NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy; Jackson LM, Parker RM, Mattison DR, editors. The Clinical Utility of Compounded Bioidentical Hormone Therapy: A Review of Safety, Effectiveness, and Use. Washington (DC): National Academies Press (US); 2020 Jul 1.

The Clinical Utility of Compounded Bioidentical Hormone Therapy: A Review of Safety, Effectiveness, and Use.
Show detailsMedications containing steroid hormones are prescribed frequently to treat symptoms associated with the normal age-related decline in circulating hormone levels. This chapter will provide an overview of compounded bioidentical hormone therapy (cBHT) preparations, including a description of their common formulations (e.g., common active and inactive ingredients), formulation methods, quality testing, and labeling. This chapter will also describe features that may affect the quality of the cBHT preparations and highlight important variations between compounding settings (i.e., 503A compounding pharmacies and 503B outsourcing facilities). Where relevant, this chapter will discuss FDA-approved hormone products and commercial drug manufacturing facilities to provide relevant comparisons and contrasts between formulation procedures and quality testing.1
For the purposes of this report, the committee maintained a focus prioritized on 10 hormones (7 unique hormone substances and 3 salt forms) that were of interest to the U.S. Food and Drug Administration (FDA). These hormones include estrone (E1), estradiol (E2), estradiol cypionate (Ec), estriol (E3), dehydroepiandrosterone (DHEA), pregnenolone (P5), progesterone (P4), testosterone (T), testosterone cypionate (Tc), and testosterone propionate (Tp). For the purposes of this chapter, the committee will focus its discussion on bioidentical compounded formulations and, where relevant, bioidentical FDA-approved products.2 However, certain cBHT preparations contain hormones not available in FDA-approved drug products.
CBHT PREPARATIONS
Potential Variances Between Preparations
Unlike with FDA-approved hormone products, the process of formulating a compounded prescription is entirely compounder specific. That is, the content and quality of the final preparation depends completely on what is described in the Master Formulation Record (MFR) as chosen or described by the compounder.3,4 Compounder-specific factors that may influence quality and performance of the final preparation include the choice of active and inactive ingredients, ingredient testing, available compounding equipment, compounder skill, quality systems, facility cleanliness, and environmental controls. Per United States Pharmacopeia (USP) standards, once completed, the compounded preparations should undergo an abbreviated release inspection that should at a minimum include a visual inspection of the formulation, but it may also include quality checks developed and reviewed solely by the compounder (USP, 2012, 2014).5
While important, the recommended postcompounding inspection process is superficial, in that these steps do not ensure that the compounded preparation contains the purported amount of active ingredient or can deliver the active ingredient to the patient and the site of action. Because of these limitations, different compounders may use different processes to compound an identical prescription, and as a result, cBHT preparations ordered with identical prescriptions and labeled with the same name will likely vary between compounders. Indeed, FDA has received adverse event reports that reveal harmful variations in compounding (FDA, 2020a; see Chapter 7).
Inadequate Labeling Requirements
Labels for compounded preparations are not required to provide guidance for patients about safe use.6 This is in contrast with requirements for FDA-approved hormone products to provide labeled instructions for patients on the proper use and storage of the product and warnings about possible risks.7 For 503A compounding pharmacies, the decision to provide labels for compounded preparations is up to the compounder. As a result, and as presented at the study's open session meetings, dispensed cBHT preparations often do not include the boxed warnings, warnings, contraindications, and detailed instructions for use similar to those included with FDA-approved hormone product labeling, despite containing the same active ingredients (NASEM, 2019c). (See Box 5-1 and Table 5-1 for additional discussion on labeling of FDA-approved hormone products.) Although the federal statutes establishing 503A and 503B compounders specifically exempted them from federal labeling provisions on adequate directions for use,8 there is no clear clinical rationale for excluding information on the potential risks associated with the use of hormone medication, particularly for those that contain estradiol and testosterone.
BOX 5-1
Labeling of FDA-Approved Hormone Products and Boxed Warnings.
Inadequate Data Collection
In addition to concerns related to labeling, there are also concerns related to data collection. Specifically, once an MFR is created, there is no central registry where the MFR must be submitted, and there is no requirement for independent outside review, as is the case for FDA-approved products. Unlike with FDA-approved products, compounded preparations do not have a National Drug Code and are not listed in any conventional shared drug product database. In contrast, basic characteristics of FDA-approved products, including active ingredients, strength, and dosage form, are described in multiple public databases, including the Orange Book (FDA, 2020b), RxNorm (NLM, 2019), International Nonproprietary Names (WHO, 2018), and others. This further underscores that compounded preparations are unique to the compounder that prepares them, and that for identical prescriptions, the final compounded preparations may differ between compounding establishments.
TABLE 5-1FDA-Approved Drug Products and Availability of Medication Guides and Boxed Warnings
| Hormone | Medication Guides | Boxed Warnings |
|---|---|---|
| Estradiol | No | Yes |
| Progesterone | No | NA |
| Testosterone | Yes | Yes |
NOTE: NA = no applicable boxed warning for this active ingredient.
SOURCES: Committee generated, using information from NLM, 2020a.
Box
Conclusion 5-1.
TYPES OF CBHT PREPARATIONS
A wide variety of cBHT dosage forms are dispensed to patients, and because of a lack of registries and surveillance of use, it is difficult to obtain specific estimates for the number of available forms of cBHT preparations. To begin to describe the breadth of cBHT preparations dispensed by compounders, the committee found it necessary to compile its own list of cBHT preparations, drawing on available resources.9 This list presents only a small sample of the universe of cBHT preparations and at best provides a snapshot and limited description of available cBHT preparations. Indeed, in November 2019, during an open session presentation to the committee, Dr. Gina Besteman, Director of Compounding and Dispensing at Women's International Pharmacy, stated that her 503A pharmacy has “compounded over 149,000 unique hormone formulations using fewer than 10 hormones” (NASEM, 2019a).
Available Dosage Forms and Routes of Administration
To support the committee's research efforts, Reed Smith LLP submitted a briefing document that contained a list of common cBHT dosage forms.10 Although this list may not be comprehensive, it describes at least 32 different types of dosage forms of cBHT formulations. In contrast, there are only 13 FDA-approved bioidentical hormone therapy dosage forms available (see Table 5-2).
