First-line treatment: Pelvic floor muscle training versus duloxetine
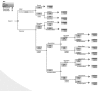
A decision tree model was developed in Microsoft Excel to compare the cost effectiveness of pelvic floor muscle training (PFMT) and duloxetine as a first-line treatment for women with moderate to severe stress UI, which is assumed to be 14 or more leakage episodes per week. Treatment effects and costs were based on a 52 week time frame. The structure of the model is shown below in . Patients are given either PFMT or duloxetine as a first-line treatment for their stress UI.
Decision tree model of PFMT versus duloxetine.
Patients in the PFMT arm can either ‘succeed’ or ‘fail’. If patients ‘fail’ on PFMT, they either continued treatment, on the basis that they derive some treatment effect, or discontinued treatment. It is assumed that there are no adverse effects from PFMT.
Patients who take duloxetine as their first-line treatment have either continued or discontinued by 12 weeks, the period for which there is most trial data.
The structure of the model allows patients on duloxetine to have an adverse event whether they continued or discontinued. However, under baseline assumptions, it is assumed that adverse events are the reason for discontinuation and that patients who continued do not experience any adverse events. Patients who continued beyond 12 weeks can, by 52 weeks, either continue on treatment or have discontinued. Again, they may have continued/discontinued with or without adverse events.
Cost parameters
Table T.1
View in own window
| Item | Cost |
|---|
| PFMT | £131 |
| Duloxetine cost per day | £1.10 |
| GP consultation | £21.00 |
| Review consultations 1 | 1 |
| Additional duloxetine-attributable consultations, weeks 12–52 | 0 |
| Drug adverse effects | £0 |
The cost of PFMT in the model is based on six sessions with a senior 1 grade physiotherapist. The first session is 1 hour and subsequent sessions last half an hour (refer to Appendix S for more details on cost derivation). The daily cost of duloxetine is derived from BNF 50. The cost of taking duloxetine also includes one review GP consultation, of 9.36 minutes duration, with the cost taken from Unit Costs of Health and Social Care 2004.932 It is additionally assumed at baseline that adverse effects of duloxetine do not impose any costs on the NHS and that there are no further review GP consultations after week 12.
Probability parameters
Table T.2
View in own window
| Item | Cost |
|---|
| PFMT successful | 50% |
| Continued PFMT if fail | 75% |
| Continued duloxetine at 12 weeks | 74% |
| Continued duloxetine at 52 weeks (if continued at 12 weeks) | 68% |
| Adverse event if continued duloxetine at 12 weeks (weeks 0–12) | 0% |
| Adverse event if continued duloxetine at 52 weeks (weeks 12–52) | 0% |
| Adverse event if discontinued duloxetine by 12 weeks (weeks 0-12) | 100% |
| Adverse event if discontinued duloxetine by 52 weeks (weeks 12–52) | 100% |
The probabilities that PFMT is successful, that patients continue PFMT if treatment fails, and that patients continue duloxetine at 12 and 52 weeks are taken from a published cost effectiveness analysis.426
Incontinence outcome parameters
Table T.3
View in own window
| Incontinence outcome | Value |
|---|
| Reduction in leakage episodes (PFMT success) | 55% |
| Reduction in leakage episodes (PFMT fail/continued) | 27.5% |
| Reduction in leakage episodes (PFMT fail/discontinued) | 0% |
| Reduction in leakage episodes (duloxetine continued) | 55% |
| Reduction in leakage episodes (duloxetine discontinued) | 0% |
| Reduction in leakage episodes (duloxetine discontinued by 12 weeks) | 42% |
| Reduction in leakage episodes (duloxetine discontinued by 52 weeks) | 55% |
| Days on duloxetine if discontinued by 12 weeks | 35 days |
| Weeks on duloxetine for those who discontinued by 52 weeks | 32 weeks |
The data above relate to the percentage reduction in leakage episodes and are taken from a published cost effectiveness analysis.426 Similarly, the days on duloxetine if discontinued by 12 weeks is also taken from this source. For those women who continued at 12 weeks but discontinued by 52 weeks, it is assumed that they stop taking duloxetine halfway through this 40 week period.
