NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Colorectal Cancer: The Diagnosis and Management of Colorectal Cancer. Cardiff: National Collaborating Centre for Cancer (UK); 2011 Nov. (NICE Clinical Guidelines, No. 131.)
This publication is provided for historical reference only and the information may be out of date.
The objectives of this chapter were to determine:
- which imaging modality most accurately determines the extent of metastases in patients with colorectal cancer and extrahepatic metastases (e.g. lung, brain, peritoneum)
- which imaging modality(s) most accurately determines the number and extent of metastases preoperatively in patients with colorectal cancer metastasised to the liver
- the effectiveness of treating metastatic disease before, after or at the same time as treating the primary tumour in patients with colorectal cancer presenting with overt synchronous metastatic disease
- the effectiveness of chemotherapy in patients with advanced and metastatic colorectal cancer
- the most effective additional treatment to systemic chemotherapy to achieve cure or long term survival in patients with apparently unresectable metastatic disease.
4.1. Management of patients presenting in stage IV
Approximately 25% of patients with colorectal cancer have metastatic disease at the time of initial presentation and it is thought that their outcome is often worse than for those patients who develop metachronous metastatic disease following apparently curative resection of their primary tumour.
The first question in managing this group of patients is whether the primary tumour needs immediate treatment because of established or impending obstructive symptoms, even in the presence of unresectable metastatic disease (see section 3.2).
The second question is whether or not both the primary tumour and the metastases are surgically resectable with curative intent. If the disease sites are considered resectable then the next questions are whether there should be preoperative or post-operative adjuvant treatments (or a combination of both) and whether the surgery should be a staged or combined procedure? Current practice varies widely including synchronous resections, staged resections with or without initial systemic treatment.
Where metastases are unresectable, currently patients fall into 2 groups:
- the extent of metastatic disease is such that although inoperable at presentation, patients might become resectable with curative intent if they have a good response to chemotherapy
- the extent of metastatic disease is such that patients are highly unlikely to be suitable for potentially curative surgery, even with a good response to chemotherapy
Advances in systemic therapy over the last 10 years have increased the potential for long-term survival and possible cure. However there remains uncertainty as to the best sequence of treatments to achieve optimal outcome.
Clinical question: In patients with colorectal cancer presenting with overt synchronous metastatic disease, what is the effectiveness of treating metastatic disease before, after or at the same time as treating the primary tumour?
Clinical evidence
There was very little evidence with which to address this topic and what was available consisted primarily of retrospective studies. There were 2 systematic reviews of retrospective studies (Hillingso and Jorgensen, 2009; Scheer et al., 2008), one randomised trial (Nordlinger et al., 2008) and 3 retrospective case series studies, two case matched (Moug et al., 2010; Benoist et al., 2005) and one non-matched case series (Mentha et al., 2008).
Synchronous resection versus staged resection
A well conducted systematic review which included 16 studies (Hillingso and Jorgensen, 2009) and a more recent case series study (Moug et al., 2010) compared outcomes in patients undergoing synchronous resection and patients undergoing staged resection of primary tumour and liver metastases. The available evidence was considered to be very low quality for all outcomes on GRADE assessment (Table 4.1).
Table 4.1
GRADE profile: Quality assessment of studies reporting length of hospital stay (days); postoperative morbidity; postoperative mortality and 5 year survival.
A pooled estimate was possible from 8/11 studies reporting on length of hospital stay. The mean difference reported was −3.10 days [95% CI: −6.76–0.56] for patients undergoing synchronous resection indicating no significant difference between the two procedures in relation to the length of hospital stay. There was however significant statistical heterogeneity when pooling the studies (I2=92%; Χ2=82.85, p<0.00001) indicating that it may not be appropriate to conduct pooled analysis.
The results of the pooled analysis show synchronous resection to be significantly better than staged resection in relation to postoperative morbidity (OR=0.68, [95% CI: 0.49–0.81]). On calculating the risk difference, there was no significant difference in the risk of mortality between the two groups (RD, 0.01, [95% CI: −0.01–0.04]). There was no significant difference in 5 year survival for patients undergoing synchronous resection versus patients undergoing staged resection.
Preoperative chemotherapy followed by surgery versus surgery alone
For chemotherapy followed by surgery versus immediate surgery, a single systematic review included only 7 studies (Scheer et al., 2008) deemed to be relevant and not all included studies were case matched meaning there was no comparison within the individual study. This, together with a non-matched case series study (Mentha et al., 2008) and a randomised trial investigating only progression free survival (Nordlinger et al., 2008) comprised the evidence base examining chemotherapy versus immediate surgery for patients with colorectal cancer and liver metastases.
Outcome data were available for length of hospital stay, tumour related complications in patients treated initially with chemotherapy, overall survival and progression free survival. The available evidence was considered to be very low to low quality for all outcomes on GRADE assessment (Table 4.2).
Table 4.2
GRADE profile: Quality assessment of studies reporting length of hospital stay (days); tumour related complications; overall survival; progression-free survival.
One retrospective case series (Benoist et al., 2005) aimed at determining the best treatment strategy for patients with asymptomatic primary tumour and irresectable metastases, reported mean hospital stay in the chemotherapy group was 11 days (SD=10 days, range=2–52 days) versus 22 days (SD=15 days, range=5–75 days) in the resection group (p=0.003).
The rate of intestinal obstruction reported in the included studies ranged from 5.6–29%; the pooled proportion of patients developing bowel obstruction was 13.9% [95% CI: 9.6–18.8%] (Scheer et al., 2008).
Haemorrhage due to primary tumour was reported in 4/7 studies included in the systematic review and ranged from 0–3.7%; the pooled proportion of patients experiencing bleeding due to primary tumour was 3% [95% CI: 0.95–6%] (Scheer et al., 2008).
Postoperative mortality ranged from 0% to 4.6%; meta-analysis of the four studies showed a mortality of 2.7% [95% CI: 1.1–5%] (Scheer et al., 2008).
Scheer et al. (2008) reported that for patients that underwent resection of the primary tumour median survival ranged from 14–23 months versus 8.2–22 months for patients treated with chemotherapy as first treatment.
Hazard ratio for progression free survival was 0.79 ([95.66% CI: 0.62–1.02], p=0.058) which corresponds to a 7.3% increase in the rate of progression free survival at 3 years from 28.1% (range 21.3–35.3) to 35.4% (range 28.1–42.7) with chemotherapy and an increase in median progression free survival from 11.7 months to 18.7 months (Nordlinger et al., 2008).
Recommendations
- Prioritise treatment to control symptoms if at any point the patient has symptoms from the primary tumour.
- If both primary and metastatic tumours are considered resectable, anatomical site-specific MDTs should consider initial systemic treatment followed by surgery, after full discussion with the patient. The decision on whether the operations are done at the same time or separately should be made by the anatomical site-specific MDTs in consultation with the patient.
Linking evidence to recommendations
The GDG considered that although overall survival is important to patients, quality of life is held in equal importance. The outcome of operative mortality was also considered important because the recommendations are aiming to prevent untimely deaths and morbidity because of the impact of this endpoint on the patient’s ability to have other treatment. Length of hospital stay was not considered a useful outcome because it was determined by local procedures and not controlled for across the studies, therefore there was the potential for bias.
The GDG noted that the evidence as assessed by GRADE methodology as very low.
Despite a lack of evidence for the specific situation of an obstructing tumour, there was GDG agreement that treatment should be given for symptom control. Due to the lack of evidence the GDG believes that at present treatment decisions of this type should be left to the MDTs in consultation with the patient.
The data on initial systemic treatment, suggested that patients presenting in stage IV with non-obstructing primary tumours might benefit in terms of quality of life and overall survival from receiving this. Therefore the GDG decided to recommend that initial systemic treatment be considered.
The GDG noted that outcomes of surgery, such as peri-operative morbidity/mortality, were similar whether the surgery was synchronous or staged. However the GDG agreed that at the individual patient level, if either procedure were high risk, it would be preferable to separate the operations even though the evidence had shown no difference in outcomes between these groups of patients
The topic was not considered a priority for health economic evaluation because there was no appropriate comparator to enable cost-effectiveness analysis to be undertaken.
4.2. Imaging hepatic metastases
Colorectal cancer that has metastasised to the liver may be amenable to surgical resection with long-term survival improvement or curative intent. The expected 5 year survival after such liver surgery now approaches 60%, with 10 year survival close to 30%. Currently, >20% of patients with hepatic colorectal cancer metastases can be considered candidates for hepatectomy with curative intent. However, hepatic resection is a costly procedure with significant morbidity; careful patient selection is crucial to achieve the best clinical outcomes.
Imaging plays three roles in patient selection:
- to detect as many liver metastases as possible and their location, in order to maximise the chance of achieving complete clearance of disease at the time of surgery
- to accurately characterise any benign liver lesions which may be present, so as to avoid unnecessary surgical procedures
- to detect other sites of metastatic disease which may themselves be amenable to treatment, or may render liver resection inappropriate (see section 4.1)
The key question is which imaging modality most accurately determines the number and extent of liver metastases preoperatively, to decide which patients are suitable for radical surgery with curative intent.
Clinical question: In a patient with colorectal cancer metastasised to the liver which imaging modality(s) most accurately determine the number and extent of metastases preoperatively?