Of the 10 hormone ingredients of focus for this report, all are available in cBHT preparations except for estradiol cypionate.11 Table 5-3 provides a summarized list of the different dosage forms available for single active ingredient cBHT preparations, as identified by the committee in its review, and compares them to available dosage forms of FDA-approved single active ingredient BHT products. In summary, the committee found that the single ingredient cBHT preparations are available in a wide variety of dosage forms and strengths, while comparatively there are fewer FDA-approved hormone products available for each of the routes of administration. For example, there are at least 13 different cBHT progesterone dosage forms, including oral (capsule, capsule sustained release, lozenge, oil, tablet), topical (cream, gel, solution, spray), injection (oil, pellets, suspension), rectal (enema), and vaginal (capsule, suppository) preparations, as compared to four different FDA-approved BHT progesterone dosage forms, including oral (capsule), topical (gel), injection (solution), and vaginal (insert). Furthermore, estriol cream and pellets containing estradiol or progesterone only exist as compounded preparations.
Conversely, there is no compounded estradiol or testosterone patch, testosterone film, or estradiol ring, perhaps a result of the complexity of manufacturing these FDA-approved dosage forms (FDA, 2018b, 2019d). Of the 10 hormone ingredients of focus for this report, only 3—DHEA, estriol, and pregnenolone—are not available as injections or implants. The three hormone salts, estradiol cypionate, testosterone cypionate, and testosterone propionate, are water insoluble and are found almost exclusively in depot oil-based injection products. These three preparations are not commonly found in other types of cBHT dosage forms, and as such are not listed in the summary Table 5-3 below. In addition, estrone and pregnenolone were only ever combined with at least one other active ingredient in the cBHT formulations found, and therefore, are also not included in Table 5-3.
TABLE 5-2Summary of Dosage Forms Available for FDA-Approved Bioidentical Hormone Therapy (BHT) Products and Compounded Bioidentical Hormone Therapy (cBHT) Preparations
| Dosage Form Available | FDA-Approved BHT Drug Products | cBHT Preparations |
|---|---|---|
| Oral | Capsule
Tablet | Capsule
Tablet triturate Troche and mini troche
Buccal tablet Soft linguet Liquid (syrup, suspension, emulsion) Sublingual drop (oil) |
| Vaginal | Gel Cream Insert
Tablet | Suppository/insert
Cream Solution (Poloxamer, etc.) |
| Topical/Transdermal | Gel
Films, extended release Spray Metered solution | Creams Gels Microemulsion gels Lotions
Suspensions |
| Injection | Oils | Aqueous Nonaqueous (e.g., sesame oil, castor oil, grapeseed oil, cosolvents) |
| Nasal | Gel, metered | Drop Spray Solution Suspension |
| Rectal | No FDA-approved option | Enema
|
| Implant | Pellet | Pellet |
NOTE: The classification of dosage forms available in FDA-approved products has been adapted from the original table submitted by Reed Smith LLP to mirror the terminology used in FDA's Orange Book.
SOURCE: Reed Smith LLP, 2019.
TABLE 5-3Available Dosage Forms for Single Active Ingredient cBHT Preparations and FDA-Approved BHT Products
| Dosage Form | Hormones | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | Estriol | Progesterone | Testosterone | DHEA | ||||||
| Preparation | ||||||||||
| C | M | C | M | C | M | C | M | C | M | |
| Capsule | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Capsule SR | ✓ | |||||||||
| Cream | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Enema | ✓ | |||||||||
| Film/Patch | ✓ | ✓ | ||||||||
| Gel | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Injection | ✓ | ✓ | ||||||||
| Insert/Ring | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Lotion | ✓ | |||||||||
| Lozenge | ✓ | ✓ | ✓ | |||||||
| Oil | ✓ | |||||||||
| Ointment | ✓ | ✓ | ✓ | |||||||
| Pellet | ✓ | ✓ | ✓ | ✓ | ||||||
| Solution | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Spray | ✓ | ✓ | ✓ | |||||||
| Suppository | ✓ | ✓ | ✓ | |||||||
| Suspension | ✓ | |||||||||
| Tablet | ✓ | ✓ | ✓ | |||||||
| Total | 11 | 7 | 4 | 0 | 13 | 4 | 9 | 4 | 6 | 1 |
NOTES: C = cBHT preparations from 503A and 503B pharmacies; M = manufactured FDA-approved product; SR = sustained release. The classification of dosage forms available in FDA-approved products has been categorized to mirror the terminology used in FDA's Orange Book. Table does not include information for estrone, pregnenolone, estradiol cypionate, testosterone cypionate, or testosterone propionate. This table does not include combinations of active ingredients.
SOURCE: FDA, 2020b.
Available Doses
For each of the dosage forms listed in Table 5-3, the compounding process generates preparations of varying strengths, adding to the number of total possible cBHT preparations available for use. For example, in considering formulations with single and multiple active ingredients, the committee identified hundreds of different formulations and strengths for cBHT preparations (see Appendixes F and G).12
To serve as an illustrative example, Figure 5-1 above compares the variety of progesterone capsule strengths against the two FDA-approved capsule strengths. The preparations with low doses of progesterone pose a clinical concern: low dose progesterone has not been found to protect the endometrium and reduce the risk of endometrial cancer induced by unopposed estrogen (Stute et al., 2016). At the committee's open session meeting in November 2019, invited panelist, Dr. Pamela Smith (Center for Personalized Medicine), described these low doses of progesterone as ineffective and something she would not prescribe (NASEM, 2019b). Nonetheless, these doses are still available and marketed to patients for use. In addition, high progesterone doses (> 200 mg) are also available in cBHT preparations, but have never been tested for safety or efficacy in FDA-approved drug products, thus raising a different set of safety concerns.
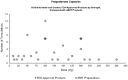
FIGURE 5-1
Comparing the strength of various compounded progesterone capsule formulations to two FDA-approved capsule strengths. SOURCES: Committee generated, using information derived from peer-reviewed literature on the use of cBHT (e.g., IJPC, 2018), cBHT preparation (more...)
In summary, compared to compounded formulations, there are fewer FDA-approved dosage forms and strengths, and in many cases there is no comparable FDA-approved drug product. Therefore, even with the limited data available to the committee, it can be assumed that there are likely tens of thousands of different cBHT preparations (dosage forms and doses) available for patient use. This includes the wide array of different dosage forms, single active ingredient preparations, multiple active ingredient preparations, and an extensive range of different active ingredient doses and dose combinations.
