This is a work of the US government and distributed under the terms of the Public Domain
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chhabra RS, Mahler J, Bristol DW, et al. NTP Genetically Modified Model Report on the Toxicology Studies of Pentaerythritol Triacrylate (Technical Grade) (CASRN 3524-68-3) in F344/N Rats, B6C3F1 Mice, and Genetically Modified (FVB Tg.AC Hemizygous) Mice (Dermal Studies): NTP GMM 04 [Internet]. Research Triangle Park (NC): National Toxicology Program; 2005 Oct.

NTP Genetically Modified Model Report on the Toxicology Studies of Pentaerythritol Triacrylate (Technical Grade) (CASRN 3524-68-3) in F344/N Rats, B6C3F1 Mice, and Genetically Modified (FVB Tg.AC Hemizygous) Mice (Dermal Studies): NTP GMM 04 [Internet].
Show detailsProcurement and Characterization
Pentaerythritol Triacrylate
Pentaerythritol triacrylate was obtained in two lots. Lot 05318JW, obtained from Aldrich Chemical Company (Milwaukee, WI), was used during the 2-week studies, and lot HCC0340, obtained from Sartomer Company (Exton, PA), was used during the 3- and 6-month studies. Identity, moisture content, purity, and stability analyses were conducted by the analytical chemistry laboratories and the study laboratory (Battelle Columbus Laboratories, Columbus, OH). Reports on analyses performed in support of the pentaerythritol triacrylate studies are on file at the National Institute of Environmental Health Sciences.
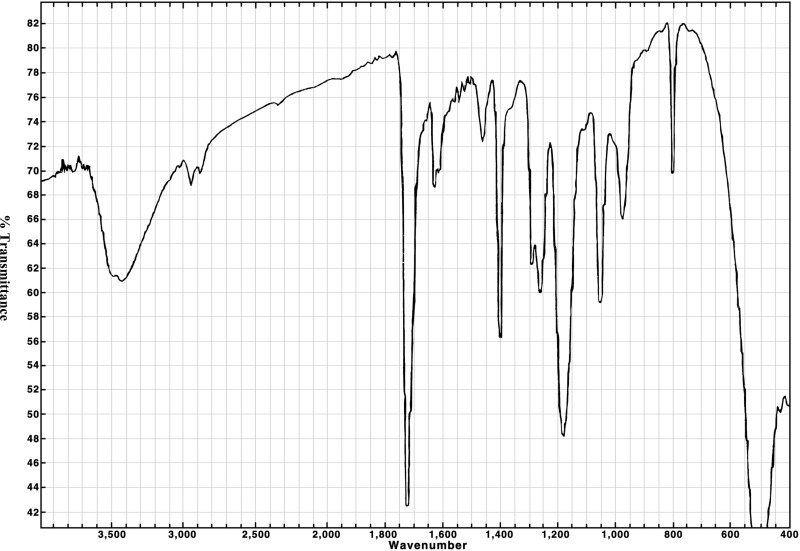
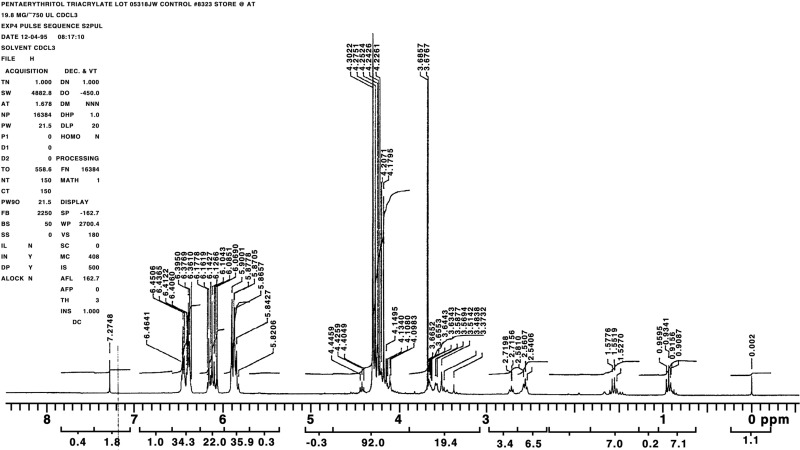
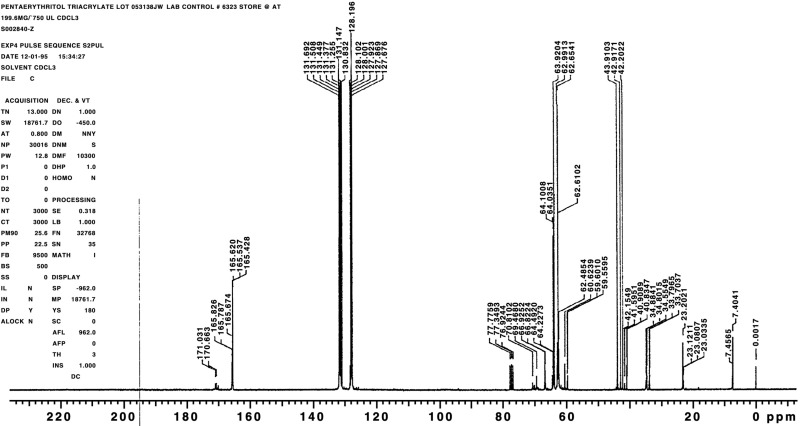
The chemical, a clear, viscous liquid, was identified as pentaerythritol triacrylate using infrared spectroscopy and proton and 13C nuclear magnetic resonance (NMR) spectroscopy. All spectra were consistent with the structure of pentaerythritol triacrylate; the infrared spectra were also consistent with a literature spectrum (Aldrich, 1985). The NMR spectra indicated a significant number of structurally related impurities in each lot. The infrared and NMR spectra are presented in Figures H1 through H6.
