This is a work of the US government and distributed under the terms of the Public Domain
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
SUMMARY
Background:
Sodium bromate is a by-product of water disinfection. We tested if sodium bromate could cause cancer in two different strains of genetically modified mice.
Methods:
We applied solutions containing sodium bromate to the backs of male and female Tg.AC mice for 6 or 9 months, and gave sodium bromate dissolved in drinking water to male and female Tg.AC and p53 mice for 6 or 10 months. The ethanol/water vehicle without any chemical was applied to the backs of control mice in the dermal studies, and animals given plain water served as the control groups for the drinking water studies. Tissues from 15 sites were examined for every animal.
Results:
Exposure to sodium bromate either through the skin or by drinking water decreased body weights in groups given the highest concentrations. No increases in tumors were seen in males or females from either strain of mice.
Conclusions:
We conclude that sodium bromate did not cause cancer in the genetically modified mice used in these studies. This chemical did cause cancer in other studies with different rodents, and thus these genetically modified mice may not be as sensitive for detecting certain cancer-causing compounds.
ABSTRACT
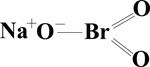
SODIUM BROMATE
CAS No. 7789-38-0
Chemical Formula: NaBrO3 Molecular Weight: 150.9
Synonyms: Bromic acid, sodium salt
Trade names: Dyetone, Neutralizer K-126, Neutralizer K-140, Neutralizer K-938
Bromate is a drinking water disinfection by-product formed during the ozonation of source water containing bromide. Sodium bromate is also used as an analytical reagent, in the oxidation of sulfur and vat dyes, and for cleaning boilers. As a mixture with sodium bromide, it is used for dissolving gold from its ores. The cosmetic industry uses sodium bromate and potassium bromate as neutralizers or oxidizers in hair wave preparations. Sodium bromate was nominated for toxicity and carcinogenicity studies in transgenic mouse models by the United States Environmental Protection Agency and the National Institute of Environmental Health Sciences. Male and female Tg.AC hemizygous mice received sodium bromate by dermal application for 26 or 39 weeks and by exposure in drinking water for 27 or 43 weeks. Male and female p53 haploinsufficient mice were exposed to sodium bromate (at least 99% pure) in drinking water for 27 or 43 weeks. Genetic toxicology studies were conducted in mouse peripheral blood erythrocytes.
26- and 39-Week Dermal Studies in Tg.AC Hemizygous Mice:
Groups of 15 male and 15 female Tg.AC hemizygous mice received dermal applications of 0, 64, 128, or 256 mg sodium bromate/kg body weight in ethanol/water, 5 days per week for 26 weeks. Additional groups of 10 male and 10 female Tg.AC hemizygous mice were dermally administered the same doses for 39 weeks. Survival of dosed groups was similar to that of vehicle control groups at 26 and 39 weeks. Mean body weights of 256 mg/kg males were less than those of the vehicle control group in both studies. Mean body weights of all dosed groups of females were less than those of the vehicle controls at 39 weeks.
Minimal decreases in hematocrit and hemoglobin concentration values occurred in 128 mg/kg females and 256 mg/kg males and females at 26 weeks. A minimal decrease in erythrocyte count also occurred in 256 mg/kg males. These decreases in erythron were accompanied by a minimal decrease in mean cell hemoglobin and mean cell hemoglobin concentration values, primarily in the females. Reticulocyte counts were significantly increased in 128 mg/kg females and 256 mg/kg males and females.
There were no increased incidences of neoplasms in male or female Tg.AC hemizygous mice exposed to sodium bromate dermally.
Relative kidney weights were significantly increased in 256 mg/kg males at 26 weeks and in all dosed groups of males at 39 weeks. Absolute testis weights in 256 mg/kg males and absolute kidney weights in 256 mg/kg females were decreased at 39 weeks. Nephropathy occurred in 14 of 15 males receiving 128 and 256 mg/kg at 26 weeks and in all 256 mg/kg females in both studies. In the thyroid gland, the incidences of follicular cell hypertrophy in all dosed groups of males and females, follicular secretory depletion in 128 and 256 mg/kg females, and lymphocytic cellular infiltrate in 256 mg/kg females were significantly increased in both studies. Splenic hematopoietic cell proliferation occurred with a significantly increased incidence in 128 and 256 mg/kg females at 26 weeks. The incidence of germinal epithelium degeneration in the testis was significantly increased in 256 mg/kg males at 39 weeks.