Multihormone cBHT Preparations
Part of the appeal of compounding bioidentical hormones is that multiple hormones can be combined into a single dosage form that does not exist in a single FDA-approved drug product. In fact, of the 741 formulations identified by the committee, 289 were formulations containing more than one active pharmaceutical ingredient (API). While it may be convenient for patients to have multiple hormones combined into a single formulation, thereby reducing their medication burden, it remains unclear whether the combinations of these hormones are necessary or sufficient to elicit the desired effects, allowing the potential for patients to be exposed to undue risk without deriving any additional benefit. Moreover, the practice of combining multiple APIs in a single formulation is a complex process requiring careful consideration of drug–drug interactions as well as the compatibility of all the active and inactive ingredients in the dosage form (Pourkavoos, 2012).
In contrast to the wide variety of available multihormone cBHT preparations, there is only one FDA-approved fixed-dose combination bioidentical hormone product, Bijuva (TherapeuticsMD, 2018). The limited FDA-approved option is likely a result of the narrow conditions under which FDA approves products with two or more active ingredients. Unlike cBHT preparations, which may combine any number of hormones without first demonstrating safety or effectiveness, FDA-approved drug products that combine multiple drugs must not only provide evidence of safety and efficacy, but they must also demonstrate the combination improves the safety over any of the individual drug components alone (see Box 5-2).13
BOX 5-2
FDA Policy and Guidance on Products Combining Multiple Drugs.
In addition, within the new drug application or abbreviated new drug application submitted for all FDA-approved drug products is a description of the manufacturing and quality processes involved,14 including the “Chemistry, Manufacturing, and Controls” (CMC) section.15 The CMC section is an extremely detailed set of documents with far more information than in an MFR. For example, the CMC section will include active and inactive ingredient potency reverification, container labeling, stability testing, and detailed manufacturing processes to control for quality attributes (Sheinin and Williams, 2002). FDA encourages that the CMC manufacturing process be further refined via the quality-by-design process through which quality is built into the drug product by identifying and controlling critical process parameters and attributes of the APIs, excipients, and final drug product. In drug manufacturing, this usually involves extensive drug product testing across batches, as well as strict control of process parameters as well as API and excipient attributes to minimize product variability (Yu et al., 2014). This is a more intensive process than what can be captured by a 503A compounding pharmacy or 503B outsourcing facility, but it provides a necessary comparative context.
CBHT ACTIVE INGREDIENTS
To prepare a cBHT formulation, the compounder must either purchase the pure active ingredient from a wholesaler or obtain the active ingredient from a suitable FDA-approved drug product, should one exist.16 The pure ingredient is referred to as the API or bulk drug substance. FDA-approved drug products are required to use APIs that have been well characterized within an application for approval or in a Drug Master File (DMF), sections of which are not available outside of FDA because they contain proprietary manufacturing information.17 As a result, the API pedigree, stability, potency, impurity profile, and other key characteristics are known and can be referenced by the drug manufacturer and FDA. This level of information far exceeds what information is routinely available to compounders when purchasing APIs because of the proprietary information contained in the DMF.
Excluding DHEA and pregnenolone, 8 of the 10 active ingredients reviewed by this committee are described in USP-National Formulary (NF) monographs for bulk drug substances (USP, 2019a). Federal law requires that APIs meet the standards of USP-NF if a monograph for that API exists.18 The existence of USP-NF monographs for estriol and estrone, which are not components of FDA-approved drug products, allows them to be compounded.
Complex Characteristics of Active Ingredients Used in cBHT Preparations
The challenges of formulating the 10 APIs covered in this report represent a complex topic that has been described in great detail over many decades (Goodman Gilman et al., 1990), and the breadth of those challenges is beyond the purview of this report. However, this topic provides useful context for the committee to consider in its examination of the clinical utility of cBHT preparations.
In all formulation processes, it is important to consider the “formulatability” of APIs, meaning how easily the API can be made into a stable dosage form capable of consistently delivering it to the sites of action in the body. As a class, steroid hormones are recognized as presenting formulation difficulties, in part because of their poor water solubility (Benet et al., 2011), potential to be metabolized in vivo (Miller and Auchus, 2011), active uptake by cellular transporters (Hammes et al., 2005), and potential variablility (FDA, 2012; see Table 5-4).
To provide a closer look at the complexity of formulating APIs, the following sections discuss six API characteristics that must be considered during formulation to more reliably deliver hormones to the active site: particle size, polymorphism, solubility, purity, in vivo metabolism, and formulation stability. A related concept, formulation release rate, will also be discussed.
TABLE 5-4Contributing Factors and Absorption Classifications of Bioidentical Hormones
| Active | Water Solubility (mg/mL) | BDDCS | USP Monograph |
|---|---|---|---|
| Dehydroepiandrosterone | 0.0635 | NR | No |
| Estradiol | 0.0036 | 1 | Yes |
| Estradiol cypionate | NA | NA | Yes |
| Estriol | 0.02734 | NR | Yes |
| Estrone | 0.03 | NR | Yes |
| Pregnenolone | 0.00706 | NR | No |
| Progesterone | 0.00881 | 2 | Yes |
| Testosterone | 0.0234 | 2 | Yes |
| Testosterone cypionate | NA | NA | Yes |
| Testosterone propionate | NA | NA | Yes |
NOTE: BDDCS = Biopharmaceutics Drug Disposition Classification System; Class 1 = high solubility; high permeability; rapid dissolution; Class 2 = low solubility; high permeability; NA = used in only injection products; NR = not reported; USP = United States Pharmacopeia.
SOURCES: Committee generated, using data from Benet et al., 2011, and NLM, 2020b.
Particle Size
API particle size and distribution strongly influence absorption, with small particles dissolving faster and more easily than large particles. Indeed, it is well recognized that hormone absorption by the body is associated highly with API particle size (Dahan et al., 2009). For example, many compounders acknowledge the importance of using “micronized” progesterone powder in cBHT preparations, but not all micronized progesterone powders are equivalent when it comes to particle size. The distribution and average of particle sizes among batches or lots of micronized progesterone API must be similar before they can be regarded as possibly equivalent (Yu et al., 2014).
Polymorphisms
Some powder hormone APIs are polymorphic, meaning they can form more than one solid crystal structure, and each crystal structure will have its own solubility characteristics (Sarkar et al., 2014; Stevenson et al., 2019), which thereby affect absorption (Censi and Di Martino, 2015).
