Abbreviations
- DCE
dynamic contrast-enhanced
- DRE
digital rectal examination
- DWI
diffusion-weighted imaging
- EQ-5D-5L
EuroQol 5-Dimensions 5-Levels Self-Report Questionnaire
- HTA
health technology assessment
- ICER
incremental cost-effectiveness ratio
- LY
life-year
- mpMRI
multi-parametric magnetic resonance imaging
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- NMA
network meta-analysis
- PI-RADS
Prostate Imaging and Reported Data System
- PSA
prostate-specific antigen
- QALY
quality-adjusted life-year
- RCT
randomized controlled trial
- SR
systematic review
- T2W
T2-weighted
- TRUS
transrectal ultrasound
- US
ultrasound
Context and Policy Issues
Prostate cancer is one of the most commonly diagnosed cancers in Canada and an estimated 21,300 Canadian patients were diagnosed with prostate cancer in 2017.1 Five-year net survival ranges widely depending on the stage at which the cancer is diagnosed, with survival for early stage prostate cancer at nearly 100% and survival for prostate cancer with distant metastases closer to 30%.1
Prostate-specific antigen (PSA) screening detects prostate cancer, but most detected cancers tend to be low-risk cancers that left untreated do not cause symptoms or death.2 Patients with elevated levels of PSA or an abnormality on digital rectal examination (DRE) undergo biopsy of the prostate, with pathology results used to diagnose prostate cancer.2,3 The Gleason grading system is used to assess prognosis based on tissue patterns observed in the biopsy samples, with numbers from 1 to 5 associated with each pattern.4 A higher number is associated with faster growth and higher likelihood of spread for a cancer.4 The Gleason score is made up of one number for the predominant histological pattern and a second number for the next most common pattern.4
Prostate biopsy is performed under guidance from transrectal ultrasound (TRUS) and 10 to 12 tissue samples (referred to as cores) are typically sampled from the prostate with a needle.4,5 If no cancer is detected by the biopsy and PSA levels continue to rise or if the results are equivocal, a repeat biopsy may be performed.5 However, the benefits of prostate biopsy must be weighed against the potential harms, such as hematuria, urinary tract infection, and sepsis.2,6
Magnetic resonance imaging (MRI) has shown promise in detecting prostate cancers and one or more functional imaging sequences, such as dynamic contrast-enhanced (DCE) imaging or diffusion weighted imaging (DWI), can be combined with T2-weighted (T2W) imaging to perform multi-parametric MRI (mpMRI).4,6 The use of mpMRI prior to performing TRUS-guided prostate biopsy could potentially reduce the number of unnecessary biopsies7 and increase the accuracy of biopsies that are performed.4,8
Information on suspicious lesion locations from MRI exams can be used to target TRUS-guided prostate biopsies. With cognitive targeting, sampling locations are determined based on a review of the previously obtained magnetic resonance (MR) images and no specialized equipment is needed.4,8 Alternatively, MR images with delineated lesions can be fused with real-time TRUS images to guide needle placement.4,8 This method requires the use of MRI-US fusion navigation systems.8
This report aims to summarize the evidence regarding the clinical effectiveness and cost-effectiveness of MRI prior to TRUS-guided prostate biopsy for the diagnosis of prostate cancer.
Research Questions
What is the clinical effectiveness of magnetic resonance imaging prior to transrectal ultrasound guided prostate biopsy for the diagnosis of prostate cancer?
What is the cost-effectiveness of magnetic resonance imaging prior to transrectal ultrasound guided prostate biopsy for the diagnosis of prostate cancer?
Key Findings
Six systematic reviews and three randomized controlled trials examining the clinical evidence of MRI prior to TRUS guided prostate biopsy for the diagnosis of prostate cancer were included in this review. There was no evidence that there was a significant difference in overall prostate cancer detection rate between a diagnostic strategy employing magnetic resonance imaging (MRI) followed by targeted transrectal US-guided (TRUS-guided) biopsy and a diagnostic strategy consisting of TRUS-guided biopsy alone. MRI-US fusion targeted TRUS-guided biopsy was had higher detection rates of patients with clinically significant prostate cancer versus standard TRUS-guided biopsy. Lower detection rates of patients with clinically insignificant prostate cancer were found for MRI-US fusion targeted TRUS-guided biopsy versus standard TRUS-guided biopsy, but not in patients undergoing repeat prostate biopsy. No clinical benefits were found for cognitive targeted TRUS-guided biopsy over standard TRUS-guided biopsy.
Four economic evaluations, which included one Canadian study, and two health technology assessments with economic evaluations were included in this review and the results suggested that including MRI before TRUS-guided biopsy was more cost-effective than standard TRUS-guided biopsy alone. MRI-US fusion targeted TRUS-guided biopsy was cost-effective in initial biopsy and repeat biopsy patients and cognitive targeted TRUS-guided biopsy was cost-effective in initial biopsy patients.
The evidence for clinical effectiveness was limited by the lack of reporting of long-term outcomes and safety outcomes, the varying quality of the systematic reviews, the heterogeneity in study characteristics, and the limited number of randomized studies. These limitations hampered the economic evaluations as the clinical inputs relied on assumptions regarding the accuracy of the diagnostic strategies and the long-term consequences of misdiagnosing patients.
Methods
Literature Search Methods
A limited literature search was conducted on key resources including PubMed, The Cochrane Library, University of York Centre for Reviews and Dissemination databases and a focused Internet search. Methodological filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, randomized controlled trials and economic studies. The search was limited to English language documents published between January 1, 2013 and August 2, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in or if they were published before 2013. Relevant systematic reviews (SRs) were excluded if all of the primary studies were reported in one or more of the other relevant SRs. Relevant randomized controlled trials (RCTs) were excluded if they were reported in an included SR. Primary studies and SRs were excluded if their populations included patients already diagnosed with prostate cancer and on active surveillance without separate reporting for these groups. Primary studies and SRs were excluded if the intervention included in-bore MRI-guided biopsy without separate reporting of TRUS-guided biopsy results.
Critical Appraisal of Individual Studies
All studies were critically appraised by one reviewer. The included SRs were critically appraised using AMSTAR II,9 network meta-analysis (NMA) was critically appraised using the ISPOR questionnaire,10 RCTs were critically appraised using the Downs and Black checklist,11 and economic studies were assessed using the Drummond checklist.12 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
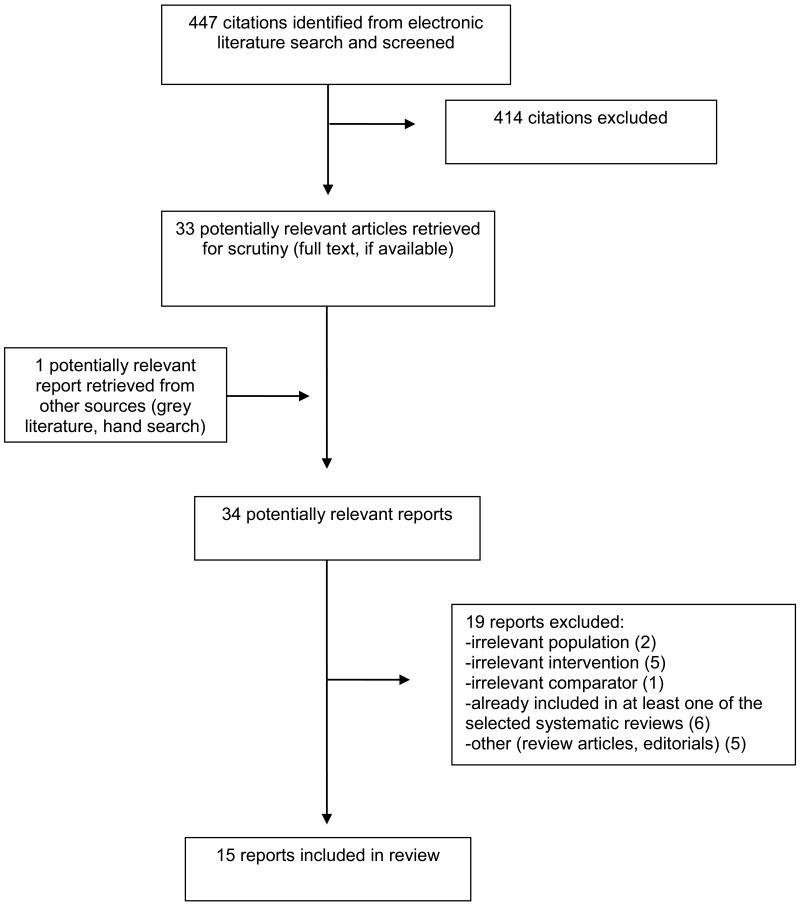
A total of 447 citations were identified in the literature search. Following screening of title and abstracts, 414 citations were excluded and 33 potentially relevant reports from the electronic search were retrieved for full-text review. One potentially relevant publication was retrieve from the grey literature search for full text review. Of these potentially relevant articles, 19 publications were excluded for various reasons, and 15 publications met the inclusion criteria and were included in this report. These comprised two health technology assessments (HTAs),6,13 six SRs,14–19 three RCTs,20–22 and four economic evaluations.23–26
Appendix 1 presents the PRISMA27 flowchart of the study selection.
Summary of Study Characteristics
Additional details regarding the characteristics of included publications are provided in Appendix 2.
Study Design
Two HTAs, both funded by the National Institute for Health Research HTA programme in the UK, were identified regarding clinical effectiveness or cost-effectiveness of MRI prior to TRUS-guided prostate biopsy for the diagnosis of prostate cancer. One HTA included one RCT and included one economic evaluation6 while the other HTA included one SR and one economic evaluation.13 The economic evaluations, but not the RCT and SR, met the selection criteria and they are described alongside the other economic evaluations included in this report.
Six SRs were identified regarding clinical effectiveness. The range of publication years was from 2014 to 2018, with search dates provided for five of the SRs. Four SRs searched from database inception to the search date, with search dates ranging from May 2014 to July 2018.15,16,18,19 One SR searched from January 2004 to February 201514 and one SR did not report the date range for the literature search.17 One SR, which had an NMA, included RCTs only.18 Two SRs included only non-randomized studies14,15 and three SRs included both RCTs and non-randomized studies.16,17,19 Three SRs specified that for non-randomized studies, both targeted and systematic biopsies had to be performed within each patient.15–17 One SR included both prospective and retrospective non-randomized studies,16 one SR included only prospective non-randomized studies,14 and three SRs did not report whether the primary studies were prospective or retrospective.15,17,19
in Appendix 5 provides a detailed description of the overlap in the primary studies between the SRs.
Three RCTs were identified that were not already reported in one of the SRs, with publication years ranging from 2015 to 2018.20–22 One RCT was a multi-centre RCT20 while the other two were single centre RCTs.21,22
Four economic evaluations were identified which assessed the cost-effectiveness of MRI prior to TRUS-guided prostate biopsy, yielding a total of six economic evaluations when combined with the two HTAs. Five studies used cost-utility analysis6,13,23,25,26 and one study reported costs and clinical benefits separately.24 The economic evaluations from the two HTAs were from the UK National Health Service perspective,6,13 one evaluation was from the Canadian provincial public health system perspective,23 and three evaluations did not state the perspective.24–26 The time horizon used was lifetime in one study,25 18 years in one study,26 five, 10, 15, and 20 years in one study,23 and 30 years in one of the HTAs.13 One study modelled the intervention and its comparator without any follow-up24 and one of the HTAs used this approach in its short-term model.6 The same HTA also evaluated a long-term model with a lifetime time horizon.6 Clinical inputs were derived from the literature in all of the studies, with one of the HTAs conducting an SR to inform some of the inputs.13 Sources of cost inputs included the UK National Health Service Reference Costs and National Tariff Payment System for the two HTAs,6,13 US Medicare or Medicaid reimbursement for two studies,24,25 the Québec Régie de l’assurance maladie and Ministère de la Santé et des Services Sociaux lists and institutional pharmacy records for one study,23 and hospital departments in the authors’ institution for one study.26 Three studies also included cost inputs from the literature.13,24,25 A decision tree model was used for the short-term model in one of the HTAs6 and two other studies.24,25 A Markov model was used in three studies,13,23,26 one HTA,13 and in the long-term model in the other HTA.6
Country of Origin
The first author was from China in three SRs,16,18,19 the Netherlands in two SRs,14,15 and France in one SR.17 One RCT was conducted at centres in Europe and North America,20 one RCT was conducted in Finland,22 and the third RCT was conducted in Italy.21 Of the economic evaluations, the two from the HTAs were conducted in the UK,6,13 two were conducted in the US,24,25 one was conducted in Canada,23 and one was conducted in the Netherlands.26
Patient Population
All six of the SRs included both patients undergoing prostate biopsy for the first time (initial biopsy or biopsy naïve patients) and patients who had a previous negative biopsy (repeat biopsy patients). Two of the SRs15,16 reported only on patients who had at least one suspicious lesion on multi-parametric magnetic resonance imaging (mpMRI) prior to biopsy.