The purity of lot 05318JW was determined by Galbraith Laboratories, Inc. (Knoxville, TN) using elemental analyses and by the analytical chemistry laboratory using gas chromatography, high-performance liquid chromatography (HPLC), and HPLC with mass spectrometry (HPLC/MS). Moisture content was determined by Galbraith Laboratories using Karl Fischer titration. The purity of lot HCC0340 was determined by the analytical chemistry and study laboratories using HPLC. Gas chromatography was performed using system A (Table H1). HPLC was performed with an Ultracarb 5 ODS (30) column (150 mm × 4.6 mm, 5-µm particle size; Phenomenex, Torrance, CA) and ultraviolet detection at 221 nm (lot 05318JW) or 220 nm (lot HCC0340). A mobile phase of (A) methanol:Milli-Q water (50:50) and (B) methanol:Milli-Q water (90:10) was used; the solvent program was a linear gradient of 100% A to 100% B over 30 minutes with a 30-minute hold, then 100% B to 100% A in 1 minute with a 10-minute hold. The flow rate was 0.8 mL/minute.
For lot 05318JW, elemental analyses for carbon, hydrogen, and oxygen were in agreement with the theoretical values for pentaerythritol triacrylate. Karl Fischer titration indicated approximately 943 ppm water. Gas chromatography indicated one major peak, five impurities with areas greater than 1% relative to the major peak area, and six impurities with relative areas between 0.5% and 1%. HPLC indicated a major peak, 18 impurities with areas greater than 1% relative to the major peak area, and five impurities with relative areas between 0.5% and 1%; the concentration of pentaerythritol triacrylate was determined to be approximately 10%. HPLC/MS indicated that 8 of the 18 impurity peaks with relative areas greater than 1% included structurally related adducts, dimers, and acrylates as well as trimethylolpropane triacrylate and its related esters and adducts. No substantial amount of 4-methoxyphenol, a stabilizer added to pentaerythritol triacrylate, was detected. The overall purity of lot 05318JW was determined to be approximately 10%, which is representative of commercial-grade pentaerythritol triacrylate.