Solubility
The low water solubility of reproductive hormones controls absorption from all routes of administration (oral, topical, injection/implant) and is compensated for many ways. For oral formulations, large daily doses are administered (Boyd et al., 2019), while with the topical route, lower doses are applied with the possible inclusion of penetration enhancers (Ng, 2018; Prausnitz and Langer, 2008) to compensate for poor aqueous solubility.
Purity
API purity can affect the efficacy of the drug (Roy, 2002). Absorption is a concentration-dependent process, so as the amount of active ingredient in an API is reduced, so will the amount absorbed into the body. Of note, 503A compounding pharmacies are not required to assess the purity of their APIs and are permitted to rely solely on the product certificate of analysis and expiry date as provided by the API manufacturer. If, at the 503A compounding pharmacy, an API becomes contaminated accidentally or degrades faster than expected because it was stored at the wrong temperature, the 503A compounding pharmacy may not detect those changes. In contrast, 503B outsourcing facilities and FDA-approved drug manufacturers are required to perform confirmatory API purity testing before using APIs in compounding.19
In Vivo Metabolism
Hormones are targets of extensive in vivo cellular metabolism (Miller and Auchus, 2011) and transport (Siiteri et al., 1982), making it difficult for them to reach their sites of action reliably once absorbed. The liver, for example, effectively metabolizes hormones that are delivered orally, another reason why oral doses are so much higher than topically delivered doses (von Schoultz, 2009). In addition, for any given route of administration (oral, topical, injected/implanted) there is significant variability between patients in the resulting blood levels of hormones and their metabolites (Kuhl, 2005), and there is even significant day-to-day variability within the same patient (Brambilla et al., 2007; Kraemer et al., 2003). For further discussion on bioavailability of cBHT preparations, see Chapter 6.
Formulation Stability
FDA-approved drug manufacturers must test their products for API stability within the formulation and for their ability to safely release hormones over time.20 Demonstrating API stability in a formulation is of obvious importance. For example, if a dissolved API crystalizes out of solution in a topical cream, it may decrease the bioavailability of the hormone, decreasing the effectiveness of the cream (Garnero et al., 2018). This scenario can happen simply because the cream loses water over time if it was not in a well-sealed package. As a second example, for many formulations, the order of ingredient mixing, inclusion of solubilizers, and other process variables are critical for formulation stability. This is especially the case for topical preparations, as microstructures are formed between ingredients within the dosage form (Jeong et al., 2003). Simple two or three ingredient topical formulations or use of a single premixed commercial base may not be adequate to produce a formulation with suitable stability. For comparison, Box 5-4 lists the 10 inactive ingredients incorporated into Estrace Cream, which are all required for prolonged product stability.
BOX 5-4
Inactive Ingredients in Estrace Cream, an FDA-Approved Drug Product.
In contrast, 503A compounding pharmacies are not required to demonstrate that APIs are stable in formulations for the life (i.e., expiry date or beyond-use date) of their products. Neither are compounders required to measure hormone release rates from cBHT preparations. For example, at a November 2019 open session meeting, Dr. Gary S. Donovitz (BioTE Medical, LLC) who uses a 503B outsourcing facility to prepare testosterone pellets for use in their practice was asked during an open session meeting whether their group tested the pellets to determine the rate of testosterone release over time. Dr. Donovitz expressed that his practice does not conduct such testing, but instead examines the peak serum testosterone levels in patients, using certain target ranges for optimization (NASEM, 2019c).
All of the factors reviewed in this section must be taken into account when developing drug formulations with hormone APIs. Formulations require rigorous testing and improvement over time, before knowing that they can reliably, over time, deliver hormones through the skin, gastrointestinal tract, and tissues. However, the materials, methods, and quality standards followed differ among pharmacies when preparing cBHT preparations, even when filling identical prescriptions.
Examples of Complexity: Formulations, Dosage Forms, and Potency
As a formulation's complexity increases, so does the potential for product failure. For example, Bi-est (formulated with estradiol and estriol, in varying amounts) and Tri-est (formulated with estradiol, estriol, and estrone, in varying amounts) are commonly prescribed cBHT formulations.21 An even more complex formulation is “Arousal Cream,” which contains six active ingredients. Unless formal stability studies on these complex formulations are conducted and disclosed, there is cause for concern regarding the stability and bioavailability of every multi-ingredient compounded preparation.
The level of potency is also an important consideration. For compounded preparations, USP has established a ±10 percent potency deviation range for most compounded preparations (USP Compounding Expert Committee, 2014). Yet, differences in the potency of cBHT preparations have been documented between compounding pharmacies. For example, when compared with product labeling, estradiol capsule potency varied from –26 percent to +5 percent, estradiol cream potency varied from –11 percent to +1.4 percent, progesterone capsule potency varied from –9 percent to +31 percent, and progesterone cream potency varied from –5 percent to +12 percent of the label (Stanczyk et al., 2019). For reference, FDA-approved products typically must maintain potency within a ±10 percent range from the label (Blessy, 2014) to maintain safety and effectiveness (FDA, 2004).
Illustrative Example of Complex cBHT Preparations: Hormone Pellets
Hormone pellet therapies are complex formulations that must be prepared to exacting specifications in order to safely, reliably, and repeatedly deliver a uniform amount of hormone over time. Pellets are implanted subcutaneously and are expected to release hormone(s) at a controlled uniform rate, typically over weeks to months (McCullough, 2014). As such, they are controlled release dosage forms, which makes them different from the more routinely compounded “immediate release” therapies such as topical creams, oral capsules, or immediate release injections.
The committee found multiple examples of cBHT pellets for estradiol, testosterone, and progesterone, available in varied strengths (see Appendix F).22 In regard to the scope of available pellet therapies, the testosterone replacement market alone has been described as a multibillion dollar industry (McCullough, 2014).
Several factors affect the ability of a pellet to reliably release hormone(s) over time, including content uniformity and pellet size and shape (length, width, surface area). Content uniformity is how well the hormone(s) and inactive ingredients are mixed together. As pellets slowly dissolve over time, and thereby slowly release hormone(s) over time, any pockets of hormone crystals (e.g., from a nonuniform mixture) within the pellet may deliver an unintended bolus of hormone(s) to the body.