Clinical evidence
There were two meta-analyses available comparing PET to MRI and CT (Bipat et al., 2005) and PET to CT (Wiering et al., 2005). In both studies, per patient analysis showed that PET has higher sensitivity than MRI and CT but this was not the case on a per lesion basis with sensitivities for all modalities being comparable. Gadolinium contrast-enhanced MRI and SPIO-contrast enhanced MRI were better than non-enhanced MRI and CT and this was more manifest in the subgroup analysis that looked at specific sizes of lesions which showed that MRI had a better sensitivity in detecting micrometastases of <1cm.
Since 2005 a number of studies have been carried out continuing to test the ever-developing technologies of MRI and CT against each other. In the last 5 years PET has been fused with CT and there are now studies looking at the performance of PET/CT and comparing it to MRI, PET and CT.
It appears that in a per-patient analysis PET-CT has consistently higher sensitivity in all the studies compared to MRI and CT and pooled analysis supports this with a summary sensitivity and accuracy for PET/CT of 94% for both compared with MRI (80% and 91% respectively) and CT (87% for both).
On per lesion analysis MRI appeared to be the modality showing higher sensitivities across individual studies compared to CT and pooled data shows comparable results with MRI having a combined sensitivity of 88% and accuracy of 87%, CT a sensitivity of 74% and accuracy of 78% and PET/CT a sensitivity of 79% and accuracy of 97%.
A number of studies carried out subgroup analyses looking at how the modalities diagnose lesions of particular sizes. Bartolozzi et al. (2004), Bhattarajha et al. (2004) and Wiering et al. (2005) all found MRI has better sensitivity at picking up the smaller lesions <1cm compared to PET/CT and CT. The majority of lesions missed by PET/CT were micrometastases of <1cm.
Chua et al. (2007) and Liu et al. (2007) reported change in management as an outcome however both studies include the diagnosis of extrahepatic metastases in their analysis. It was not possible to extract data for this relating to hepatic metastases only.
A systematic review and meta-analysis of data comparing the diagnostic accuracy of different imaging modalities for the diagnosis of colorectal liver metastases was available (Floriani et al., 2010). Pairwise comparisons suggested that MRI performed significantly better than CT for the detection of metastatic lesions (sensitivity OR: 0.66 [95%CI: 0.55–0.80] p<0.0001) but the data were highly heterogeneous. The superiority of MRI differed between the various CT techniques in per lesion analysis which probably accounts for the observed heterogeneity. MRI was also better than CT in a per patient analysis (sensitivity OR: 0.69 [95%CI: 0.47–0.99] p=0.05) which is a more reliable indicator. FDG-PET and ultrasound performed similarly to CT, although significant between studies heterogeneity may well have confounded these results.
From a prospective case series of 34 patients (Mainenti et al., 2010) comparing MRI, PET/CT and CT, ROC analysis showed no significant difference between Gadolinium- and SPIO-enhanced MRI and showed that both forms of MRI performed significantly better than all other modalities (p<0.05). For lesions ≥10mm, the performance of PET/CT was significantly better than contrast enhanced CT (p<0.05). No significant difference was observed between the modalities when considering the groups of lesion <10mm.
Recommendation
Linking evidence to recommendations
The GDG considered sensitivity and specificity of the investigations to be the most important outcomes. They noted that the overall quality of the diagnostic studies was poor because there was poor reporting of study design parameters, varied study design and possible risk of bias.
The GDG acknowledged that the diagnosis of liver metastases is derived from a CT scan performed as part of the original staging or during follow-up after potentially curative surgery for the primary cancer. The question is: what imaging and what sequence should then be done to confirm the patient is suitable for surgery and to determine the surgical strategy?
The GDG acknowledged that evidence showed CT and MRI are comparable at detecting liver metastases, with sensitivities over 75%. They also noted that from the evidence PET-CT reported consistently higher sensitivity (90%) than the other modalities. However they were aware that PET-CT is more expensive and less widely available than the other modalities and that not all tumours are FDG avid.
The available evidence is unclear whether MRI or PET-CT should be used after a CT scan to confirm the patient with liver metastases suitable for surgery. Therefore the GDG recommended that the opinion of a hepatobiliary MDT is sought. This would then allow a specialist to make the decision on what additional imaging to use, striking a balance between missing patients with resectable disease and excessive inappropriate laparotomies.
Because of this uncertainty, the GDG decided to recommend further research in this area. The focus of this question was on the use of imaging modalities (CT, PET-CT, MRI or ultrasound) for the detection of liver metastases to inform a decision about resectability. An economic analysis of this topic would need to take into account not only accuracy of the imaging modality in detecting metastases, but also downstream consequences on treatment decisions and patient outcomes. An initial search of the clinical literature revealed that most of the relevant studies identified do not report information on resectability or change in patient management in relation to the information obtained by the imaging test. As the decision to resect is based on a number of different considerations, there is insufficient information to model the link between the imaging results and the treatment decision. Therefore the feasibility of conducting a comprehensive cost-effectiveness analysis based on currently available data is limited and the GDG agreed not to pursue development of an economic model for this topic.
Research recommendation
- A prospective trial should be conducted to investigate the most clinically effective and cost-effective sequence in which to perform MRI and PET-CT, after an initial CT scan, in patients with colorectal cancer that has metastasised to the liver, to determine whether the metastasis is resectable. The outcomes of interest are reduction in inappropriate laparotomies and improvement in overall survival.
4.3. Imaging extra-hepatic metastases
Historically, patients with extra-hepatic metastatic colorectal cancer were considered incurable, treatment was either with palliative intent or best supportive care, and life expectancy was short (typically a few months). Modern chemotherapy, combined with newer interventions in surgery and radiology offer improvements in survival that can be measured in years, and occasionally the possibility of cure.
Extra-hepatic metastases can be suspected at first diagnosis of colorectal cancer, either in the elective or emergency setting (patients presenting with stage IV disease). Alternatively, following apparently curative surgery for primary colorectal cancer, extra-hepatic metastases can be diagnosed during either routine follow-up or between follow-up appointments during investigation of new symptoms.
The issues that determine appropriate treatment for patients with extra-hepatic metastases are:
- patient specific (age, fitness, mode of presentation with colorectal cancer)
- lesion specific: whether or not the detected abnormality represents metastatic cancer or is a benign co-incidental finding
- disease specific (anatomic site(s) of disease, extent of tumour burden)
- the ability to determine the extent and location of their tumour burden.
The common sites of extra-hepatic metastases are distant lymph nodes, peritoneum and lungs. Rare sites of metastases include adrenal glands, central nervous system and bones. Previously, following apparently curative surgery for primary colorectal cancer, extra-hepatic metastases have been detected during follow-up using a combination of clinical examination, blood CEA estimations, endoscopic surveillance and liver ultrasound scans with occasional chest X-ray examinations. Over the past decade and a half there has been a move towards contrast-enhanced CT scanning of chest, abdomen and pelvis. Further information has also been obtained using MRI and PET-CT, both in lesion characterisation and also evaluation of extent and site of extra-hepatic tumour burden.
Having detected extra-hepatic disease, it is important to determine the extent of disease to offer the appropriate treatment strategy. There is uncertainty about the role of metastasectomy for the treatment of resectable lung metastases and this is being investigated in the PulMiCC trial. However, little is known as to which is the most useful investigation or the correct sequence of investigations to accurately determine the extent of tumour burden in patients with extra-hepatic metastatic colorectal cancer.
Clinical question: In a patient with colorectal cancer and extrahepatic metastases (e.g. lung, brain, peritoneum), which imaging modality most accurately determines the extent of metastases?
Clinical evidence
The evidence base for this question comprises one systematic review of observational studies (Wiering et al., 2005) and nine retrospective case series (Desai et al., 2003; Imdahl et al., 2000; Potter et al., 2009; Schmidt et al., 2009; Selzner et al., 2004; Squillaci et al., 2008; Tanaka et al., 2002; Valk et al., 1999; Votrubova et al., 2006). None of the studies were designed to directly compare the effectiveness of the imaging techniques in detecting extrahepatic metastases.
FDG-PET versus CT
Wiering et al. (2005) found that FDG-PET had a higher sensitivity and specificity (91.5% and 95.4%) than CT scan (60.9% and 91.1%) in detecting extrahepatic metastases. Using only the highest weighted studies from the meta-analysis, the pooled sensitivity and specificity for FDG-PET were 91.2% and 98.4% respectively and for CT the sensitivity and specificity were 55.3% and 95.6%. Tanaka et al. (2002) reported that FDG-PET also had higher accuracy and sensitivity (78% and 88%) than CT (44% and 38%) in diagnosing peritoneal metastases, but the study numbers were very low (n=23). Valk et al. (1999) reported sensitivity and specificity for detecting extrahepatic metastases of 92% and 99% for FDG-PET compared with 61% and 96% for CT. The authors also added that FDG-PET had a significantly higher specificity than CT in detecting lung metastases.
Potter et al. (2009) found no significant difference in diagnostic accuracy between FDG-PET and CT/MRI but the study provided some information with regard to the role of the reader, since a significant difference in accuracy and sensitivity was found between the three individuals who interpreted the CT/MRI scans.
PET/CT versus MRI
Schmidt et al. (2009) found that PET/CT had higher sensitivity than whole body MRI in the detection of distant metastasis (80% versus 78%) but there was no difference in specificity (95%) and accuracy was similar (PET/CT: 87%, whole body MRI: 86%). Squillaci et al. (2008) did not report sensitivity or specificity but suggested that both modalities were equivalent in detecting extrahepatic metastases. Both studies concluded that PET/CT detected more lung metastases than whole body MRI.
