This is a work of the US government and distributed under the terms of the Public Domain
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Leakey JEA, Allaben WT, Dunnick JK, et al. NTP Genetically Modified Model Report on the Toxicology and Carcinogenicity Studies of 3’-Azido-3’-Deoxythymidine (CASRN 30516-87-1) in Genetically Modified C3B6.129F1-Trp53tm1Brd N12 Haploinsufficient Mice (In Utero and Postnatal Gavage Study): NTP GMM 14 [Internet]. Research Triangle Park (NC): National Toxicology Program; 2013 Oct.

NTP Genetically Modified Model Report on the Toxicology and Carcinogenicity Studies of 3’-Azido-3’-Deoxythymidine (CASRN 30516-87-1) in Genetically Modified C3B6.129F1-Trp53tm1Brd N12 Haploinsufficient Mice (In Utero and Postnatal Gavage Study): NTP GMM 14 [Internet].
Show detailsProcurement and Characterization
AZT
3′-Azido-3′-deoxythymidine (AZT) was obtained from Cipla Ltd., Mumbai Central (Mumbai, India) in one lot (FX4159) used in the 30- and 45-week studies and the 45-week stop-study. Identity and purity analyses were conducted by the study laboratory at the National Center for Toxicological Research (NCTR; Jefferson, AR) and Galbraith Laboratories, Inc. (Knoxville, TN). Reports on analyses performed in support of the AZT studies are on file at the NCTR.
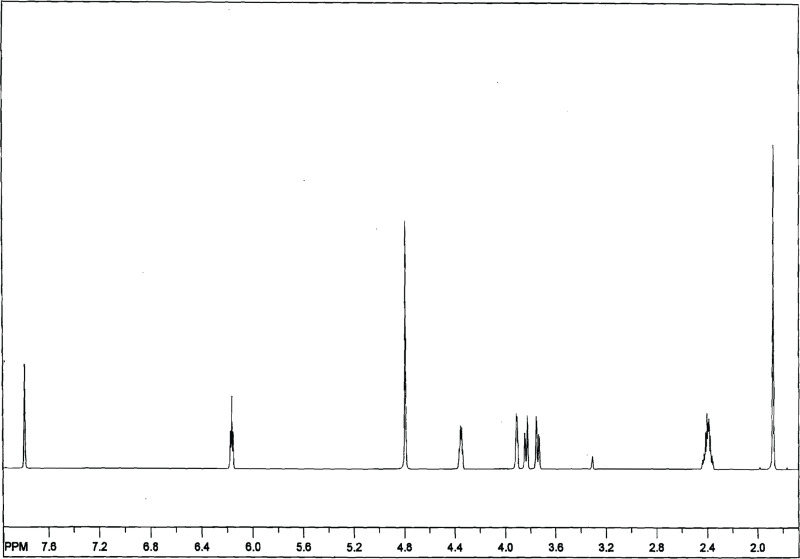
Lot FX4159 of the chemical, a white crystalline powder, was identified as AZT by the study laboratory using proton nuclear magnetic resonance (NMR) spectroscopy, direct exposure probe-electron ionization mass spectrometry, and high-performance liquid chromatography (HPLC) coupled with electrospray ionization mass spectrometry. All spectra were consistent with literature spectra and the structure of AZT. A representative proton NMR spectrum is presented in Figure F1. The melting point range of lot FX4159 was determined to be 122.5° to 123.4° C by Galbraith Laboratories, Inc.; these values were consistent with those reported in the literature for AZT crystallized from water (Merck, 2006).
Karl Fischer titration and elemental analyses of lot FX4159 were performed by Galbraith Laboratories, Inc., and the study laboratory determined the purity of the bulk chemical by HPLC. HPLC was conducted with a Waters Millennium® system using photodiode array detection at 254 nm (Waters Corporation, Milford, MA). The analytical column was a Nova-Pak® (3.9 mm × 150 mm, 4 μm particle size) C18 column (Waters Corporation). The mobile phase (flow rate 1 mL/minute) was held at 5% acetonitrile:95% water for 5 minutes and then linearly changed to 95% acetonitrile:5% water over 20 minutes, followed by a final 5-minute hold.