For most pellets, the size, shape, and surface area of the pellet must be uniform among all pellets of the same dose, and the pellet surface area must be proportional between different dose pellets. That is because the dose delivered is proportional to the surface area of the pellet (McCullough, 2014). Whether compounded or FDA approved, testosterone pellets for men are usually implanted in sets of 10 or more (Glaser and York, 2019; McMahon et al., 2017), and each is expected to deliver hormone(s) at the same rate (McCullough, 2014). Small deviations in length or width will change the rate of hormone delivery. Note that pellets are much smaller than the products a pharmacist typically prepares. For example, a Testopel pellet is 3 mm wide and 8 mm long (McCullough, 2014), which is about the size of a single grain of rice. To dispense a pellet in response to a patient prescription, a pharmacist would have to own and know how to operate special equipment that can mix, dry, extrude, package, and sterilize the pellets. Such equipment is simply not found in the majority of pharmacies. It also cannot be made “on demand.” Also, to be sure the pellets are of uniform size, the pharmacist would have to be able to measure small differences in length and width, on the scale of ≤ 0.3 mm, among same dose pellets.
To prepare pellets simply requires special compounding equipment that is only found in pharmaceutical manufacturing facilities. Also to test a batch of pellets to show they are the correct size, shape, have proper content uniformity, and deliver hormones at a uniform prespecified rate, requires both complex physicochemical testing (specialized dissolution testing) and analytical test methods. Again, such testing is only available in specialized pharmaceutical analytical testing facilities.
Because hormone pellets deliver hormones slowly over an extended period of time, including up to 2 to 5 months (Glaser and York, 2019; McCullough, 2014), specialized bioavailability study designs are required to show how reliable and consistent the delivery happens in humans. That is, pellets require the types of bioavailability study designs applied to depot injection products, which deliver active ingredients over weeks and months. As will be discussed in Chapter 6, compounded preparations are not required to be tested for bioavailability. Also the standard in vitro batch release tests do not capture information required to have assurance that a hormone pellet can safely and uniformly deliver hormones over long time periods. This may explain why there is evidence of significant adverse reactions, indicative of hormone overdose, in groups of patients who have received hormone pellet therapy (Jiang, 2019).23 For example, a higher incidence of mood swings, breast tenderness, hair pattern changes, acne, weight gain, and dyslipidemias were reported in woman who received compounded pellets containing estradiol or testosterone, when compared with woman receiving an FDA-approved hormone product. Also in the same study, woman receiving pellet products had significantly higher and more variable serum concentrations of estradiol and testosterone, when compared with woman receiving an FDA-approved product (Jiang, 2019). There is also evidence of compounding pharmacies substituting inactive ingredients into pellet products (e.g., a cholesterol base for a steric acid base), which would completely change the release characteristics of the pellet,24 a formulation change that could clearly put patients at risk.
Difficult to Compound
Given the complexity of the compounding process as discussed above, the Federal Food, Drug, and Cosmetic Act (FDCA) asks FDA to develop regulations identifying drugs and drug categories that “present demonstrable difficulties for compounding” and to establish a Difficult to Compound List, which would preclude the use of listed drugs in compounded preparations.25 When considering whether or not a drug is difficult to formulate, both the active ingredient and the dosage form should be considered. Relevant to this report, most of the cBHT APIs and certain cBHT formulations exhibit one or more of the complexities discussed in the criteria for the Difficult to Compound List (see Box 5-3).
BOX 5-3
Difficult to Compound List.
Box
Conclusion 5-2.
INACTIVE INGREDIENTS
All FDA-approved products and those compounded by 503B outsourcing facilities are required to list their inactive ingredients on their container labeling according to the FDCA's provisions regarding misbranded drugs.26 While there is no explicit requirement in Section 503A to include inactive ingredients on the label, the FDCA section on misbranded drugs that applies to all drugs (manufactured or compounded) includes a requirement that drugs list inactive ingredients on the container label.27,28 However, 503A pharmacies and other health care facilities may not have the infrastructure to provide appropriate information on inactive ingredients, and as a result, inactive ingredients labeling may not be routinely followed for compounded drugs (ISMP, 2018). Therefore, it may not be possible for many patients to know what inactive ingredients were used in the formulation of a given cBHT preparation. If a patient were to have an allergic reaction to a compounded preparation, it may be difficult to review the inactive ingredients contained therein when considering what the patient may have reacted to. This seems unbalanced, especially as a primary rationale for compounding is to provide products for patients who may have allergies to FDA-approved products (McBane et al., 2019). In contrast, compounded preparations made at 503B outsourcing facilities are required to list inactive ingredients directly on the label, and given FDA's increased oversight role of 503B facilities, the agency would likely detect noncompliance with this requirement (see Box 5-4).29
TABLE 5-5Inactive Ingredients in Estrace Cream and Their Function
| Ingredient | Purpose |
|---|---|
| Water | Solvent |
| Propylene glycol | Humectant; preservative; solvent or cosolvent |
| Stearyl alcohol | Stiffening agent; emollient; weak emulsifying agent |
| White ceresin wax | Stiffening agent |
| Mono and diglycerides | Emulsifier; stabilizer; emollient; plasticizer |
| Hypromellose | Gelling agent |
| Sodium lauryl sulfate | Emulsifier; solubilizer |
| Methylparaben | Preservative |
| Edetate disodium | Chelating agent; preservative |
| Tertiary-butylhydroquinone | Antioxidant |
Some compounding suppliers offer premixed commercial bases (solutions, creams, semisolids) to which active ingredients can be added to prepare compounded formulations. A few examples include VersaBase Cream, VersaBase Gel, Lipoderm, and MucoLox (Clark and Hover, 2016). These make compounding easier by reducing the number of ingredients and steps needed to prepare a compounded product. In some cases, base suppliers offer some documentation to aid in preparing the MFR, along with limited data implying that the base delivers the hormone through the skin or mucous membranes. As an example, instructions for preparing progesterone in Versabase Cream are available online (Allen, 2017). The product contains only three ingredients: micronized progesterone, pentylene glycol, and Versabase Cream. Many of these base mixtures, however, are not specialized for specific formulations (Medisca, n.d.) as are FDA-approved drug products, and they are not required to be tested for their ability to deliver hormones through the skin or mucous membranes with the same rigor as FDA-approved drug products.
Some cBHT preparations are described in USP monographs. However, the inactive ingredients are not always precisely defined within those USP monographs, and the compounder is permitted to apply his or her skill and art when selecting inactive ingredients. For example, Estradiol Vaginal Cream USP describes the cream component as “a stable cream base.” Similarly, Estradiol Cypionate Injection USP instructs the compounder to use “a suitable oil” as the injection vehicle (USP, 2019b).