PET/CT versus CT
Selzner et al. 2004 found no difference in the ability of PET/CT or contrast enhanced CT to detect the presence of extrahepatic metastases but PET/CT was more sensitive than CT in the detection of lung metastases (100% versus 78%). PET/CT was also more sensitive than CT for portal and para-aortic lymph node metastasis (77% versus 46%) although these differences were not statistically significant.
Others
Votrubova et al. (2006) showed PET/CT was superior (sensitivity 95%, specificity 100%, accuracy 100%) to FDG uptake (sensitivity 74%, specificity 88%, diagnostic accuracy 88%) for the diagnosis of extra abdominal and/or hepatic recurrence of colorectal cancer and in the diagnosis of any form of colorectal cancer recurrence (p<0.05).
Desai et al. (2003) presented no data on the effect of PET on surgical decision making in patients with metastatic or recurrent colorectal cancer but observed that the information provided by PET complemented that provided by the CT scan. Imdahl et al. (2000) reported a higher sensitivity and specificity for PET (94% and 100%) compared with chest X-ray (64% and 98%) for the detection of pulmonary metastases.
Two studies (Metser et al., 2010; Choi et al., 2010) were identified during updates as providing evidence for the topic though both studies were case series studies and neither were specifically designed to answer the question of which modality is best for identifying number and extent of extrahepatic metastases.
Choi et al. (2010) evaluated the role of chest CT on preoperative staging of rectal cancer to assess the impact on treatment strategy though the study was of a low quality and it was difficult to draw any conclusions as to the effectiveness of chest CT on the preoperative staging of pulmonary metastases when compared with standard chest X-ray.
Metser et al. (2010) compared the detection of tumour recurrence and metastases with FDG-PET/CT with contrast enhanced multi-detector CT in patients with colorectal cancer and elevated CEA levels and reported that on event based analysis (number of lesions) PET/CT was significantly more sensitive that multi-detector CT (p=0.002) but there was no difference in specificity (p=1.0) of the two modalities for detection or recurrence or metastases. Tumour based analysis showed that PET/CT was significantly better than multi-detector CT for the detection of recurrence and metastases (p<0.0001) though again there was no difference in specificity (p=0.56).
Recommendations
- Offer contrast-enhanced CT of the chest, abdomen and pelvis to patients being assessed for metastatic colorectal cancer.
- If intracranial disease is suspected, offer contrast-enhanced MRI scan of the brain. Do not offer imaging of the head, neck and limbs unless involvement of these sites is suspected clinically.
- Discuss all imaging with the patient following review by the appropriate anatomical site-specific MDT.
- If the CT scan shows the patient may have extra-hepatic metastases that could be amenable to further radical surgery, an anatomical site-specific MDT should decide whether a positron emission tomography-CT (PET-CT) scan of the whole body is appropriate.
- If the diagnosis of extra-hepatic recurrence remains uncertain, keep the patient under clinical review and offer repeat imaging at intervals agreed between the healthcare professional and patient.
Linking evidence to recommendations
The GDG considered sensitivity and specificity of the investigations to be the most important outcomes. They noted that there was limited, poor-quality evidence to address this topic. The GDG also observed that imaging technology is improving all the time and it can sometimes be unclear whether results from older imaging studies are generalisable to modern clinical practice.
The GDG noted that CT of the thorax, abdomen and pelvis has high specificity and modest sensitivity for the detection of extra-hepatic metastases, as it covers the organs and viscera at greatest risk for recurrence or metastases from colorectal cancer. The GDG were also aware that CT is widely available throughout the NHS, inexpensive relative to the other modalities and applicable to almost all patients. Therefore the GDG recommended that CT be used initially to determine the extent of extrahepatic metastases.
The GDG considered that isolated asymptomatic metastasis from colorectal cancer to the head, neck or limbs was unusual and therefore did not warrant routine imaging. However, when there is suspicion of intracranial disease, a contrast-enhanced MRI provides greatest sensitivity and specificity and is the investigation of choice.
Because of the relative high cost and limited availability of PET-CT, the GDG considered this was an inappropriate first investigation for detecting extra-hepatic metastases. However, for patients with extra-hepatic metastases thought to be amenable to radical surgery, the GDG considered the increased sensitivity provided by PET-CT could be useful in the avoidance of non-beneficial surgery. Given the lack of studies directly addressing this issue, the GDG agreed it was more appropriate for the decision on whether or not to perform a PET-CT to be left to the site-specialist MDT.
The limited evidence published to date is insufficient to fully define the benefits and limitations of PET-CT in this specialized area of practice. PET-CT is considerably more costly than the other imaging modalities, and in the UK, is available only at a small number of specialist centres. It has increased sensitivity for detecting extra-hepatic metastases beyond that of MRI and CT, but it is unclear whether this benefit has a sufficient impact on patient management to justify the cost. Therefore the GDG decided to recommend further research in this area.
The GDG acknowledged that the pelvis is a common site for recurrence of colorectal cancer. When pelvic recurrence is suspected on CT scan, they agreed that MRI has increased specificity to discriminate between recurrent tumour and complications of treatment. Furthermore it better demonstrates the anatomic relationships of recurrent tumour to pelvic viscera, major blood vessels and bony structures, and thus facilitates the selection for radical surgery with curative intent.
The GDG acknowledged that the use of MRI in addition to CT scanning for pelvic disease was likely to incur substantially higher costs. However the GDG agreed that this balanced against improved patient selection for radical surgery.
The additional use of PET-CT incurs a further substantial increase in cost, but the trade-off is further improved patient selection when radical surgery is being considered, in particular the avoidance of non-beneficial surgery and the costs and complications associated with this.
Research recommendation
- A prospective, multi-centre observational study of the quality, sensitivity, specificity and cost-effectiveness of using PET-CT in the management of patients with colorectal cancer should be conducted.
4.4. Chemotherapy for advanced and metastatic colorectal cancer
The management of locally advanced and metastatic adenocarcinoma of the colon and rectum has advanced markedly over the past 10 years. The introduction of a number of new chemotherapeutic and biological agents has led to significant increases in progression free and overall survival. The clinical efficacy of these agents has been the subject of a number of previous NICE technology appraisals (TA). It is recognised that management of advanced colorectal cancer encompasses a spectrum of no treatment, monotherapy (see section 4.4.3) and combination therapy. This section is specifically focused on combination chemotherapy.
Both oxaliplatin and irinotecan have developed important roles in the management of colorectal cancer – both in combination with fluoropyrimidines and also, for irinotecan, as a single agent.
Over 50,000 patients have now been treated in trials looking at optimal combinations of oxaliplatin and a fluoropyrimidines (5-flourouracil or capecitabine). These data confirm the value of this combination in terms of trial endpoints when compared against single agent fluoropyrimidines. When combinations of oxaliplatin and a fluoropyrimidine are compared against irinotecan combinations then generally the results are equal, albeit with differing toxicities.
Irinotecan appears to have activity both in combination with a fluoropyrimidine and as a single agent. The combination regimens seem to have less toxicity, and a trend to better outcomes than when used as a single agent.
Currently, for patients with advanced metastatic disease, both oxaliplatin and irinotecan can be used to extend disease-free and overall survival. There are a number of less frequent circumstances (for example liver-limited metastatic disease) (see sections 4.1–4.3) where alternative strategies are used but these are with the intention of long-term disease control, rather than palliation. Defining the optimal strategy for sequencing of these agents remains a difficult trial endpoint.
Recommendations on the use of oxaliplatin, irinotecan and raltitrexed were made in NICE TA9311. However, since the publication of TA93 in 2005 there has been an expansion in the amount of published trial data and therefore TA93 is being updated within this guideline. The GDG accepted the recommendations of TA93 for the use of irinotecan and oxaliplatin but also wished to address the following issues:
- The value of combining irinotecan with an oral fluoropyrimidine.
- The optimal sequencing of oxaliplatin and irinotecan combinations.
- The value of raltitrexed in patients who cannot tolerate 5FU/FA based regimens of for whom these are inappropriate.
Due to a lack of trial data on direct comparisons between all relevant drug sequences a Mixed Treatment Comparison (MTC) was chosen to address the optimal sequencing question. This technique allows data from indirect comparisons to be used as evidence (see Figure 4.1).
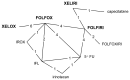
Figure 4.1
MTC network of evidence used to inform response rate and progression-free survival for first-line treatments. Treatments in bold text are of primary interest to the analysis. A line between two treatments indicates a head-to-head comparison (RCT) exists; (more...)
This update of TA93 does not cover the value of biological agents since recommendations have already been made on their use in TA17612 and TA21213.
4.4.1. Oxaliplatin and irinotecan in combination with fluoropyrimidines
Clinical question: What is the effectiveness of oxaliplatin and irinotecan-based chemotherapy regimens for patients with advanced and metastatic colorectal cancer?
This clinical question includes both an update to identify new evidence that has become available after TA9314 was issued (August 2005) and an expansion to the guideline scope to address the following issues that were deemed by the GDG to be relevant to recent developments in clinical practice:
- the use of irinotecan or oxaliplatin in combination with the oral fluoropyrimidine capecitabine
- sequencing of combination chemotherapy (first and second line)
Although there are data on the choice of chemotherapy regimens to treat patients with advanced and metastatic colorectal cancer, none of the studies identified by the systematic review provided a comprehensive analysis with which to directly answer the review question.