The three RCTs included biopsy naïve patients and in two of the RCTs,20,22 mean age ranged from 62 to 64.5 years. In one RCT, median PSA level (concentration of PSA in blood serum) ranged from 6.50 ng/mL to 6.75 ng/mL20 and in another RCT, mean PSA level ranged from 6.1 ng/mL to 6.2 ng/mL.22 Baseline characteristics were not provided one of the RCTs, though PSA level had to be above 4 ng/mL for inclusion in the study.21 Settings for the procedures were not specified, but all study centres in the RCTs were affiliated with a university or hospital.
Four economic evaluations were conducted in a biopsy naïve population6,23,25,26 and two in a repeat biopsy population.13,24 The base case in three studies specified patients’ age, which was 65 years in one study,25 a range of 60 to 65 years in another study,23, and either 60 years or 70 years in the third study.13 In the other studies, patients were defined by cancer category6 or cancer prevalence (25%).24,26
Interventions and Comparators
All of the SRs compared mpMRI and TRUS-guided biopsy with a standard TRUS-guided biopsy. The MRI arm specifically included MRI-US fusion targeted TRUS-guided biopsy in five SRs14,16–19. In three of these SRs, results were also reported for the comparison of combined MRI-US fusion targeted and standard TRUS-guided biopsy versus standard TRUS-guided biopsy alone.16–18 In one SR, cognitive targeted TRUS-guided biopsy was also considered as an intervention, alone or in combination with standard TRUS-guided biopsy.17 Standard TRUS-guided biopsy, was specified as involving 10 to 14 cores in one SR16 and 8 to 12 cores in another.15 Two SRs specified that the biopsy had to be performed using the transrectal approach,15,16 one SR included studies using either the transrectal or transperineal approach,14 and three SRs did not specify the approach.17–19
In the multi-centre RCT, the intervention was mpMRI with T2-weighted (T2W) imaging, diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) imaging followed by cognitive or MRI-US fusion targeted TRUS-guided biopsy in patients with at least one suspicious lesion on MRI.20 The approach was transrectal or transperineal and patients with a negative MRI (no suspicious lesions) did not undergo biopsy. In the control group, all patients underwent 10- to 12-core standard TRUS-guided biopsy. In one single-centre RCT, the intervention was mpMRI with DCE imaging, DWI, and apparent diffusion coefficient mapping followed by combined cognitive targeted and standard TRUS-guided biopsy.22 In the targeted biopsy, up to 3 suspicious lesions were sampled with up to 4 cores taken per lesion. The comparator was standard 10- to 12-core TRUS-guided biopsy alone. In the other single-centre RCT, the intervention was mpMRI with T2W imaging, DWI, and DCE imaging followed by combined cognitive targeted and 14-core standard TRUS-guided biopsy.21 One lesion was chosen based on the MRI exam for the targeted biopsy and 2 cores were taken from it. The control group underwent 14-core TRUS-guided standard biopsy alone.
In the economic evaluations, the intervention in two studies was mpMRI followed by MRI-US fusion targeted TRUS-guided biopsy for suspicious lesions and no biopsy in the absence of suspicious lesions.24,26 In one study, different scenarios were investigated where the intervention was MRI followed by either cognitive or MRI-US fusion targeted TRUS-guided biopsy for detected lesions and either standard TRUS-guided biopsy or no biopsy performed in the absences of detected lesions.25 In another study, the intervention was MRI followed by combined cognitive targeted and standard TRUS-guided biopsy.23 The economic evaluation in one of the HTAs assessed interventions involving permutations of mpMRI, TRUS-guided standard biopsy, and template mapping biopsy.6 This report summarizes the results for interventions where mpMRI was performed ahead of TRUS-guided biopsy either as an initial strategy or following a negative TRUS-guided biopsy. In the initial biopsy setting, TRUS-guided biopsy could be performed for all cancers or only for cancer that were considered clinically significant on mpMRI. The intervention in the other HTA was MRI (with various sequences assessed) followed by combined MRI-US fusion targeted and standard TRUS-guided biopsy in patients with suspicious lesions and no biopsy in the absence of suspicious lesions.13 In all the economic evaluations, the comparator was standard TRUS-guided biopsy. Extended-cores systematic TRUS-guided biopsy was specified for one of the HTAs.13
Outcomes
One SR reported the proportion of patients with any detected prostate cancer.17 One SR reported proportions of patients with any detected prostate cancer as well as with clinically significant detected prostate cancer.14 Four SRs reported proportions of patients in which any cancer, clinically significant cancer, or clinically insignificant cancer was detected.15,16,18,19 Criteria for clinically significant caner were not specified in the SRs, except for one SR which defined it as a minimum Gleason score of 7.16 The presence of cancer was determined by pathology results from the biopsy cores.
One RCT reported the proportion of patients with clinically significant prostate cancer as the primary outcome with proportions of patients who had clinically insignificant prostate cancer, avoided biopsies, health-related quality of life measured on the EuroQol 5-Dimensions 5-Levels Self-Report Questionnaire (EQ-5D-5L), and adverse events reported as secondary outcomes.20 One RCT reported the proportion of patients with prostate cancer as the primary outcome and proportions of patients with clinically significant prostate cancer, clinically insignificant prostate cancer, and complications reported as secondary outcomes. The third RCT reported the numbers of patients with prostate cancer and with prostate cancer with a Gleason score of at least 6.
Costs and quality-adjusted life-years (QALYs) were reported in five of the economic evaluations.6,13,23,25,26 Of these economic evaluations, net health benefit (NHB) was reported in one study25 and incremental cost-effectiveness ratio (ICER) was reported in one HTA.13 One economic evaluation reported costs, number of biopsies avoided, number of patients with detected prostate cancer, and the number of missed cancers.24
Summary of Critical Appraisal
Systematic Reviews
Five SRs14–17,19 were critically appraised using AMSTAR 2.9 Strengths common to the five SRs14–17,19 were that the PICO components were described in the research questions and inclusion criteria for the review, keywords for the literature search were provided and the included studies were described in adequate detail. In four of the SRs,14–16,19 the literature search included at least two databases, study selection was performed in duplicate, reasons for excluding studies were given, and the review authors reported no conflicts of interest. These strengths indicate that the SR authors used appropriate methods to conduct systematic literature searches that were relevant to the selection criteria for this report. Risk of bias was assessed in four SRs14,15,18,19, with QUADAS, QUADAS-2, or the Cochrane Risk of Bias Tool used. Reasons for high risk of bias were discussion in two SRs,14,19 but potential impacts were not discussed in any of the SRs. In the three SRs that conducted meta-analyses,15,16,19 appropriate methods for statistical combination of results were used and no publications bias was found when Egger’s test was used. Limitations common to the five SRs14–17,19 were the lack of clarity regarding whether review methods were established prior to the conduct of the review, trial registries and grey literature were not searched, data extraction was not performed in duplicate, and sources of funding were not reported for the primary studies. It is unclear whether the systematic literature searches identified all relevant primary studies, post hoc analyses were conducted, or if there was potential bias from sources of funding. Sources of heterogeneity and their impact were not discussed in four of the SRs.15–17,19 Potential impacts on results from risk of bias15 or heterogeneity14,15,19 were discussed in only some of the SRs, making it difficult to assess the internal validity of the results.
The SR with the NMA18 was critically appraised using the ISPOR questionnaire.10 The NMA included outcomes relevant to this report, though the population was not clearly defined. The researchers attempted to include all relevant studies and the studies were connected within one evidence network. The risk of bias assessment identified a high risk of bias for allocation concealment and high or unclear risk of bias for random sequence generation in the included studies. While appropriate methods were used to conduct the NMA, consistency between direct and indirect evidence was not assessed for all relevant pairwise comparisons, no rationale was provided for the use of a random-effects model, and assumptions about heterogeneity in the random-effects model and heterogeneity in the results were not discussed. Lack of reporting of patient characteristics and study characteristics for the included studies meant that it was unclear whether there were systematic differences in effect modifiers across comparisons and subgroup or meta-regression analyses were not conducted. The results were reported appropriately as odds ratios and rank probabilities, but the conclusions did not reflect the results and the limitations of the NMA.
Randomized Controlled Trials
Strengths common to all three RCTs were that the study objective, inclusion and exclusion criteria, and interventions being compared were described clearly. In two of the RCTs, the main outcomes, potential confounders, and main findings were also clearly described.20,22 Patient characteristics were not reported in one RCT.21 External validity was another common strength in the RCTs, since patients were representative of the populations from which they were recruited and staff, places, and facility were representative of the treatment received by the source population. Regarding internal validity, patients and investigators were not blinded to intervention allocation, though investigators were blinded to the MRI results of until the systematic biopsies were completed in the intervention arm.22 It was not clear in any of the RCTs whether outcomes assessors, namely pathologists, were blinded to allocation. Patients all three RCTs were randomized to the groups and patients lost to follow-up were accounted for in two RCTs.20,22 In the latter two RCTs, the reasons for patients not undergoing the assigned intervention differed between groups, but these patients made up less than 10% of each group.20,22 Statistical tests were conducted in two of the RCTs20,22 and were appropriate with any post hoc analyses clearly indicated. One of the two RCTs had sufficient power to demonstrate non-inferiority,20 while it was unclear whether the other RCT met the sample size criteria.22 In the two RCTs with statistical analyses20,22, the main issue was the unclear risk of bias from lack of blinding of investigators and potential lack of blinding of outcomes assessors to intervention allocation. In the other RCT,21 important information was not reported (e.g., patient characteristics, disposition and adverse events) and the same issue with blinding as for the other RCTs was also present.
Economic Evaluations
There were no limitations identified in the economic evaluations in the two HTAs,6,13 though costs and QALYs were not reported in aggregated form and incremental analysis was not reported for all assessed diagnostic strategies in one HTA.6 In the other four economic evaluations,23–26 common strengths were that the research questions, its economic importance, and the rationale for choosing the interventions were stated, the alternatives being compared were clearly described, the form of economic evaluation was stated and justified, sources for effectiveness data and health utilities were stated, details of the model were given, discounting was used for long-term models, currencies were stated, and the conclusions followed from the results with appropriate limitations identified. The time horizon was stated and outcomes were reported in disaggregated and aggregated form along with incremental analysis in three of the studies.23,25,26 A limitation common to the four economic studies23–26 was that sources of clinical data were cited, but details of the sources were not given. In three of the four studies, the viewpoint of the analysis was not clearly stated.24–26 Details on the interventions were not provided in two studies25,26 and sensitivity analyses were not clearly described in two studies.23,26
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Summary of Findings
Appendix 4 presents a table of the main study findings and authors’ conclusions. In SRs using narrative evidence synthesis, results are only presented for primary studies not already reported in the other SRs. in Appendix 5 provides a detailed description of the overlap in the primary studies between the SRs.
Clinical Effectiveness of MRI Prior to TRUS-Guided Prostate Biopsy
Detection of any Prostate Cancer
In the four SRs with meta-analysis15,16,18,19 and three RCTs,20–22 no significant differences in the proportion of patients with any prostate cancer detected were found between either of the targeted biopsy approaches alone (MRI-US fusion or cognitive TRUS-guided biopsy) and standard TRUS-guided biopsy. When the combination of targeted and standard TRUS-guided biopsy was compared with standard TRUS-guided biopsy alone, one SR16 with meta-analysis found that combined fusion targeted and standard biopsy was superior to standard biopsy alone for detection of any prostate cancer (regardless of whether it is an initial or repeat biopsy), while one SR with an NMA18 and two RCTs21,22 did not find significant differences between the combined targeted and standard biopsy approach and standard biopsy in initial biopsy patients.