For compounded USP monograph preparations, this lack of specificity around inactive ingredients may lead to different cream base products being used by different compounders. In these cases, an estradiol cream preparation purchased from one compounding pharmacy may have different inactive ingredients than a product with the same name purchased from a second compounding pharmacy. Differences in inactive ingredients can affect the availability and absorption of cBHT active ingredients, given their low solubility and high metabolism (Panakanti and Narang, 2012), which would result in patients being exposed to differing amounts of the active ingredient.
Similarly for preparations without USP monographs, such as cBHT pellets, the inactive ingredients used may vary between compounders. For example a 25 mg estradiol pellet from one compounder may contain different ingredients than an estradiol pellet provided by a second compounder. There is a reported case where the same pharmacy used a different base to compound testosterone pellets, without knowing the effect of the formulation change on stability.30
Box
Conclusion 5-3.
REFERENCES
- Allen LJ. Progesterone 50 mg/g in versabase cream. US Pharmicist. 2017;42(9):47–48.
- Benet LZ, Broccatelli F, Oprea TI. BDDCs applied to over 900 drugs. AAPS Journal. 2011;13(4):519–547. [PMC free article: PMC3231854] [PubMed: 21818695]
- Blessy M, Patel RD, Prajapati PN, Agrawal YK. Development of forced degradation and stability indicating studies of drugs—a review. Journal of Pharmaceutical Analysis. 2014;4(3):159–165. [PMC free article: PMC5761119] [PubMed: 29403878]
- Boyd BJ, Bergström CAS, Vinarov Z, Kuentz M, Brouwers J, Augustijns P, Brandl M, Bernkop-Schnürch A, Shrestha N, Préat V, Müllertz A, Bauer-Brandl A, Jannin V. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. European Journal of Pharmaceutical Sciences. 2019;137:104967. [PubMed: 31252052]
- Brambilla DJ, O'Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clinical Endocrinology (Oxford). 2007;67(6):853–862. [PubMed: 18052942]
- Censi R, Di Martino P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules. 2015;20(10):18759–18776. [PMC free article: PMC6331817] [PubMed: 26501244]
- Chang R-K, Raw A, Lionberger R, Yu L. Generic development of topical dermatologic products: Formulation development, process development, and testing of topical dermatologic products. AAPS Journal. 2013;15(1):41–52. [PMC free article: PMC3535108] [PubMed: 23054971]
- Clark D, Hover S. Compounding options in women's health. Paper read at HRT Symposium; Savannah, GA: 2016.
- Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: Provisional BCS classification of the world's top oral drugs. AAPS Journal. 2009;11(4):740–746. [PMC free article: PMC2782078] [PubMed: 19876745]
- FDA (U.S. Food and Drug Administration). Guidance for industry: Q1E evaluation of stability data. 2004. [June 1, 2020]. https://www
.fda.gov/media/71722/download. - FDA. Early development considerations for innovative combination products. 2006. [June 1, 2020]. https://www
.fda.gov/regulatory-information /search-fda-guidance-documents /early-development-considerations-innovative-combination-products. - FDA. Draft guidance on progesterone. 2012. [June 23, 2020]. https://www
.accessdata .fda.gov/drugsatfda_docs /psg/Progesterone _insertvag_22057_RC09-12.pdf. - FDA. Codevelopment of two or more new investigational drugs for use in combination. 2013. [June 1, 2020]. https://www
.fda.gov/regulatory-information /search-fda-guidance-documents /codevelopment-two-or-more-new-investigational-drugs-use-combination. - FDA. Testosterone information. 2015. [February 28, 2020]. https://www
.fda.gov/drugs /postmarket-drug-safety-information-patients-and-providers /testosterone-information. - FDA. MedWatch. 2018a. [June 3, 2020]. https://www
.fda.gov/safety /medwatch-fda-safety-information-and-adverse-event-reporting-program. - FDA. FYs 2013-2017 GDUFA science and research report: Transdermal drug products. 2018b. [June 12, 2020]. https://www
.fda.gov/industry /generic-drug-user-fee-amendments /fys-2013-2017-gdufa-science-and-research-report-transdermal-drug-products. - FDA. Email from G. Cosel to National Academies staff regarding aggregated volume output of products containing ingredients of interest to compounded bioidentical hormone therapy study. May 30, 2020 (available through the National Academies Public Access File. 2019a.
- FDA. Drug master files (DMFs). 2019b. [February 28, 2020]. https://www
.fda.gov/drugs /forms-submission-requirements /drug-master-files-dmfs. - FDA. Medication guides. 2019c. [December 31, 2019]. https://www
.accessdata .fda.gov/scripts/cder/daf/index .cfm?event=medguide.page. - FDA. Upcoming product-specific guidances for complex generic drug product development. 2019d. [February 28, 2020]. https://www
.fda.gov/drugs /guidances-drugs /upcoming-product-specific-guidances-complex-generic-drug-product-development. - FDA. Compounding: Inspections, recalls, and other actions. 2020a. [January 4, 2020]. https://www
.fda.gov/drugs /human-drug-compounding /compounding-inspections-recalls-and-other-actions. - FDA. Orange Book: Approved drug products with therapeutic equivalence evaluations. 2020b. [March 26, 2020]. https://www
.accessdata .fda.gov/scripts/cder/ob/index.cfm. - Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Delivery. 2015;22(8):969–987. [PubMed: 24456019]
- Garnero C, Zoppi A, Aloisio C, Longhi MR. Chapter 7—technological delivery systems to improve biopharmaceutical properties. In. In: Grumezescu AM, editor. Nanoscale fabrication, optimization, scale-up and biological aspects of pharmaceutical nanotechnology. Norwich, NY: William Andrew Publishing; 2018. pp. 253–299.
- Glaser RL, York AE. Subcutaneous testosterone anastrozole therapy in men: Rationale, dosing, and levels on therapy. International Journal of Pharmaceutical Compounding. 2019;23(4):325–339. [PubMed: 31315085]
- Goodman Gilman A, Rall TW, Nies AS, Taylor P. Goodman and Gilman's the pharmacological basis of therapeutics. 8th ed. New York: McGraw-Hill; 1990.