For lot FX4159, Karl Fischer titration indicated less than 0.46% water. Elemental analyses for carbon, hydrogen, nitrogen, and oxygen were in agreement with the theoretical values for AZT. HPLC detected no impurity peaks by comparisons to spectra from previously characterized AZT standards, and the purity of lot FX4159 was determined to be 100% under the conditions of the assay.
Dosing Vehicle
The vehicle used for dose formulations in the 30- and 45-week studies and the 45-week stop-study was a 0.2% methylcellulose/0.1% Tween® 80 aqueous solution. Methylcellulose was obtained from Sigma-Aldrich® Corporation (St. Louis, MO) in one lot (014K0081). Identity studies of lot 014K0081 were performed by the study laboratory using proton and carbon-13 NMR spectroscopy; the results of these analyses were consistent with those obtained previously for a methylcellulose standard obtained from Fischer Scientific (Pittsburgh, PA). Tween® 80 was obtained from Sigma-Aldrich® Corporation in one lot (073K00641).
Preparation and Analysis of Dose Formulations
The dose formulations were prepared by mixing AZT with the dosing vehicle to give the required concentrations (Table F1). The dose formulations were stored at room temperature in sealed amber glass bottles for up to 29 days.
Stability studies of 0.05 and 0.20 mg/mL formulations were performed by the study laboratory with HPLC using the same Waters chromatography equipment utilized for determination of test chemical purity. For the stability studies however, two mobile phases were used: mobile phase A consisted of 5% methanol:95% water, and mobile phase B consisted of 90% methanol:10% water; both mobile phases contained 0.005 M sodium phosphate monobasic and 0.003 M sodium pentanesulfonic acid and were adjusted to pH 2.5 with phosphoric acid. The HPLC solvent program (flow rate 1.0 mL/minute) was a linear gradient from 100% A to 100% B in 3 minutes, followed by a 7.5-minute hold. Stability was confirmed for at least 29 days for formulations stored in sealed amber glass bottles at room temperature.
Periodic analyses of the dose formulations of AZT were conducted by the study laboratory using the HPLC system utilized for the dose formulation stability studies; accuracy of dose delivery from the dosing apparatus was also periodically determined using this HPLC system. Of the dose formulations analyzed and used during the studies, 209 of 211 were within 10% of the target concentrations (Table F2). For the dose accuracy of delivered doses, 17 of the 20 samples analyzed were within 10% of the target doses (Table F3).