27- and 43-Week Drinking Water Studies in Tg.AC Hemizygous Mice:
Groups of 15 male and 15 female Tg.AC hemizygous mice were exposed to drinking water containing 0, 80, 400, or 800 mg/L sodium bromate for 27 weeks (equivalent to average daily doses of approximately 13, 63, and 129 mg/kg to male mice and 15, 72, and 148 mg/kg to female mice). Additional groups of 10 male and 10 female Tg.AC hemizygous mice were exposed to drinking water containing 0, 80, 400, or 800 mg/L sodium bromate for 43 weeks (equivalent to average daily doses of approximately 11, 52, and 131 mg/kg to male mice and 15, 65, and 152 mg/kg to female mice). Survival of exposed groups was similar to that of control groups at 27 weeks. Survival was decreased in 400 mg/L females and 800 mg/L males and females at 43 weeks. Mean body weights of 400 mg/L males and 800 mg/L males and females were less than those of the control groups in both studies. Water consumption by exposed mice was generally similar to that by control groups throughout both studies.
Minimal decreases in hematocrit, hemoglobin concentration, and erythrocyte count values occurred primarily in 400 and 800 mg/kg females at 27 weeks. There were also decreases in mean cell hemoglobin and mean cell hemoglobin concentration values, but these occurred primarily in treated males. Reticulocyte counts were increased in 400 mg/kg males and 800 mg/kg males and females.
There were no increased incidences of neoplasms in male or female Tg.AC hemizygous mice exposed to sodium bromate in the drinking water.
Absolute kidney weights were significantly decreased in 800 mg/L females and relative kidney weights were increased in 400 and 800 mg/L males at 27 weeks. Absolute testis weights were significantly decreased in 800 mg/L males at 43 weeks. Thyroid gland follicular cell hypertrophy and follicular secretory depletion occurred in most 400 and 800 mg/L males and females at 27 weeks and in most exposed females at 43 weeks, and the incidences of thyroid gland follicular cell hypertrophy were significantly increased in all exposed groups of males at 43 weeks. The incidences of thyroid gland lymphocytic cellular infiltrates were significantly increased in 400 and 800 mg/L females in both studies and in 800 mg/L males at 43 weeks. The incidences of nephropathy were significantly increased in all exposed groups of males and in 400 and 800 mg/L females at 27 weeks. Renal tubule degeneration occurred with significantly increased incidences in 800 mg/L males and females in both studies. The incidences of renal tubule hypertrophy were significantly increased in 400 and 800 mg/L females at 27 weeks and in 800 mg/L males and females at 43 weeks. Pituitary gland pars distalis hypertrophy occurred with a significantly increased incidence in 800 mg/L females in both studies. The incidence of hyperkeratosis of the forestomach epithelium was significantly increased in 800 mg/L females at 43 weeks. The incidences of tubule degeneration of the epididymis and germinal epithelium degeneration of the testis were significantly increased in 800 mg/L males at 43 weeks.
27- and 43-Week Drinking Water Studies in P53 Haploinsufficient Mice:
Groups of 15 male and 15 female p53 haploinsufficient mice were exposed to drinking water containing 0, 80, 400, or 800 mg/L sodium bromate for 27 weeks (equivalent to average daily doses of approximately 8, 39, and 74 mg/kg to males and 13, 72, and 136 mg/kg to females). Additional groups of 10 male and 10 female p53 haploinsufficient mice were exposed to drinking water containing 0, 80, 400, or 800 mg/L sodium bromate for 43 weeks (equivalent to average daily doses of approximately 7, 37, and 65 mg/kg to males and 11, 58, and 107 mg/kg to females). In both studies, survival of exposed groups was similar to that of control groups. Mean body weights of 400 and 800 mg/L females were less than those of the control groups during most of the studies. Water consumption by exposed mice was generally similar to that by control groups in both studies. No neoplasms or nonneoplastic lesions in male or female p53 haploinsufficient mice were attributed to exposure to sodium bromate in either study.
