38) Cohort 3: 66 (53) | Delta (July 2021 – Oct 2021), Alpha (Feb 2021 – May 2021), Beta, and Gamma | 5,833,003 |
Reinfection After first 3 months: aHR=0.05 (95% CI 0.05, 0.05) After 9-20 months (Delta): aHR=0.07 (95% CI 0.06, 0.08) Hospitalization After first 3 months: aHR=0.13 (95% CI 0.11, 0.16) After 9-20 months (Delta): aHR=0.22 (95% CI 0.15, 0.34) | Up to 20 months |
| Nyberg, 2022 | UK | ~ 2 months | Omicron, Delta |
Total: 1,516,702 Delta: 448,843 Omicron: 1,067,859 |
Reinfection cases: Overall 108940 (7.2%) Delta 102,957 (1.3%) Omicron 102,957 (9.6%) | NR |
Appendix C. Version History
Version 3 – Fills evidence gaps.
Version 2 – Provides an update for findings on Key Questions 2 and 3.
Version 1 – Synthesizes available evidence (through July 2022) on prevalence of anti-SARS-CoV-2 antibodies following SARS-CoV-2 infection.
Afterword
Recognized for excellence in conducting comprehensive systematic reviews, the Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Center (EPC) program is developing a range of rapid evidence products to assist end-users in making specific decisions in a limited timeframe.
The AHRQ EPC Program recognizes that people are struggling with urgent questions on how to address the COVID-19 pandemic. To shorten timelines, reviewers make strategic choices about which review processes to abridge. The adaptations made for expediency may limit the certainty and generalizability of the findings from the review, particularly in areas with a large literature base. Transparent reporting of the methods used and the resulting limitations of the evidence synthesis are extremely important.
If you have comments or have unpublished data to share related to this report, they may be sent by mail to the Task Order Officer named below at: Agency for Healthcare Research and Quality, 5600 Fishers Lane, Rockville, MD 20857, or by email to vog.shh.qrha@cpe and will be considered in the next version of the report.
- Robert Otto Valdez, Ph.D.DirectorAgency for Healthcare Research and Quality
- Craig A. Umscheid M.D., M.S.DirectorEvidence-based Practice Center ProgramCenter for Evidence and Practice ImprovementAgency for Healthcare Research and Quality
- Arlene Bierman, M.D., M.S.DirectorCenter for Evidence and Practice ImprovementAgency for Healthcare Research and Quality
- Christine Chang, M.D., M.P.H.Task Order OfficerEvidence-based Practice Center ProgramCenter for Evidence and Practice Improvement
Report and Appendix References
- 1.
- Helfand M, Fiordalisi C, Wiedrick J, et al. Risk for Reinfection After SARS-CoV-2: A Living, Rapid Review for American College of Physicians Practice Points on the Role of the Antibody Response in Conferring Immunity Following SARS-CoV-2 Infection. Annals of Internal Medicine. 2022;M21–4245. doi: 10.7326/M21-4245. PMID: 35073157. [PMC free article: PMC8791447] [PubMed: 35073157] [CrossRef]
- 2.
- Arkhipova-Jenkins I, Helfand M, Armstrong C, et al. Antibody Response After SARS-CoV-2 Infection and Implications for Immunity : A Rapid Living Review. Annals of Internal Medicine. 2021 Mar 16;174(6):811–21. doi: 10.7326/M20-7547. PMID: 33721517. [PMC free article: PMC8025942] [PubMed: 33721517] [CrossRef]
- 3.
- Clarke KJ, JM, Deng Y, et al,. . Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies — United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep 2022;71:606–608. 2022. doi: 10.15585/mmwr.mm7117e3external. [PMC free article: PMC9098232] [PubMed: 35482574] [CrossRef]
- 4.
- Center for Disease Control and Prevention. Nationwide COVID-19 Infection- and Vaccination-Induced Antibody Seroprevalence (Blood donations). 2022. https://covid
.cdc.gov /covid-data-tracker /#nationwide-blood-donor-seroprevalence. - 5.
- U.S. Food and Drug Administration. Independent Evaluations of COVID-19 Serological Tests. 2022. https://open
.fda.gov /apis/device/covid19serology/ - 6.