Table F1Preparation and Storage of Dose Formulations in the In Utero/Postnatal Gavage Studies of AZT
| Preparation |
|---|
| AZT was added to an aqueous solution of 0.2% methylcellulose and 0.1% Tween® 80 and stirred with a magnetic stir bar to form a suspension; suspensions were then filtered through a 0.2 μm filter. The dose formulations were prepared approximately weekly. |
| Chemical Lot Number |
| FX4159 |
| Maximum Storage Time |
| 29 days |
| Storage Conditions |
| Stored in sealed amber glass bottles at room temperature |
| Study Laboratory |
| National Center for Toxicological Research (Jefferson, AR) |
Table F2Results of Analyses of Dose Formulations Administered to Heterozygous F1 p53+/− Mice in the 30-Week, 45-Week, and 45-Week Stop-Exposure In Utero/Postnatal Gavage Studies of AZT
| Date Analyzed | Target Concentration (mg/mL) | Determined Concentration (mg/mL)a | Difference From Target (%) |
|---|---|---|---|
| November 29, 2004 | 8 | 8.08 ± 0.10 | +1 |
| 16 | 15.9 ± 0.8 | -1 | |
| 24 | 22.9 ± 0.2 | -5 | |
| December 06, 2004 | 8 | 8.78 ± 0.11 | +10 |
| 16 | 17.4 ± 0.1 | +9 | |
| 24 | 24.1 ± 0.2 | 0 | |
| December 20, 2004 | 8 | 7.92 ± 0.10 | -1 |
| 16 | 16.5 ± 0.3 | +3 | |
| 24 | 23.7 ± 0.1 | -1 | |
| December 27, 2004 | 8 | 7.98 ± 0.06 | 0 |
| 8 | 7.96 ± 0.03 | 0 | |
| 16 | 16.7 ± 0.1 | +4 | |
| 16 | 16.7 ± 0.2 | +4 | |
| 24 | 23.6 ± 0.1 | -2 | |
| December 29, 2004 | 8 | 7.96 ± 0.22 | 0 |
| 16 | 16.5 ± 0.1 | +3 | |
| 24 | 23.5 ± 0.3 | -2 | |
| January 05, 2005 | 8 | 7.85 ± 0.19 | -2 |
| 16 | 16.0 ± 0.4 | 0 | |
| 24 | 23.4 ± 0.3 | -2 | |
| January 11, 2005 | 8 | 7.72 ± 0.02 | -3 |
| 16 | 15.8 ± 0.3 | -1 | |
| 24 | 22.9 ± 0.5 | -5 | |
| January 12, 2005 | 8 | 7.83 ± 0.20 | -2 |
| 16 | 16.6 ± 0.1b | +4 | |
| 24 | 23.3 ± 0.1 | -3 | |
| January 19, 2005 | 8 | 8.17 ± 0.04 | +2 |
| 16 | 16.5 ± 0.2 | +3 | |
| 24 | 23.4 ± 0.3 | -3 | |
| January 25, 2005 | 8 | 8.21 ± 0.07 | +3 |
| 16 | 16.6 ± 0.2 | +4 | |
| 24 | 23.4 ± 0.1 | -3 | |
| January 28, 2005 | 16 | 17.1 ± 0.3 | +7 |
| 24 | 23.9 ± 0.2 | 0 | |
| February 01, 2005 | 8 | 8.17 ± 0.12 | +2 |
| 16 | 16.6 ± 0.2c | +2 | |
| 24 | 22.1 ± 0.4 | -8 | |
| February 07, 2005 | 8 | 8.50 ± 0.13 | +6 |
| 16 | 16.1 ± 0.3 | +1 | |
| 24 | 24.0 ± 1.2 | 0 | |
| February 14, 2005 | 8 | 8.16 ± 0.13 | +2 |
| 16 | 16.4 ± 0.1 | +2 | |
| 24 | 24.4 ± 0.1 | +2 | |
| February 18, 2005 | 8 | 8.21 ± 0.09 | +3 |