In the absence of direct, comparative evidence an indirect modelling exercise known as a Mixed Treatment Comparison was conducted to address these issues and make use of all available data. The outcome of this exercise was to inform decision-making regarding optimal combinations and sequences of chemotherapy for the management of advanced colorectal cancer. Full details of this analysis can be found in Appendix 2. Mixed Treatment Comparisons that draw on both direct and indirect evidence have become an important method to address decision problems that, often for feasibility reasons, cannot be practically answered by conducting further randomised controlled trials.
Clinical evidence (see also Appendix 2)
After a review of the available evidence for this topic and consultation with the GDG, the following chemotherapy regimens were considered relevant to include within this clinical question:
- FOLFOX (oxaliplatin in combination with 5-flourouracil and folinic acid)
- FOLFIRI (irinotecan in combination with 5-flourouracil and folinic acid)
- XELOX (oxaliplatin in combination with capecitabine)
- XELIRI (irinotecan in combination with capecitabine)
- irinotecan as a single agent
The GDG identified ten sequences based on these chemotherapy regimens that were considered relevant to current clinical practice (Table 4.3). Sequences were limited to two lines of treatment.
Table 4.3
Summary of ten chemotherapy treatment sequences of interest.
The search for evidence included randomised controlled trials (RCTs) that reported on response, progression-free survival and overall survival for one or more of the chemotherapy regimens of interest as first-line treatment, second-line treatment or as part of a prospectively sequenced trial. Head-to-head RCTs were not available to inform all comparisons of interest. In addition, overall survival is likely to be influenced by the sequence of chemotherapy treatments; data on overall survival that was reported from studies conducted only in first line (with limited information about subsequent treatment) or only in second line (with limited information about prior treatment) was regarded with caution, thus further limiting the number of head-to-head comparisons available to inform this endpoint.
In order to facilitate a comparative analysis of all ten chemotherapy sequences, it was necessary to consider evidence that enabled indirect comparison of the treatments of interest. For example, if an RCT existed comparing two treatments A vs B, and another RCT existed comparing B vs C, however no RCT was identified comparing A vs C, then the evidence from the RCTs comparing A vs B and B vs C can be used to produce an indirect estimate of the relative effectiveness of A vs C. For the analysis of first-line treatment effects, both head-to-head trials (direct comparisons) as well as indirect comparisons were simultaneously considered as part of the evidence base to inform the estimate of effect size between 2 or more treatments of interest, therefore the analysis for first line is referred to as a Mixed Treatment Comparison (MTC).
A total of twenty-three studies formed the evidence network for the analysis of response rate and progress-free survival for first-line treatment (Colucci et al., 2005; Comella et al., 2005; Comella et al., 2009; Cunningham et al., 2009; Diaz-Rubio et al., 2007; Douillard et al., 2000; Ducreux et al., 2010; Falcone et al., 2007; Gennatas et al., 2006; Giachetti et al., 2000; Goldberg et al., 2004; Goldberg et al., 2006; de Gramont et al., 2000; Hochster et al., 2008; Kohne et al., 2005; Kohne et al., 2008; Koopman et al., 2007; Martoni et al., 2006; Porschen et al., 2007, Saltz et al., 2000; Seymour et al., 2007; Souglakos et al., 2006; Tournigand et al., 2004). The evidence network is shown in Figure 4.1.
For the analysis of effectiveness of second-line treatment, the search for RCTs identified four studies in which two treatments of interest had been compared specifically as second-line chemotherapy (Haller et al., 2008; Kim et al., 2009; Rothenberg et al., 2008; Rougier et al., 1998). However upon examination of the inclusion criteria for these studies, it was noted that all patients in these trials had received either single agent irinotecan or singe agent 5-fluorouracil as first-line treatment for advanced colorectal cancer. Therefore, these studies did not reflect the specific treatment sequences of interest to the current review and were excluded from the analysis.
The only other source of data on second-line response rates, PFS and overall survival for the treatment sequences of interest was from prospectively sequenced studies. Three prospectively sequenced trials were available (Tournigand et al., 2004; Koopman et al., 2007; Seymour et al., 2007) and reported data on response rate and PFS after first and second line. However, Seymour et al. 2007 did not compare any sequences of interest or any sequences common to the other two trials, and was therefore excluded from the evidence space. The remaining trials provide evidence on only three of the ten sequences of interest and do not form a connected evidence network. In order to facilitate the analysis, two important assumptions were explored:
- the oral and iv fluoropyrimidine formulations (capecitabine and 5-FU) are equally effective when used as part of a combination treatment
- the first-line study by Cunningham et al. 2009 could be considered a quasi-sequenced study because the protocol pre-specified that patients who progressed on first-line treatment should be offered irinotecan as second-line treatment
The validity of these two assumptions was explored using statistical methods and through discussion with GDG members. Using these key assumptions for the analysis, a network of evidence was constructed for the relevant sequences of treatment as shown in Figure 4.2. Each comparison was informed by using either direct evidence from a head-to-head trial or indirect evidence via a common comparator, but not by both types of evidence simultaneously. Therefore the second-line analysis is more accurately referred to as an indirect (rather than mixed) treatment comparison.

Figure 4.2
Network of sequenced studies to inform second-line response rate, progression-free survival and overall survival (assuming equivalent effect of capecitabine and 5-FU).
Quality of the evidence
All studies that were identified for inclusion in the mixed or indirect treatment comparison were RCTs and were assessed using the NICE methodology checklist for randomised trials All studies included were considered to be methodologically sound. The quality assessment for this topic cannot be produced in GRADE as the software cannot yet accommodate the issues surrounding indirect treatment comparisons. GRADE has been designed to assess the quality of the total body of evidence for a given outcome rather that the methodological quality of individual studies included in the analysis. While this is certainly a more informative and useful way in which to assess the quality of evidence, an indirect treatment comparison presents a particular problem in that the information used to inform the model includes, where possible, direct evidence, but in many cases will also include data from studies which do not directly assess the interventions of interest against each other and is so considered indirect evidence. Using a MTC method however, will allow for inbuilt considerations in the model in order to account for the indirectness of the data.
First-line treatment response rate and progression-free survival
The results of the MTC analysis for first-line treatments are shown in Table 4.3.
Table 4.3
Summary of response rates and PFS for first-line treatments.
Second-line treatment response rates, progression-free survival and overall survival
The results of the indirect treatment comparison for sequences are shown in Table 4.4.
Table 4.4
Summary of response rates and PFS for second-line treatments (given as part of a sequence) and overall survival for sequences of treatment.
In first-line treatment, the results of the mixed treatment comparison suggest that FOLFOX was associated with a higher probability of being the most effective regimen with respect to both response rate and PFS. The small benefit in favour of FOLFOX was also evident when comparing second-line response rates, however was not the case with respect to second-line PFS.
For the endpoint overall survival, the indirect treatment comparison suggests no differences between the treatment sequences of interest.
Toxicity
Toxicity data was reported in a number of studies, though the consistency of reporting was variable. Commonly reported toxicities included nausea and vomiting, diarrhoea, neutropenia, febrile neutropenia, anaemia, palmar-plantar erythrodysesthesia (PPE), peripheral neuropathy and toxic death. MTC methods were not applied to toxicity data as there was insufficient data to inform the analysis.
For first-line regimens, the grade 3/4 toxicities that were most commonly reported across all treatments included diarrhoea (from 15.6% for FOLFOX to 30.3% for XELIRI), neutropenia (from 7.6% for XELOX to 28.7% for FOLFOX) and peripheral neuropathy (from 16.3% for FOLFOX to 18.3% for XELOX).
In second-line treatment, grade 3/4 neutropenia was one of the most commonly reported toxicities (from 22% for irinotecan to 33% for FOLFOX). It was also noted that single agent irinotecan was associated with a higher rate of grade 3/4 diarrhoea (22%) than the other treatments. Data should be interpreted with caution as only a small number of studies were available to inform regimen-specific toxicity rates in most cases.
Quality of Life
Quality of life was included as an outcome in a total of seven studies; four were first-line studies (Comella et al., 2009; Falcone et al., 2007; Douillard et al., 2000; DeGramont et al., 2000); two were second-line studies (Cunningham et al, 1999; Rougier et al, 1998) and one was a sequenced study (Koopman et al., 2007).
Only one trial compared two treatments of interest (FOLFOX and XELOX) and only in first line (Comella at al., 2009). To compare quality of life between arms, baseline questionnaires were filled in by a total of 312 patients (97% of total patient population) and again at 8 weeks, 16 weeks and 24 weeks following treatment (EORTC-QLQ-C30 version 3). The baseline single item and global health status/quality of life scores did not differ significantly between the two arms. No significant differences in the change of single scores were observed between the two arms apart from constipation (p=0.001) and financial item score (p=0.004). At the predetermined time point for the comparison, a preservation of the quality of life was observed in 47% of patients in either arm. A higher proportion of patients in the XELOX arm showed a deterioration of the global health status/quality of life score after 16 weeks and 24 weeks though the differences were not statistically significant.