For lot HCC0340, HPLC indicated a major peak, seven impurity peaks with areas greater than 1% of the major peak area, and nine impurity peaks with relative areas between 0.5% and 1%. By comparison of the retention times of these impurity peaks to those in the HPLC/MS analysis of lot 05318JW, the impurities were tentatively identified as structurally related adducts, dimers, and acrylates as well as trimethylolpropane triacrylate and its related esters and adducts. The overall purity of lot HCC0340 was determined to be approximately 45%.
To ensure stability, the bulk chemical was stored at room temperature, protected from light, in amber glass bottles with Teflon®-lined lids. Stability was monitored throughout the studies with gas chromatography by systems similar to systems A and B. No degradation of the bulk chemical was detected.
12-O-Tetradecanoylphorbol-13-acetate
12-O-Tetradecanoylphorbol-13-acetate was obtained from Sigma Chemical Company (St. Louis, MO) in one lot (48H1178) for use in the 6-month study. Identity and purity analyses were conducted by the analytical chemistry laboratory, Research Triangle Institute (Research Triangle Park, NC). The bulk chemical was stored in its original containers, protected from light, at −20° C or less.
The chemical was identified as 12-O-tetradecanoylphorbol-13-acetate by infrared and proton NMR spectroscopy. All spectra were consistent with the structure of 12-O-tetradecanoylphorbol-13-acetate.
The purity was determined with HPLC using a Zorbax Rx C8 column (250 mm × 4.6 mm, 5-µm particle size; DuPont, Wilmington, DE) and ultraviolet detection at 232 nm. A mobile phase of water:acetonitrile (10:90) (isocratic) was used; the flow rate was 1.0 mL/minute. HPLC indicated a major peak, one impurity peak with an area of approximately 0.11% of the total peak area, and two minor impurities with areas less than 0.1% of the total peak area. The overall purity was determined to be greater than 99%.
Acetone
Acetone was obtained in two lots from Honeywell Burdick and Jackson (Muskegon, MI) (lots BK792 and BL631) and in five lots from Spectrum Chemical Manufacturing Corporation (Gardena, CA) (lots JE342, KP206, LS0051, MI0172, and NE0173). Lots BK792, BL631, and JE342 were used in the 2-week studies, lots KP206 and LS0051 were used in the 3-month studies, and lots MI0172 and NE0173 were used in the 6-month study. Identity and purity analyses of lots BL631 and JE342 and all lots used in the 3- and 6-month studies were conducted by the analytical chemistry laboratory (Midwest Research Institute, Kansas City, MO) and the study laboratory.
The chemical, a clear liquid, was identified as acetone by the analytical chemistry laboratory (lots BL631, JE342, KP206, and LS0051) or the study laboratory (lots MI0172 and NE0173) using infrared spectroscopy. The purity was analyzed by the analytical chemistry laboratory (lots BL631, JE342, KP206, and LS0051) or the study laboratory (lots MI0172 and NE0173) using gas chromatography system C. No significant impurities were detected in any lot. The overall purity of each lot was determined to be greater than 99%.
To ensure stability, the bulk chemical was stored in amber glass bottles at room temperature. No degradation of the acetone was detected.
Preparation and Analysis of Dose Formulations
The dose formulations were prepared twice (2-week studies) or approximately every 4 weeks by mixing pentaerythritol triacrylate and acetone to give the required concentration (Table H2). The dose formulations were stored for up to 35 days for all studies at room temperature in amber glass bottles with Teflon®-lined lids except dose formulations prepared on September 9 and 12, 1996 (3-month studies), which were stored in amber (rat) or clear (mouse) glass vials with Teflon® septa. Positive control formulations for the 6-month study were prepared twice by mixing 12-O-tetradecanoylphorbol-13-acetate with acetone to provide a concentration of 12.5 µg/mL.