In the two SRs that synthesized evidence narratively,14,17 the studies not already included in the other SRs showed numerically higher detection rates of cancer14,17 with fusion targeted biopsy compared with standard TRUS biopsy in three studies. In one of the SRs using narrative synthesis,17 one study showed a numerically larger proportion of patients with prostate cancer detected using cognitive targeted biopsy or combined cognitive and standard biopsy (P value not reported), one study showed a statistically significantly larger proportion of patients with prostate cancer detected using combined cognitive targeted and standard biopsy, and one study showed a numerically smaller proportion of patients with prostate cancer using cognitive targeted biopsy versus 26- to 32-core systematic biopsy (P value not reported).
Detection of Clinically Significant Prostate Cancer
Clinically significant prostate cancers were detected in larger proportions of patients undergoing MRI-US fusion targeted biopsy in three15,16,19 of the four SRs with meta-analysis. Two of the SRs15,19 included a mix of initial and repeat biopsy patients while the other SR16 analyzed these populations both separately and together. In two of the SRs,15,16 only patients who had at least one suspicious lesion on MRI were included. No difference between fusion targeted biopsy (alone or in combination with standard biopsy) and standard biopsy was found in the NMA for this outcome.18 One SR also compared cognitive targeted TRUS-guided biopsy with standard TRUS-guided biopsy for the detection of significant cancer and found no significant difference between the interventions.15 In one SR that synthesized evidence narratively,17 targeted (fusion or cognitive) biopsy detected clinically significant prostate cancer in a numerically higher proportion of patients versus standard biopsy in the three studies in which the outcome was reported.
Two RCTs compared combined cognitive targeted and random TRUS-guided biopsy with random biopsy alone in biopsy naïve patients.21,22 Combined targeted and random biopsy detected clinically significant prostate cancer in more patients when compared with random biopsy alone in one RCT21 (statistical testing not performed) while the other RCT did not find a significant difference between groups.22 One RCT20 allowed investigators to use either MRI-fusion or cognitive targeted TRUS-guided biopsy in the mpMRI group, which was compared against a standard TRUS-guided biopsy group in biopsy naïve patients. Clinically significant cancer was detected in a significantly larger proportion of patients who underwent mpMRI with targeted biopsy for suspicious lesions than who underwent standard TRUS biopsy.
Detection of Clinically Insignificant Prostate Cancer
MRI-US fusion targeted biopsy detected significantly smaller proportions of patients with clinically insignificant prostate cancer than standard biopsy in all patients and initial biopsy patients but not repeat biopsy patients in one SR16 and in two SRs analyzing a mix of initial and repeat biopsy patients.15,19 One of the SRs also compared cognitive targeted biopsy with standard biopsy15 and found no significant difference between groups for this outcome. One RCT20 reporting this outcome found a significantly smaller proportion of patients with clinically insignificant prostate cancer in the targeted biopsy (using either method in patients with a suspicious lesion) group compared with the standard biopsy group. The other RCT22 reporting this outcome found no significant difference between the combined cognitive targeted and random biopsy group and the random biopsy group.
Health-Related Quality of Life
One RCT20 measured health-related quality of life using the EQ-5D-5L and found no significant differences between the group undergoing targeted biopsy (fusion or cognitive) for suspicious lesions and the group undergoing standard TRUS-guided biopsy.
Adverse Events and Complications
In one RCT, the proportion of patients experiencing blood in urine, blood in semen, pain at site of procedure, rectal bleeding, or erectile dysfunction was numerically greater in patients in the standard TRUS-guided biopsy arm compared with patients in the MRI and targeted biopsy arm.20 In this study, patients without suspicious lesions on MRI did not undergo biopsy. In another RCT, one patient collapsed following biopsy (intervention group not reported) and no urinary tract infections were reported.22
Cost-Effectiveness of MRI Prior to TRUS-Guided Prostate Biopsy
MRI-US Fusion Targeted TRUS-Guided Biopsy
In one cost utility study comparing fusion targeted biopsy with standard biopsy in initial biopsy patients,25 fusion biopsy was more cost effective according to NHB at a willingness-to-pay (WTP) threshold of $50,000/QALY, regardless of whether standard biopsy was performed for patients with a negative MRI. In one cost utility study comparing fusion targeted biopsy with standard biopsy in repeat biopsy patients,26 fusion biopsy was cost-effective at a WTP of €80,000 with an incremental cost-effectiveness ratio (ICER) of 1,386 €/QALY. In this study, the results were most sensitive to survival after treatment of clinically significant prostate cancer and survival with untreated clinically insignificant prostate cancer. In one of the HTAs,13 the cost utility analysis compared combined fusion targeted and standard biopsy against systematic extended-cores biopsy in repeat biopsy patients using various MRI strategies. The targeted strategy was cost-effective at a WTP threshold of ₤30,000 when T2W MRI was used as well as when MRS was used in 60-year-old patients. Other MRI sequences or combinations were not cost-effective. T2W MRI dominated at lower cancer prevalence rates and MRI strategies were not cost-effective when patients with negative MRI findings underwent extended-cores systematic biopsy.
One cost-effectiveness study24 in repeat biopsy patients found that, based on a simulated cohort of 100 patients, the costs for mpMRI followed by fusion biopsy for patients with suspicious lesions were less than for standard biopsy, with most patients in the mpMRI arm being able to avoid biopsy. On the other hand, the number of missed prostate cancers was higher in the mpMRI arm. The results became less favourable when the assumed prostate cancer prevalence rate was increased.
Cognitive Targeted TRUS-Guided Biopsy
In one cost utility study comparing cognitive targeted biopsy with standard biopsy in initial biopsy patients,25 cognitive targeted biopsy was more cost effective according to NHB at a WTP threshold of $50,000/QALY, regardless of whether standard biopsy was performed for patients with a negative MRI. In a cost utility study comparing combined cognitive targeted and standard biopsy with standard biopsy alone in initial biopsy patients, cognitive targeted biopsy was dominant for time horizons ranging from 5 to 20 years in a Canadian setting.23
MRI-US Fusion or Cognitive Targeted TRUS-Guided Biopsy
One HTA performed a cost utility analysis comparing mpMRI followed by targeted TRUS-guided biopsy with TRUS-guided biopsy.6 The performance of targeted TRUS-guided biopsy was informed by the SR by Schoots et al.15 and the estimate included a mix of studies using cognitive and fusion targeted biopsy (with one in-bore MRI-guided biopsy study). Incremental analysis results were not available for all diagnostic strategies, but the targeted biopsy strategies were associated with higher costs and more QALYs than standard TRUS-guided biopsy.
Limitations
A major limitation of the body of evidence synthesized in this report is the lack of evidence for long-term clinical outcomes. For example, the consequences of cancers that potentially remained undetected could not be ascertained from the included studies. Also, in the three SRs14,15,19 that assessed risk of bias using QUADAS or QUADAS-2, the risk of bias from the reference standard was consistency rated high for all of the included studies as standard TRUS-guided biopsy or combined targeted and standard biopsy was not expected to correctly classify the target condition. These limitations also affected the economic evaluations as the long-term clinical inputs for the models relied on assumptions of the true accuracy of the diagnostic strategies being compared. There was no evidence found comparing adverse events or complications between the diagnostic strategies. There was also no evidence (aside from one RCT20) found comparing health-related quality of life or other patient-reported outcomes between strategies. While two of the included RCTs included 500 and 1,170 patients, respectively, most of the studies included in the SRs had fewer than 200 patients.
There was heterogeneity among the studies in the diagnostic strategies that was not addressed in the SRs. Sources of heterogeneity included the combination of MRI sequences employed, the field strength of the MRI systems, whether or not an endorectal coil was used for MRI, the scale used for grading suspicious areas on MR images, the number of cores taken in any of the biopsy strategies, whether or not targeted cores were taken in conjunction with systematic or random cores, and the MRI-US fusion platform used. Operator experience, especially for targeted biopsies, may bias cancer detection rates and this information was not reported in most studies. Methods may become more standardized as optimal mpMRI and biopsy sampling strategies are identified and more investigators adopt a standard such as the Prostate Imaging and Reported Data System (PI-RADS) for grading lesions on mpMRI.
Four of the six SRs assessed risk of bias of the primary studies.14,15,18,19 Of these, only two14,19 discussed reasons for risk of bias and even these SRs did not discuss the potential impact on the results.
Two of the SRs with meta-analyses15,16 only included patients who had at least one suspicious lesion on mpMRI. This inclusion criterion could have enriched the populations with patients with cancer and clinically significant cancer.
Five of the SRs included non-randomized studies,14–17,19 with three SRs15–17 specifying that both targeted and standard biopsy had to be performed in each patient. Therefore, there was a possibility that one biopsy method could have influenced the other. Also, the comparison of combined targeted and standard biopsy with standard biopsy in these studies would be fundamentally different than in an RCT.
There was overlap in the included studies of the six SRs and some primary studies were represented in more SRs than other studies. It is unclear how this affected the overall body of evidence.
One of the economic evaluations was conducted for the Canadian setting, and cognitive targeted TRUS biopsy was the intervention of interest. Treatment allocation assumptions also varied between the economic evaluations and it is not clear how generalizable they were to the Canadian setting.
Conclusions and Implications for Decision or Policy Making
A total of 15 relevant publications were identified, which comprised two HTAs with economic evaluations,6,13 six SRs,14–19 three RCTs,20–22 and four economic studies.23–26
There was no evidence found for a significant difference in prostate cancer detection rate between a diagnostic strategy employing MRI followed by either cognitive or MRI-US fusion targeted TRUS-guided biopsy and a diagnostic strategy consisting of TRUS-guided biopsy alone.
The results of three SRs with meta-analysis15,16,19 and one RCT20 consistently demonstrated higher detection rates of patients with clinically significant prostate cancer for MRI-US fusion targeted biopsy versus standard TRUS-guided biopsy alone. However, two of the SRs15,16 only included patients with suspicious lesions detected on MRI prior to biopsy and the results may not be generalizable to the larger population of patients undergoing TRUS-guided biopsy. The RCT20 compared a diagnostic strategy of mpMRI followed by either fusion or cognitive targeted TRUS-guided biopsy for suspicious lesions against a strategy of standard TRUS-guided biopsy alone.
The evidence for cognitive targeted TRUS-guided biopsy versus standard TRUS-guided biopsy was limited. Out of one SR15 and two RCTs21,22 comparing detection rates of patients with clinically significant prostate cancer between the two methods, cognitive targeted biopsy was favoured in one RCT21 which did not statistically test this comparison.
MRI-US fusion TRUS-guided biopsy consistently demonstrated lower detection rates of patients with clinically insignificant prostate cancer, except for when it was used in repeat biopsy patients.15,16,19 The same result was also demonstrated for targeted TRUS-guided biopsy involving either MRI-US fusion or cognitive targeting.20 There was no evidence for a difference between cognitive targeted TRUS-guided biopsy and standard TRUS-guided biopsy alone for the detection of clinically insignificant prostate cancer.15,22
Overall, the economic evaluations suggested that including MRI before TRUS-guided biopsy was more cost-effective than standard TRUS-guided biopsy alone despite the testing costs associated with the former being higher. MRI-US fusion targeted TRUS-guided biopsy was cost-effective in initial biopsy and repeat biopsy patients while cognitive targeted TRUS-guided biopsy was cost-effective in initial biopsy patients but not evaluated in repeat biopsy patients. One study performed an analysis from the point of view of the Canadian provincial public health system perspective and found that cognitive targeted TRUS-guided biopsy dominated standard TRUS-guided biopsy with time horizons of 5 to 20 years.23
The evidence for clinical effectiveness was limited by the lack of reporting of long-term outcomes. Also, the SRs identified high risk of bias in the primary studies due to the lack of an accurate reference standard. These limitations also hampered the economic evaluations as the long-term clinical inputs relied on assumptions regarding the accuracy of the diagnostic strategies compared and the downstream effects of misdiagnosing patients. The SRs were almost exclusively informed by non-randomized studies and some SRs only included studies in which both targeted and standard biopsy cores were taken in each patient. Therefore, it was not possible to compare long-term outcomes and harms between the two biopsy strategies.