| 16 | 16.7 ± 0.3 | +4 | |
| 24 | 24.7 ± 0.4 | +3 | |
| February 23, 2005 | 8 | 8.11 ± 0.28 | +1 |
| 16 | 16.7 ± 0.1 | +5 | |
| 24 | 24.7 ± 0.4 | +3 | |
| March 01, 2005 | 8 | 8.38 ± 0.14 | +5 |
| 16 | 17.1 ± 0.3 | +7 | |
| 24 | 24.9 ± 0.1 | +4 | |
| March 03, 2005 | 8 | 8.30 ± 0.04 | +4 |
| 16 | 17.2 ± 0.2 | +7 | |
| 24 | 24.2 ± 0.8 | +1 | |
| March 08, 2005 | 8 | 8.08 ± 0.14 | +1 |
| 16 | 16.5 ± 0.6 | +3 | |
| 24 | 24.9 ± 0.1 | +4 | |
| March 14, 2005 | 8 | 8.32 ± 0.22 | +4 |
| 16 | 17.2 ± 0.6 | +8 | |
| 24 | 24.7 ± 0.9 | +3 | |
| March 18, 2005 | 8 | 8.22 ± 0.10 | +3 |
| 16 | 16.8 ± 0.2 | +5 | |
| 24 | 24.6 ± 0.4 | +2 | |
| March 22, 2005 | 8 | 8.49 ± 0.13 | +6 |
| 16 | 16.3 ± 0.1 | +2 | |
| 24 | 22.6 ± 1.4 | -6 | |
| March 29, 2005 | 24 | 23.2 ± 0.2 | -2 |
| March 31, 2005 | 8 | 8.28 ± 0.10 | +3 |
| 16 | 16.0 ± 0.2 | 0 | |
| 24 | 22.3 ± 0.4 | -7 | |
| April 05, 2005 | 24 | 23.4 ± 0.0 | -2 |
| April 08, 2005 | 8 | 8.14 ± 0.16 | +2 |
| 16 | 16.2 ± 0.2 | +1 | |
| 24 | 23.2 ± 0.3 | -3 | |
| April 13, 2005 | 8 | 8.51 ± 0.10 | +6 |
| 16 | 16.4 ± 0.1 | +2 | |
| 24 | 23.3 ± 0.1 | -3 | |
| April 18, 2005 | 8 | 8.27 ± 0.20 | +3 |
| 16 | 15.4 ± 0.8 | -4 | |
| 24 | 23.0 ± 0.5 | -4 | |
| April 26, 2005 | 8 | 8.53 ± 0.09 | +7 |
| 16 | 16.4 ± 0.2 | +3 | |
| 24 | 23.3 ± 0.2 | -3 | |
| 24 | 23.7 ± 0.2 | -1 | |
| April 29, 2005 | 8 | 8.37 ± 0.06 | +5 |
| 16 | 16.4 ± 0.1 | +2 | |
| May 04, 2005 | 8 | 8.49 ± 0.02 | +6 |
| 16 | 16.1 ± 0.2 | +1 | |
| 24 | 23.1 ± 0.1 | -4 | |
| May 10, 2005 | 8 | 8.51 ± 0.05 | +6 |
| 16 | 16.0 ± 0.2 | 0 | |
| 24 | 22.9 ± 0.4 | -5 | |
| May 16, 2005 | 8 | 8.46 ± 0.18 | +6 |
| 16 | 13.9 ± 0.1 | -13 | |
| 24 | 22.7 ± 0.0 | -5 | |
| May 19, 2005 | 8 | 8.55 ± 0.29 | +7 |
| 16 | 16.7 ± 0.1 | +4 | |
| 24 | 23.3 ± 0.3 | -3 | |
| May 25, 2005 | 8 | 8.19 ± 0.05 | +2 |
| 16 | 16.1 ± 0.1 | +1 | |
| 24 | 23.0 ± 0.1 | -4 | |
| June 01, 2005 | 8 | 7.90 ± 0.15 | -1 |
| 16 | 16.2 ± 0.2 | +1 | |
| 24 | 23.1 ± 0.1 | -4 | |
| June 08, 2005 | 8 | 8.48 ± 0.08 | +6 |
| 16 | 16.6 ± 0.1 | +4 | |
| 24 | 23.8 ± 0.3 | -1 | |
| June 14, 2005 | 8 | 7.38 ± 0.04 | -8 |
| 16 | 14.3 ± 0.1 | -10 | |
| 24 | 21.0 ± 0.0 | -13 | |
| June 20, 2005 | 8 | 8.19 ± 0.08 | +2 |
| 16 | 16.4 ± 0.1 | +2 | |
| 24 | 23.3 ± 0.1 | -3 | |
| June 22, 2005 | 8 | 7.84 ± 0.04 | -2 |
| 16 | 15.8 ± 0.1 | -1 | |
| 24 | 22.5 ± 0.2 | -6 | |
| June 30, 2005 | 8 | 8.44 ± 0.09 | +6 |
| 16 | 13.6 ± 0.2b | -15 | |
| 24 | 22.4 ± 0.4 | -7 | |
| July 01, 2005 | 16 | 16.3 ± 0.5 | +2 |
| July 06, 2005 | 8 | 8.26 ± 0.02 | +3 |