Stability studies of the 6.25 and 100 mg/mL dose formulations for the 2-week studies and a 400 µg/mL formulation were performed by the study laboratory with gas chromatography by systems similar to system A (6.25 and 100 mg/mL) or B (400 µg/mL). Stability was confirmed for at least 35 days for dose formulations stored in amber glass bottles with Teflon®-lined lids or septa, with minimal headspace, at temperatures up to 25° C and for 3 hours under animal room conditions, periodically or continually exposed to air and light.
Periodic analyses of the dose formulations of pentaerythritol triacrylate were conducted by the study laboratory using gas chromatography by systems similar to systems A and B. During the 2-week studies, the dose formulations were analyzed once; all dose formulations for rats and mice were within 10% of the target concentration with no value greater than 110% of the target concentration (Table H3). Animal room samples of these dose formulations were also analyzed; all animal room samples for rats and three of five for mice were within 10% of the target concentration. During the 3-month studies, the dose formulations were analyzed at the beginning, midpoint, and end of the studies; animal room samples of these dose formulations were also analyzed (Table H4). Of the dose formulations analyzed, all 15 for rats and 14 of 15 for mice were within 10% of the target concentration, with no value greater than 104% of the target concentration; 10 of 15 animal room samples for rats and nine of 15 for mice were within 10% of the target concentration. During the 6-month study, the dose formulations were analyzed every 8 or 9 weeks (Table H5). Of the dose formulations analyzed, 14 of 15 were within 10% of the target concentration, with no value greater than 103% of the target concentration; all five animal room samples were also within 10% of the target concentration. The single dose formulation in each of the 3- and 6-month studies that was not within 10% of the target concentration was remixed and reanalyzed and was found to be within 10% of the target concentration. The positive control formulations were analyzed by the analytical chemistry laboratory using HPLC with a system similar to that described for the positive control purity analysis and were found to be within 10% of the target concentration (data not shown).
Figure H1Infrared Absorption Spectrum of Pentaerythritol Triacrylate (Approximately 10% Pure Pentaerythritol Triacrylate-Technical Grade) Used in the 2-Week Studies
Figure H2Infrared Absorption Spectrum of Pentaerythritol Triacrylate (Approximately 45% Pure Pentaerythritol Triacrylate-Technical Grade) Used in the 3- and 6-Month Studies
Figure H3Proton Nuclear Magnetic Resonance Spectrum of Pentaerythritol Triacrylate (Approximately 10% Pure Pentaerythritol Triacrylate-Technical Grade) Used in the 2-Week Studies