Economic evaluation (see also Appendix 2)
A decision tree was constructed to reflect key events in the treatment pathway for advanced colorectal cancer patients in order to compare costs and health effects for the ten sequences of chemotherapy. In first line, patients receive one of four possible irinotecan or oxaliplatin-based combination chemotherapy regimens. Following first-line treatment, the model allows for a proportion of patients to discontinue treatment. The remaining proportion of patients go on to receive one of five possible second-line treatments (Figure 4.3).
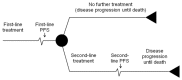
Figure 4.3
Basic structure of the cost-effectiveness model. The same structure was applied to all ten treatment sequences in the analysis.
The main effectiveness outcome in the model is quality-adjusted life years (QALYs). The model assumes a lifetime time horizon. Survival time is partitioned in the model using the progression-free survival and overall survival results of the MTC analysis. While receiving chemotherapy and prior to the onset of progressive disease, patients are assumed to be in a stable disease state. Following the point of progression in the model, patients are assumed to be in a progressive disease state with a lower overall quality of life. The model does not explore survival conditional on best response to treatment. This is because there was insufficient detail reported in the clinical literature to facilitate survival analysis dependent on tumour response.
The impact of chemotherapy-related toxicities was taken into account in the model both in terms of disutility to the patient as well as cost associated with management. Toxicities in the cost-effectiveness model were limited to those with most clinical relevance as well as data to support estimates of both the impact on patient well-being and cost. This included febrile neutropenia and grade 3 and 4 diarrhoea and vomiting.
The sources of data inputs for key parameters in the model are summarised briefly in Table 4.5. The model was made probabilistic to take into account the impact of parameter uncertainty on results.
Table 4.5
Key parameters and sources of data inputs for the cost-effectiveness model.
The results of the mixed and indirect treatment comparisons were used as inputs to conduct a cost-effectiveness analysis. This allowed the sequences to be ranked in order of cost-effectiveness. The total costs and total QALYs in the base case analysis for each of the ten sequences of chemotherapy are summarised in Table 4.6. Costs ranged from £16,285 for FOLFOX - irinotecan up to £18,568 for FOLFOX – XELIRI. Total QALYs ranged from 0.819 for XELIRI – XELOX up to 0.941 for FOLFOX – FOLFIRI.
Table 4.6
Total costs and effectiveness by treatment strategy.
Taking FOLFOX – irinotecan as the reference (least expensive) strategy, all other strategies were shown to be less effective and also more costly (i.e. dominated) except the sequence FOLFOX – FOLFIRI. Compared to the reference strategy, the sequence FOLFOX – FOLFIRI produces 0.019 more QALYs and incurs £2,051 in additional costs. This yields an incremental cost-effectiveness ratio (ICER) of £109,604/QALY.
Results presented above reflect the expected costs and effectiveness estimates for the treatment sequences of interest, however given uncertainty associated with many parameters in the model, we are also interested in the distribution over incremental costs, incremental effectiveness and the joint cost-effectiveness distribution. This is particularly relevant in the present analysis given that the differences in total QALYs between several strategies are very small. Taking into account parameter uncertainty, probabilistic sensitivity analysis showed there is a non-negligible probability that some sequences other than FOLFOX – FOLFIRI may also be equivalent or even more effective than the reference strategy. Cost-effectiveness acceptability curves (CEAC) can be used to show the probability of the various treatment options being cost effective over a range of willingness to pay (WTP) thresholds. The CEACs (see Appendix 2) show that FOLFOX – irinotecan is consistently the strategy with the highest probability of being cost-effective, however as the WTP threshold increases, so does the probability that the sequences FOLFOX-FOLFIRI and XELOX-FOLFIRI are cost-effective.
Sensitivity analysis – drug discounts
Currently available data on the impact of price discounts for generic pharmaceutical products across the NHS were applied to the economic analysis (see Table A2.24 in Appendix 2) and these results are presented in Table 4.7.
Table 4.7
Cost-effectiveness results for non-dominated strategies taking into account price discounts for generic pharmaceutical products.
Subsequent probabilistic sensitivity analysis using these discounted drug prices showed there is greater uncertainty about which strategy has the highest probability of being cost effective. This is shown by the intersecting cost-effectiveness acceptability curves for FOLFOX-irinotecan, FOLFOX-FOLFIRI and XELOX-FOLFIRI over the range of WTP thresholds between approximately £20,000 and £50,000/QALY (Figure 4.4).

Figure 4.4
Cost-effectiveness acceptability curves using discounted drug prices.
In this sensitivity analysis, when discounted prices for non-proprietary drugs were taken into account, the ICER for FOLFOX – FOLIRI vs FOLFOX-irinotecan fell to £47,800/QALY. Therefore taking parameter uncertainty and drug discounts into account, the treatment strategies FOLFOX-irinotecan, FOLFOX-FOLFIRI and XELOX-FOLFIRI were associated with the highest probability of being cost effective.
Conclusion
The results of the mixed and indirect treatment comparisons were used as inputs to conduct a cost-effectiveness analysis. The cost-effectiveness analysis showed that when survival was quality-adjusted (taking into account both disease status and toxicities), the difference in total QALYs between the various sequential treatment strategies was in most cases modest. Taking FOLFOX-irinotecan as the reference (least costly) strategy, all other treatment sequences were found to be less effective (in terms of QALYs) and more costly except the sequence FOLFOX-FOLFIRI. The ICER comparing FOLFOX-FOLFIRI to FOLFOX-irinotecan was of £110K/QALY. When drug discounts were taken into account, the ICER for FOLFOX – FOLIRI vs FOLFOX-irinotecan fell to approximately £48K/QALY. Because of the small differences in total QALYs between strategies, it was important to consider how uncertainty may impact the results of the cost-effectiveness analysis. Taking parameter uncertainty and drug discounts into account, three strategies (FOLFOX-irinotecan, FOLFOX-FOLFIRI and XELOX-FOLFIRI) were associated with the highest probability of being cost effective.
Full details of the methods and results for the mixed treatment comparison and economic evaluation for this topic can be found in Appendix 2.
Recommendations
- When offering multiple chemotherapy drugs to patients with advanced and metastatic colorectal cancer consider one of the following sequences of chemotherapy unless they are contraindicated:
- FOLFOX (folinic acid plus fluorouracil plus oxaliplatin) as first-line treatment then single agent irinotecan as second-line treatment or
- FOLFOX as first-line treatment then FOLFIRI (folinic acid plus fluorouracil plus irinotecan15) as second-line treatment or
- XELOX (capecitabine plus oxaliplatin) as first-line treatment then FOLFIRI (folinic acid plus fluorouracil plus irinotecan15) as second-line treatment.
- Decide which combination and sequence of chemotherapy to use after full discussion of the side effects and the patient’s preferences.
- 15
At the time of publication (November 2011), irinotecan did not have UK marketing authorisation for second-line combination therapy. Informed consent should be obtained and documented.
Linking evidence to recommendations
The GDG considered the outcomes of progression-free survival, and overall survival to be particularly important. The GDG also considered response rate, toxicity and quality of life to be informative. However they noted that data on quality of life were limited. Cost-effectiveness was also considered to be important.
The GDG noted that there was little difference in clinical effectiveness between the sequences of interest. The GDG used Mixed Treatment Comparison (MTC) techniques to inform the clinical and economic analysis for this topic. The rationale for this type of analysis is detailed in Appendix 2.
The quality assessment of the individual trials included in the mixed treatment comparison showed that they were all of high methodological quality. The quality assessment for this MTC cannot be produced in GRADE as the software cannot yet accommodate the issues surrounding indirect treatment comparisons. GRADE has been designed to assess the quality of the total body of evidence for a given outcome rather that the methodological quality of individual studies included in the analysis. While this is certainly a more informative and useful way in which to assess the quality of evidence, an indirect treatment comparison presents a particular problem in that the information used to inform the model includes, where possible, direct evidence, but in many cases will also include data from studies which do not directly assess the interventions of interest against each other and is so considered indirect evidence. Using a MTC method however, will allow for inbuilt considerations in the model in order to account for the indirectness of the data.
The GDG also noted from the base case health economic analysis that FOLFOX – irinotecan emerged as the least costly treatment of the 10 sequences investigated. All other strategies were dominated except FOLFOX – FOLFIRI. However the GDG recognised that because the difference in QALYs between sequences was small, even small changes to the difference in costs had a substantial impact on the ICER. The mean ICER for each sequence within the model was considered by the GDG. However given the uncertainty around these estimates the GDG considered the probability of each sequence being cost effective as the more significant determinant when making their recommendations.
Probabilistic sensitivity analysis using discounted drug prices showed that there is uncertainty about which sequence has the highest probability of being cost effective around a willingness to pay threshold of £20,000 – £30,000/QALY. The GDG also recognised that these discounted drug prices were based on currently available estimates which may change.
Given this uncertainty the GDG could not be sure that the reference strategy (FOLFOX – irinotecan) was the most cost effective at a willingness to pay threshold of £30,000 per QALY gained. They therefore decided to recommend that the three sequences shown by the probabilistic sensitivity analysis to have the highest probability of being cost effective (FOLFOX – irinotecan, FOLFOX – FOLFIRI and XELOX – FOLFIRI) be considered for the treatment of patients with advanced and metastatic colorectal cancer, unless clinically contraindicated.