There was heterogeneity among the studies in the diagnostic strategies in the SRs, contributing to uncertainty in the results. As more research is conducted into the optimal methods for mpMRI and biopsy sampling strategies, the heterogeneity among studies may decrease.
One of the six included economic evaluations was conducted from a Canadian perspective and it is unclear how generalizable the results of the other evaluations were to the Canadian setting as allocation of patients to treatment and costs associated with diagnosis and treatment may vary between countries.
Potential effects of adding MRI before TRUS-guided prostate biopsy in terms of additional burdens on health care systems were summarized in one of the HTAs.13 If mpMRI and targeted TRUS-guided biopsy are to be performed, radiologists and urologists would require training. Also, new equipment and software to document lesions for biopsy and for MRI-US fusion guidance would need to be purchased. While the identification of more patients with intermediate- or high-risk prostate cancer requiring treatment may increase with addition of MRI before biopsy, it is also possible this would be balanced by reduced detection of patients with low-risk prostate cancer. Finally, more reliable identification of low-risk prostate cancer patients could increase uptake of active surveillance, requiring more capacity for PSA testing, interval biopsies, and follow-up clinics.
References
- 1.
- 2.
- 3.
Rendon
RA, Mason
RJ, Marzouk
K, et al. Canadian Urological Association recommendations on prostate cancer screening and early diagnosis.
Can Urol Assoc J. 2017;11(10):298–309. [
PMC free article: PMC5659858] [
PubMed: 29381452]
- 4.
Litwin
MS, Tan
HJ. The diagnosis and treatment of prostate cancer: a review.
JAMA. 2017;317(24):2532–2542. [
PubMed: 28655021]
- 5.
- 6.
Brown
LC, Ahmed
HU, Faria
R, et al. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: the PROMIS study.
Health Technol Assess. 2018;22(39):1–176. [
PMC free article: PMC6077599] [
PubMed: 30040065]
- 7.
Ahmed
HU, El-Shater Bosaily
A, Brown
LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study.
Lancet. 2017;389(10071):815–822. [
PubMed: 28110982]
- 8.
- 9.
- 10.
Jansen
JP, Trikalinos
T, Cappelleri
JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report.
Value Health. 2014;17(2):157–173. [
PubMed: 24636374]
- 11.
- 12.
- 13.
Mowatt
G, Scotland
G, Boachie
C, et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: a systematic review and economic evaluation.
Health Technol Assess. 2013;17(20):vii–xix, 1–281. [
PMC free article: PMC4781459] [
PubMed: 23697373]
- 14.
Gayet
M, van der Aa
A, Beerlage
HP, Schrier
BP, Mulders
PF, Wijkstra
H. The value of magnetic resonance imaging and ultrasonography (MRI/US)-fusion biopsy platforms in prostate cancer detection: a systematic review.
BJU Int. 2016;117(3):392–400. [
PubMed: 26237632]
- 15.
Schoots
IG, Roobol
MJ, Nieboer
D, Bangma
CH, Steyerberg
EW, Hunink
MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis.
Eur Urol. 2015;68(3):438–450. [
PubMed: 25480312]
- 16.
Tang
Y, Liu
Z, Tang
L, et al. Significance of MRI/transrectal ultrasound fusion three-dimensional model-guided, targeted biopsy based on transrectal ultrasound-guided systematic biopsy in prostate cancer detection: a systematic review and meta-analysis.
Urol Int. 2018;100(1):57–65. [
PubMed: 29084410]
- 17.
van Hove
A, Savoie
PH, Maurin
C, et al. Comparison of image-guided targeted biopsies versus systematic randomized biopsies in the detection of prostate cancer: a systematic literature review of well-designed studies.
World J Urol. 2014;32(4):847–858. [
PubMed: 24919965]
- 18.
Wang
Y, Zhu
J, Qin
Z, et al. Optimal biopsy strategy for prostate cancer detection by performing a Bayesian network meta-analysis of randomized controlled trials.
J Cancer. 2018;9(13):2237–2248. [
PMC free article: PMC6036722] [
PubMed: 30026819]
- 19.
Wu
J, Ji
A, Xie
B, et al. Is magnetic resonance/ultrasound fusion prostate biopsy better than systematic prostate biopsy? An updated meta- and trial sequential analysis.
Oncotarget. 2015;6(41):43571–43580. [
PMC free article: PMC4791251] [
PubMed: 26498362]
- 20.
- 21.
Panebianco
V, Barchetti
F, Sciarra
A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study.
Urol Oncol. 2015;33(1):17.e11–17.e17. [
PubMed: 25443268]
- 22.
Tonttila
PP, Lantto
J, Paakko
E, et al. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy-naive men with suspected prostate cancer based on elevated prostate-specific antigen values: results from a randomized prospective blinded controlled trial.
Eur Urol. 2016;69(3):419–425. [
PubMed: 26033153]
- 23.
Cerantola
Y, Dragomir
A, Tanguay
S, Bladou
F, Aprikian
A, Kassouf
W. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer.
Urol Oncol. 2016;34(3):119.e111–119. [
PubMed: 26602178]
- 24.
Lotan
Y, Haddad
AQ, Costa
DN, Pedrosa
I, Rofsky
NM, Roehrborn
CG. Decision analysis model comparing cost of multiparametric magnetic resonance imaging vs. repeat biopsy for detection of prostate cancer in men with prior negative findings on biopsy.
Urol Oncol. 2015;33(6):266.e269–216. [
PubMed: 25858102]
- 25.
Pahwa
S, Schiltz
NK, Ponsky
LE, Lu
Z, Griswold
MA, Gulani
V. Cost-effectiveness of MR imaging-guided strategies for detection of prostate cancer in biopsy-naive men.
Radiology. 2017;285(1):157–166. [
PMC free article: PMC5621719] [
PubMed: 28514203]
- 26.
Venderink
W, Govers
TM, de Rooij
M, Futterer
JJ, Sedelaar
JPM. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic transrectal ultrasound, direct in-bore MRI, and image fusion.
Am J Roentgenol. 2017;208(5):1058–1063. [
PubMed: 28225639]
- 27.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Systematic Reviews and Meta-Analyses
View in own window
| First Author, Publication Year, Country | Study Designs and Numbers of Primary Studies Included | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes |
|---|
Tang, 2018, China
Search date: February 3, 2017 | 9 prospective and 3 retrospective non-randomized studies in which both targeted and standard TRUS-guided biopsy were performed in the same session in each patient | Increased serum PSA or positive DRE No proven PCa and not on active surveillance ≥ 1 suspicious lesion on prostate MRI prior to biopsy Separate analyses for initial and repeat biopsy patients
| Intervention:
Comparator:
Notes:
| Proportion of patients with:
|
Wang, 2018, China
Search date: July 2017 | 20 RCTs in the network-meta-analyses | Patients undergoing prostate biopsy for PCa detection | Prostate biopsy methods compared:
| Proportion of patients with:
|
Gayet, 2016, Netherlands
Search dates: January 1, 2004 to February 17, 2015 | 11 prospective, non-randomized studies | Initial biopsy or previous negative biopsy Not on active surveillance Clinical suspicion of PCa due to raised PSA and/or abnormal DRE
| Intervention:
Comparator:
Notes:
Transrectal or transperineal approach Lesions were scored on mpMRI using PI-RADS, a Likert scale, or NIH score
| Proportion of patients with:
|
Schoots, 2015, Netherlands
Search date: May 23, 2014 | 15 non-randomized studies in which both targeted and systematic TRUS-guided biopsy were performed in the in each patient | | Intervention / index test:
Comparator:
Reference test:
Notes:
| Proportion of patients with:
|
Wu, 2015, China
Search date: May 1, 2015 | 1 RCT and 15 non-randomized studies | Patients referred for prostate biopsy with clinical suspicion of PCa due to raised PSA and/or abnormal DRE | Intervention:
Comparator:
Notes:
| Proportion of patients with:
|
Van Hove, 2014, France
Search date(s) not reported | 2 RCTs and 13 non-randomized studies in which both targeted and standard TRUS-guided biopsy were performed in the same session in each patient | | Intervention:
Cognitive or MRI-US fusion targeted TRUS-guided biopsy (may or may not include systematic biopsy) Other targeting methods were summarized separately (i.e. US elastography, contrast-enhanced US, and histoscanning)
Comparator:
Notes:
| Proportion of patients with PCa |
DRE = digital rectal examination; mpMRI = multiparametric magnetic resonance imaging; MRI = magnetic resonance imaging; NIH = National Institutes of Health; PCa = prostate cancer; PI-RADS = Prostate Imaging – Reporting and Data System; PSA = prostate-specific antigen; RCT = randomized controlled trial; TRUS = transrectal ultrasound; US = ultrasound.
Table 3Characteristics of Included Randomized Controlled Trials
View in own window
| First Author, Publication Year, Country | Study Design | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes |
|---|
| Kasivisvanathan, 2018, UK (multiple sites in Europe and North America) | Multi-centre RCT | N = 500 Biopsy and treatment naïve Mean age: 64.4 and 64.5 years in each group Median PSA, ng/mL: 6.75 and 6.50 in each group
| Intervention:
Comparator:
| Primary outcome: Proportion of patients with clinically significant PCa (Gleason score of 3 + 4 or greater)
Secondary outcomes: Proportion of patients with clinically insignificant PCa, proportion of patients in mpMRI group who did not undergo biopsy, EQ-5D-5L, adverse events after procedures |
| Tontilla, 2016, Finland | Single-centre RCT | | Intervention:
mpMRI (DCE imaging, DWI, and ADC mapping) with images scored from 1 to 4 for likelihood of cancer Combined 10- to 12-core TRUS-guided random biopsy and cognitive targeted biopsy (1 to 2 cores per lesion; maximum 2 lesions) based a diagrammatic report
Comparator:
| Primary outcome: Proportion of patients with PCa
Secondary outcomes: proportion of patients with clinically significant PCa (Gleason score > 3 + 3, > 2 positive cores, or maximum cancer core length ≥ 3 mm), proportion of patients with clinically insignificant PCa, complications |
| Panebianco, 2015, Italy | Single-centre RCT |
Inclusion/exclusion criteria:
Biopsy naïve Total PSA level > 4 ng/mL PSA density > 0.15 PSA velocity > 0.75 ng/mL/year Free-to-total PSA ratio < 0.10 when total PSA level between 4 and 10 ng/mL
| Intervention:
mpMRI (T2W imaging, DWI, and DCE imaging) Index lesion was determined on mpMRI by higher PI-RADS score (and lower ADC in the event of a tie) If suspicious lesion detected on mpMRI: combined 12-core TRUS-guided random and cognitive targeted biopsy (10 cores from the peripheral zone [4 cores from the base, 4 cores from the mid gland, and 2 cores from the apex] and 2 cores from the index lesion) If no suspicious lesion detected on mpMRI: 14-core TRUS-guided random biopsy
Comparator:
| Number of patients with PCa, number of patients with PCa with Gleason score at least 6 |
ADC = apparent diffusion coefficient; CDR = cancer detection rate; DCE = dynamic contrast-enhanced; DRE = digital rectal examination; DWI = diffusion-weighted imaging; EQ-5D = EuroQol 5 Dimensions 5 Levels Self-Report Questionnaire; mpMRI = multiparametric magnetic resonance imaging; PCa = prostate cancer; PI-RADS = Prostate Imaging – Reporting and Data System; PSA = prostate-specific antigen; RCT = randomized controlled trial; TRUS = transrectal ultrasound; T2W = T2-weighted.
