NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.

PDQ Cancer Information Summaries [Internet].
Show detailsThis PDQ cancer information summary has current information about the treatment of endometrial cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Date Last Modified") is the date of the most recent change. The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
General Information About Endometrial Cancer
Key Points for This Section
Endometrial cancer is a disease in which malignant (cancer) cells form in the tissues of the endometrium.
The endometrium is the lining of the uterus, a hollow, muscular organ in a woman’s pelvis. The uterus is where a fetus grows. In most nonpregnant women, the uterus is about 3 inches long. The lower, narrow end of the uterus is the cervix, which leads to the vagina.
Anatomy of the female reproductive system. The organs in the female reproductive system include the uterus, ovaries, fallopian tubes, cervix, and vagina. The uterus has a muscular outer layer called the myometrium and an inner lining called the endometrium.
Cancer of the endometrium is different from cancer of the muscle of the uterus, which is called sarcoma of the uterus. See the PDQ summary on Uterine Sarcoma Treatment for more information about uterine sarcoma.
Obesity and having metabolic syndrome may increase the risk of endometrial cancer.
Anything that increases your chance of getting a disease is called a risk factor. Having a risk factor does not mean that you will get cancer; not having risk factors doesn't mean that you will not get cancer. Talk to your doctor if you think you may be at risk for endometrial cancer.Risk factors for endometrial cancer include the following:
- Taking estrogen-only hormone replacement therapy (HRT) after menopause.
- Having metabolic syndrome.
- Having type 2 diabetes.
- Exposure of endometrial tissue to estrogen made by the body. This may be caused by:
- -
Never giving birth.
- -
Menstruating at an early age.
- -
Starting menopause at a later age.
- Having polycystic ovary syndrome.
- Having a family history of endometrial cancer in a first-degree relative (mother, sister, or daughter).
- Having endometrial hyperplasia.
Older age is the main risk factor for most cancers. The chance of getting cancer increases as you get older.
Taking tamoxifen for breast cancer or taking estrogen alone (without progesterone) can increase the risk of endometrial cancer.
Endometrial cancer may develop in breast cancer patients who have been treated with tamoxifen. A patient who takes this drug and has abnormal vaginal bleeding should have a follow-up exam and a biopsy of the endometrial lining if needed. Women taking estrogen (a hormone that can affect the growth of some cancers) alone also have an increased risk of endometrial cancer. Taking estrogen combined with progesterone (another hormone) does not increase a woman’s risk of endometrial cancer.
Signs and symptoms of endometrial cancer include unusual vaginal bleeding or pain in the pelvis.
These and other signs and symptoms may be caused by endometrial cancer or by other conditions. Check with your doctor if you have any of the following:
Tests that examine the endometrium are used to diagnose endometrial cancer.
Because endometrial cancer begins inside the uterus, it does not usually show up in the results of a Pap test. For this reason, a sample of endometrial tissue must be removed and checked under a microscope to look for cancer cells. One of the following procedures may be used:
- Endometrial biopsy: The removal of tissue from the endometrium (inner lining of the uterus) by inserting a thin, flexible tube through the cervix and into the uterus. The tube is used to gently scrape a small amount of tissue from the endometrium and then remove the tissue samples. A pathologist views the tissue under a microscope to look for cancer cells.
- Dilatation and curettage: A procedure to remove samples of tissue from the inner lining of the uterus. The cervix is dilated and a curette (spoon-shaped instrument) is inserted into the uterus to remove tissue. The tissue samples are checked under a microscope for signs of disease. This procedure is also called a D&C.
- Hysteroscopy: A procedure to look inside the uterus for abnormal areas. A hysteroscope is inserted through the vagina and cervix into the uterus. A hysteroscope is a thin, tube-like instrument with a light and a lens for viewing. It may also have a tool to remove tissue samples, which are checked under a microscope for signs of cancer.