Genetic Toxicology:
Sodium bromate exposure resulted in significantly increased frequencies of micronucleated erythrocytes in male and female Tg.AC hemizygous and p53 haploinsufficient mice administered the chemical in drinking water for 27 weeks or by dermal application for 26 weeks. Tg.AC hemizygous mice were treated by both routes; p53 haploinsufficient mice were exposed only through drinking water. In all three micronucleus tests, a clear dose response was observed in male and female mice. Significant increases in the percentage of polychromatic erythrocytes among total erythrocytes were observed in male and female Tg.AC hemizygous mice exposed via drinking water and in male Tg.AC hemizygous mice dosed dermally with sodium bromate. The percentage of polychromatic erythrocytes was not significantly altered in male or female p53 mice.
Conclusions:
Under the conditions of these drinking water studies, there was no evidence of carcinogenic activity* of sodium bromate in male or female p53 haploinsufficient mice exposed to 80, 400, or 800 mg/L for 27 or 43 weeks.
No treatment-related neoplasms were seen in male or female Tg.AC hemizygous mice exposed dermally to 64, 128, or 256 mg sodium bromate/kg body weight for 26 or 39 weeks.
No treatment-related neoplasms were seen in male or female Tg.AC hemizygous mice exposed by drinking water to 80, 400, or 800 mg sodium bromate/L for 27 or 43 weeks.
In drinking water and dermal studies in Tg.AC hemizygous mice there were increased incidences of nonneoplastic lesions in the thyroid gland and kidney.
Summary of the 26- and 39-Week Dermal and Genetic Toxicology Studies of Sodium Bromate in Tg.AC Hemizygous Mice
| Males | Females | |||
|---|---|---|---|---|
| 26 Weeks | 39 Weeks | 26 Weeks | 39 Weeks | |
| Doses in ethanol/water | Vehicle control, 64, 128, or 256 mg/kg | Vehicle control, 64, 128, or 256 mg/kg | Vehicle control, 64, 128, or 256 mg/kg | Vehicle control, 64, 128, or 256 mg/kg |
| Body weights | 256 mg/kg group less than the vehicle control group | 128 and 256 mg/kg groups less than the vehicle control group | Dosed groups similar to the vehicle control group | Dosed groups less than the vehicle control group |
| Survival rates | 12/15, 11/15, 13/15, 15/15 | 8/10, 8/10, 8/10, 8/10 | 11/15, 10/15, 11/15, 11/15 | 7/10, 7/10, 8/10, 8/10 |
| Nonneoplastic effects | Thyroid gland: follicular cell hypertrophy (0/15, 7/15, 10/15, 14/15) Kidney: nephropathy (8/15, 8/15, 14/15, 14/15) | Thyroid gland: follicular cell hypertrophy (0/10, 9/10, 8/10, 8/10) Testes: gernimal epithelium degeneration (1/19, 2/10, 3/10, 6/10) | Thyroid gland: follicular cell hypertrophy (1/15, 9/15, 12/15, 13/15); follicular secretory depletion (6/15, 11/15, 13/15, 14/15); lymphocyte cellular infiltrates (0/15, 6/15, 3/15, 12/15) Kidney: nephropathy (8/15, 7/15, 13/15, 15/15) Spleen: hematopoietic cell proliferation (3/15, 5/15, 9/15, 10/15) | Thyroid gland: follicular cell hypertrophy (1/9, 9/10, 9/10, 10/10); follicular secretory depletion (5/9, 8/10, 10/10, 10/10); lymphocyte cellular infiltrates (0/9, 2/10, 5/10, 10/10) Kidney: nephropathy (5/9, 6/10, 8/10, 10/10) |
| Neoplastic effects | None | None | None | None |
| Genetic toxicology | ||||
| Micronucleated erythrocytes | ||||
| Mouse peripheral blood in vivo: | Positive in male and female Tg.AC hemizygous mice | |||
Summary of the 27- and 43-Week Drinking Water and Genetic Toxicology Studies of Sodium Bromate in Tg.AC Hemizygous Mice