Table 4Characteristics of Included Economic Evaluations
View in own window
| First Author, Publication Year, Country | Type of Analysis, Time Horizon, Perspective | Decision Problem | Population Characteristics | Intervention and Comparator(s) | Approach | Clinical and Cost Data Used in Analysis | Main Assumptions |
|---|
| Brown, 2018, UK | Cost-utility analyses, short-term (no follow-up) and long-term (lifetime) time horizons, UK NHS perspective | To identify the most cost-effective diagnostic strategy among combinatio ns of TRUS-guided biopsy, mpMRI, and template mapping biopsy for in patients with suspected PCa | Patients with suspected prostate cancer being referred for further investigation (i.e., biopsy naïve) Patients were sampled from a cohort with no cancer (N = 159), low-risk cancer (N = 98), intermediat e-risk cancer (N = 301), or high-risk cancer (N = 18) as defined in the PIVOT trial (reported by Wilt et al.)
| Intervention: mpMRI followed by TRUS-guided biopsy or template mapping biopsy in patients with either any cancerous lesions or lesions suspected to be clinically significant (based on a 5-point Likert scale, score ≥ 2 for base case)
Comparator: TRUS-guided biopsy | Decision tree model for short-term analysis and Markov model for long-term analysis | Clinical data
From the PROMIS trial: diagnostic test accuracy of mpMRI, standard and TRUS-guided biopsy From the literature: survival data; diagnostic test accuracy of mpMRI and targeted TRUS biopsy (estimate from Schoots et al. includes one study using in-bore MRI guidance); impact of each test on health-related quality of life; rates of adverse events; utility decrements
Cost data
Costs associated with procedures and adverse events from NHS Reference Costs 2014-2015 General practitioner costs from Curtis Costs associated with treatment and associated AEs from 2014/15 National Tariff Payment System and Lord et al.
| Patients diagnosed with clinically significant cancer receive immediate radical treatment Patients diagnosed with clinically insignificant cancer receive active surveillance Long-term outcomes of patients with PCa that was not detected were assumed to be equivalent to those of men allocated to active surveillance Intermediate-risk patients misclassified as low-risk will not receive radical treatment
|
| Pahwa, 2017, US | Cost-utility analysis, entire lifetime, perspective not stated | To evaluate the cost-effectivene ss of mpMRI followed by MR imaging-guided biopsy strategies in the detection of prostate cancer in biopsy-naïve men | 65-year-old (additional analyses in subgroups of 41 to 50 years, 51 to 60 years, and 61 to 70 years), biopsy-naïve male patients with elevated PSA levels or clinically significant DRE findings | Intervention: MRI followed by cognitive or fusion targeted biopsy for detected lesions. Different scenarios were modelled:
Comparator: Standard TRUS biopsy with 12 to 16 cores | Decision tree model | Clinical data
From the literature: prevalence of prostate cancer; probability of detecting clinically significant cancer, sensitivity, and specificity of MRI and standard biopsy; complication rates of biopsy procedures; sensitivity of MRI-targeted biopsy for detection of clinically significant and insignificant cancer; probability of patients choosing a given treatment pathway; lifetime QALYs for various treatments Lifetime QALYs for untreated cancer and androgen deprivation therapy assumed by author
Cost data
Diagnostic procedure costs were derived from the physician fee schedule from Medicare/Medicaid Cost of losing a day of work was derived from the Bureau of Labor Statistics Lifetime costs of treatment procedures were estimated from the literature
| A tumour confined to the prostate with volume < 0.5 cm3 and Gleason score of ≤ 6 was clinically insignificant WTP of $50,000 Lifetime QALYs for untreated clinically insignificant PCa is the same as for watchful waiting
|
| Venderink, 2017, Netherlands | Cost-utility analysis, 18-year time horizon, perspective not stated | To evaluate the differences in cost-effectivene ss of in- bore MR-guided biopsy, MRI-TRUS fusion biopsy, and TRUS biopsy for the detection of clinically significant prostate cancer | Biopsy-naïve patients with elevated serum PSA or abnormal DRE finding. Prevalence of PCa was set at 25%, with half of tumours being clinically significant. | Intervention: mpMRI followed by MRI-TRUS fusion biopsy for detected lesions
Comparator: Systematic TRUS biopsy | Decision tree and Markov model | Clinical data
Transition probabilities for TRUS biopsy, MRI-TRUS fusion biopsy, and mpMRI; utility data; survival data from the literature Distribution in initial treatment, prevalence of clinically significant tumours and specificity of TRUS for any prostate cancer based on expert opinion
Cost data
Diagnostic, treatment, active surveillance, follow-up, and urine incontinence costs from hospital departments
| WTP threshold of €80,000 Falsely negative (or insignificant) PCas would eventually be detected A Gleason score of 3 + 4 or greater was clinically significant Specificity of targeted or systematic TRUS biopsy for any prostate cancer is 100%
|
| Cerantola, 2016, Canada | Cost-utility analysis, 5-, 10-, 15-, and 20-year time horizon, provincial public health system perspective | To assess the added initial costs and benefits related to prostate MRI and cognitive targeted biopsy | Biopsy-naïve male patients 60 to 65 years of age with PSA values of 4 to 10 µg/L or abnormal DRE finding | Intervention: MRI followed by cognitive targeted TRUS biopsy if at least one lesion had PI-RADS score of 3 to 5 (1 to 4 targeted cores in addition to 12-core standard biopsy)
Comparator: Standard 12-core TRUS biopsy | Markov model | Clinical data
Detection rates of cancer and significant cancer and false-negative rates; biochemical recurrence and survival data; utility values from the literature Treatment allocation rates were based on expert opinion and confirmed by Mowatt et al. (HTA)
Cost data
| WTP threshold of $50,000/QALY gained Utility value of 0.92 assumed for remission Costs for radiation therapy are based on intensity-modulated radiation therapy Costs related to complications were not considered Patients with negative MRI with persistent clinical suspicion of prostate cancer had TRUS systematic biopsy within 3 years Treatment allocation same regardless of diagnostic test
|
| Lotan, 2015, US | Cost-effectiveness analysis, no follow-up period, perspective not stated | To compare the cost of an mpMRI strategy to inform the need for a repeated TRUS biopsy with the cost of performing TRUS biopsy in all patients | Repeat biopsy patients with persistent indication for PCa. Prevalence of PCa was set at 25%. | Intervention: mpMRI followed by MRI-US fusion targeted TRUS biopsy in patients with suspicious lesions (no biopsy for patients with negative mpMRI)
Comparator: 14-core systematic TRUS biopsy in all patients | Decision tree model | Clinical data
Cost data
Costs of performing office-based TRUS biopsy and pathology based on 2014 Medicare reimbursement Costs of sepsis from the literature Loss of wages from US Labor Department MRI fusion workstation purchase price
| |
| Mowatt, 2013, UK | Cost-utility analysis, 30-year time horizon, NHS and personal social services perspective | To assess the cost-effectivene ss of using difference MRI sequences (T2W imaging, MRS, DCE imaging, and DWI) to direct prostate biopsy following a previous negative biopsy | | Intervention: MRI followed by combined MRI-US fusion targeted and systematic TRUS biopsy (no biopsy for patients with negative MRI)
Comparator: Extended-cores (14 to 16 cores) systematic TRUS biopsy alone | Markov model | Clinical data
Prevalence of disease states; complication rates arising from testing and treatment; relative risk of metastases; utility values from the literature Diagnostic accuracy of MRI from a systematic review conducted as part of the HTA (from elsewhere for extended-core systematic TRUS biopsy)
Cost data
NHS reference costs for biopsies, complications Unit Costs of Health and Social Care (with clinician input for time estimates) for MRI tests Combination of the above sources with an HTA for treatment costs
| PCa prevalence of 24% False negatives would have persistently elevated PSA level (monitored every 6 months) and would be offered saturation biopsy 12 months later No further biopsies for patients without PCa if repeat biopsy was negative Diagnosed patients had reduced risk of progression to metastases in line with that for radical prostatectomy Untreated disease was assumed to occur at the rate observed for patients receiving external beam radiation therapy alone
|
AS = active surveillance; DCE = dynamic contrast-enhanced; DRE = digital rectal examination; DWI = diffusion-weighted imaging; HTA = health technology assessment; mpMRI = multiparametric magnetic resonance imaging; MR = magnetic resonance; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; MSSS = Ministere de la Santé et des Services sociaux; NHS = National Health Service; PCa = prostate cancer; PI-RADS = Prostate Imaging – Reporting and Data System; PSA = prostate-specific antigen; RAMQ = Regie de l’assurance maladie du Quebec; SR = systematic review; T2W = T2-weighted; WTP = willingness-to-pay.
Appendix 3. Critical Appraisal of Included Publications
Table 5Strengths and Limitations of Systematic Reviews and Meta-Analyses using AMSTAR 29
View in own window
| Strengths | Limitations |
|---|
| Tang, 201816 |
|---|
The research questions and inclusion criteria for the review included the components of PICO Two databases were searched and keywords were provided for the literature search Study selection was performed in duplicate Reasons for excluding studies were given (without an accompanying list of studies) Included studies were described in adequate detail Appropriate methods for statistical combination of results were used (random effects model used when I2 ≥ 50%) No publication bias was found when Egger’s test was used (also assessed using funnel plot) The review authors reported no competing interests
| It is unclear whether review methods were established prior to the conduct of the review No explanation was given for the inclusion of cohort study designs only The literature search did not include trial registries, grey literature, or a search of reference lists in included studies Data extraction was not performed in duplicate Risk of bias in the individual studies was not assessed Sources of funding were not reported for the included studies Heterogeneity in some of the results was not explained
|
| Gayet, 201614 |
|---|
The research questions and inclusion criteria for the review included the components of PICO Neither RCTs nor non-randomized studies were excluded Multiple databases were searched and keywords were provided for the literature search Study selection was performed in duplicate Reasons for excluding studies were given (without an accompanying list of studies) Included studies were described in adequate detail QUADAS-2 was used to assess the risk of bias of included studies Reasons for high risk of bias were discussed Heterogeneity in the results and its likely sources were discussed The review authors reported no conflicts of interest
| It is unclear whether review methods were established prior to the conduct of the review The literature search did not include trial registries, grey literature, or a search of reference lists in included studies Data extraction was not performed in duplicate The review authors did not assess the potential impact of risk of bias in individual studies on the results of the meta-analysis Sources of funding were not reported for the included studies
|
| Schoots, 201515 |
|---|
The research questions and inclusion criteria for the review included the components of PICO The review authors explained their use of cohort study designs in which patients received both interventions Multiple databases were searched, keywords were provided for the literature search, and reference lists of included studies were searched Study selection was performed in duplicate Reasons for excluding studies were given (without an accompanying list of studies) Included studies were described in adequate detail QUADAS was used to assess the risk of bias of included studies Appropriate methods for statistical combination of results were used The review authors accounted for study quality when discussing the results Pre-specified subgroup analyses were performed Heterogeneity in the results and its likely sources and impacts were discussed The potential impact of risk of bias in individual studies on the results of the meta-analysis was discussed No publication bias was found when Egger’s test was used (also assessed using funnel plot) The review authors reported no competing interests
| It is unclear whether review methods were established prior to the conduct of the review The literature search did not include trial registries or grey literature It is unclear whether data extraction was performed in duplicate Sources of funding were not reported for the included studies
|
| Wu, 201519 |
|---|
The research questions and inclusion criteria for the review included the components of PICO Neither RCTs nor non-randomized studies were excluded Multiple databases were searched, keywords were provided for the literature search, and reference lists of included studies were searched Study selection and data extraction were performed in duplicate Reasons for excluding studies were given (without an accompanying list of studies) Included studies were described in adequate detail QUADAS-2 was used to assess the risk of bias of included studies Appropriate methods for statistical combination of results were used Studies with a high risk of bias using the QUADAS-2 tool were excluded Risk of bias was noted in the discussion due to the use of TRUS-guided biopsy as the reference standard (see Limitations) Heterogeneity was discussed for results with I2 ≥ 50% No publication bias was found when Egger’s test and Begg’s test were used (also assessed using funnel plot) The review authors reported no conflicts of interest
| It is unclear whether review methods were established prior to the conduct of the review The literature search did not include trial registries or grey literature Sources of funding were not reported for the included studies The review authors did not assess the potential impact of risk of bias in individual studies on the results of the meta-analysis
|
| Van Hove, 201417 |
|---|
The research questions and inclusion criteria for the review included the components of PICO The review authors explained their use of RCTs or cohort study designs in which patients received both interventions Medical subject heading terms were provided for the PubMed search and reference lists of included studies were searched Included studies were described in adequate detail
| It is unclear whether review methods were established prior to the conduct of the review The literature search did not include multiple databases, trial registries, or grey literature It is unclear whether study selection or data extraction were performed in duplicate A list of excluded studies was not provided There was no risk of bias assessment of the individual studies Heterogeneity in the results was not discussed Sources of funding were not reported for the included studies One of the review authors reported honoraria from medical imaging system manufacturers without mentioning how potential conflicts of interest were managed
|
RCT = randomized controlled trial.