Other tests and procedures used to diagnose endometrial cancer include the following:
- Physical exam and health history: An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
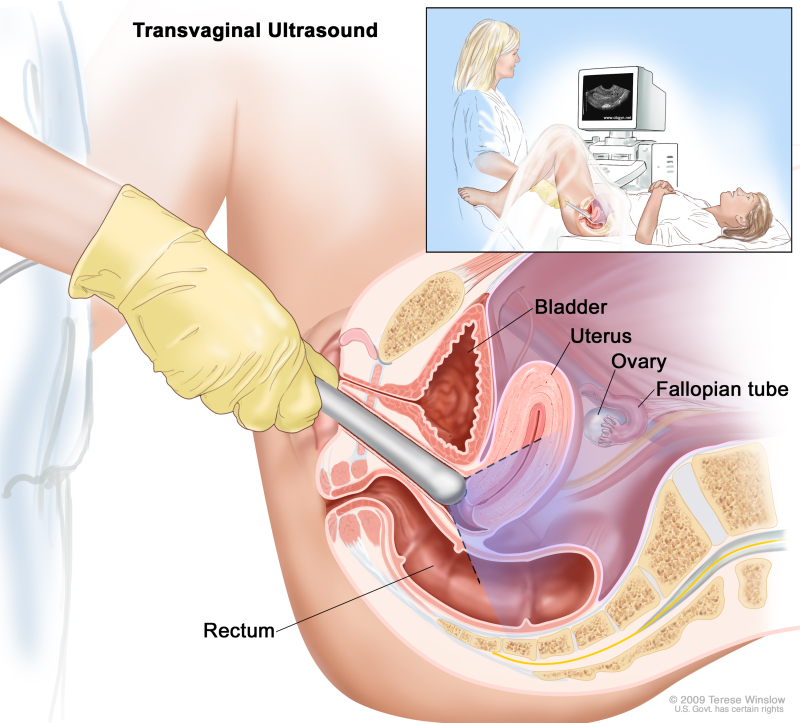
- Transvaginal ultrasound exam: A procedure used to examine the vagina, uterus, fallopian tubes, and bladder. An ultrasound transducer (probe) is inserted into the vagina and used to bounce high-energy sound waves (ultrasound) off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram. The doctor can identify tumors by looking at the sonogram.
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis and treatment options depend on the following:
- The stage of the cancer (whether it is in the endometrium only, involves the uterus wall, or has spread to other places in the body).
- How the cancer cells look under a microscope.
- Whether the cancer cells are affected by progesterone.
Endometrial cancer can usually be cured because it is usually diagnosed early.
Stages of Endometrial Cancer
Key Points for This Section
After endometrial cancer has been diagnosed, tests are done to find out if cancer cells have spread within the uterus or to other parts of the body.
The process used to find out whether the cancer has spread within the uterus or to other parts of the body is called staging. The information gathered from the staging process determines the stage of the disease. It is important to know the stage in order to plan treatment. Certain tests and procedures are used in the staging process. A hysterectomy (an operation in which the uterus is removed) will usually be done to treat endometrial cancer. Tissue samples are taken from the area around the uterus and checked under a microscope for signs of cancer to help find out whether the cancer has spread.
The following procedures may be used in the staging process:
- Pelvic exam: An exam of the vagina, cervix, uterus, fallopian tubes, ovaries, and rectum. A speculum is inserted into the vagina and the doctor or nurse looks at the vagina and cervix for signs of disease. A Pap test of the cervix is usually done. The doctor or nurse also inserts one or two lubricated, gloved fingers of one hand into the vagina and places the other hand over the lower abdomen to feel the size, shape, and position of the uterus and ovaries. The doctor or nurse also inserts a lubricated, gloved finger into the rectum to feel for lumps or abnormal areas.
Pelvic exam. A doctor or nurse inserts one or two lubricated, gloved fingers of one hand into the vagina and presses on the lower abdomen with the other hand. This is done to feel the size, shape, and position of the uterus and ovaries. The vagina, cervix, fallopian tubes, and rectum are also checked.
- Chest x-ray: An x-ray of the organs and bones inside the chest. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
- CT scan (CAT scan): A procedure that makes a series of detailed pictures of areas inside the body, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
- MRI (magnetic resonance imaging): A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body. This procedure is also called nuclear magnetic resonance imaging (NMRI).