- Qaseem A, Yost J, Etxeandia-Ikobaltzeta I, et al. What Is the Antibody Response and Role in Conferring Natural Immunity After SARS-CoV-2 Infection? Rapid, Living Practice Points From the American College of Physicians (Version 1). Annals of internal medicine. 2021;174(6):828–35. doi: 10.7326/M20-7569. PMID: 33721518. [PMC free article: PMC8017476] [PubMed: 33721518] [CrossRef]
- 7.
- Agency for Healthcare Research and Quality. Immunity After COVID-19. Research Protocol for a Living Rapid Review. https:
//effectivehealthcare .ahrq.gov/products /immunity-after-covid/protocol. 2020. doi: https: //effectivehealthcare .ahrq.gov/products /immunity-after-covid/protocol. - 8.
- Hansen K, Caliendo A, Arias C, et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: Serologic testing. Clinical Infectious Diseases. 2020;ciaa1343. doi: 10.1093/cid/ciaa1343. PMID: 32918466. [PMC free article: PMC7543294] [PubMed: 32918466] [CrossRef]
- 9.
- Cervinski MA, Parnas ML, Buxton BP, et al. Current Testing Strategies for SARS-CoV-2 in the United States. Clin Chem. 2021 Jul 6;67(7):935–40. doi: 10.1093/clinchem/hvab046. PMID: 33822921. [PMC free article: PMC8083522] [PubMed: 33822921] [CrossRef]
- 10.
- Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med. 2020 Sep 1;383:1724–34. doi: 10.1056/NEJMoa2026116. PMID: 32871063. [PMC free article: PMC7494247] [PubMed: 32871063] [CrossRef]
- 11.
- Dehgani-Mobaraki P, Zaidi AK, Yadav N, et al. Longitudinal observation of antibody responses for 14 months after SARS-CoV-2 infection. Clinical Immunology. 2021;230:108814. doi: 10.1016/j.clim.2021.108814. PMID: 34343708. [PMC free article: PMC8325385] [PubMed: 34343708] [CrossRef]
- 12.
- Haveri A, Ekström N, Solastie A, et al. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection. medRxiv. 2021, July 16;Preprint. doi: 10.1101/2021.07.13.21260426. [PMC free article: PMC8646652] [PubMed: 34580856] [CrossRef]
- 13.
- Kučinskaitė-Kodzė I, Simanavičius M, Šimaitis A, et al. Persistence of SARS-CoV-2-Specific Antibodies for 13 Months after Infection. Viruses. 2021;13(11):2313. doi: 10.3390/v13112313. PMID: 34835119. [PMC free article: PMC8622371] [PubMed: 34835119] [CrossRef]
- 14.
- Petersen LR, Sami S, Vuong N, et al. Lack of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a large cohort of previously infected persons. Clinical Infectious Diseases. 2021;73(9):e3066–e73. doi: 10.1093/cid/ciaa1685. PMID: 33147319. [PMC free article: PMC7665429] [PubMed: 33147319] [CrossRef]
- 15.
- Shang Y, Liu T, Li J, et al. Factors affecting antibody response to SARS-CoV-2 in patients with severe COVID-19. Journal of Medical Virology. 2020 2020/00;93(2):612–4. doi: 10.1002/jmv.26379. PMID: 33289107. [PubMed: 33289107] [CrossRef]
- 16.
- Wang B, Van Oekelen O, Mouhieddine TH, et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. medRxiv. 2020, June 05 Jun 05;Preprint (Version 1). doi: 10.1101/2020.06.04.20122846. PMID: 32577702. [PMC free article: PMC7359431] [PubMed: 32664919] [CrossRef]
- 17.
- Agarwal A, Baghmar S, Qureshi S, et al. Persistent Antibody Responses to SARS-CoV-2 Infection in Cancer Patients: A Single-Center Retrospective Observational Study. Indian Journal of Medical and Paediatric Oncology. 2021;42(02):123–9. doi: 10.1055/s-0041-1733823. [CrossRef]
- 18.
- Marra A, Generali D, Zagami P, et al. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Annals of Oncology. 2021;32(1):113–9. doi: 10.1016/j.annonc.2020.10.473. PMID: 33098994. [PMC free article: PMC7577226] [PubMed: 33098994] [CrossRef]
- 19.