QALY parameters
Table T.4
View in own window
| Outcome | QALYs |
|---|
| QALY gain – pre-treatment to continent | 0.063 |
| PFMT success | 0.035 |
| PFMT fail – continued | 0.017 |
| PFMT fail – discontinued | 0.0 |
| Duloxetine continued at 12 weeks | 0.008 |
| Duloxetine continued at 12–52 weeks | 0.027 |
| Duloxetine discontinued by 12 weeks | 0.003 |
| Duloxetine discontinued at 52 weeks | 0.013 |
| Duloxetine adverse effects by 12 weeks | −0.003 |
| Duloxetine adverse effects by 52 weeks | −0.013 |
The QALY gain of treatment was derived from a published cost effectiveness analysis426 and from information submitted to guideline developers within the stakeholder process. In a cost–utility analysis of TVT versus colposuspension, QALYs were derived from women who completed an EQ-5D questionnaire at baseline and 6 months after hospital discharge.931 For TVT, the baseline estimate of QOL was 0.778 (0.785 for colposuspension) and at 6 months this had risen to 0.806. The cure rate for TVT patients was 66% and this can be used to estimate the QOL of a cure, as not all patients are dry at 6 months:
A published HTA report reviewing evidence on the clinical and cost effectiveness of TVT reports this QOL data, including the fact that a cure is associated with a QOL of 0.82. However, in its own cost effectiveness model, it uses QALY values of 0.85 and 0.80 for continent and incontinent women respectively.716
The published cost effectiveness analysis of duloxetine includes surgery as a follow-up treatment for patients in whom conservative management is unsuccessful.426 The authors assume that in such patients there is a pre-surgery disutility of 0.05. However, these pre-surgery patients have had conservative management, which it is assumed has led to some reduction in leakage episodes, with a concomitant utility gain. The overall utility gain is calculated thus:
A brief explanation of this formula is as follows:
post-PFMT disutility is one and the same as pre-surgery disutility (0.05)
pre-surgery patients:
75% continued with PFMT and had a 27.5% reduction in leakage episodes
25% did not continue with PFMT and had a 0% reduction in leakage episodes
weighted reduction in leakage episodes = (0.75 × 0.275) + (0.25 × 0) = 0.21
therefore the post-PFMT disutility is only 79% (i.e., 1 - 0.21 = 0.79) of the pre-PFMT disutility and therefore the disutility of moderate to severe UI prior to any reduction in leakage episodes is 0.063.
The other QALY parameters are derived in a linear fashion from the percentage reduction in leakage episodes associated with the particular outcome (each terminal node on the tree) and the maximum QALY gain attainable from pretreatment to continent. In other words, if the QALY gain in achieving continence is 0.063 then a 55% reduction in leakage episodes is assumed to produce a 0.035 (i.e., 0.063 × 0.55) gain in QALYs. On the assumption that adverse effects are the main cause of discontinuation, it seems a reasonable approximation to say that the disutility from adverse event must be at least as great as any utility gain from reduced UI symptoms.
Results
Table T.5
View in own window
| Treatment | Cost for 52 weeks | QALY | Incremental cost | Incremental QALY | ICER |
|---|
| PFMT | £131 | 0.024 | | 0.004 | Dominates |
| Duloxetine | £291 | 0.019 | £160 | | |
Using baseline assumptions, PFMT ‘dominates’ duloxetine. This means that it is both more effective and less costly.
Sensitivity analysis
Sensitivity analysis is used in economic evaluation to assess how sensitive the results of the model are to the assumptions made about the model parameters, particularly those parameters where considerable uncertainty exists as to their actual value.
One-way sensitivity analysis involves altering the value of a single parameter, holding all the others constant, to determine how sensitive the cost effectiveness conclusion is to the assumptions made about that particular parameter. Multi-way sensitivity analysis means that several default parameters are changed simultaneously, although one of the difficulties with this technique is the huge number of possible permutations that exist.
The results of some sensitivity analyses for this model are shown below. As the default shows PFMT to be dominant (produces more benefit for less cost), parameter values have been varied in favour of duloxetine. The rationale for this is that confidence in the robustness of the default conclusion – that PFMT is more cost effective – will be strengthened if the conclusion holds under less favourable scenarios for PFMT.