| 16 | 15.5 ± 0.1 | -3 | |
| 24 | 22.8 ± 0.2 | -5 | |
| July 13, 2005 | 8 | 8.23 ± 0.06 | +3 |
| 16 | 15.5 ± 0.2 | -3 | |
| 24 | 22.8 ± 0.1 | -5 | |
| July 20, 2005 | 8 | 8.36 ± 0.09 | +5 |
| 16 | 15.5 ± 0.1 | -3 | |
| 24 | 22.8 ± 0.2 | -5 | |
| July 27, 2005 | 8 | 8.15 ± 0.06 | +2 |
| 16 | 14.8 ± 0.6 | -8 | |
| 24 | 22.3 ± 0.9 | -7 | |
| August 01, 2005 | 8 | 8.49 ± 0.13 | +6 |
| 16 | 16.1 ± 0.1 | +1 | |
| 24 | 23.9 ± 0.3 | 0 | |
| August 02, 2005 | 16 | 15.6 ± 0.2 | -3 |
| 24 | 23.2 ± 0.2 | -3 | |
| August 04, 2005 | 8 | 8.12 ± 0.06 | +2 |
| 16 | 15.6 ± 0.2 | -2 | |
| 24 | 22.8 ± 0.4 | -5 | |
| August 09, 2005 | 8 | 8.24 ± 0.04 | +3 |
| 16 | 15.5 ± 0.0 | -3 | |
| 24 | 22.9 ± 0.1 | -5 | |
| 24 | 22.9 ± 0.3 | -5 | |
| 24 | 22.9 ± 0.2 | -5 | |
| August 18, 2005 | 8 | 8.33 ± 0.11 | +4 |
| 16 | 15.4 ± 0.1 | -3 | |
| 24 | 22.5 ± 0.3 | -6 | |
| August 25, 2005 | 8 | 8.01 ± 0.13 | 0 |
| 16 | 14.8 ± 0.4 | -8 | |
| 24 | 22.9 ± 0.2 | -5 | |
| August 31, 2005 | 8 | 8.02 ± 0.06 | 0 |
| 16 | 15.3 ± 0.1 | -4 | |
| 24 | 24.0 ± 0.2 | 0 | |
| September 08, 2005 | 8 | 8.00 ± 0.11 | 0 |
| 16 | 16.7 ± 0.1 | +5 | |
| 16 | 16.8 ± 0.3 | +5 | |
| 24 | 24.9 ± 0.2 | +4 | |
| September 15, 2005 | 8 | 7.89 ± 0.22 | -1 |
| 16 | 16.8 ± 0.1 | +5 | |
| 24 | 24.9 ± 0.2 | +4 | |
| September 22, 2005 | 8 | 8.03 ± 0.16 | 0 |
| 16 | 16.4 ± 1.1 | +2 | |
| 24 | 24.2 ± 1.1 | +1 | |
| September 30, 2005 | 8 | 8.27 ± 0.08 | +3 |
| 16 | 15.8 ± 0.0 | -1 | |
| 24 | 23.1 ± 0.2 | -4 | |
| October 03, 2005 | 8 | 8.49 ± 0.28 | +6 |
| 16 | 16.4 ± 0.0 | +3 | |
| 24 | 24.3 ± 0.1 | +1 | |
| October 06, 2005 | 8 | 8.57 ± 0.11 | +7 |
| 16 | 16.3 ± 0.1 | +2 | |
| 24 | 23.7 ± 0.1 | -1 | |
| October 12, 2005 | 8 | 8.05 ± 0.08 | +1 |
| 16 | 15.5 ± 0.1 | -3 | |
| 24 | 22.7 ± 0.3 | -5 | |
| October 19, 2005 | 8 | 8.38 ± 0.08 | +5 |
| 16 | 15.8 ± 0.1 | -1 | |
| 24 | 23.3 ± 0.1 | -3 | |
| October 26, 2005 | 8 | 8.10 ± 0.26 | +1 |
| 16 | 16.2 ± 0.4 | +1 | |
| 24 | 23.4 ± 0.5 | -2 | |
| November 02, 2005 | 8 | 8.25 ± 0.08 | +3 |
| 16 | 15.8 ± 0.3 | -2 | |
| 24 | 22.8 ± 0.3 | -5 | |
| November 09, 2005 | 8 | 8.10 ± 0.41 | +1 |
| 16 | 16.1 ± 0.2 | +1 | |
| 24 | 23.5 ± 0.3 | -2 | |
| November 16, 2005 | 8 | 8.36 ± 0.04 | +5 |
| 16 | 15.9 ± 0.1 | 0 | |
| 24 | 23.0 ± 0.4 | -4 | |
| December 05, 2005 | 8 | 8.41 ± 0.17 | +5 |
| 16 | 16.3 ± 0.1 | +2 | |
| 24 | 24.1 ± 0.1 | 0 | |
| December 12, 2005 | 8 | 8.29 ± 0.18 | +4 |
| 16 | 16.4 ± 0.2 | +3 | |
| 24 | 23.9 ± 0.4 | -1 | |
| December 15, 2005 | 8 | 8.38 ± 0.28 | +5 |