4.4.2. Raltitrexed
Clinical question: What is the most effective treatment for advanced colorectal cancer patients when 5-FU/FA based regimens are not tolerated or inappropriate
Clinical evidence
There is no good quality evidence with which to address this question with the body of evidence comprising one randomised trial comparing raltitrexed to 5FU/LV from which the results of the raltitrexed arm will provide indirect evidence (Popov et al., 2008), one randomised phase II trial (Feliu et al., 2005) comparing raltitrexed + oxaliplatin with raltitrexed + irinotecan and a small number of non-randomised phase II trials (Aparicio et al., 2005; Chiara et al., 2005; Cortinovis et al., 2004; Feliu et al., 2004; Laudani et al., 2004; Maroun et al., 2006; Santini et al., 2004; Vyzula et al., 2006).
For patients receiving treatment with raltitrexed, serious adverse events were reported in 16.3% of patients, deaths related to treatment were reported for 2.2% (n=20). Of 20 deaths considered related to raltitrexed, 11 were associated with a major protocol deviation. The 5-year recurrence free survival rate was 47.8% [95% CI: 42.3–53%] for patients receiving raltitrexed. In the intention to treat population, the 5-year survival rate was 61.9% [95% CI: 55.4–66.1%] (Popov et al., 2008).
Recommendations
- Consider raltitrexed only for patients with advanced colorectal cancer who are intolerant to 5-fluorouracil and folinic acid or for whom these drugs are not suitable (for example, patients who develop cardiotoxicity). Fully discuss the risks and benefits of raltitrexed with the patient.
- Prospectively collect data on quality of life, toxicity, response rate, progression free survival and overall survival for all patients taking raltitrexed.
Linking evidence to recommendations
The GDG recognised that the population for this question must be “patients who are not able to tolerate 5FU/FA based regimens, or for whom 5FU/FA based regimens are inappropriate”, to match the population used in TA93. Whilst this is the licensed indication for raltitrexed and therefore the population of interest, the GDG noted that it is currently not possible to identify those patients who are intolerant to 5FU/FA before they actually receive the drug. Therefore it is also not possible to randomise 5FU/FA intolerant patients to the interventions of interest. Consequently, there will never be any directly relevant evidence with which to answer this question.
Since the GDG agreed the efficacy of raltitrexed was likely to be the same for both 5FU/FA tolerant and intolerant patients, and TA93 had used ‘indirect’ evidence (from raltitrexed arms of trials comparing raltitrexed with 5FU/FA), a similar ‘indirect’ approach was used to update the raltitrexed part of TA93.
The GDG acknowledged that the review of the evidence had highlighted that the evidence base for raltitrexed has not changed significantly since TA93 and there was no good quality evidence to address the question being investigated. This lack of good quality evidence also meant it was not possible to conduct robust cost-effectiveness analysis for the use of raltitrexed.
The GDG highlighted that if patients who are intolerant to 5FU/FA are not able to receive raltitrexed, this will severely limit the potential treatment options for this group of patients. Both TA33 and TA93 have recommended that the use of raltitrexed is confined to appropriately designed clinical studies. However trials of raltitrexed in 5FU/FA intolerant patients have not happened so far and are unlikely to happen. The GDG were therefore concerned that the use of raltitrexed is being denied to a specific subgroup in which it is impossible to obtain direct evidence of effectiveness. Consequently they agreed to recommend that raltitrexed can be considered for the subgroup of patients with advanced colorectal cancer who are intolerant to 5FU/FA, so long as the risks and benefits are discussed with the patient and audit data are collected.
4.4.3. Capecitabine and tegafur with uracil
The recommendations in this section are from ‘Guidance on the use of capecitabine and tegafur with uracil for metastatic colorectal cancer’, NICE technology appraisal guidance 61 (NICE, 2003)
Recommendations
- Oral therapy with either capecitabine or tegafur with uracil (in combination with folinic acid) is recommended as an option for the first-line treatment of metastatic colorectal cancer.
- The choice of regimen (intravenous 5-fluorouracil and folinic acid or one of the oral therapies) should be made jointly by the individual and the clinician(s) responsible for treatment. The decision should be made after an informed discussion between the clinician(s) and the patient; this discussion should take into account contraindications and the side-effect profile of the agents as well as the clinical condition and preferences of the individual.
- The use of capecitabine or tegafur with uracil to treat metastatic colorectal cancer should be supervised by oncologists who specialise in colorectal cancer.
Linking evidence to recommendations
These recommendations are from ‘Guidance on the use of capecitabine and tegafur uracil for metastatic colorectal cancer’, NICE technology appraisal guidance 61 (NICE, 2003). They were formulated by the technology appraisal and not by the guideline developers. They have been incorporated into this guideline in line with NICE procedures for developing clinical guidelines, and the evidence to support these recommendations can be found at www.nice.org.uk/TA61.
4.5. Biological agents in metastatic colorectal cancer
Recommendations on ‘Bevacizumab in combination with oxaliplatin and either fluorouracil plus folinic acid or capecitabine for the treatment of monastic colorectal cancer’ can be found in NICE technology appraisal guidance 21216.
Recommendations on the use of ‘Cetuximab for the first-line treatment of metastatic colorectal cancer’ can be found in NICE technology appraisal guidance 17617.
NICE’s advice on the use of ‘Cetuximab for the treatment of metastatic colorectal cancer following failure of oxaliplatin-containing chemotherapy (terminated appraisal)’ can be found at http://guidance.nice.org.uk/TA150.
Recommendations on the use of ‘Bevacizumab and cetuximab for the treatment of metastatic colorectal cancer’ can be found in NICE technology appraisal guidance 11818.
4.6. Adjuncts to chemotherapy in unresectable metastatic disease
Up to 50% of patients with colorectal cancer will develop liver and/or lung metastases at some time during the course of their disease. Metastases can also arise at other sites in the body. The peritoneum may be the predominant or only route of spread in 10–15% of patients with colorectal cancer. Surgery for metastases is not always possible and, for example, only 10–20% of patients with liver metastases will have disease suitable for liver resection. Where metastatic disease is considered unresectable, systemic combination chemotherapy, with or without biological agents, is the standard of care. Systemic therapy alone can prolong median survival to approximately 2 years, but long term cure is unlikely.
Provided a good response is seen in patients with unresectable liver, lung or peritoneal disease following chemotherapy, then local procedures can be attempted to try to prolong the disease-free interval. These local procedures have been most applied to the liver where radiofrequency ablation (RFA) is the most commonly used local treatment, although conclusive data on the benefits have not yet been published. There are even less data on alternative local procedures such as microwave, laser, cryotherapy, radio-embolisation or stereotactic body radiotherapy (SBRT). Some of these local procedures can also be applied to lung metastases, depending on the size and position of individual lesions. Therefore, for those patients whose metastatic disease is considered unresectable but who have chemo-sensitive disease, the question remains what benefit is there to adding local treatment to consolidate chemotherapy response.
Clinical question: What is the most effective additional treatment to systemic chemotherapy to achieve cure or long term survival in patients with apparently unresectable metastatic disease?
Clinical evidence
This topic aimed to determine whether patients originally identified as being incurable and with poor long term prognosis due to the presence of unresectable metastatic disease can achieve cure or long-term survival through treatment with systemic chemotherapy with or without additional treatments. There was no comparative evidence with which to address this topic.
A systematic review of the literature identified no studies comparing any combination of the interventions of interest for this topic and although a small number of non-comparative studies, investigating individual interventions were identified, it was considered that the evidentiary benefits of including such studies was low and would not inform any recommendations regarding the best form of treatment for this patient group.
Research Recommendations
- Prospective studies should investigate and compare the effectiveness of techniques for refining local ablation (radiofrequency ablation, radioembolisation, microwave, cryotherapy, laser and stereotactic radiotherapy) in patients with metastatic colorectal cancer. Outcomes of interest are technical feasibility, local control, disease-free survival, overall survival, toxicity and quality of life.
- Consider patients for entry into NCRN approved studies on local ablative therapies.
- Novel techniques for the treatment of metastatic disease, including peritoneal carcinomatosis, should be carefully audited so that case-mix adjusted outcome data may be collected and evaluated.
Linking evidence to recommendations
The GDG acknowledged that there is currently no evidence available to answer this question. Therefore, the GDG could not recommend a particular treatment to achieve cure or long-term survival in patients with apparently unresectable metastatic disease.
The GDG noted that currently these treatments are being widely used without evidence of valid outcomes and an increasing number of patients are being considered for these interventions. Ongoing trials in this area have had difficulties in recruiting sufficient numbers of patients but with modern practice and increasing availability of these techniques (in a standardised form), the GDG believed there is value in recommending further research because trial recruitment is more likely to be successful.