| Males | Females | |||
|---|---|---|---|---|
| 27 Weeks | 43 Weeks | 27 Weeks | 43 Weeks | |
| Concentrations in water | 0, 80, 400, or 800 mg/L | 0, 80, 400, or 800 mg/L | 0, 80, 400, or 800 mg/L | 0, 80, 400, or 800 mg/L |
| Body weights | 400 and 800 mg/L groups less than the control group | 400 and 800 mg/L groups less than the control group | 800 mg/L group less than the control group | 80 and 800 mg/L groups less than the control group |
| Survival rates | 13/15, 13/15, 14/15, 14/15 | 6/10, 7/10, 6/10, 4/10 | 10/15, 9/15, 10/15, 12/15 | 7/10, 5/10, 4/10, 2/10 |
| Nonneoplastic effects | Thyroid gland: follicular cell hypertrophy (1/15, 2/14, 12/15, 15/15); follicular secretory depletion (4/15, 6/14, 15/15, 15/15) Kidney: nephropathy (1/15, 7/15, 10/15, 14/15); renal tubule degeneration (0/15, 0/15, 0/15, 10/15) | Thyroid gland: follicular cell hypertrophy (0/10, 6/10, 8/10, 8/9); lymphocytic cellular infiltrates (0/10, 0/10, 0/10, 4/9) Kidney: renal tubule degeneration (0/10, 0/10, 1/10, 8/10); renal tubule hypertrophy (0/10, 0/10, 1/10, 6/10) Epididymis: degeneration (1/10, 1/10, 0/10, 7/10) Testes: germinal epithelium degeneration (0/10, 0/10, 3/10, 8/10) | Thyroid gland: follicular cell hypertrophy (2/15, 2/13, 11/13, 13/15); follicular secretory depletion (7/15, 7/13, 11/13, 14/15); lymphocytic cellular infiltrates (0/15, 0/13, 5/13, 11/15) Kidney: nephropathy (2/15, 2/15, 10/15, 13/15); renal tubule degeneration (0/15, 0/15, 2/15, 8/15); renal tubule hypertrophy (0/15, 1/15, 5/15, 12/15) Pituitary gland: pars distalis hypertrophy (0/15, 0/15, 0/15, 6/15) | Thyroid gland: follicular cell hypertrophy (0/10, 8/9, 10/10, 10/10); follicular secretory depletion (1/10, 8/9, 9/10, 10/10); lymphocytic cellular infiltrates (0/10, 2/9, 7/10, 8/10) Kidney: renal tubule degeneration (0/10, 0/10, 0/10, 7/10); renal tubule hypertrophy (0/10, 0/10, 2/10, 5/10) Pituitary gland: pars distalis hypertrophy (0/10, 0/10, 2/9, 6/10) |
| Neoplastic effects | None | None | None | None |
| Genetic toxicology | ||||
| Micronucleated erythrocytes | ||||
| Mouse peripheral blood in vivo: | Positive in male and female Tg.AC hemizygous mice | |||
Summary of the 27- and 43-Week Carcinogenesis and Genetic Toxicology Studies of Sodium Bromate in p53 Haploinsufficient Mice
| Males | Females | |||
|---|---|---|---|---|
| 27 Weeks | 43 Weeks | 27 Weeks | 43 Weeks | |
| Concentrations in water | 0, 80, 400, or 800 mg/L | 0, 80, 400, or 800 mg/L | 0, 80, 400, or 800 mg/L | 0, 80, 400, or 800 mg/L |
| Body weights | Exposed groups similar to the control group | Exposed groups similar to the control group | 400 and 800 mg/L groups less than the control group | 400 and 800 mg/L groups less than the control group |
| Survival rates | 14/15, 14/15, 14/15, 14/15 | 9/10, 8/10, 8/10, 9/10 | 14/15, 15/15, 15/15, 14/15 | 9/10, 9/10, 10/10, 10/10 |
| Nonneoplastic effects | None | None | None | None |
| Neoplastic Effects | None | None | None | None |
| Level of evidence of carcinogenic activity | No evidence | No evidence | No evidence | No evidence |
| Genetic toxicology | ||||
| Micronucleated erythrocytes | ||||
| Mouse peripheral blood in vivo: | Positive in male and female p53 haploinsufficient mice | |||
- *
Explanation of Levels of Evidence of Carcinogenic Activity is on page 11. A summary of the Technical Reports Review Subcommittee comments and the public the discussion on this Report appears on page 13.