| 16 | 16.4 ± 0.3 | +3 | |
| 24 | 23.5 ± 0.2 | -2 | |
| December 21, 2005 | 8 | 8.35 ± 0.02 | +4 |
| 16 | 16.2 ± 0.4 | +1 | |
| 24 | 21.8 ± 0.4 | -9 | |
| December 26, 2005 | 8 | 8.25 ± 0.07 | +3 |
| 16 | 15.8 ± 0.3 | -1 | |
| 24 | 23.2 ± 0.0 | -3 | |
| January 10, 2006 | 16 | 15.8 ± 0.1 | -1 |
| January 18, 2006 | 16 | 16.8 ± 0.4 | +5 |
| 24 | 24.9 ± 0.6 | +4 | |
| February 01, 2006 | 16 | 16.1 ± 0.2 | 0 |
| February 15, 2006 | 16 | 14.6 ± 0.3 | -9 |
| February 21, 2006 | 16 | 15.5 ± 0.4 | -3 |
- a
Results of triplicate analyses (mean ± standard deviation). For pups [postnatal days (PND) 1 to 10], dosing volume=5 mL/kg; 8 mg/mL=40 mg/kg, 16 mg/mL=80 mg/kg, 24 mg/mL=120 mg/kg. For dams (gestational days 12 to 18) and pups after PND 10, dosing volume=10 mL/kg; 8 mg/mL=80 mg/kg, 16 mg/mL=160 mg/kg, 24 mg/mL=240 mg/kg.
- b
Not used in the animal studies
- c
n=2
Table F3Accuracy of Doses Administered to Heterozygous F1 p53+/− Mice in the 30-Week, 45-Week, and 45-Week Stop-Exposure In Utero/Postnatal Gavage Studies of AZT
| Date Analyzed | Target Dose (mg) | Actual Dose (mg)a | Difference From Target (%) |
|---|---|---|---|
| November 29, 2004 | 4.87 | 4.44 ± 0.09 | -9 |
| 9.67 | 8.92 ± 0.33 | -8 | |
| December 06, 2004 | 0.48 | 0.47 ± 0.01 | -2 |
| 3.248 | 3.05 ± 0.10 | -6 | |
| December 09, 2004 | 1.44 | 1.43 ± 0.03 | -1 |
| 7.20 | 6.89 ± 0.25 | -4 | |
| January 11, 2005 | 1.44 | 1.36 ± 0.02 | -5 |
| 7.20 | 6.69 ± 0.10 | -7 | |
| March 03, 2005 | 1.44 | 1.40 ± 0.05 | -3 |
| 7.20 | 6.75 ± 0.09 | -6 | |
| March 16, 2005 | 1.44 | 1.44 ± 0.04 | 0 |
| 7.20 | 6.98 ± 0.23 | -3 | |
| April 29, 2005 | 1.44 | 1.37 ± 0.02 | -5 |
| 7.20 | 6.62 ± 0.07 | -8 | |
| July 07, 2005 | 1.44 | 1.34 ± 0.04 | -7 |
| 7.20 | 6.43 ± 0.24 | -11 | |
| September 14, 2005 | 7.20 | 6.20 ± 0.16 | -14 |
| September 21, 2005 | 7.20 | 6.80 ± 0.08 | -5 |
| December 09, 2005 | 7.20 | 6.44 ± 0.07 | -10 |
| January 25, 2006 | 7.20 | 7.02 ± 0.12 | -2 |
- a
Results of triplicate analyses (mean ± standard deviation).
- CHEMICAL CHARACTERIZATION AND DOSE FORMULATION STUDIES - NTP Genetically Modifie...CHEMICAL CHARACTERIZATION AND DOSE FORMULATION STUDIES - NTP Genetically Modified Model Report on the Toxicology and Carcinogenicity Studies of 3’-Azido-3’-Deoxythymidine (CASRN 30516-87-1) in Genetically Modified C3B6.129F1-Trp53tm1Brd N12 Haploinsufficient Mice (In Utero and Postnatal Gavage Study)
Your browsing activity is empty.
Activity recording is turned off.
See more...