Figure H4Proton Nuclear Magnetic Resonance Spectrum of Pentaerythritol Triacrylate (Approximately 45% Pure Pentaerythritol Triacrylate-Technical Grade) Used in the 3- and 6-Month Studies
Figure H513C Nuclear Magnetic Resonance Spectrum of Pentaerythritol Triacrylate (Approximately 10% Pure Pentaerythritol Triacrylate-Technical Grade) Used in the 2-Week Studies
Figure H613C Nuclear Magnetic Resonance Spectrum of Pentaerythritol Triacrylate (Approximately 45% Pure Pentaerythritol Triacrylate-Technical Grade) Used in the 3- and 6-Month Studies
Table H1Gas Chromatography Systems Used in the Dermal Studies of Pentaerythritol Triacrylate
| Detection System | Column | Carrier Gas | Oven Temperature Program |
|---|---|---|---|
| System A | |||
| Flame ionization | DB-5, 30 m × 0.32 mm, 0.25-μm film thickness (J&W Scientific, Folsom, CA) | Helium at 5 mL/minute | 70° C for 2.5 minutes, then 9° C/minute to 210° C, held 10 minutes |
| System B | |||
| Flame ionization | RTX-5, 30 m × 0.25 mm, 0.25-μm film thickness (Restek, Bellefonte, PA) | Helium at approximately 3 mL/minute | 70° C for 2.5 minutes, then 9° C/minute to 210° C, held 10 minutes |
| System C | |||
| Flame ionization | 20% SP-2401/0.1% Carbowax 1500 on 100/120 Supelcoport, 2.4 m × 2 mm | Nitrogen or helium at approximately 30 mL/minute | 40° C for 4 minutes, then 10° C/minute to 170° C |
Table H2Preparation and Storage of Dose Formulations in the Dermal Studies of Pentaerythritol Triacrylate
| 2-Week Studies | 3-Month Studies | 6-Month Study |
|---|---|---|
| Preparation | ||
| Pentaerythritol triacrylate was manually shaken or sonicated with acetone. Dose formulations were prepared twice. | Same as 2-week studies. Dose formulations were prepared every 4 weeks. | Same as 3-month studies |
| Chemical Lot Number | ||
| 05318JW | HCC0340 | HCC0340 |
| Maximum Storage Time | ||
| 35 days | 35 days | 35 days |
| Storage Conditions | ||
| Stored in amber glass bottles with Teflon® - lined lids at room temperature | Formulations prepared on September 9 and 12, 1996, were stored in amber or clear glass vials with Teflon® septa at room temperature; other formulations were stored same as 2-week studies. | Same as 2-week studies |
| Study Laboratory | ||
| Battelle Columbus Laboratories (Columbus, OH) | Battelle Columbus Laboratories (Columbus, OH) | Battelle Columbus Laboratories (Columbus, OH) |
Table H3Results of Analyses of Dose Formulations Administered to Rats and B6C3F1 Mice in the 2-Week Dermal Studies of Pentaerythritol Triacrylate
| Date Prepared | Date Analyzed | Target Concentration (mg/mL) | Determined Concentrationa (mg/mL) | Difference from Target (%) |
|---|---|---|---|---|
| Rats | ||||
| May 17, 1996 | May 18, 1996 | 25 | 25.79 | +3 |
| 50 | 52.92 | +6 | ||
| 100 | 108.7 | +9 | ||
| 200 | 219.5 | +10 | ||
| 400 | 431.8 | +8 | ||
| June 13, 14, and 17, 1996b | 25 | 26.61 | +6 | |
| 50 | 54.15 | +8 | ||
| 100 | 103.6 | +4 | ||
| 200 | 207.0 | +4 | ||
| 400 | 421.2 | +5 | ||
| Mice | ||||