Contents
- FOREWORD
- CONTRIBUTORS
- EXPLANATION OF LEVELS OF EVIDENCE OF CARCINOGENIC ACTIVITY
- NATIONAL TOXICOLOGY PROGRAM BOARD OF SCIENTIFIC COUNSELORS TECHNICAL REPORTS REVIEW SUBCOMMITTEE
- SUMMARY OF TECHNICAL REPORTS REVIEW SUBCOMMITTEE COMMENTS
- INTRODUCTION
- MATERIALS AND METHODS
- RESULTS
- 26-Week Dermal Study in Tg.AC Hemizygous Mice
- 39-Week Dermal Study in Tg.AC Hemizygous Mice
- 27-Week Drinking Water Study in Tg.AC Hemizygous Mice
- 43-Week Drinking Water Study in Tg.AC Hemizygous Mice
- 27-Week Drinking Water Study in p53 Haploinsufficient Mice
- 43-Week Drinking Water Study
- Genetic Toxicology
- DISCUSSION AND CONCLUSIONS
- REFERENCES
- APPENDIX A. SUMMARY OF LESIONS IN Tg.AC HEMIZYGOUS MICE IN THE DERMAL STUDIES OF SODIUM BROMATE
- APPENDIX B. SUMMARY OF LESIONS IN Tg.AC HEMIZYGOUS MICE IN THE DRINKING WATER STUDIES OF SODIUM BROMATE
- APPENDIX C. SUMMARY OF LESIONS IN p53 HAPLOINSUFFICIENT MICE IN THE DRINKING WATER STUDIES OF SODIUM BROMATE
- APPENDIX D. GENETIC TOXICOLOGY
- APPENDIX E. CLINICAL PATHOLOGY RESULTS
- APPENDIX F. ORGAN WEIGHTS AND ORGAN-WEIGHT-TO-BODY-WEIGHT RATIOS
- APPENDIX G. CHEMICAL CHARACTERIZATION AND DOSE FORMULATION STUDIES
- APPENDIX H. WATER AND COMPOUND CONSUMPTION IN THE 27- AND 43-WEEK DRINKING WATER STUDIES OF SODIUM BROMATE
About the Series
- NLM CatalogRelated NLM Catalog Entries
- NTP Genetically Modified Model Report on the Toxicology Studies of Sodium Bromat...NTP Genetically Modified Model Report on the Toxicology Studies of Sodium Bromate (CASRN 7789-38-0) in Genetically Modified (FVB Tg.AC Hemizygous) Mice (Dermal and Drinking Water Studies) and Carcinogenicity Studies of Sodium Bromate in Genetically Modified [B6.129-Trp53tm1Brd (N5) Haploinsufficient] Mice (Drinking Water Studies)
Your browsing activity is empty.
Activity recording is turned off.
See more...
![Cover of NTP Genetically Modified Model Report on the Toxicology Studies of Sodium Bromate (CASRN 7789-38-0) in Genetically Modified (FVB Tg.AC Hemizygous) Mice (Dermal and Drinking Water Studies) and Carcinogenicity Studies of Sodium Bromate in Genetically Modified [B6.129-Trp53tm1Brd (N5) Haploinsufficient] Mice (Drinking Water Studies)](/corehtml/pmc/pmcgifs/bookshelf/thumbs/th-ntpgmmr6-lrg.png)