- PET scan (positron emission tomography scan): A procedure to find malignant tumor cells in the body. A small amount of radioactive glucose (sugar) is injected into a vein. The PET scanner rotates around the body and makes a picture of where glucose is being used in the body. Malignant tumor cells show up brighter in the picture because they are more active and take up more glucose than normal cells do.
- Lymph node dissection: A surgical procedure in which the lymph nodes are removed from the pelvic area and a sample of tissue is checked under a microscope for signs of cancer. This procedure is also called lymphadenectomy.
There are three ways that cancer spreads in the body.
Cancer can spread through tissue, the lymph system, and the blood:
- Tissue. The cancer spreads from where it began by growing into nearby areas.
- Lymph system. The cancer spreads from where it began by getting into the lymph system. The cancer travels through the lymph vessels to other parts of the body.
- Blood. The cancer spreads from where it began by getting into the blood. The cancer travels through the blood vessels to other parts of the body.
Cancer may spread from where it began to other parts of the body.
When cancer spreads to another part of the body, it is called metastasis. Cancer cells break away from where they began (the primary tumor) and travel through the lymph system or blood.
- Lymph system. The cancer gets into the lymph system, travels through the lymph vessels, and forms a tumor (metastatic tumor) in another part of the body.
- Blood. The cancer gets into the blood, travels through the blood vessels, and forms a tumor (metastatic tumor) in another part of the body.
The metastatic tumor is the same type of cancer as the primary tumor. For example, if endometrial cancer spreads to the lung, the cancer cells in the lung are actually endometrial cancer cells. The disease is metastatic endometrial cancer, not lung cancer.
metastasis: how cancer spreads
Many cancer deaths are caused when cancer moves from the original tumor and spreads to other tissues and organs. This is called metastatic cancer. This animation shows how cancer cells travel from the place in the body where they first formed to other parts of the body.
The following stages are used for endometrial cancer:
Stage I
Stage IA and stage IB endometrial cancer. In stage IA, cancer is in the endometrium only or less than halfway through the myometrium (the muscle layer of the uterus). In stage IB, cancer has spread halfway or more into the myometrium.
In stage I, cancer is found in the uterus only. Stage I is divided into stages IA and IB, based on how far the cancer has spread.
- Stage IA: Cancer is in the endometrium only or less than halfway through the myometrium (muscle layer of the uterus).
Stage II
Stage II endometrial cancer. Cancer has spread into connective tissue of the cervix, but has not spread outside the uterus.
In stage II, cancer has spread into connective tissue of the cervix, but has not spread outside the uterus.
Stage III
In stage III, cancer has spread beyond the uterus and cervix, but has not spread beyond the pelvis. Stage III is divided into stages IIIA, IIIB, and IIIC, based on how far the cancer has spread within the pelvis.
- Stage IIIA: Cancer has spread to the outer layer of the uterus and/or to the fallopian tubes, ovaries, and ligaments of the uterus.
- Stage IIIB: Cancer has spread to the vagina and/or to the parametrium (connective tissue and fat around the uterus).
- Stage IIIC: Cancer has spread to lymph nodes in the pelvis and/or around the aorta (largest artery in the body, which carries blood away from the heart).
Endometrial cancer may be grouped for treatment as follows:
Low-risk endometrial cancer
Grades 1 and 2 tumors are usually considered low-risk. They usually do not spread to other parts of the body.
High-risk endometrial cancer
Grade 3 tumors are considered high-risk. They often spread to other parts of the body. Uterine papillary serous, clear cell, and carcinosarcoma are three subtypes of endometrial cancer that are considered grade 3.
Endometrial cancer can recur (come back) after it has been treated.
The cancer may come back in the uterus, the pelvis, in lymph nodes in the abdomen, or in other parts of the body.
Treatment Option Overview
Key Points for This Section
There are different types of treatment for patients with endometrial cancer.
Different types of treatment are available for patients with endometrial cancer. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Five types of standard treatment are used:
Surgery
Surgery (removing the cancer in an operation) is the most common treatment for endometrial cancer. The following surgical procedures may be used:
- Total hysterectomy: Surgery to remove the uterus, including the cervix. If the uterus and cervix are taken out through the vagina, the operation is called a vaginal hysterectomy. If the uterus and cervix are taken out through a large incision (cut) in the abdomen, the operation is called a total abdominal hysterectomy. If the uterus and cervix are taken out through a small incision (cut) in the abdomen using a laparoscope, the operation is called a total laparoscopic hysterectomy.