Table 6Strengths and Limitations of Network Meta-Analyses using the ISPOR Questionnaire10
View in own window
| Strengths | Limitations |
|---|
| Wang, Year18 |
|---|
Relevance
Credibility
Researchers attempted to include all relevant RCTs The trials for the interventions of interest form one connected network of RCTs There was low risk of selective reporting bias in all included studies Within-study randomization was preserved in the meta-analyses Methods to evaluate consistency between direct and indirect comparisons were described The evidence network was provided which included information on the number of RCTs per direct comparison Individual study results are reported Odds ratios between interventions obtained from the network meta-analysis were reported along with 95% credible intervals Rank probabilities for each intervention and outcome were given The authors declared no competing interests
| Relevance
Credibility
Most included RCTs had a high risk of bias for allocation concealment and high or unclear risk of bias for random sequence generation It is unclear whether there were systematic differences in treatment effect modifiers across different treatment comparisons as patient characteristics were not reported and very few study characteristics were reported Methods to evaluate consistency between direct and indirect comparisons were not applied to all relevant pairwise comparisons (including any comparisons with TRUS-guided biopsy) No rationale was provided for the use of a random effects model for network meta-analysis Assumptions about heterogeneity in the random effects model were not explored or discussed Heterogeneity results were not reported and no subgroup analyses or meta-regression analysis was performed The results of direct comparisons, indirect comparisons, and network meta-analysis are reported separately for only select pairwise comparisons The conclusions do not reflect the results of the network meta-analysis and its limitations
|
PCa = prostate cancer; RCT = randomized controlled trial; TRUS = transrectal ultrasound-guided.
Table 7Strengths and Limitations of Clinical Studies using the Downs and Black Checklist11
View in own window
| Strengths | Limitations |
|---|
| Kasivisvanathan, 201820 |
|---|
Reporting
The objective of the study, main outcomes, inclusion and exclusion criteria, interventions being compared, potential confounders, and main findings are clearly described A list of complications to be recorded was cited but not provided 95% confidence intervals and exact P values are reported for the main outcomes
External validity
Patients asked to participate were representative of the population from which they were recruited Staff, places, and facilities were representative of the treatment received by the source population
Internal validity
Post hoc analyses were clearly indicated Follow-up was similar between groups (biopsy pathology) Statistical tests for the main outcomes were appropriate, including adjustment for centre The main outcome measures were valid and reliable (cancers and significant cancers on pathology) Patients in both groups were recruited from the same population over the same time period Patients were randomized to intervention groups Patients lost to follow-up were accounted for The study had sufficient power to demonstrate non-inferiority
| Reporting
External validity
Internal validity
Patients and investigators were not blinded to intervention allocation It is unclear whether outcomes assessors (pathologists) were blinded to intervention allocation Some patients did not undergo assigned intervention (< 10% in each group) and reasons differed between the groups
|
| Tontilla, 201622 |
|---|
Reporting
The objective of the study, main outcomes, inclusion and exclusion criteria, interventions being compared, potential confounders, and main findings are clearly described Interquartile ranges and exact P values are reported for the main outcomes Reasons for exclusion and available biopsy results were reported for patients excluded due to protocol violations
External validity
Patients asked to participate were representative of the population from which they were recruited Staff, places, and facilities were representative of the treatment received by the source population
Internal validity
Random biopsies were conducted with urologists blinded to MRI results Post hoc analyses were not conducted Follow-up was similar between groups (biopsy pathology) Statistical tests for the main outcomes were appropriate Compliance with the interventions was reliable The main outcome measures were valid and reliable (cancers and significant cancers on pathology) Patients in both groups were recruited from the same population over the same time period Patients were randomized to intervention groups Patients lost to follow-up were accounted for
| Reporting
External validity
Internal validity
Patients were not blinded to the intervention they received It is unclear whether outcomes assessors (pathologists) were blinded to intervention allocation It was unclear whether the study met the calculated minimum sample size (one group did and the other group did not)
|
| Panebianco, 201521 |
|---|
Reporting
External validity
Patients asked to participate were representative of the population from which they were recruited Staff, places, and facilities were representative of the treatment received by the source population
Internal validity
Post hoc analyses were not conducted Follow-up was similar between groups (biopsy pathology) Compliance with the interventions was reliable The main outcome measure was valid and reliable (significant cancers on pathology) Patients in both groups were recruited from the same population over the same time period Patients were randomized to intervention groups
| Reporting
The main outcome is not clearly describe in the Methods section Potential confounders and main findings are not clearly described Patient characteristics are not described Statistical tests comparing groups were not performed Adverse events or complications are not reported
External validity
Internal validity
Patients and investigators were not blinded to intervention allocation It is unclear whether outcomes assessors (pathologists) were blinded to intervention allocation Patients lost to follow-up are not described
|
Table 8Strengths and Limitations of Economic Studies using the Drummond Checklist12
View in own window
| Strengths | Limitations |
|---|
| Brown, 20186 |
|---|
The research question, its economic importance, the viewpoint of the analysis, and the rationale for choosing the interventions are stated The alternatives being compared are clearly described The form of economic evaluation is stated and justified in relation to the questions addressed The sources of effectiveness estimates used are stated The effectiveness study supplying diagnostic accuracy data for mpMRI and TRUS-guided biopsy (PROMIS trial) is described in detail The primary outcome measure for the economic evaluation is clearly stated Sources and methods used for health state utilities are stated Methods for estimating quantities and costs are described The currency used for all costs (2006 Great British Pounds) was stated Details of the models are given and the key parameters are justified The time horizon is stated A standard discount rate of 3.5% was used for costs according NICE guidance Details of statistical test and confidence intervals are given for stochastic data The approaches to sensitivity analyses are given The choice of variables for sensitivity analysis is justified Ranges for sensitivity analysis are given Relevant alternatives are compared Incremental analysis is reported for the most cost effective diagnostic strategies Major outcomes are presented in disaggregated (cost and QALYs) for all strategies and aggregated (cost/QALY) form for the most cost effective diagnostic strategies The conclusions follow from the data reported and are clearly stated with appropriate limitations identified
| |
| Pahwa, 201725 |
|---|
The research question, its economic importance, and the rationale for choosing the interventions are stated The alternatives being compared are clearly described (see limitations) The form of economic evaluation is stated and justified in relation to the questions addressed The sources of effectiveness estimates used are stated The primary outcome measure for the economic evaluation is clearly stated Methods to value health states and other benefits are stated or cited Methods for estimating costs are described The currency used (2016 US dollars) was stated Costs were adjusted for inflation, though the rate is not stated (some data from the literature was already discounted at a rate of 3%) or justified Details of the model are given and the key parameters are justified The time horizon is stated Details of statistical tests and confidence intervals are given for stochastic data One-way and probabilistic sensitivity analyses are described, with ranges given or threshold analysis performed The choice of variables for sensitivity analysis is justified Relevant alternatives are compared Incremental analysis is reported Major outcomes are presented in disaggregated (lifetime cost and lifetime QALYs) and aggregated (NHB) form The conclusions follow from the data reported and are clearly stated with limitations identified
| The viewpoint of the analysis is not clearly stated Details on sources of clinical data are not given Details on cognitive or MRI-TRUS fusion biopsy were not provided (i.e., whether targeted lesions were biopsied alone or together with standard biopsy) Details of currency conversion for literature-derived costs are not given
|
| Venderink, 201726 |
|---|
The research question, its economic importance, and the rationale for choosing the interventions are stated The alternatives being compared are clearly described (see limitations) The form of economic evaluation is stated and justified in relation to the questions addressed The sources of effectiveness estimates used are stated The primary outcome measure for the economic evaluation is clearly stated Sources for health state utilities are cited Methods for estimating costs are described The currency used (Euros) was stated, though the year was not given Costs were adjusted for inflation at a rate of 4% according to Dutch pharmacoeconomic guidelines Details of the model are given and the key parameters are justified The time horizon is stated Relevant alternatives are compared (though details are lacking, see limitations) Incremental analysis is reported Major outcomes are presented in disaggregated (cost and QALYs) and aggregated (cost/QALY) form The conclusions follow from the data reported and are clearly stated with limitations identified
| The viewpoint of the analysis is not clearly stated Details on sources of clinical data are not given Details on the standard TRUS biopsy, MRI-TRUS fusion biopsy, and in-bore MRI-guided biopsy arms were not provided (i.e., whether targeted lesions were biopsied alone or together with standard biopsy; number of cores for standard biopsy) Deterministic sensitivity analyses are mentioned, but parameters and ranges were not described
|
| Cerantola, 201623 |
|---|
The research question, its economic importance, the viewpoint of the analysis, and the rationale for choosing the interventions are stated The alternatives being compared are clearly described The form of economic evaluation is stated and justified in relation to the questions addressed The sources of effectiveness estimates used are stated The primary outcome measure for the economic evaluation is clearly stated Sources for most health state utilities are cited Methods for estimating costs are described The currency used for all costs (2014 Canadian dollars) was stated A standard discount rate of 5% was used for costs and outcomes (though the choice of rate was not justified) Details of the model are given and the key parameters are justified The time horizon is stated Relevant alternatives are compared Incremental analysis is reported Major outcomes are presented in disaggregated (cost and QALYs) and aggregated (cost/QALY) form Ranges for sensitivity analysis are given The conclusions follow from the data reported and are clearly stated with appropriate limitations identified
| Details on sources of clinical data are not given One of the health state utilities was assumed without a rationale given The approach to sensitivity analysis is not given and the choice of variables for sensitivity analysis is not justified
|
| Lotan, 201524 |
|---|
The research question, its economic importance, and the rationale for choosing the interventions are stated The alternatives being compared are clearly described The form of economic evaluation is justified in relation to the questions addressed The sources of effectiveness estimates used are stated Methods for estimating quantities and unit costs are described The currency used for all costs (2014 US dollars) was stated Details of the model are given and the key parameters are justified The approach to sensitivity analysis is given Relevant alternatives are compared The conclusions follow from the data reported and are clearly stated with appropriate limitations identified
| The viewpoint of the analysis is not clearly stated The form of economic evaluation is not explicitly stated The primary outcome measure for the economic evaluation is not clearly stated Details on sources of clinical data are not given Productivity changes are not reported separately and their relevance is not discussed The time horizon is not explicitly stated, though it is clear that only the MRI and biopsy procedures and associated sepsis complications are modelled The ranges over which some variables are varied are not stated Incremental analysis is not reported Major outcomes are presented in disaggregated form but not aggregated form
|
| Mowatt, 201313 |
|---|
The research question, its economic importance, the viewpoint of the analysis, and the rationale for choosing the interventions are stated The alternatives being compared are clearly described The form of economic evaluation is stated and justified in relation to the questions addressed The sources of effectiveness estimates used are stated The HTA includes an SR used to inform diagnostic accuracy of MRI and TRUS biopsy The primary outcome measure for the economic evaluation is clearly stated Sources and methods used for health state utilities are stated Methods for estimating quantities and costs are described The currency used for all costs (2009-2010 Great British Pounds) was stated Details of the models are given and the key parameters are justified The time horizon is stated A standard discount rate of 3.5% was used for costs according NICE guidance Details of statistical test and confidence intervals are given for stochastic data The approaches to sensitivity analyses are given The choice of variables for sensitivity analysis is justified Ranges for sensitivity analysis are given Relevant alternatives are compared Incremental analysis is reported Major outcomes are presented in disaggregated (cost, life years, and QALYs) for all strategies and aggregated (cost / life year or cost/QALY) form The conclusions follow from the data reported and are clearly stated with appropriate limitations identified
| No limitations were identified |
HTA = health technology assessment; mpMRI = multi-parametric magnetic resonance imaging; MRI = magnetic resonance imaging; NICE = National Institute for Health and Care Excellence; QALY = quality-adjusted life year; SR = systematic review; TRUS = transrectal ultrasound.