Cost differential between PFMT and duloxetine
Table T.6
View in own window
| Duloxetine ICER cost – PFMT cost | (cost/QALY) | Comment |
|---|
| £160 | PFMT dominates | PFMT more cost effective |
| £140 | PFMT dominates | PFMT more cost effective |
| £120 | PFMT dominates | PFMT more cost effective |
| £100 | PFMT dominates | PFMT more cost effective |
| £80 | PFMT dominates | PFMT more cost effective |
| £60 | PFMT dominates | PFMT more cost effective |
| £40 | PFMT dominates | PFMT more cost effective |
| £20 | PFMT dominates | PFMT more cost effective |
| £0 | PFMT dominates | PFMT more cost effective |
| −£10 | £2,097 | PFMT more cost effectivea |
| −£20 | £4,282 | PFMT more cost effectivea |
| −£30 | £6,466 | PFMT more cost effectivea |
| −£40 | £8,923 | PFMT more cost effectivea |
| −£50 | £11,107 | PFMT more cost effectivea |
| −£60 | £13,291 | PFMT more cost effectivea |
| −£70 | £15,476 | PFMT more cost effectivea |
| −£80 | £17,660 | PFMT more cost effectivea |
| −£90 | £19,900 | Borderline – NICE ICER threshold |
PFMT is always the more effective treatment. The ICER is for PFMT relative to duloxetine. Keeping all the other model parameter values constant, the annual cost of duloxetine would have to fall to £41 a year (i.e., drug costs would have to fall to £0.08 per day from their current level of £1.10) for the relative cost effectiveness of PFMT to be called into question.
Continued duloxetine at 12 weeks
Table T.7
View in own window
| Duloxetine ICER cost – PFMT cost | (cost/QALY) | Comment |
|---|
| 75% | PFMT dominates | PFMT more cost effective |
| 80% | PFMT dominates | PFMT more cost effective |
| 85% | PFMT dominates | PFMT more cost effective |
| 90% | PFMT dominates | PFMT more cost effective |
| 95% | £227,000 | PFMT more cost effectivea |
| 100% | £105,000 | PFMT more cost effectivea |
Holding all other parameter values constant, it is necessary for 92% of patients on duloxetine to continue at 12 weeks in order for duloxetine to generate more QALYs than PFMT. However, even for a zero discontinuation rate at 12 weeks, the additional benefit falls a long way short of being cost effective because of the large cost differential between the two strategies.
Continued duloxetine at 52 weeks
Table T.8
View in own window
| Continued duloxetine at 52 weeks | (cost/QALY) | Comment |
|---|
| 70% | PFMT dominates | PFMT more cost effective |
| 75% | PFMT dominates | PFMT more cost effective |
| 80% | PFMT dominates | PFMT more cost effective |
| 85% | PFMT dominates | PFMT more cost effective |
| 90% | PFMT dominates | PFMT more cost effective |
| 95% | £229,000 | PFMT more cost effectivea |
| 100% | £108,000 | PFMT more cost effectivea |
Similarly, 91% of patients who continued at 12 weeks must still be on duloxetine at 52 weeks (0.74 × 0.91 = 67% of all patients) for duloxetine to generate more QALYs than PFMT. However, even if there is no discontinuation after 12 weeks, the small gain in QALYs (0.002) is considered poor value at an incremental cost of £200 per patient.
Continued PFMT if fail
Table T.9
View in own window
| Continued PFMT if fail | ICER (cost/QALY) | Comment |
|---|
| 70% | PFMT dominates | PFMT more cost effective |
| 60% | PFMT dominates | PFMT more cost effective |
| 50% | PFMT dominates | PFMT more cost effective |
| 40% | PFMT dominates | PFMT more cost effective |
| 30% | PFMT dominates | PFMT more cost effective |
| 20% | £588,000 | PFMT more cost effectivea |
| 10% | £140,000 | PFMT more cost effectivea |
| 0% | £80,000 | PFMT more cost effectivea |
Duloxetine is more effective than PFMT for low values of this parameter. Therefore, the ICER is calculated for duloxetine relative to PFMT.
The conclusion that PFMT is cost effective is not sensitive to the assumption made about those who fail with PFMT but continue with their pelvic floor exercises, if all other parameter values are held constant.
Reduction in leakage episodes if continue with PFMT after ‘failure’
Table T.10
View in own window
| Reduction in leakage episodes if PFMT fail/continued | ICER (cost/QALY) | Comment |
|---|
| 25% | PFMT dominates | PFMT more cost effective |
| 20% | PFMT dominates | PFMT more cost effective |
| 15% | PFMT dominates | PFMT more cost effective |
| 10% | PFMT dominates | PFMT more cost effective |
| 5% | £194,000 | PFMT more cost effectivea |
| 0% | £80,000 | PFMT more cost effectivea |
The conclusion that PFMT is cost effective is not sensitive to the assumption made about the reduction in leakage episodes for those who continue pelvic floor exercises after PFMT has failed, if all other parameter values are held constant.