Hysterectomy. The uterus is surgically removed with or without other organs or tissues. In a total hysterectomy, the uterus and cervix are removed. In a total hysterectomy with salpingo-oophorectomy, (a) the uterus plus one (unilateral) ovary and fallopian tube are removed; or (b) the uterus plus both (bilateral) ovaries and fallopian tubes are removed. In a radical hysterectomy, the uterus, cervix, both ovaries, both fallopian tubes, and nearby tissue are removed. These procedures are done using a low transverse incision or a vertical incision.
- Radical hysterectomy: Surgery to remove the uterus, cervix, and part of the vagina. The ovaries, fallopian tubes, or nearby lymph nodes may also be removed.
- Lymph node dissection: A surgical procedure in which the lymph nodes are removed from the pelvic area and a sample of tissue is checked under a microscope for signs of cancer. This procedure is also called lymphadenectomy.
After the doctor removes all the cancer that can be seen at the time of the surgery, some patients may be given radiation therapy or hormone treatment after surgery to kill any cancer cells that are left. Treatment given after the surgery, to lower the risk that the cancer will come back, is called adjuvant therapy.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. There are two types of radiation therapy:
- External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
- Internal radiation therapy uses a radioactive substance sealed in needles, seeds, wires, or catheters that are placed directly into or near the cancer.
The way the radiation therapy is given depends on the type and stage of the cancer being treated. External and internal radiation therapy are used to treat endometrial cancer, and may also be used as palliative therapy to relieve symptoms and improve quality of life.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping the cells from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). When chemotherapy is placed directly into the cerebrospinal fluid, an organ, or a body cavity such as the abdomen, the drugs mainly affect cancer cells in those areas (regional chemotherapy).
The way the chemotherapy is given depends on the type and stage of the cancer being treated.
Hormone therapy
Hormone therapy is a cancer treatment that removes hormones or blocks their action and stops cancer cells from growing. Hormones are substances made by glands in the body and circulated in the bloodstream. Some hormones can cause certain cancers to grow. If tests show that the cancer cells have places where hormones can attach (receptors), drugs, surgery, or radiation therapy is used to reduce the production of hormones or block them from working.
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do. Monoclonal antibodies, mTOR inhibitors, and signal transduction inhibitors are three types of targeted therapy used to treat endometrial cancer.
- Monoclonal antibody therapy: Monoclonal antibodies are immune system proteins made in the laboratory to treat many diseases, including cancer. As a cancer treatment, these antibodies can attach to a specific target on cancer cells or other cells that may help cancer cells grow. The antibodies are able to then kill the cancer cells, block their growth, or keep them from spreading. Monoclonal antibodies are given by infusion. They may be used alone or to carry drugs, toxins, or radioactive material directly to cancer cells. Bevacizumab is used to treat stage III, stage IV, and recurrent endometrial cancer.
monoclonal antibodies: how monoclonal antibodies treat cancer
How do monoclonal antibodies work to treat cancer? This video shows how monoclonal antibodies, such as trastuzumab, pembrolizumab, and rituximab, block molecules cancer cells need to grow, flag cancer cells for destruction by the body’s immune system, or deliver harmful substances to cancer cells.
- mTOR inhibitor therapy: mTOR inhibitors block a protein called mTOR, which helps control cell division. mTOR inhibitors may keep cancer cells from growing and prevent the growth of new blood vessels that tumors need to grow. Everolimus and ridaforolimus are used to treat stage III, stage IV, and recurrent endometrial cancer.
New types of treatment are being tested in clinical trials.
Information about clinical trials is available from the NCI website.
Treatment for endometrial cancer may cause side effects.
For information about side effects caused by treatment for cancer, visit our Side Effects page.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
As you go through treatment, you will have follow-up tests or check-ups. Some tests that were done to diagnose or stage the cancer may be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back).
Treatment of Stage I and Stage II Endometrial Cancer
For information about the treatments listed below, see the Treatment Option Overview section.
