NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998.

Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline.
Show detailsThis report is the second in a series of publications resulting from the comprehensive effort being undertaken by the Food and Nutrition Board's (FNB) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (DRI Committee) and its panels and subcommittees.
ORIGIN
This initiative began in June 1993, when the FNB organized a symposium and public hearing entitled “Should the Recommended Dietary Allowances Be Revised?” Shortly thereafter, to continue its collaboration with the larger nutrition community on the future of the Recommended Dietary Allowances (RDAs), the FNB took two major steps: (1) it prepared, published, and disseminated the concept paper “How Should the Recommended Dietary Allowances Be Revised?” (IOM, 1994), which invited comments regarding the proposed concept, and (2) it held several symposia at nutrition-focused professional meetings to discuss the FNB's tentative plans and to receive responses to this initial concept paper. Many aspects of the conceptual framework of the DRIs came from the United Kingdom's Dietary Reference Values for Food Energy and Nutrients for the United Kingdom report (COMA, 1991).
The five general conclusions presented in the FNB's 1994 concept paper are as follows:
- Sufficient new information has accumulated to support a reassessment of the RDAs.
- Where sufficient data for efficacy and safety exist, reduction in the risk of chronic degenerative disease is a concept that should be included in the formulation of future recommendations.
- Upper levels of intake should be established where data exist regarding risk of toxicity.
- Components of food of possible benefit to health, although not meeting the traditional concept of a nutrient, should be reviewed, and if adequate data exist, reference intakes should be established.
- Serious consideration must be given to developing a new format for presenting future recommendations.
Subsequent to the symposium and the release of the concept paper, the FNB held workshops at which invited experts discussed many issues related to the development of nutrient-based reference values, and FNB members have continued to provide updates and engage in discussions at professional meetings. In addition, the FNB gave attention to the international uses of the earlier RDAs and the expectation that the scientific review of nutrient requirements should be similar for comparable populations.
Concurrently, Health Canada and Canadian scientists were reviewing the need for revision of the Recommended Nutrient Intakes (RNIs) (Health Canada, 1990). Consensus after a symposium for Canadian scientists cosponsored by the Canadian National Institute of Nutrition and Health Canada in April 1995 was that the Canadian government should pursue the extent to which involvement with the developing FNB process would be of benefit to both Canada and the United States in terms of leading toward harmonization.
On the basis of extensive input and deliberations, the FNB initiated action to provide a framework for the development and possible international harmonization of nutrient-based recommendations that would serve, where warranted, for all of North America. To this end, in December 1995 the FNB began a close collaboration with the government of Canada and appointed the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (DRI Committee). It is hoped that representatives from Mexico will join in future deliberations.
THE CHARGE TO THE COMMITTEE
In 1995 the DRI Committee was appointed by the Institute of Medicine, National Academy of Sciences, to oversee and conduct this project. To accomplish this task over a period of 5 years, the DRI Committee devised a plan involving the work of seven or more expert nutrient group panels and two overarching subcommittees (Figure A-1). The process described below for this report is expected to be used for subsequent reports.
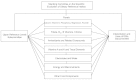
FIGURE A-1
Structure of Dietary Reference Intakes project.
The Panel on Folate, Other B Vitamins, and Choline, composed of experts on those nutrients, has been responsible for reviewing the scientific literature concerning the B vitamins and choline for each stage of the lifespan, considering the roles of nutrients in decreasing the risk of chronic and other diseases and conditions, and interpreting the current data on intakes in North American population groups.
The panel had additional tasks that are specifically related to this group of nutrients and are thus not necessarily part of the DRI process: an analysis of information specific to the prevention of neural tube defects, an analysis of information specific to the diagnosis and prevention of pernicious anemia and vitamin B12 deficiency, and the identification of a research agenda to provide a basis for public policy decisions related to recommended intakes and ways to achieve those intakes.
The panel was charged with analyzing the literature, evaluating possible criteria or indicators of adequacy, and providing substantive rationales for their choices of each criterion. By using the criterion chosen for each stage of the lifespan, the panel was to estimate the average requirement for each nutrient or food component reviewed, assuming that adequate data were available to do so. As the panel members reviewed data on Tolerable Upper Intake Levels (ULs), they also interacted with the Subcommittee on Upper Reference Levels of Nutrients, which assisted the panel in applying the risk assessment model to each B vitamin and choline. The DRI values in this report are a product of the joint efforts of the DRI Committee, the Panel on Folate, Other B Vitamins, and Choline, and the Subcommittee on Upper Reference Levels of Nutrients. The Subcommittee on Interpretation and Uses of Dietary Reference Intakes had not yet been appointed and thus did not participate in the development of this report.
REFERENCES
- COMA (Committee on Medical Aspects of Food Policy). Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. London: HMSO; 1991. Report on Health and Social Subjects, No. 41.
- Health Canada. Nutrition Recommendations. The Report of the Scientific Review Committee. Ottawa: Canadian Government Publishing Centre; 1990.
- IOM (Institute of Medicine). How Should the Recommended Dietary Allowances Be Revised? Washington, DC: National Academy Press; 1994. [PubMed: 25101385]
- PubMedLinks to PubMed
- Origin and Framework of the Development of Dietary Reference Intakes - Dietary R...Origin and Framework of the Development of Dietary Reference Intakes - Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline
Your browsing activity is empty.
Activity recording is turned off.
See more...