References
- Aparicio J, Vincent JM, Maestu I, Bosch C, Galan A. First line treatment with irinotecan and raltitrexed in metastatic colorectal cancer. Mature results of a multicentre phase II study. Oncology. 2005;68(1):58–63. [PubMed: 15809521]
- Bartolozzi C, Donati F, Cioni D, Procacci C, Morana G, Chiesa A, Grazioli L, Cittadini G, Cittadini G, Giovagnoni A, Gandini G, Maass J, Lencioni R. Detection of colorectal liver metastases: a prospective multicenter trial comparing unenhanced MRI, MnDPDP-enhanced MRI, and spiral CT. European Radiology. 2004;14(1):14–20. [PubMed: 14730384]
- Benoist S, Pautrat K, MItry E, Rougier P, Penna C, Nordlinger B. Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. British Journal of Surgery. 2005;92:1155–1160. [PubMed: 16035135]
- Bhattaharjya SB. Prospective study of contrast-enhanced computed tomography, computed tomography during arterioportography, and magnetic resonance imaging for staging colorectal liver metastases for liver resection. British Journal of Surgery. 2004;91:1361–1369. [PubMed: 15376205]
- Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, Stoker J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis. Meta-analysis (DARE structured abstract). Radiology. 2005;237:123–131. [PubMed: 16100087]
- Bipat S, van Leeuwen MS, IJzermans JN, Comans EF, Planting AS, Bossuyt PM, Greve JW, Stoker J. Evidence based guideline on management of colorectal liver metastases in the Netherlands (Review). Netherlands Journal of Medicine. 2007;65(1):5–14. [PubMed: 17293634]
- Chiara S, Nobile MT, Tomasello L, Acquati M. Phase II trial of irinotecan and raltitrexed in chemotherapy-naive advanced colorectal cancer. Anticancer Research. 2005;25(2B):1391–1396. [PubMed: 15865096]
- Choi D, Kwak J, Kim J, Woo SU, Kim SH. Preoperative chest computerised tomography in patients with locally advance mid or low rectal cancer: its role in staging and impact on treatment strategy. Journal of Surgical Oncology. 2010;102(6):588–592. [PubMed: 20607759]
- Chua SC, Groves AM, Kayani I, Menezes L, Gacinovic S, Du Y, Bomanji JB, Ell PJ. The impact of F-18-FDG PET/CT in patients with liver metastases. European Journal of Nuclear Medicine and Molecular Imaging. 2007;34:1906–1914. [PubMed: 17713766]
- Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L, Borsellino N, Misino A, Romito S, Durini E, Cordio S, Di Seri M, Lopez M, Maiello E, Montemurro S, Cramarossa A, Lorusso V, Di Bisceglie M, Chiarenza M, Valerio MR, Guida T, Leonardi V, Pisconti S, Rosati G, Carrozza F, Nettis G, Valdesi M, Filippelli G, Fortunato S, Mancarella S, Brunetti C. Gruppo Oncologico Dell’Italia Meridionale. Phase III Randomised Trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicentre study of the Gruppo Oncologico Dell’ Italia Meridonale. Journal of Clinical Oncology. 2005;23(22):4866–4875. [PubMed: 15939922]
- Comella P, Massidda B, Filipelli G, Farris A, Natale D, Barberis G, Maiorino L, Palmeri S, Cannone M, Condemi G. Southern Italy Cooperative Oncology Group. Randomised trial comparing biweekly oxaliplatin plus oral capecitabine versus oxaliplatin plus iv bolus fluorouracil/leucovorin in metastatic colorectal cancer patients: results of the Southern Italy Cooperative Oncology Study 0401. Journal of Cancer Research and Clinical Oncology. 2009;135:217–26. [PubMed: 18719941]
- Comella P, Massidda B, Filippelli G, Palmeri S, Natale D. Oxaliplatin plus high dose folinic acid and 5 fluorouracil i.v. bolus (OXAFAFU) versus irinotecan plus high dose folinic acid and 5-fluorouracil i.v. bolus (IRIFAFU) in patients with metastatic colorectal carcinoma: a Southern Italy Cooperative Oncology Group phase III trial. Annals of Oncology. 2005;16(6):878–886. [PubMed: 15837702]
- Cortinovis D, Bajetta E, Di Bartolomeo M, Dogini G. Raltitrexed plus oxaliplatin in the treatment of metastatic colorectal cancer. Tumori. 2004;90(2):186–191. [PubMed: 15237580]
- Cunningham D, Sirohi B, Pluzanska A, Utracka-Hutka B, Zaluski J, Glynne-Jones R, Koralewski P, Bridgewater J, Mainwaring P, Wasan H, Wang JY, Szczylik C, Clingan P, Chan RT, Tabah-Fisch I, Cassidy J. Two different first line 5 fluorouracil regimens with or without oxaliplatin in patients with metastatic colorectal cancer. Annals of Oncology. 2009;20:244–250. [PubMed: 18854549]
- Cunningham D, Glimelius B. A phase III study of irinotecan (CPT-11) versus best supportive care in patients with metastatic colorectal cancer who have failed 5-fluorouracil therapy. V301 study group. Seminars in Oncology. 1999;26(1 Suppl 5):6–12. [PubMed: 10213009]
- Desai D, Zervos E, Arnold M, Burak W, Mantil J, Martin E. Positron emission tomography affects surgical management in recurrent colorectal cancer patients. Annals of Surgical Oncology. 2003;10(1):59–64. [PubMed: 12513962]
- Diaz-Rubio E, Tabernero J, Gomez-Espana A, Massutí B, Sastre J, Chaves M, Abad A, Carrato A, Queralt B, Reina JJ, Maurel J, González-Flores E, Aparicio J, Rivera F, Losa F, Aranda E. Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. Phase III study of capecitabine plus oxaliplatin compared with continuous infusion fluorouracil as first line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the treatment if digestive tumours trial. Journal of Clinical Oncology. 2007;25(27):4224–4230. [PubMed: 17548839]
- Douillard J, Cunnigham D, Roth A, Navarro, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. [PubMed: 10744089]
- Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, Adenis A, Faroux R, Rebischung C, Bergougnoux L, Kockler L, Douillard JY. GI Group of the French Anti-Cancer Centers. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first line treatments for metastatic colorectal cancer. International Journal of Cancer. 2010;128(3):682–690. [PubMed: 20473862]
- Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G. Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) as first line treatment for metastatic colorectal cancer: the GRUPPO Ocologico Nord Ovest. Journal of Clinical Oncology. 2007;25(13):1670–1676. [PubMed: 17470860]
- Feliu J, Castanon C, Salud A, Mel JR, Escudero P, Pelegrin A, Lopez-Gomez L, Ruiz M, Gonzalez E, Juarez F, Lizon J, Castro J, Gonzalez-Baron M. Phase II randomised trial of raltitrexed-oxaliplatin versus raltitrexed-irinotecan as first line treatment in advanced colorectal cancer. British Journal of Cancer. 2005;93:1230–1235. [PMC free article: PMC2361515] [PubMed: 16265344]
- Feliu J, Salud A, Escudero P, Lopez-Gomez L. Irinotecan plus raltitrexed as first-line treatment in advanced colorectal cancer: a phase II study. British Journal of Cancer. 2004;90(8):1502–1507. [PMC free article: PMC2409728] [PubMed: 15083176]
- Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. Journal of Magnetic Resonance Imaging. 2010;31(1):19–31. [PubMed: 20027569]
- Gennatas C, Papaxoninis G, Michalaki V, Mouratidou D, Andreadis C, Tsavaris N, Pafiti A. A prospective randomised study of irinotecan (CPT-11), leucovorin (LV) and 5-fluorouracil (5FU) versus leucovorin and 5-fluorouracil in patients with advanced colorectal carcinoma. Journal of Chemotherapy. 2006;18(5):538–544. [PubMed: 17127232]
- Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Lévi F. Phase III multicenter randomised trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first line treatment of metastatic colorectal cancer. Journal of Clinical Oncology. 2000;18(1):136–147. [PubMed: 10623704]
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts S. Randomised controlled trial of reduced dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: A North American intergroup trial. Journal of Clinical Oncology. 2006;24(21):3347–3353. [PubMed: 16849748]
- Goldberg R, Sargent D, Morton R, Fuchs C, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomised controlled trial of fluourouracil plus leucovorin, irinotecan and oxaliplation combinations in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology. 2004;22(1):23–30. [PubMed: 14665611]
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and Flourouracil with or without oxaliplatin as first line treatment in advanced colorectal cancer. Journal of Clinical Oncology. 2000;18:2938–2947. [PubMed: 10944126]
- Haller DG, Rothenberg ML, Wong AO, Koralewski PM, Miller WH Jr, Bodoky G, Habboubi N, Garay C, Olivatto LO. Oxaliplatin plus irinotecan compared with irinotecan alone as second line treatment after single agent fluoropyrimidine therapy for metastatic colorectal carcinoma. Journal of Clinical Oncology. 2008;26(28):4544–4550. [PubMed: 18824706]
- Hillingso J, Wille-Jorgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer – a systematic review. Colorectal Disease. 2009;11(1):3–10. [PubMed: 18637099]
- Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, Schwartzberg L, Hedrick E. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first line treatment of metastatic colorectal cancer: results of the TREE study. Journal of Clinical Oncology. 2008;26(21):3523–3529. [PubMed: 18640933]
- Kim GP, Sargent DJ, Mahoney MR, Rowland KMJ, Philip PA, Mitchell E, Matthews AD, Fitch TR, Goldberg RM, Alberts SR, Pitot HC. Phase III noninferiority trial comparing irinotecan with oxaliplatin, fluorouracil and leucovorin in patients with advanced colorectal carcinoma previously treated with fluorouracil: N9841. Journal of Clinical Oncology. 2009;27(17):2848–2854. [PMC free article: PMC2698019] [PubMed: 19380443]