- Hammes A, Andreassen T, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert F, Luppa P, Nykjaer A, Willnow T. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–762. [PubMed: 16143106]
- ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use). Evaluation for stability data Q1E. (February 2003). 2003. [December 31, 2019]. https://database
.ich .org/sites/default/files/Q1E_Guideline .pdf. - IJPC (International Journal of Pharmaceutical Compounding). IJPC. Edmond, OK: International Journal of Pharmaceutical Compounding; 2018. Compounding for bioidentical hormone replacement therapy patients. Purchased compiled peer-reviewed articles from 1997-2018. In.
- ISMP (Institute for Safe Medication Practices). FDA guidance needed to assure safe labeling practices by 503A and 503B compounders. 2018. [April 29, 2020]. https://www
.ismp.org /resources/fda-guidance-needed-assure-safe-labeling-practices-503a-and-503b-compounders. - Jeong JC, Lee J, Cho K. Effects of crystalline microstructure on drug release behavior of poly(epsilon-caprolactone) microspheres. Journal of Controlled Release. 2003;92(3):249–258. [PubMed: 14568406]
- Jiang X. Postmenopausal pellet versus FDA approved hormonal therapy: An assessment of serum estradiol and testosterone levels. Paper presented at North American Menopause Society 2019 Annual Meeting; Chicago, IL: 2019.
- Kraemer GR, Kraemer RR, Ogden BW, Kilpatrick RE, Gimpel TL, Castracane VD. Variability of serum estrogens among postmenopausal women treated with the same transdermal estrogen therapy and the effect on androgens and sex hormone binding globulin. Fertility and Sterility. 2003;79(3):534–542. [PubMed: 12620436]
- Kuhl H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. [PubMed: 16112947]
- Lostritto R. Content uniformity (CU) testing for the 21st century: CDER perspective. [February 28, 2020]. https://www
.fda.gov/media/85340/download. - McBane SE, Coon SA, Anderson KC, Bertch KE, Cox M, Kain C, LaRochelle J, Neumann DR, Philbrick AM. Rational and irrational use of nonsterile compounded medications. Journal of the American College of Clinical Pharmacy. 2019;2(2):189–197.
- McCullough A. A review of testosterone pellets in the treatment of hypogonadism. Current Sexual Health Reports. 2014;6(4):265–269. [PMC free article: PMC4431706] [PubMed: 25999802]
- McMahon CG, Shusterman N, Cohen B. Pharmacokinetics, clinical efficacy, safety profile, and patient-reported outcomes in patients receiving subcutaneous testosterone pellets 900 mg for treatment of symptoms associated with androgen deficiency. Journal of Sexual Medicine. 2017;14(7):883–890. [PubMed: 28673432]
- Medisca. Cream base reference chart. n.d. [February 28, 2020]. https://www
.medisca.com /Files/ReferenceCharts /Cream%20& %20Gel%20Bases%20Reference %20Chart%20-%20MUS.pdf. - Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrinology Review. 2011;32(1):81–151. [PMC free article: PMC3365799] [PubMed: 21051590]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Perspectives from Compounding Pharmacists. November 12, 2019 (available through the National Academies Public Access File. 2019a. Gina Besteman, R.Ph.
- NASEM. Pamela M.D. Smith M.P.H., M.S. Perspectives from Providers. November 12, 2019 (available through the National Academies Public Access File. 2019b.
- NASEM. Peter Koshland, Pharm.D. Perspectives from Compounding Pharmacists. November 12, 2019 (available through the National Academies Public Access File). 2019c.
- Ng KW. Penetration enhancement of topical formulations. Pharmaceutics. 2018;10(2):51. [PMC free article: PMC6027320] [PubMed: 29673184]
- NLM (National Library of Medicine). RxNorm Database. 2019. [December 6, 2019]. https://www
.nlm.nih.gov /research/umls/rxnorm/index.html. - NLM. Dailymed. 2020a. [February 11, 2020]. https://dailymed
.nlm.nih.gov/dailymed. - NLM. Pubchem. 2020b. [June 4, 2020]. https://pubchem
.ncbi.nlm.nih.gov. - Panakanti R, Narang AS. Impact of excipient interactions on drug bioavailability from solid dosage forms. Pharmaceutical Research. 2012;29(10):2639–2659. [PubMed: 22610283]
- Pourkavoos N. Unique risks, benefits, and challenges of developing drug-drug combination products in a pharmaceutical industrial setting. Combination Products in Therapy. 2012;2(1):2.
- Prausnitz MR, Langer R. Transdermal drug delivery. Nature Biotechnology. 2008;26(11):1261–1268. [PMC free article: PMC2700785] [PubMed: 18997767]
- Reed Smith LLP. Comparison chart of 503A and 503B dosage forms, provided to the committee by Reed Smith LLP on behalf of a client, a coalition of six compounding facilities. November 7, 2019 (available through the National Academies Public Access File. 2019.
- Rowe R, Sheskey P, Owen S. Handbook of pharmaceutical excipients. 5th ed. London, UK: Pharmaceutical Press and American Pharmacists Association; 2006.
- Roy J. Pharmaceutical impurities—a mini-review. AAPS PharmSciTech. 2002;3(2):E6. [PMC free article: PMC2750308] [PubMed: 12916943]
- Sarkar A, Ragab D, Rohani S. Polymorphism of progesterone: A new approach for the formation of form ii and the relative stabilities of form i and form ii. Crystal Growth & Design. 2014;14(9):4574–4582.
- Sheinin E, Williams R. Chemistry, manufacturing, and controls information in NDAs and ANDAs, supplements, annual reports, and other regulatory filings. Pharmaceutical Research. 2002;19(3):217–226. [PubMed: 11934225]
- Siiteri PK, Murai JT, Raymoure WJ, Kuhn RW, Hammond GL, Nisker JA. The serum transport of steroid hormones. In. Greep RO, editor. Boston, MA: Academic Press; Proceedings of the 1981 Laurentian Hormone Conference. 1982;38:457–510.
- Stanczyk FZ, Niu C, Azen C, Mirkin S, Amadio JM. Determination of estradiol and progesterone content in capsules and creams from compounding pharmacies. Menopause. 2019;26(9):966. [PMC free article: PMC6738624] [PubMed: 31453957]
- Stefanick ML. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the U.S. Food and Drug Administration. American Journal of Medicine. 2005;118(Suppl 12B):64–73. [PubMed: 16414329]
- Stevenson EL, Lancaster RW, Buanz ABM, Price LS, Tocher DA, Price SL. The solid state forms of the sex hormone 17-β-estradiol. CrystEngComm. 2019;21(13):2154–2163.