Multi-way sensitivity analysis
In the following example all of the following have been changed:
Table T.11
View in own window
| Parameter | Default | New value |
|---|
| Duloxetine cost per day £1.10 | £1.10 | £0.90 |
| Review consultations | 1 | 0 |
| PFMT successful | 50% | 40% |
| Continue PFMT if fail | 75% | 50% |
| Continued duloxetine at 12 weeks | 74% | 80% |
| Continued duloxetine at 80 weeks | 68% | 80% |
Under this scenario, the ICER for duloxetine is £27,000 per QALY. According to the NICE threshold, this would suggest that duloxetine was borderline cost effective. However, this figure has only been achieved by biasing all the changes to parameter values in favour of duloxetine.
Clearly, it is possible to set parameter values in the model so that duloxetine is cost effective. However, the plausibility of such values is contingent on duloxetine being considerably more efficacious than PFMT, and this is not supported by the best available evidence at this time.
Second-line treatment: surgery versus duloxetine
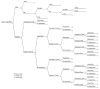
Given the finding that PFMT dominated duloxetine as a first-line treatment, a further decision tree model was developed, using TreeAge Pro 2006, to compare the cost effectiveness of surgery versus duloxetine for women with moderate to severe stress UI in whom first-line treatment with PFMT has been unsuccessful. A 2 year time frame was used for this model to reflect the fact that surgery has long-lasting effects that are not contingent on recurrent treatment costs. The decision tree for this model is shown in .
Decision tree model of surgery versus duloxetine.
Patients in the surgery arm have primary surgery that can either ‘succeed’ or ‘fail’. A proportion of patients in whom primary surgery fails will choose to have a second operation or even a third if the second also fails. The model does not include complications arising from surgery, most of which would be minor. Although they are extremely rare (less than 1 in 10 000 cases), severe complications (for example transfusion, ITU admission, death) may occur.
The duloxetine ‘sub-tree’ is the same as in the first-line treatment model, with the addition of continue/discontinued branches at 2 years for those still taking the drug at 52 weeks. As with the first-line model, the decision tree structure for duloxetine includes patient pathways that allow for continuation on therapy with adverse events and for discontinuation in the absence of adverse events. However, the simplifying default assumptions for model parameters is that adverse events cause discontinuation and that patients who continued with duloxetine did not experience any adverse events.
Cost parameters
Table T.12
View in own window
| Resource item | Value |
|---|
| Surgery (TVT) | £1,014 |
| Gynaecology outpatient consultation | £124 |
| Urodynamics | £140 |
| Urodynamics prior to primary surgery | 1 |
| Urodynamics prior to secondary surgery | 1 |
| Duloxetine cost per day | £1.10 |
| GP consultation | £21.00 |
| Review consultations for duloxetine | 1 |
| Drug adverse effects | £0 |
The cost of surgery is based on a published economic evaluation of TVT.716 It is assumed that a patient will have a gynaecology outpatient consultation prior to primary surgery and following a ‘failed’ operation. The cost of a gynaecology outpatient consultation is based on the mean value reported in the 2004 NHS Reference Costs for a first attendance for an outpatient gynaecology consultation. It is additionally assumed that urodynamics will be undertaken prior to primary or secondary surgery, reflecting current practice. The cost of urodynamics is taken from the mean unit cost for urodynamics reported in the 2003 NHS Reference Costs. The daily cost of duloxetine is derived from BNF 50. The cost of taking duloxetine also includes one review GP consultation, of 9.36 minutes duration, with the cost taken from Unit Costs of Health and Social Care 2004.932 It is additionally assumed at baseline that adverse effects of duloxetine do not impose any costs on the NHS and that there are no further review GP consultations after week 12. In accordance with NICE methodology, costs occurring in the second year are discounted at 3.5%.
Probability parameters
The adverse event probabilities are a simplifying assumption of this model. The other probabilities are taken from a published cost effectiveness study.426 The surgery success rate parameter is taken from an RCT of open colposuspension versus TVT.659,660
Table T.13
View in own window
| Event | Probability |
|---|
| Surgery (TVT) successful | 66% |
| Have second surgery if primary surgery fails | 75% |
| Have third surgery if second surgery fails | 30% |
| Adverse event if continued duloxetine | 0% |
| Adverse event if discontinued duloxetine | 100% |
| Continued duloxetine at 12 weeks | 74% |
| Continued duloxetine at 52 weeks | 68%a |
| Continued duloxetine at 2 years | 90%a |
- a
Expressed as a proportion of those continuing from the previous period.