Low-risk endometrial cancer (grade 1 or grade 2)
Treatment of low-risk stage I endometrial cancer and stage II endometrial cancer may include the following:
- Surgery (total hysterectomy and bilateral salpingo-oophorectomy). Lymph nodes in the pelvis and abdomen may also be removed and viewed under a microscope to check for cancer cells.
- Surgery (total hysterectomy and bilateral salpingo-oophorectomy, with or without removal of lymph nodes in the pelvis and abdomen) followed by internal radiation therapy. In certain cases, external radiation therapy to the pelvis may be used in place of internal radiation therapy.
- Radiation therapy alone for patients who cannot have surgery.
- A clinical trial of a new chemotherapy regimen.
If cancer has spread to the cervix, a radical hysterectomy with bilateral salpingo-oophorectomy may be done.
High-risk endometrial cancer (grade 3)
Treatment of high-risk stage I endometrial cancer and stage II endometrial cancer may include the following:
- Surgery (radical hysterectomy and bilateral salpingo-oophorectomy). Lymph nodes in the pelvis and abdomen may also be removed and viewed under a microscope to check for cancer cells.
- Surgery (radical hysterectomy and bilateral salpingo-oophorectomy) followed by chemotherapy and sometimes radiation therapy.
- A clinical trial of a new chemotherapy regimen.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Stage III, Stage IV, and Recurrent Endometrial Cancer
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage III endometrial cancer, stage IV endometrial cancer, and recurrent endometrial cancer may include the following:
- Surgery (radical hysterectomy and removal of lymph nodes in the pelvis so they can be viewed under a microscope to check for cancer cells) followed by adjuvant chemotherapy and/or radiation therapy.
- Chemotherapy and internal and external radiation therapy for patients who cannot have surgery.
- Hormone therapy for patients who cannot have surgery or radiation therapy.
- Targeted therapy with mTOR inhibitors (everolimus or ridaforolimus) or a monoclonal antibody (bevacizumab).
- A clinical trial of a new treatment regimen that may include combination chemotherapy, targeted therapy, such as an mTOR inhibitor (everolimus) or signal transduction inhibitor (metformin), and/or hormone therapy, for patients with advanced or recurrent endometrial cancer.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
To Learn More About Endometrial Cancer
For more information from the National Cancer Institute about endometrial cancer, see the following:
For general cancer information and other resources from the National Cancer Institute, visit:
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of endometrial cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Endometrial Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/uterine/patient/endometrial-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389334]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.
- Review Endometrial Cancer Prevention (PDQ®): Patient Version.[PDQ Cancer Information Summari...]Review Endometrial Cancer Prevention (PDQ®): Patient Version.PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Endometrial Cancer Screening (PDQ®): Patient Version.[PDQ Cancer Information Summari...]Review Endometrial Cancer Screening (PDQ®): Patient Version.PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Melanoma Treatment (PDQ®): Patient Version.[PDQ Cancer Information Summari...]Review Melanoma Treatment (PDQ®): Patient Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Gallbladder Cancer Treatment (PDQ®): Patient Version.[PDQ Cancer Information Summari...]Review Gallbladder Cancer Treatment (PDQ®): Patient Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Urethral Cancer Treatment (PDQ®): Patient Version.[PDQ Cancer Information Summari...]Review Urethral Cancer Treatment (PDQ®): Patient Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Endometrial Cancer Treatment (PDQ®) - PDQ Cancer Information SummariesEndometrial Cancer Treatment (PDQ®) - PDQ Cancer Information Summaries
- AGENCOURT_13322109 NICHD_XGC_Tad1 Xenopus laevis cDNA clone IMAGE:6880130 3', mR...AGENCOURT_13322109 NICHD_XGC_Tad1 Xenopus laevis cDNA clone IMAGE:6880130 3', mRNA sequencegi|29479840|gnl|dbEST|17305392|gb|C 10.1|Nucleotide
- Endometrial Cancer Screening (PDQ®) - PDQ Cancer Information SummariesEndometrial Cancer Screening (PDQ®) - PDQ Cancer Information Summaries
Your browsing activity is empty.
Activity recording is turned off.
See more...