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 9Summary of Findings for Included Systematic Reviews and Meta-Analyses
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Tang, 201816 |
|---|
3D MRI-US fusion targeted TRUS biopsy + standard TRUS biopsy vs. standard TRUS biopsy
Proportion of patients with PCa
Cohorts with initial biopsy patients only: OR of 1.38 (95% CI, 1.21 to 1.57); N = 1823 Cohorts with repeat biopsy patients only: OR of 1.92 (95% CI, 1.45 to 2.54); N = 528 Cohorts with both initial and repeat biopsy patients: OR of 1.68 (95% CI, 1.39 to 2.03); N = 874 All biopsy cohorts: OR of 1.52 (95% CI, 1.38 to 1.68); N = 3225
3D MRI-US fusion targeted TRUS biopsy alone vs. standard TRUS biopsy
All biopsy cohorts
Proportion of patients with PCa: OR of 1.08 (95% CI, 0.92 to 1.27); N = 3225 Proportion of patients with Gleason score ≥ 7: OR of 1.36 (95% CI, 1.20 to 1.55); N = 2573 Proportion of patients with Gleason score < 7: OR of 0.64 (95% CI, 0.55 to 0.75); N = 2573
Initial biopsy cohorts (N = 1823)
Proportion of patients with PCa: OR of 0.89 (95% CI, 0.78 to 1.02) Proportion of patients with Gleason score ≥ 7: OR of 1.24 (95% CI, 1.07 to 1.43) Proportion of patients with Gleason score < 7: OR of 0.60 (95% CI, 0.51 to 0.72)
Repeat biopsy cohorts (N = 528)
Proportion of patients with PCa: OR of 1.33 (95% CI, 0.99 to 1.77) Proportion of patients with Gleason score ≥ 7: OR of 1.89 (95% CI, 1.32 to 2.72) Proportion of patients with Gleason score < 7: OR of 0.74 (95% CI, 0.48 to 1.13)
Cohorts with both initial and repeat biopsy patients
Proportion of patients with PCa: OR of 1.44 (95% CI, 0.96 to 2.16); N = 874 Proportion of patients with Gleason score ≥ 7: OR of 1.95 (95% CI, 1.29 to 2.96); N = 222 Proportion of patients with Gleason score < 7: OR of 0.93 (95% CI, 0.55 to 1.57); N = 222
Notes:
| “In the population scheduled for prostate biopsy for increased serum PSA or/and abnormal DRE with suspicious lesion on MRI but non-previous evidence of cancer, MRI/TRUS fusion 3D-Tb [three-dimensional targeted biopsy] combined with Sb [standard biopsy] significantly improves the PCa detection rate compared to each of them alone; MRI/TRUS fusion 3D-Tb detects a significantly more high-Gleason-score PCa, and tends to detect more PCa in the population with previous negative biopsy, but has no significant superiority in overall PCa detection.” [p. 64] |
| Wang, 201818 |
|---|
TRUS-guided biopsy vs. MRI-US fusion targeted TRUS biopsy + TRUS-guided biopsy
Proportion of patients with PCa: OR of 0.59 (95% CrI, 0.24 to 1.4) Proportion of patients with significant PCa: OR of 0.47 (95% CrI, 0.14 to 1.6) Proportion of patients with insignificant PCa: OR of 0.98 (95% CrI, 0.33 to 3.0)
TRUS-guided biopsy vs. MRI-US fusion targeted biopsy
Proportion of patients with PCa: OR of 0.66 (95% CrI, 0.36 to 1.2) Proportion of patients with significant PCa: OR of 0.42 (95% CrI, 0.16 to 1.0) Proportion of patients with insignificant PCa: OR of 1.6 (95% CrI, 0.71 to 4.0)
Notes:
| “In summary, the outcomes of the present network meta-analysis shed light on that FUS-GB [fusion-guided biopsy] or FUS-GB plus TRUS-GB [TRUS-guided biopsy] showed their superiority, compared with other puncture methods, in the detection of PCa. Besides, TPUS [transperineal ultrasound] or TRUS-GB was more easily associated with the harms of unnecessary biopsies and over-diagnosis.” [p. 2247] |
| Gayet, 201614 |
|---|
Evidence was synthesized narratively and all studies except for two (Salami et al. and Shoji et al.) were included in meta-analyses already described in this report. In both studies, all patients had at least one suspicious lesion on mpMRI.
MRI-US fusion targeted TRUS biopsy vs. systematic TRUS-guided biopsy
Salami et al. 2015 (140 repeat biopsy patients)
Shoji et al. 2015 (20 biopsy naïve patients)
| “Although MRI/US-fusion TB [targeted biopsy] has proved its value in men with prior negative biopsies, general use of this technique in the diagnosis of prostate cancer should only be performed after critical consideration because in our present analysis, no clear advantage of MRI/US fusion-guided TB could be found for CDRs of all prostate cancers; however, MRI/US fusion guided TBs tended to give a higher CDR for clinically significant prostate cancers.” [p. 399] |
| Schoots, 201515 |
|---|
Cognitive targeted biopsy vs. TRUS-guided biopsy
Sensitivity for PCa: RR of 1.08 (95% CI, 0.88 to 1.33) Sensitivity for significant PCa: RR of 1.03 (95% CI, 0.91, 1.16) Sensitivity for insignificant PCa: RR of 0.17 (95% CI, 0.03 to 1.02)
MRI-US fusion targeted biopsy vs. TRUS-guided biopsy
Sensitivity for PCa: RR of 1.09 (95% CI, 0.88 to 1.35) Sensitivity for significant PCa: RR of 1.29 (95% CI, 1.16, 1.43) Sensitivity for insignificant PCa: RR of 0.71 (95% CI, 0.55 to 0.92)
Notes:
| “In men with clinical suspicion of prostate cancer and a suspicious lesion seen on multiparametric MRI, MRI-TBx [targeted biopsy guided by prior MRI] and TRUS-Bx [TRUS-guided biopsy] did not differ in overall detection of prostate cancer. However, MRI-TBx had a higher rate of detection of potentially significant prostate cancer and a lower rate of detection of insignificant prostate cancer compared to the standard TRUS-Bx.” [p. 448]
“However, we found significant heterogeneity, which limits the strengths of the conclusions that can be made. Furthermore, as a consequence of underlying methodological flaws of MRI-TBx, the comparison to standard systematic biopsy needs to be regarded with caution.” [p. 448] |
| Wu, 201519 |
|---|
MRI-US fusion targeted biopsy vs. TRUS-guided systematic biopsy
Proportion of patients with PCa: RR of 1.06 (95% CI, 1.01 to 1.12); N = 3013 and 3105 in each group
Low MRI suspicion (PI-RADS score of 2 or 3) subgroup: RR of 0.36 (95% CI, 0.26 to 0.49); N = 253 and 554 in each group Moderate/high MRI suspicion (PI-RADS score of 4 or 5) subgroup: RR of 1.46 (95% CI, 1.28, 1.67); N = 354 and 554 in each group
Proportion of patients with clinically significant PCa: RR of 1.19 (95% CI, 1.10 to 1.29); N = 2481 and N = 2583 in each group Proportion of patients with clinically insignificant PCa: RR of 0.68 (95% CI, 0.59 to 0.79); N = 2395 and N = 2494 in each group
Notes:
| “We found that, although more evidence is needed, MR/US fusion prostate biopsy alone detected more prostate cancers than systematic biopsy and was better than systematic biopsy in detecting clinically significant prostate cancers. For those men with moderate/high suspicion in mp-MRI, MR/US fusion biopsy showed a great advantage.” [p. 43578] |
| Van Hove, 201417 |
|---|
Evidence was synthesized narratively and all studies except for 4 were included in meta-analyses already described in this report. In all 4 of the studies, all patients were undergoing repeat biopsy.
Labanaris et al. 2010 (N = 260)
Pepe et al. 2013 (N = 78)
Single cohort Cognitive targeted TRUS biopsy vs. 26- to 32- core systematic TRUS-guided biopsy vs. combined targeted/systematic
Sciarra et al. 2010 (N = 180)
Lee et al. 2012 (N = 87)
Single cohort MRI-US fusion targeted TRUS biopsy vs. 12-core systematic TRUS-guided biopsy vs. combined targeted/systematic
| “Based on well-designed, controlled studies no clear advantage of targeted biopsies over the current standard of systematic biopsies can be observed, if the overall detection rate is considered as an outcome. […] In the initial biopsy setting, image-targeted biopsies are often associated with inferior prostate cancer detection rates relative to systematic biopsies, whereas in the repeat biopsy setting image-targeted biopsies can provide superior prostate cancer detection rates relative to systematic biopsies” [p. 856] |
CI = confidence interval; CrI = credible interval; DRE = digital rectal examination; mpMRI = multiparametric magnetic resonance imaging; MRI = magnetic resonance imaging; OR = odds ratio; PCa = prostate cancer; PI-RADS = Prostate Imaging – Reporting and Data System; PSA = prostate-specific antigen; RCT = randomized controlled trial; RR = relative risk; TRUS = transrectal ultrasound; US = ultrasound.