- Cattaneo C, Cancelli V, Imberti L, et al. Production and persistence of specific antibodies in COVID-19 patients with hematologic malignancies: role of rituximab. Blood Cancer Journal. 2021;11(9):151. doi: 10.1038/s41408-021-00546-9. PMID: 34521813. [PMC free article: PMC8438656] [PubMed: 34521813] [CrossRef]
- 20.
- Liu Y, Xiao Y, Wu S, et al. People living with HIV easily lose their immune response to SARS-CoV-2: result from a cohort of COVID-19 cases in Wuhan, China. BMC Infectious Diseases. 2021;21(1):1029 doi: 10.1186/s12879-021-06723-2. PMID: 34598701. [PMC free article: PMC8485113] [PubMed: 34598701] [CrossRef]
- 21.
- Becchetti C, Broekhoven AG, Dahlqvist G, et al. Humoral response to SARS-CoV-2 infection among liver transplant recipients. Gut. 2022;71(4):746–56. doi: 10.1136/gutjnl-2021-326609. PMID: 34987065. [PubMed: 34987065] [CrossRef]
- 22.
- Caballero-Marcos A, Salcedo M, Alonso-Fernández R, et al. Changes in humoral immune response after SARS-CoV-2 infection in liver transplant recipients compared to immunocompetent patients. American Journal of Transplantation. 2021;21(8):2876–84. doi: 10.1111/ajt.16599. PMID: 33835707. [PMC free article: PMC8251470] [PubMed: 33835707] [CrossRef]
- 23.
- Søfteland JM, Gisslén M, Liljeqvist JÅ, et al. Longevity of anti-spike and anti-nucleocapsid antibodies after COVID-19 in solid organ transplant recipients compared to immunocompetent controls. American Journal of Transplantation. 2021;22(4):1245–52. doi: 10.1111/ajt.16909. PMID: 34860447. [PMC free article: PMC9906230] [PubMed: 34860447] [CrossRef]
- 24.
- Caballero-Marcos A, Citores MJ, Alonso-Fernández R, et al. Decreased Long-Term Severe Acute Respiratory Syndrome Coronavirus 2-Specific Humoral Immunity in Liver Transplantation Recipients 12 Months After Coronavirus Disease 2019. Liver Transplantation. 2022;28(6):1039–50. doi: 10.1002/lt.26389. [PubMed: 34919762] [CrossRef]
- 25.
- Favà A, Donadeu L, Sabé N, et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. American Journal of Transplantation. 2021. doi: 10.1111/ajt.16570. [PMC free article: PMC8251492] [PubMed: 33756051] [CrossRef]
- 26.
- Favà A, Donadeu L, Jouve T, et al. A comprehensive assessment of long-term SARS-CoV-2–specific adaptive immune memory in convalescent COVID-19 Solid Organ Transplant recipients. Kidney international. 2022;101(5):1027–38. doi: 10.1016/j.kint.2021.12.029. [PMC free article: PMC8813192] [PubMed: 35124011] [CrossRef]
- 27.
- Johannesen CK, Rezahosseini O, Gybel-Brask M, et al. Risk Factors for Being Seronegative following SARS-CoV-2 Infection in a Large Cohort of Health Care Workers in Denmark. Microbiology Spectrum. 2021;9(2):e00904–21. doi: 10.1128/Spectrum.00904-21. PMID: 34668738. [PMC free article: PMC8528102] [PubMed: 34668738] [CrossRef]
- 28.
- Masiá M, Telenti G, Fernández M, et al. SARS-CoV-2 seroconversion and viral clearance in patients hospitalized with COVID-19: viral load predicts antibody response. Open Forum Infectious Diseases. 2021;8(2):ofab005. doi: 10.1093/ofid/ofab005. PMID: 33614814. [PMC free article: PMC7881755] [PubMed: 33614814] [CrossRef]
- 29.
- Thiruvengadam R, Chattopadhyay S, Mehdi F, et al. Longitudinal serology of SARS-CoV-2-infected individuals in India: a prospective cohort study. Am J Trop Med Hyg. 2021;105(1):66–72. doi: 10.4269/ajtmh.21-0164. PMID: 34003792. [PMC free article: PMC8274753] [PubMed: 34003792] [CrossRef]
- 30.