| May 13 and 17, 1996 | May 18, 1996 | 6.25 | 6.314 | +1 |
| 12.5 | 13.09 | +5 | ||
| 25 | 25.79 | +3 | ||
| 50 | 52.92 | +6 | ||
| 100 | 108.7 | +9 | ||
| June 13, 14, and 17, 1996b | 6.25 | 6.626 | +6 | |
| 12.5 | 13.02 | +4 | ||
| 25 | 25.84c | +3 | ||
| 50 | 56.65 | +13 | ||
| 100 | 114.6 | +15 | ||
- a
Results of duplicate analyses. For rats, dosing volume=0.5 mL/kg; 25 mg/mL=12.5 mg/kg; 50 mg/mL=25 mg/kg; 100 mg/mL=50 mg/kg; 200 mg/mL=100 mg/kg; 400 mg/mL=200 mg/kg. For mice, dosing volume=2.0 mL/kg; 6.25 mg/mL=12.5 mg/kg; 12.5 mg/mL=25 mg/kg; b 25 mg/mL=50 mg/kg; 50 mg/mL=100 mg/kg; 100 mg/mL=200 mg/kg
- b
Animal room samples
- c
Results of quadruplicate analyses
Table H4Results of Analyses of Dose Formulations Administered to Rats and B6C3F1 Mice in the 3-Month Dermal Studies of Pentaerythritol Triacrylate
| Date Prepared | Date Analyzed | Target Concentration (mg/mL) | Determined Concentrationa (mg/mL) | Difference from Target (%) |
|---|---|---|---|---|
| Rats | ||||
| September 9, 1996 | September 10-12, 1996 | 1.5 | 1.552 | +3 |
| 3 | 3.117 | +4 | ||
| 6 | 6.269 | +4 | ||
| 12 | 12.37 | +3 | ||
| 24 | 24.75 | +3 | ||
| October 18, 1996b | 1.5 | 1.488 | -1 | |
| 3 | 3.111 | +4 | ||
| 6 | 6.351 | +6 | ||
| 12 | 12.57 | +5 | ||
| 24 | 25.10 | +5 | ||
| November 4, 1996 | November 5, 1996 | 1.5 | 1.562 | +4 |
| 3 | 3.078 | +3 | ||
| 6 | 6.046 | +1 | ||
| 12 | 11.85 | -1 | ||
| 24 | 24.15 | +1 | ||
| December 12-13, 1996b | 1.5 | 1.766 | +18 | |
| 3 | 5.231 | +74 | ||
| 6 | 7.219 | +20 | ||
| 12 | 15.62 | +30 | ||
| 24 | 28.05 | +17 | ||
| December 2, 1996 | December 3, 1996 | 1.5 | 1.563 | +4 |
| 3 | 3.105 | +4 | ||
| 6 | 6.142 | +2 | ||
| 12 | 12.30 | +3 | ||
| 24 | 24.46 | +2 | ||
| January 2, 1997b | 1.5 | 1.485 | -1 | |
| 3 | 2.942 | -2 | ||
| 6 | 5.987 | 0 | ||
| 12 | 12.21 | +2 | ||
| 24 | 23.03 | -4 | ||
| Mice | ||||
| September 9, 1996 | September 10, 1996 | 0.375 | 0.3535c | -6 |
| 0.75 | 0.4043c | -46 | ||
| 1.5 | 1.552 | +3 | ||
| 3 | 3.117 | +4 | ||
| 6 | 6.269 | +4 | ||
| September 12, 1996 | September 12, 1996 | 0.75 | 0.7473d | 0 |
| October 18, 1996b | 0.375 | 0.3333 | -11 | |
| 0.75 | 0.7502 | 0 | ||
| 1.5 | 1.539 | +3 | ||
| 3 | 3.153 | +5 | ||
| 6 | 6.528 | +9 | ||
| November 4, 1996 | November 5, 1996 | 0.375 | 0.3883 | +4 |
| 0.75 | 0.7628 | +2 | ||
| 1.5 | 1.562 | +4 | ||
| 3 | 3.078 | +3 | ||
| 6 | 6.046 | +1 | ||
| December 12-13, 1996b | 0.375 | 0.4420 | +18 | |
| 0.75 | 0.9022 | +20 | ||
| 1.5 | 1.857 | +24 | ||
| 3 | 3.620 | +21 | ||
| 6 | 8.960 | +49 | ||
| December 2, 1996 | December 3, 1996 | 0.375 | 0.3842 | +2 |
| 0.75 | 0.7695 | +3 | ||
| 1.5 | 1.563 | +4 | ||
| 3 | 3.105 | +4 | ||
| 6 | 6.142 | +2 | ||
| January 2, 1997b | 0.375 | 0.3456 | -8 | |
| 0.75 | 0.7414 | -1 | ||
| 1.5 | 1.504 | 0 | ||
| 3 | 2.914 | -3 | ||
| 6 | 6.004 | 0 | ||
- a