Incontinence outcome parameters
Table T.14
View in own window
| Incontinence outcome | Value |
|---|
| Reduction in leakage episodes (surgery success) | 100% |
| Reduction in leakage episodes (surgery fail) | 50% |
| Reduction in leakage episodes (duloxetine continued) | 55% |
| Reduction in leakage episodes (duloxetine discontinued) | 0% |
| Reduction in leakage episodes (duloxetine discontinued by 12 weeks) | 42% |
| Reduction in leakage episodes (duloxetine discontinued after 12 weeks) | 55% |
| Days on duloxetine if discontinued by 12 weeks | 35 days |
| Weeks on duloxetine for those who discontinue by 52 weeks | 32 weeks |
| Weeks on duloxetine for those who discontinue by 2 years | 78 weeks |
It is assumed that women who stop taking duloxetine between 12 and 52 weeks, and between the first and second year, do so at the midpoint of these time intervals.
QALY parameters
Table T.15
View in own window
| Outcome | QALYs |
|---|
| QALY gain – pre-treatment to continent | 0.063 |
| Duloxetine continued at 12 weeks | 0.008 |
| Duloxetine continued at 12–52 weeks | 0.026 |
| Duloxetine continued at 2 yearsa | 0.069 |
| Duloxetine discontinued by 12 weeks | 0.003 |
| Duloxetine discontinued by 52 weeks | 0.013 |
| Duloxetine discontinued by 2 yearsa | 0.052 |
| Duloxetine adverse effects at 12 weeks | −0.003 |
| Duloxetine adverse effects at 52 weeks | −0.013 |
| Duloxetine adverse effects at 2 yearsa | −0.017 |
| Surgery successa | 0.126 |
| Surgery long-term faila | 0.063 |
Again, it is assumed that adverse effects are the main cause of discontinuation and that the disutility from adverse event must be at least as great as any utility gain from reduced UI symptoms. The other QALY values are derived by assuming a linear relationship between QALY gain and the reduction in leakage episodes.
QALYs occurring in the second year of the model are discounted at 3.5% in accordance with NICE guidance.
Results
Table T.16
View in own window
| Treatment | Cost | QALY | Incremental cost | Incremental QALY | ICER |
|---|
| Duloxetine | £477 | 0.0345 | | | |
| Surgery | £1,655 | 0.1143 | £1,178 | 0.0798 | £14,765 |
Using baseline assumptions, surgery would be considered as the more cost effective treatment with an ICER well within the £20,000 per QALY threshold for cost effectiveness suggested by NICE.
Sensitivity analysis
A series of one-way sensitivity analyses was undertaken to establish the parameter thresholds to achieve a £20,000 cost per QALY. Given the baseline result, this means varying parameter values in favour of duloxetine.
QALY parameters
Table T.17
View in own window
| Parameter | Value at which surgery cost per QALY = £20,000 |
|---|
| Cost of surgery (TVT) | £1,450 |
| Cost of duloxetine per day | £0.09 |
| Surgery ‘success’ | 48% |
| Reduction in leakage episodes for surgery ‘success’ | 80% |
| Reduction in leakage episodes for duloxetine ‘success’ | 100% |
| QALY gain from cure | 0.0465 |
For all other parameter values, the ICER remains below £20,000 per QALY.
Discussion
This model suggests that surgery is more cost effective than duloxetine as a second-line treatment for stress UI in women who have failed PFMT. Sensitivity analysis suggested that this result was not greatly affected by the assumptions used to inform parameter values.
The model was restricted to a 2 year follow-up because of a lack of long-term effectiveness data, particularly for duloxetine. Although, the effectiveness of surgery may decline over time, the limited time frame of the model still represents a considerable bias against surgery, as it does not allow for long-lasting effects and the continuing costs that would be required for medical therapy. However, this bias is offset to some extent by the decision not to include complications arising from surgery, to simplify the model.
The surgery success rate parameter is taken from an RCT of open colposuspension versus TVT,659,660 but this is a lower value than published case series; our assumptions may therefore underestimate the success of primary surgery. While it is probably inaccurate to say that the success of surgery does not decline with subsequent procedures, the assumption that this is the case simplifies the model.
However, the model also shows duloxetine to be a much cheaper strategy than surgery and therefore it could be considered as a second-line treatment for women who would choose it in preference to surgery, as lower cost care does not impose opportunity costs on the NHS.