- Wei J, Matthews PC, Stoesser N, et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nature communications. 2021;12(1):6250. doi: 10.1038/s41467-021-26479-2. PMID: 34716320. [PMC free article: PMC8556331] [PubMed: 34716320] [CrossRef]
- 31.
- Staines HM, Kirwan DE, Clark DJ, et al. IgG seroconversion and pathophysiology in severe acute respiratory syndrome coronavirus 2 infection. Emerging infectious diseases. 2021;27(1):85–91. doi: 10.3201/eid2701.203074. PMID: 33256890. [PMC free article: PMC7774532] [PubMed: 33256890] [CrossRef]
- 32.
- Lustig Y, Mendelson E, Mandelboim M, et al. Existence of immunological memory response in true sero-negative individuals post COVID-19 molecular diagnosis. Clinical Infectious Diseases. 2022;ciac196. doi: 10.1093/cid/ciac196. PMID: 35271690. [PubMed: 35271690] [CrossRef]
- 33.
- Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. New England Journal of Medicine. 2022 2022 Mar 31;386(13):1288–90. doi: 10.1056/NEJMc2200133. PMID: 35139269. [PMC free article: PMC8849180] [PubMed: 35139269] [CrossRef]
- 34.
- Gazit S, Shlezinger R, Perez G, et al. The Incidence of SARS-CoV-2 Reinfection in Persons With Naturally Acquired Immunity With and Without Subsequent Receipt of a Single Dose of BNT162b2 Vaccine : A Retrospective Cohort Study. Ann Intern Med. 2022 Feb 15:M21–4130. doi: 10.7326/m21-4130. PMID: 35157493. [PMC free article: PMC8855786] [PubMed: 35157493] [CrossRef]
- 35.
- SARS-CoV-2 variants of concern and variants under investigation in England. Technical Briefing 34. UK Health Security Agency; 2022.
- 36.
- Kim P, Gordon SM, Sheehan MM, et al. Duration of SARS-CoV-2 Natural Immunity and Protection against the Delta Variant: A Retrospective Cohort Study. Clin Infect Dis. 2021 Dec 3;ciab999. doi: 10.1093/cid/ciab999. PMID: 34864907. [PMC free article: PMC8690283] [PubMed: 34864907] [CrossRef]
- 37.
- Michlmayr D, Hansen CH, Gubbels SM, et al. Observed Protection Against SARS-CoV-2 Reinfection Following a Primary Infection: A Danish Cohort Study Using Two Years of Nationwide PCR-Test Data. Available at SSRN: 4054807. 2022, March 10;Preprint. doi: 10.2139/ssrn.4054807. [PMC free article: PMC9245510] [PubMed: 35791335] [CrossRef]
- 38.
- Pulliam J, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022 Mar 15:eabn4947. doi: 10.1126/science.abn4947. PMID: 35289632. [PMC free article: PMC8995029] [PubMed: 35289632] [CrossRef]
- 39.
- Ferguson N, Ghani A, Cori A, et al. Report 49: Growth, population distribution and immune escape of Omicron in England Imperial College London. 2021.
- 40.
- Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. New England Journal of Medicine. 2022;386(13):1207–20. doi: 10.1056/NEJMoa2118691. PMID: 35172051. [PMC free article: PMC8908850] [PubMed: 35172051] [CrossRef]
- 41.
- Nyberg T, Ferguson N, Nash S, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. The Lancet. 2022;399(10332):1303–12. doi: 10.1016/S0140-6736(22)00462-7. PMID: 35305296. [PMC free article: PMC8926413] [PubMed: 35305296] [CrossRef]
- 42.
- Krutikov M, Stirrup O, Nacer-Laidi H, et al. Outcomes of SARS-CoV-2 Omicron infection in residents of Long-Term Care. medRxiv. 2022, January 27;Preprint (Version 2). doi: 10.1101/2022.01.21.22269605. [PMC free article: PMC9067940] [PubMed: 35531432] [CrossRef]
- 43.