Results of duplicate analyses. For rats, dosing volume=0.5 mL/kg; 1.5 mg/mL=0.75 mg/kg; 3 mg/mL=1.5 mg/kg; 6 mg/mL=3 mg/kg; 12 mg/mL=6 mg/kg; 24 mg/mL=12 mg/kg. For mice, dosing volume=2.0 mL/kg; 0.375 mg/mL=0.75 mg/kg; 0.75 mg/mL=1.5 mg/kg; b 1.5 mg/mL=3 mg/kg; 3 mg/mL=6 mg/kg; 6 mg/mL=12 mg/kg
- b
Animal room samples
- c
Remixed; not used in study
- d
Results of remix
Table H5Results of Analyses of Dose Formulations Administered to Tg.AC Hemizygous Mice in the 6-Month Dermal Study of Pentaerythritol Triacrylate
| Date Prepared | Date Analyzed | Target Concentration (mg/mL) | Determined Concentrationa (mg/mL) | Difference from Target (%) |
|---|---|---|---|---|
| July 13, 1998 | July 13-14, 1998 | 0.227 | 0.2288 | +1 |
| 0.455 | 0.4606 | +1 | ||
| 0.909 | 0.9139 | +1 | ||
| 1.82 | 1.772 | -3 | ||
| 3.64 | 3.623 | 0 | ||
| August 24-25, 1998b | 0.227 | 0.2339 | +3 | |
| 0.455 | 0.4511 | -1 | ||
| 0.909 | 0.9422 | +4 | ||
| 1.82 | 1.979 | +9 | ||
| 3.64 | 3.680 | +1 | ||
| September 8, 1998 | September 8-10, 1998 | 0.227 | 0.1904c | -16 |
| 0.455 | 0.4149 | -9 | ||
| 0.909 | 0.8249 | -9 | ||
| 1.82 | 1.722 | -5 | ||
| 3.64 | 3.575 | -2 | ||
| September 13, 1998 | September 14, 1998 | 0.227 | 0.2224d | -2 |
| November 30, 1998 | December 1-2, 1998 | 0.227 | 0.2220 | -2 |
| 0.455 | 0.4464 | -2 | ||
| 0.909 | 0.9247 | +2 | ||
| 1.82 | 1.880 | +3 | ||
| 3.64 | 3.694 | +1 |
- a
Results of duplicate analyses. Dosing volume=3.3 mL/kg; 0.227 mg/mL=0.75 mg/kg; 0.455 mg/mL=1.5 mg/kg; 0.909 mg/mL=3 mg/kg; b 1.82 mg/mL=6 mg/kg; 3.64 mg/mL=12 mg/kg
- b
Animal room samples
- c
Remixed; not used in study
- d
Results of remix
- CHEMICAL CHARACTERIZATION AND DOSE FORMULATION STUDIES - NTP Genetically Modifie...CHEMICAL CHARACTERIZATION AND DOSE FORMULATION STUDIES - NTP Genetically Modified Model Report on the Toxicology Studies of Pentaerythritol Triacrylate (Technical Grade) (CASRN 3524-68-3) in F344/N Rats, B6C3F1 Mice, and Genetically Modified (FVB Tg.AC Hemizygous) Mice (Dermal Studies)
- MATERIALS AND METHODS - NTP Genetically Modified Model Report on the Toxicology ...MATERIALS AND METHODS - NTP Genetically Modified Model Report on the Toxicology Studies of Sodium Bromate (CASRN 7789-38-0) in Genetically Modified (FVB Tg.AC Hemizygous) Mice (Dermal and Drinking Water Studies) and Carcinogenicity Studies of Sodium Bromate in Genetically Modified [B6.129-Trp53tm1Brd (N5) Haploinsufficient] Mice (Drinking Water Studies)
- transmembrane protein 43 isoform 7 [Homo sapiens]transmembrane protein 43 isoform 7 [Homo sapiens]gi|2246031141|ref|NP_001394208.1|Protein
- UNVERIFIED: Choloepus didactylus isolate T1722 matrix metallopeptidase 20 (MMP20...UNVERIFIED: Choloepus didactylus isolate T1722 matrix metallopeptidase 20 (MMP20) pseudogene, partial sequencegi|2425613777|gb|OP966268.1|Nucleotide
- Sisoridae cytochrome oxidase subunit I (COI) gene, partial cds; mitochondrial.Sisoridae cytochrome oxidase subunit I (COI) gene, partial cds; mitochondrial.PopSet: 113056588PopSet
Your browsing activity is empty.
Activity recording is turned off.
See more...