- Kohne CH, De Greve J, Hartmann JT, Lang I, Vergauwe P, Becker K, Braumann D, Joosens E, Müller L, Janssens J, Bokemeyer C, Reimer P, Link H, Späth-Schwalbe E, Wilke HJ, Bleiberg H, Van Den Brande J, Debois M, Bethe U, Van Cutsem E. Irinotecan combined with infusional 5-fluorouracil/folinic acid or Capecitabine plus celecoxib or placebo in the first line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Annals of Oncology. 2008;19:920–926. [PubMed: 18065406]
- Kohne CH, van Cutsem E, Wils J, Bokemeyer C, El-Serafi M, Lutz MP, Lorenz M, Reichardt P, Rückle-Lanz H, Frickhofen N, Fuchs R, Mergenthaler HG, Langenbuch T, Vanhoefer U, Rougier P, Voigtmann R, Müller L, Genicot B, Anak O, Nordlinger B. European Organisation for Research and Treatment of Cancer Gastrointestinal Group. Phase III study of weekly high dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European organisation for research and treatment of cancer gastrointestinal group study 40986. Journal of Clinical Oncology. 2005;23(22):4856–4865. [PubMed: 15939923]
- Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G, Loosveld OJ, van Bochove A, Sinnige HA, Creemers GJ, Tesselaar ME, Slee PH, Werter MJ, Mol L, Dalesio O, Punt CJ. Sequential versus combination chemotherapy with Capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007;370(9582):135–142. [PubMed: 17630036]
- Imdahl A, Reinhardt MJ, Nitzche EU, Mix M, Dingeldey A, Einert A, Baier P, Farthmann EH. Imapct of 18F-FDG-positron emission tomography for decision making in colorectal cancer recurrences. Langenbeck’s Archives of Surgery. 2000;385:129–134. [PubMed: 10796051]
- Laudani A, Gebbia V, Leonardi V, Savio G. Activity and Toxicity of oxaliplatin plus raltitrexed in 5 fluorouracil refractory metastatic colorectal adenocarcinoma. Anticancer Research. 2004;24(2C):1139–1142. [PubMed: 15154638]
- Liu YN, Huang MX, An Q, Wei JM. The Impact of PET/CT on therapeutic strategy of patients with colorectal cancer metastasis. Hepatogastroenterology. 2009;56:968–970. [PubMed: 19760922]
- Mainenti P, Mancini M, Mainolfi C, Camera L, Maurea S, Manchia A, Tanga M, Persico F, Addeo P, D’Antonio D, Speranza A, Bucci L, Persico G, Pace L, Salvatore M. Detection of colorectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdominal Imaging. 2010;35(5):511–521. [PubMed: 19562412]
- Maroun JA, Jonker D, Seymour L, Goel R, Vincent M, Kocha W, Cripps C, Fisher B, Lister D, Malpage A, Chiritescu G. National Cancer Institute of Canada Clinical Trials Group. A National Cancer Institute of Canada Clinical Trials Group Study--IND.135: Phase I/II study of irinotecan (camptosar), oxaliplatin and raltitrexed (tomudex) (COT) in patients with advanced colorectal cancer. European Journal of Cancer. 2006;42(2):193–199. [PubMed: 16330204]
- Martoni AA, Pinto C, Di Fabio F, Lelli G, Rojas Llimpe FL, Gentile AL, Mutri V, Ballardini P, Giaquinta S, Piana E. Capecitabine plus oxaliplatin (XELOX) versus protracted 5-fluorouracil venous infusion plus oxaliplatin (PVIFOX) as first line treatment in advanced colorectal cancer: A GOAM phase II randomised study (FOCA trial). European Journal of Cancer. 2006;42(18):3161–3168. [PubMed: 17098421]
- Mentha G, Roth A, Terraz S, Giostra E, Gervaz P, Andres A, Morel P, Rubbia-Brandt L, Majno PE. ‘Liver First’ Approach in the treatment of colorectal cancer with synchronous liver metastases. Digestive Surgery. 2008;25:430–435. [PubMed: 19212115]
- Metser U, You J, McSweeny S, Freeman M, Hendler A. Assessment of Tumour Recurrence in Patients with colorectal cancer and elevated carcinoembryonic antigen level: FDG PET/CT versus contrast enhanced 64-MDCT of the chest and abdomen. American Journal of Roentgenology. 2010;194:766–771. [PubMed: 20173157]
- Moug SJ, Smith D, Leen E, Roxburgh C, Horgan PG. Evidence for a synchronous operative approach in the treatment of colorectal cancer with hepatic metastases: A case matched study. European Journal of Surgical Oncology. 2010;36(4):365–370. [PubMed: 20034757]
- Nordlinger B, Sorbye H, Glimlius B, Poston G, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T. EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. [PMC free article: PMC2277487] [PubMed: 18358928]
- Popov I, Carrato A, Sobrero A, Vincent M, Kerr D. Raltitrexed (Tomudex) versus standard leucovorin modulated bolus 5-fluorouracil: results from the randomised phase III Pan-European trial in adjuvant colon cancer 01 (PETACC-1). European Journal of Cancer. 2008;44(15):2204–2211. [PubMed: 18707870]
- Porschen R, Arkenau HT, Kubica S, Greil R, Seufferlein T, Freier W, Kretzschmar A, Graeven U, Grothey A, Hinke A, Schmiegel W, Schmoll HJ. AIO Colorectal Study Group. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: A final report of the AIO colorectal study group. Journal of Clinical Oncology. 2007;25(27):4217–4223. [PubMed: 17548840]
- Potter KC, Husband JE, Houghton SL, Brown G. Diagnostic accuracy of serial CT/magnetic resonance imaging review vs. positron emission tomography/CT in colorectal cancer patients with suspected and known recurrence. Diseases of the Colon and Rectum. 2009;52(2):253–259. [PubMed: 19279420]
- Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, Gollins S, Siu LL, Laguerre S, Cunningham D. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second line therapy in metastatic colorectal cancer: a randomised phase III noninferiority study. Annals of Oncology. 2008;19(10):1720–6. [PubMed: 18550577]
- Rougier P, van Custem E, Bajetta E, Nierderle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998;352(9138):1407–1412. [PubMed: 9807986]
- Saltz L, Cox J, Blanke C, Rosen L, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2000;343(13):905–915. [PubMed: 11006366]
- Santini D, Massacesi C, D’Angelillo RM, Marcucci M. Raltitrexed plus weekly oxaliplatin as first line chemotherapy in metastatic colorectal cancer: a multi centre non-randomised phase II study. Medical Oncology. 2004;21:1:59–66. [PubMed: 15034215]
- Scheer MG, Sloots CE, van der Wilt GJ, Ruers TJM. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Annals of Oncology. 2008;19(11):1829–1835. [PubMed: 18662955]
- Schmidt GP, Baur-Melnyk A, Haug A, Utzschneider S, Becker CR, Tiling R, Reiser MF, Hermann KA. Whole-body MRI at 1.5 T and 3 T compared with FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. European Radiology. 2009;19(6):1366–78. [PubMed: 19190917]
- Selzner M, Hany T, Wildbreet P, McCormack L, Zakiyah K, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metstatic colorectal cancer of the liver. Annals of Surgery. 2004;240(6):1027–1036. [PMC free article: PMC1356518] [PubMed: 15570208]
- Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, Smith DB, Shepherd S, Maraveyas A, Ferry DR, Meade AM, Thompson L, Griffiths GO, Parmar MK, Stephens RJ. FOCUS Trial Investigators; National Cancer Research Institute Colorectal Clinical Studies Group. Different strategies of sequential and combination chemotherapuy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007;370(9582):143–152. [PubMed: 17630037]
- Souglakos J, Androulakis N, Sygrigos K, Polysos A. FOFLFOXIRI (folinic acid, 5 fluorouracil, oxaliplatin and irinotecan) versus (folinic acid, 5 fluorouracil and irinotecan) as first line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). British Journal of Cancer. 2006;94(6):798–805. [PMC free article: PMC2361370] [PubMed: 16508637]
- Squillaci E, Maneti G, Mancino S, Ciccio C, Calabria F, Danieli R, Schillaci O, Simonetti G. Staging of colon cancer: whole body MRI vs. whole body PET-CT – initial clinical experience. Abdominal Imaging. 2008;33:676–688. [PubMed: 18373114]
- Tanaka T, Kawai Y, Kanai M, Taki Y, Nakamoto Y, Takabayashi A. Usefulness of FDG-positron emission tomography in diagnosing peritoneal recurrence of colorectal cancer. The American Journal or Surgery. 2002;184:433–436. [PubMed: 12433608]
- Tournigand C, Andre T, Achille R, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomised GERCOR study. Journal of Clinical Oncology. 2004;22(15):229–237. [PubMed: 14657227]
- Valk P, Abella-Culmna E, Haseman M, Pounds T, Tesar R, Myers R, Greiss H, Hofer G. Whole-body PET imaging with [F18] fluorodeoxyglucose in management of recurrent colorectal cancer. Archives of Surgery. 1999;134:503–511. [PubMed: 10323422]
- Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, Sedlackova E, Lipska L, Stahalova V. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33:779–784. [PubMed: 16565845]
- Vyzula R, Kocakova I, Demlova R, Kiss I. Raltitrexed plus oxaliplatin in the second line treatment of metastatic colorectal cancer. Neoplasma. 2006;53(2):119–127. [PubMed: 16575467]
- Wiering B, Krabbe P, Jager G, Oyen W, Ruers T. The impact of fluor-18-deoxyglucose-poitron emission tomography in the management of colorectal liver metastases; a systematic review and meta-analysis. Cancer. 2005;104(12):2658–2670. [PubMed: 16315241]
- Management of metastatic disease - Colorectal CancerManagement of metastatic disease - Colorectal Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...