- Gallais F, Gantner P, Bruel T, et al. Anti-SARS-CoV-2 Antibodies Persist for up to 13 Months and Reduce Risk of Reinfection. medRxiv. 2021, May 17;Preprint (Version 3). doi: 10.1101/2021.05.07.21256823. [CrossRef]
- 44.
- Lumley SF, Rodger G, Constantinides B, et al. An Observational Cohort Study on the Incidence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and B.1.1.7 Variant Infection in Healthcare Workers by Antibody and Vaccination Status. Clinical Infectious Diseases. 2021;74(7):1208–19. doi: 10.1093/cid/ciab608. PMID: 34216472. [PMC free article: PMC8994591] [PubMed: 34216472] [CrossRef]
- 45.
- Krutikov M, Palmer T, Tut G, et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 Long Term Care Facilities (VIVALDI study). Lancet Healthy Longev. 2021;2(6):e362–e70. doi: 10.1016/S2666-7568(21)00093-3. PMID: 34104901. [PMC free article: PMC8175048] [PubMed: 34104901] [CrossRef]
- 46.
- Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021 Apr 17;397(10283):1459–69. doi: 10.1016/s0140-6736(21)00675-9. PMID: 33844963. [PMC free article: PMC8040523] [PubMed: 33844963] [CrossRef]
- 47.
- Jeffery-Smith A, Iyanger N, Williams SV, et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill. 2021 Feb 4;26(5):2100092. doi: 10.2807/1560-7917.ES.2021.26.5.2100092. PMID: 33541486. [PMC free article: PMC7863231] [PubMed: 33541486] [CrossRef]
- 48.
- Abo-Leyah H, Gallant S, Cassidy D, et al. The protective effect of SARS-COV-2 antibodies in Scottish healthcare workers. ERJ Open Res. 2021 06/07/2021;7(2):00080–2021. doi: 10.1183/23120541.00080-2021. PMID: 34104643. [PMC free article: PMC8164012] [PubMed: 34104643] [CrossRef]
- 49.
- Abu-Raddad LJ, Chemaitelly H, Coyle P, et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine. 2021 2021/05/01/;35:100861. doi: 10.1016/j.eclinm.2021.100861. PMID: 33937733. [PMC free article: PMC8079668] [PubMed: 33937733] [CrossRef]
- 50.
- Finch E, Lowe R, Fischinger S, et al. SARS-CoV-2 antibodies protect against reinfection for at least 6 months in a multicentre seroepidemiological workplace cohort. PLoS Biology. 2022;20(2):e3001531. doi: 10.1371/journal.pbio.3001531. PMID: 35143473. [PMC free article: PMC8865659] [PubMed: 35143473] [CrossRef]
- 51.
- Chen Q, Zhu K, Liu X, et al. The Protection of Naturally Acquired Antibodies Against Subsequent SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Emerging Microbes & Infections. 2022 2022/12/31;11(1):793–803. doi: 10.1080/22221751.2022.2046446. PMID: 35195494. [PMC free article: PMC8920404] [PubMed: 35195494] [CrossRef]
- 52.
- Leidi A, Berner A, Dumont R, et al. Occupational risk of SARS-CoV-2 infection and reinfection during the second pandemic surge: a cohort study. Occupational and Environmental Medicine. 2022;79(2):116–9. doi: 10.1136/oemed-2021-107924. PMID: 34880045. [PubMed: 34880045] [CrossRef]
- 53.
- Maier HE, Kuan G, Saborio S, et al. Clinical spectrum of SARS-CoV-2 infection and protection from symptomatic re-infection. Clin Infect Dis. 2021 Aug 19;ciab717. doi: 10.1093/cid/ciab717. PMID: 34411230. [PMC free article: PMC8499752] [PubMed: 34411230] [CrossRef]
- 54.
- Wilkins JT, Hirschhorn LR, Gray EL, et al. Serologic status and SARS-CoV-2 infection over 6 months of follow up in healthcare workers in Chicago: a cohort study. Infection Control & Hospital Epidemiology. 2021 Aug 9:1–9. doi: 10.1017/ice.2021.367. PMID: 34369331. [PMC free article: PMC8438416] [PubMed: 34369331] [CrossRef]
- 55.