Table 10Summary of Findings of Included Randomized Controlled Trials
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Kasivisvanathan, 201820 |
|---|
mpMRI group (cognitive or fusion targeted) vs. standard biopsy group
Proportion of patients with clinically significant PCa: 38% vs. 26%; adjusted difference of 12% (95% CI, 4% to 20%; P = 0.005)
Proportion of patients with clinically insignificant PCa: 9% vs. 22%; adjusted difference of −13% (95% CI, −19% to −7%; P < 0.001 after post hoc Bonferroni correction) Biopsies avoided due to negative MRI: 28% (71 of 252 patients in the mpMRI group) Health-related quality of life (EQ-5D-5L index score and VAS) 24 hours and 30 days after biopsy: no significant difference between groups Adverse events (% of patients)
Blood in urine: 30% vs. 63% Blood in semen 32% vs. 60% Pain at site of procedure: 13% vs. 23% Rectal bleeding: 14% vs. 22% Erectile dysfunction (11% vs. 16%) Serious adverse events: 2% of both groups
Notes:
| “In conclusion, in men with a clinical suspicion of prostate cancer, we found that a diagnostic pathway including risk assessment with MRI before biopsy and MRI-targeted biopsy in the presence of a lesion suggestive of cancer was superior to the diagnostic pathway of standard transrectal ultrasonography–guided biopsy.” [p. 1776] |
| Tontilla, 201622 |
|---|
mpMRI followed by random + cognitive targeted biopsy vs. random biopsy alone
Proportion of patients with PCa: 64% vs. 57%; difference of 7.5% (95% CI, −10% to 25%; P = 0.5) Proportion of patients with clinically significant PCa: 55% vs. 45%, difference of 9.7% (95% CI, −8.5% to 27%; P = 0.8) Proportion of patients with clinically insignificant PCa: 9.4% vs. 12%, difference of −2.2% (95% CI, −14% to 10%; P = 0.5) Median number of biopsy cores: 12 (IQR, 12 to 14) vs. 12 (IQR, 10 to 12)
Complications
Notes:
| “This randomized blinded controlled trial demonstrated that the addition of cognitive MP-MRI/TRUS-fusion TB [targeted biopsy] to routine TRUS-guided RB [random biopsy] did not improve prostate CDR [cancer detection rate]in men with suspected PCa based on PSA values. Cognitive MP-MRI/TRUS fusion seems to be reliable [i.e., agreement between random and targeted biopsies] in terms of positive TBs.” [p. 424] |
| Panebianco, 201521 |
|---|
mpMRI followed by cognitive targeted + random biopsy vs. random biopsy alone
Notes:
| “The proportion of men with clinically significant PCa is higher among those randomized to mp-MRI/biopsy vs. those randomized to TRUS-guided biopsy” [p. 17.e6] |
CI = confidence interval; EQ-5D-5L = EuroQol 5 Dimensions 5 Levels Self Report Questionnaire; IQR = interquartile range; mpMRI = multiparametric MRI;MR = magnetic resonance imaging; PCa = prostate cancer; PSA = prostate-specific antigen; TRUS = transrectal ultrasound
Table 11Summary of Findings of Included Economic Evaluations
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Brown, 20186 |
|---|
Initial biopsy
mpMRI followed by TRUS-guided biopsy for clinically significant PCa
Testing cost: 581 (95% CI, 573 to 588) Cost following diagnosis: 4329 (95% CI, 3900 to 4814) Overall QALYs: 8.45 (95% CI, 8.15 to 8.78)
mpMRI followed by TRUS-guided biopsy for all PCa
Testing cost: 596 (95% CI, 592 to 600) Cost following diagnosis: 4351 (95% CI, 3926 to 4834) Overall QALYs: 8.46 (95% CI, 8.16 to 8.78)
TRUS biopsy alone
Testing cost: 415 (95% CI, 412 to 420) Cost following diagnosis: 4038 (95% CI, 3602 to 4537) Overall QALYs: 8.41 (95% CI, 8.11 to 8.74)
Repeat biopsy (no cancer detected on initial TRUS biopsy)
mpMRI followed by TRUS-guided biopsy for clinically significant PCa
Testing cost (includes initial biopsy): 707 (95% CI, 683 to 730) Cost following diagnosis: 4646 (95% CI, 4235 to 5077) Overall QALYs: 8.52 (95% CI, 8.23 to 8.82)
TRUS biopsy alone
Testing cost (includes initial biopsy): 627 (95% CI, 610 to 644) Cost following diagnosis: 4324 (95% CI, 3900 to 4798) Overall QALYs: 8.44 (95% CI, 8.14 to 8.75)
Notes
Costs are in £ Targeted biopsy could be cognitive or fusion biopsy Patients with at least one lesion on mpMRI with a minimum score of 2 out of 5 undergo TRUS-guided biopsy Clinically significant cancer is defined for mpMRI as lesion volume of ≥ 0.2 mL and/or Gleason score of ≥ 3 + 4 Clinically significant cancer is defined for TRUS-guided biopsy as dominant Gleason pattern of ≥ 4 and/or any Gleason pattern of ≥ 5 and/or cancer core length of ≥ 6 mm
| “The results from PROMIS suggest that a diagnostic strategy that incorporates mpMRI as an initial test in unscreened men referred for prostate biopsy may be useful in three ways. First, it is likely to reduce the proportion of men having unnecessary biopsies. Second, fewer men with clinically important prostate cancer will be missed. Third, the incorporation of mpMRI may enhance the cost-effectiveness of the prostate cancer diagnostic and therapeutic pathway.” [p. 107] |
| Pahwa, 201725 |
|---|
Strategy for MRI-detected lesions / strategy when no lesions detected on MRI
Standard biopsy without MRI
Cognitive biopsy / no biopsy
Fusion biopsy / no biopsy
Cognitive biopsy / standard biopsy
Fusion biopsy / standard biopsy
Sensitivity analyses
Targeted biopsy strategies outperformed standard biopsy strategy regardless of Gleason grade thresholds for clinically significant cancer Small improvements in sensitivity and specificity of MRI in the detection of clinically significant cancer made the use of contrast-enhanced MRI cost effective Analysis by age groups yielded similar results
Notes
NHB = Lifetime QALYs – (Lifetime cost / WTP threshold) A positive NHB indicates the intervention is cost effective given the WTP threshold A higher NHB indicates that an intervention is more cost effective WTP threshold was $50,000 Initial biopsy setting
| “In conclusion, our study evaluated MR imaging–guided strategies for the initial detection of prostate cancer. It shows that improvement in the detection of clinically significant prostate cancer by using MR imaging provides substantial benefit to the patient as measured by NHB” [p. 165] |
| Venderink, 201726 |
|---|
TRUS biopsy alone (reference case)
Cost: €2596 QALYs: 12.8162
MRI-US fusion targeted TRUS biopsy
Sensitivity analyses
Varying the assumptions based on expert opinion (cost and diagnostic accuracy parameters) did not change the outcome Varying the utility did not significantly change the outcome The outcome was most sensitive to survival after treatment of clinically significant prostate cancer and survival with untreated clinically insignificant prostate cancer (TRUS biopsy more cost-effective with yearly survival of 93.2% for the former or 96.5% for the latter)
Note: Repeat biopsy setting | “Taking the limitations into consideration, we conclude that MRI-TRUS fusion biopsy seems more cost-effective than TRUS-guided biopsy in a Dutch health care setting.” [p. 1063] |
| Cerantola, 201623 |
|---|
Cognitive targeted TRUS biopsy vs. TRUS biopsy alone
5-year time horizon
Cost: $7,231 vs. $8,027 QALYs: 4.29 vs. 4.25
10-year time horizon
15-year time horizon
20-year time horizon
MRI with cognitive targeted biopsy is the dominant strategy for the base case at each time horizon.
Sensitivity analyses
Varying discount rate (0 to 10%), active surveillance rate (10% to 25%), possibility of recurrence in intermediate-high-risk group (2.9% to 7.7%), and sets of utility values did not change the outcome (MRI with cognitive targeted biopsy still dominant)
Note: Initial biopsy setting | “The present model suggests that the integration of MRI and MRGTB [MRI-guided cognitive targeted biopsy] in PCa [prostate cancer] diagnosis and management is a cost-effective measure, with the MRGTB pathway being the dominant strategy at 5-, 10-, 15-, and 20-year horizon. Our study suggests that the adoption of MRI and MRGTB in clinical practice produces clinical benefits for patients at reduced costs for the health care system.” [p. 119.e8] |
| Lotan, 201524 |
|---|
mpMRI strategy with MRI-US fusion biopsy vs. TRUS systematic biopsy alone
PCa prevalence of 24% (base case)
Cost per 100 patients: $87,700 vs. $90,400 Number of biopsies avoided in mpMRI arm: 73.1 Number of patients with detected PCa: 16 vs. 20.4 Number of missed PCas: 8 vs. 3.6
PCa prevalence of 10% (lower bound of one-way sensitivity analysis)
Cost per 100 patients: $79,400 vs. $90,400 Number of biopsies avoided in mpMRI arm: 81.8 Number of patients with detected PCa: 6.7 vs. 8.5 Number of missed PCas: 3.3 vs. 1.5
PCa prevalence of 50% (upper bound of one-way sensitivity analysis)
Cost per 100 patients: $103,000 vs. $90,400 Number of biopsies avoided in mpMRI arm: 57 Number of patients with detected PCa: 33.3 vs. 42.5 Number of missed PCas: 16.7 vs. 7.5
Note: Repeat biopsy setting | “The use of MP-MRI to select patients for repeat biopsy reduced the number of biopsies needed by 73% and resulted in a small number of cancers being missed at almost equivalent cost compared with the TRUS biopsy arm. Further studies are required to determine whether those cancers missed represent clinically significant tumors.” [p. 266.e14] |
| Mowatt, 201313 |
|---|
Patients aged 60 years
Patients aged 60 years
Sensitivity analyses
When prevalence was assumed to be 10% (instead of 24%), T2-MRI dominated systematic TRUS-guided biopsy MRI is not cost-effective (at a WTP threshold of £30,000/QALY) in 70-year-olds when prevalence is 50% T2-MRI dominates and MRS is cost-effective compared to systematic TRUS biopsy in most sensitivity analyses MRI is not cost-effective when MRI-negative patients receive extended-cores systematic TRUS-guided biopsy
Notes:
| “To summarise, the level of uncertainty surrounding model inputs and structural assumptions makes it difficult to arrive at a definitive conclusion on the cost-effectiveness of using MRS/MRI techniques to aid the localisation of prostate abnormalities for biopsy. […] Data from subgroup analysis would also suggest that the use of more sensitive and more expensive sequences is more likely to be cost-effective in subgroups of patients who are more likely to be harbouring cancer.” [p. 87] |
CI = confidence interval; DCE = dynamic contrast-enhanced; DWI = diffusion-weighted imaging; ICER = incremental cost-effectiveness ratio; LY = life year; mpMRI = multiparametric magnetic resonance imaging; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; NHB: net health benefit; PCa = prostate cancer; QALY = quality-adjusted life year; TRUS = transrectal ultrasound; T2W = T2-weighted; WTP = willingness-to-pay.
Appendix 5. Overlap between Included Systematic Reviews
Table 12Primary Study Overlap between Included Systematic Reviews
View in own window
| Primary Study Citation | Systematic Review Citation |
|---|
| Tang, 201816 | Wang, 201818 | Gayet, 201614 | Schoots, 201515 | Wu, 201519 | Van Hove, 201417 |
|---|
| Alberts, 2016 | | ▪ | | | | |
| Baco, 2015 | | ▪ | | | ▪ | |
| Belas, 2012 | | | | ▪ | | |
| Borkowetz, 2015 | | | ▪ | | ▪ | |
| Cool, 2016 | ▪ | | | | | |
| Costa, 2013 | | | | ▪ | | |
| de Gorski, 2015 | | | | | ▪ | |
| Delongchamps, 2013 | | | ▪ | | ▪ | ▪ |
| Delongchamps, 2016 | ▪ | | | | | |
| Durmus, 2013 | | | | ▪ | | |
| Fiard, 2013 | ▪ | | ▪ | ▪ | ▪ | ▪ |
| Haffner, 2011 | | | | ▪ | | ▪ |
| Junker, 2015 | ▪ | | ▪ | | ▪ | |
| Kuru, 2013 | | | ▪ | | ▪ | |
| Labanaris, 2010 | | | | | | ▪ |
| Lee, 2012 | | | | | | ▪ |
| Mendhiratta, 2015 | ▪ | | | | | |
| Meng, 2016 | ▪ | | | | | |
| Miyagawa, 2010 | | | | | ▪ | ▪ |
| Mozer, 2014 | | | ▪ | ▪ | ▪ | ▪ |
| Park, 2008 | | | | ▪ | | |
| Park, 2011 | | | | ▪ | | ▪ |
| Pepe, 2013 | | | | | | ▪ |
| Peter, 2011 | | ▪ | | | | |
| Porpiglia, 2016 | | ▪ | | | | |
| Portalez, 2012 | | | | ▪ | | |
| Puech, 2013 | ▪ | | | ▪ | ▪ | ▪ |
| Rastinehad, 2014 | | ▪ | ▪ | ▪ | ▪ | ▪ |
| Rud, 2012 | | | | ▪ | | |
| Salami, 2015 | | | ▪ | | | |
| Sciarra, 2010 | | | | | | ▪ |
| Shoji, 2015 | | | ▪ | | | |
| Siddiqui, 2013 | ▪ | | | ▪ | | |
| Siddiqui, 2015 | ▪ | | ▪ | | ▪ | |
| Sonn, 2013 | | | | ▪ | ▪ | ▪ |
| Taverna, 2016 | | ▪ | | | | |
| Ukimura, 2015 | ▪ | | | | ▪ | |
| Vourganti, 2012 | ▪ | | | | ▪ | ▪ |
| Wysock, 2013 | | | ▪ | ▪ | ▪ | ▪ |
Appendix 6. Additional References of Potential Interest
Relevant Randomized Studies Reported in the Included Systematic Reviews and Therefore Excluded
Baco
E, Rud
E, Eri
LM, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy.
Eur Urol. 2016;69(1):149–156. [
PubMed: 25862143]
Porpiglia
F, Manfredi
M, Mele
F, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naive patients with suspected prostate cancer.
Eur Urol. 2017;72(2):282–288. [
PubMed: 27574821]
Taverna
G, Bozzini
G, Grizzi
F, et al. Endorectal multiparametric 3-tesla magnetic resonance imaging associated with systematic cognitive biopsies does not increase prostate cancer detection rate: a randomized prospective trial.
World J Urol. 2016;34(6):797–803. [
PubMed: 26481226]
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Magnetic resonance imaging for prostate assessment: a review of clinical and cost-effectiveness. Ottawa: CADTH; 2018 Sep. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.