- Shields AM, Faustini S, Kristunas C, et al. Longitudinal protection following natural SARS-CoV-2 infection and early vaccine responses: insights from a cohort of community based dental health care professionals. medRxiv. 2021, February 26;Preprint. doi: 10.1101/2021.02.24.21252368. [CrossRef]
- 56.
- Clarke CL, Prendecki M, Dhutia A, et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney International. 2021;99(6):1470–7. doi: 10.1016/j.kint.2021.03.009. PMID: 33774082. [PMC free article: PMC7992297] [PubMed: 33774082] [CrossRef]
- 57.
- Letizia AG, Ge Y, Vangeti S, et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study. The Lancet Respiratory Medicine. 2021;9(7):712–20. doi: 10.1016/S2213-2600(21)00158-2. PMID: 33865504. [PMC free article: PMC8049591] [PubMed: 33865504] [CrossRef]
- 58.
- Havervall S, Ng H, Jernbom Falk A, et al. Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19. Journal of Internal Medicine. 2022;291(1):72–80. doi: 10.1111/joim.13387. PMID: 34459525. [PMC free article: PMC8661920] [PubMed: 34459525] [CrossRef]
- 59.
- Schuler IV CF, Gherasim C, O’Shea K, et al. Mild SARS-CoV-2 illness is not associated with reinfections and provides persistent spike, nucleocapsid, and virus-neutralizing antibodies. Microbiology Spectrum. 2021;9(2):e0008721. doi: 10.1128/Spectrum.00087-21. PMID: 34468184. [PMC free article: PMC8557889] [PubMed: 34468184] [CrossRef]
- 60.
- Kohler P, Güsewell S, Seneghini M, et al. Impact of baseline SARS-CoV-2 antibody status on syndromic surveillance and the risk of subsequent COVID-19—a prospective multicenter cohort study. BMC Medicine. 2021;19(1):270. doi: 10.1186/s12916-021-02144-9. PMID: 34649585. [PMC free article: PMC8514323] [PubMed: 34649585] [CrossRef]
- 61.
- Muir L, Jaffer A, Rees-Spear C, et al. Neutralizing antibody responses after SARS-CoV-2 infection in end-stage kidney disease and protection against reinfection. Kidney International Reports. 2021;6(7):1799–809. doi: 10.1016/j.ekir.2021.03.902. PMID: 33942026. [PMC free article: PMC8081267] [PubMed: 33942026] [CrossRef]
- 62.
- Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. The Lancet Infectious Diseases. 2022. doi: 10.1016/S1473-3099(22)00143-8. PMID: 35366962. [PMC free article: PMC8971363] [PubMed: 35366962] [CrossRef]
- 63.
- Johanna Briggs Institute. Critical Appraisal Checklist for Cohort Studies. 2017. doi: https://jbi
.global/sites /default/files/2019-05 /JBI_Critical_Appraisal-Checklist _for _Cohort_Studies2017_0.pdf. - 64.
- Slavin RE. Best evidence synthesis: an intelligent alternative to meta-analysis. Journal of clinical epidemiology. 1995;48(1):9–18. doi: 10.1016/0895-4356(94)00097-a. PMID: 7853053. [PubMed: 7853053] [CrossRef]
- 65.
- Berkman ND, Lohr KN, Ansari M, et al. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the Agency for Healthcare Research and Quality: an update. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); November 18, 2013.PMID: 24404627. [PubMed: 24404627]
- 66.
- Slezak J, Bruxvoort K, Fischer H, et al. Rate and severity of suspected SARS-Cov-2 reinfection in a cohort of PCR-positive COVID-19 patients. Clinical Microbiology and Infection. 2021;27(12):1860. e7-.e10. doi: 10.1016/j.cmi.2021.07.030. [PMC free article: PMC8373524] [PubMed: 34419576] [CrossRef]
- 67.
- Chemaitelly H, Bertollini R, Abu-Raddad LJ. Efficacy of Natural Immunity against SARS-CoV-2 Reinfection with the Beta Variant. New England Journal of Medicine. 2021;385(27):2585–6. doi: 10.1056/NEJMc2110300. [PMC free article: PMC8693689] [PubMed: 34910864] [CrossRef]
- 68.
- Shrestha NK, Burke PC, Nowacki AS, et al. Necessity of Coronavirus Disease 2019 (COVID-19) Vaccination in Persons Who Have Already Had COVID-19. Clin Inf Dis. 2022. doi: 10.1093/cid/ciac022. [PMC free article: PMC8807217] [PubMed: 35028662] [CrossRef]
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- CDC
Centers for Disease Control and Prevention
- COVID-19
Coronavirus disease 2019
- EHR
Electronic health records
- FDA
Food and Drug Administration
- HIPPA
Health Insurance Portability and Accountability Act
- IgG
Immunoglobulin G
- JBI
Joanna Briggs Institute
- PCR
Polymerase Chain Reaction
- RT-PCR
Reverse transcription polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SIREN SARS-CoV-2
Immunity and Reinfection Evaluation
- SoE
Strength of Evidence
- WHO
World Health Organization
Version 3.0
Suggested citation:
Holmer HK, Mackey K, Fiordalisi CV, Armstrong C, Gean E, Arkhipova-Jenkins I, Helfand M. Antibody Response Following SARS-CoV-2 Infection and Implications for Immunity: Final Update of a Rapid, Living Review. (Prepared by the Scientific Resource Center under Contract No. 290-2017-0003). AHRQ Publication No. 22(23)-EHC037. Rockville, MD: Agency for Healthcare Research and Quality. October 2022. Posted final reports are located on the Effective Health Care Program search page. DOI: https://doi.org.10.23970/AHRQEPCCOVIDIMMUNITY3.
Disclaimers: This report is based on research conducted by the Scientific Resource Center for the AHRQ Effective Health Care program under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2017-0003). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
None of the investigators have any affiliations or financial involvement that conflicts with the material presented in this report.
The information in this report is intended to help healthcare decision makers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information, i.e., in the context of available resources and circumstances presented by individual patients.
This report is made available to the public under the terms of a licensing agreement between the author and the Agency for Healthcare Research and Quality. This report may be used and reprinted without permission except those copyrighted materials that are clearly noted in the report. Further reproduction of those copyrighted materials is prohibited without the express permission of copyright holders.
AHRQ or U.S. Department of Health and Human Services endorsement of any derivative products that may be developed from this report, such as clinical practice guidelines, other quality enhancement tools, or reimbursement or coverage policies, may not be stated or implied.
Role of the Funding Source: Funding for this review was provided by AHRQ. The funding source assigned the topic and provided comments on draft manuscripts, but was not involved in data collection, analysis, manuscript preparation, or addressing comments from reviewers.
AHRQ appreciates appropriate acknowledgment and citation of its work. Suggested language for acknowledgment: This work was based on an evidence report, Antibody Response Following SARS-CoV-2 Infection and Implications for Immunity: Final Update of a Rapid, Living Review, by the Scientific Resource Center for the Evidence-based Practice Center Program at the Agency for Healthcare Research and Quality (AHRQ).
- Effectiveness of Telehealth for Women's Preventive Services
- Use of Telehealth During the COVID-19 Era
- Masks for Prevention of COVID-19 in Community and Healthcare Settings: Surveillance Report
- Resource Allocation and Pandemic Response: An Evidence Synthesis To Inform Decision Making
- No-Touch Modalities for Disinfecting Patient Rooms in Acute Care Settings
- The Evidence Base for Telehealth: Reassurance in the Face of Rapid Expansion During the COVID-19 Pandemic
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Antibody Response Following SARS-CoV-2 Infection and Implications for Immunity: ...Antibody Response Following SARS-CoV-2 Infection and Implications for Immunity: Final Update of a Rapid, Living Review
- Mus musculus poly (ADP-ribose) polymerase family, member 3 (Parp3), transcript v...Mus musculus poly (ADP-ribose) polymerase family, member 3 (Parp3), transcript variant 2, mRNAgi|902967392|ref|NM_145619.3|Nucleotide
- Fomes fomentarius CIRM-BRFM 1821 transcriptome - 1821_RNAFomes fomentarius CIRM-BRFM 1821 transcriptome - 1821_RNAbiosample
Your browsing activity is empty.
Activity recording is turned off.
See more...