- Stute P, Neulen J, Wildt L. The impact of micronized progesterone on the endometrium: A systematic review. Climacteric. 2016;19(4):316–328. [PubMed: 27277331]
- TherapeuticsMD. Bijuva (estradiol and progesterone) capsules, for oral use. 2018. [March 5, 2020]. https://www
.accessdata .fda.gov/drugsatfda_docs /label/2018/210132s000lbl.pdf. - Upsher-Smith Laboratories. Vogelxo (testosterone) gel, for topical use. 2014. [March 3, 2020]. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2014/204399Orig1s000Lbl.pdf. - USP (United States Pharmacopeia). <1163> quality assurance in pharmaceutical compounding, edited by United States Pharmacopeia. Rockville, MD: United States Pharmacopeia; 2012.
- USP. The United States Pharmacopeial Convention, edited by United States Pharmacopeia. Rockville, MD: USP; 2014. <795> pharmaceutical compounding—nonsterile preparations. In.
- USP. USP-NF reference standards. Rockville, MD: United States Pharmacopeia; 2019a.
- USP. USP compounding compendium. 2019b. [May 12, 2020]. https://www
.usp.org/products /usp-compounding-compendium. - USP Compounding Expert Committee. Strength and stability testing for compounded preparations. Rockville, MD: United States Pharmacopeia; 2014.
- von Schoultz B. Oestrogen therapy: Oral versus non-oral administration. Gynecology and Endocrinology. 2009;25(9):551–553. [PubMed: 19479596]
- Warner Chilcott. Estrace cream. 2005. [March 5, 2020]. https://www
.accessdata .fda.gov/drugsatfda_docs /label/2005/86069s018lbl.pdf. - WHO (World Health Organization). International nonproprietary names. 2018. [June 4, 2020]. https://www
.who.int/medicines /services/inn/en. - Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, Woodcock J. Understanding pharmaceutical quality by design. AAPS Journal. 2014;16(4):771–783. [PMC free article: PMC4070262] [PubMed: 24854893]
Footnotes
- 1
As a result of the minimal oversight of the compounding process, cBHT preparations are not produced with the same level of quality assurance as FDA-approved products, and they are not required to demonstrate safety and effectiveness in clinical trials before being dispensed to a patient (Federal Food, Drug, and Cosmetic Act. 21 U.S. Code Chapter 9). See Chapter 3 for an overview of the regulation and oversight of compounded preparations.
- 2
As Chapter 4 noted, a bioidentical hormone is a term that describes a hormone that is chemically and structurally identical to those produced by the human body, with the implication that an identical structure translates to an identical physiologic response as endogenous hormones.
- 3
As discussed in Chapter 2, a compounder can begin to prepare the patient-specific cBHT preparation as described by the prescription (or clinic order for 503Bs) when an MFR and a compounding record are available.
- 4
MFRs are an important component of quality control during the formulation of a drug. If a compounding record deviates from what is written in an MFR without proper documentation for such a change, it is a breach in the quality control that aims to provide patients with consistent formulations of their prescription. As of April 2020, MFRs are only required for certain types of compounding, though similar documentation is also required in current good manufacturing practice regulations. See Written procedures; deviations. 21 CFR 211.100 (April 1, 2019).
- 5
As discussed in Chapter 3 of this report, USP itself does not have regulatory or enforcement authority, but certain USP standards are enforceable in some states under state law.
- 6
Federal Food, Drug, and Cosmetic Act. 21 U.S. Code Chapter 9.
- 7
Requirements on content and format of labeling for human prescription drug and biological products. 21 CFR § 201.56 (December 4, 2014).
- 8
Federal Food, Drug, and Cosmetic Act. 21 U.S. Code Chapter 9.
- 9
Resources included peer-reviewed literature on use of cBHT (e.g., IJPC, 2018), cBHT preparation adverse event reports (FDA, 2018a, 2020a), information provided by compounding practitioners (see the National Academies Public Access File), recent biannual outsourcing facility preparation reports (FDA, 2019a), and online marketing information from compounding pharmacies.
- 10
Reed Smith LLP is a law firm that represents a coalition of 503B outsourcing facilities, that at the time of this report, incudes Belmar Pharmacy; Fagron North America, which includes Fagron Inc., Humco, B&B Pharmaceuticals; AnazaoHealth Corporation; Women's International Pharmacy; BioTE Medical; and Carie Boyd's Prescription Shop.
- 11
At the time of this report, FDA's Orange Book does include an FDA-approved estradiol cypionate 5 mg/mL injection (Depo-estradiol) product, which may or may not be related to the inability to find a corresponding cBHT preparation.
- 12
Resources included peer-reviewed literature on use of cBHT (e.g., IJPC, 2018), cBHT preparation adverse event reports (FDA, 2018a, 2020a), information provided by compounding practitioners (see the National Academies Public Access File), recent biannual outsourcing facility preparation reports (FDA, 2019a), and online marketing information from compounding pharmacies.
- 13
Fixed-combination prescription drugs for humans. 21 CFR § 300.50 (January 5, 1999).
- 14
The new drug application (NDA) and the abbreviated new drug application (ANDA) are the mechanisms through which a drug manufacturer presents evidence to request that FDA approve its drug product. More information on this process can be found in Chapter 3.
- 15
Content and format of an NDA. 21 CFR § 314.50 (February 22, 1985).
- 16
Federal Food, Drug, and Cosmetic Act. 21 U.S. Code Chapter 9.
- 17
DMFs are submissions to FDA used to provide confidential, detailed information about facilities, processes, and articles used in the manufacturing, processing, packaging, and storing of APIs (FDA, 2019b).
- 18
Federal Food, Drug, and Cosmetic Act. 21 U.S. Code Chapter 9.
- 19
Testing and approval or rejection of components, drug product containers, and closures. 21 CFR § 211.84 (September 8, 2008).
- 20
Special testing requirements. 21 CFR § 211.167.
- 21
As discussed in Chapters 2 and 8, it is difficult to secure quantitative data on the prescription rates and dispensing practices for specific cBHT formulations, doses, and dosage forms; however, available data suggest that Bi-est and Tri-est, available in varying amounts and dosage forms, are commonly compounded preparations (IJPC, 2018; NABP, 2019).
- 22
Resources included peer-reviewed literature on the use of cBHT (e.g., IJPC, 2018), cBHT preparation adverse event reports (FDA, 2018a, 2020a), information provided by compounding practitioners (see the National Academies Public Access File), recent biannual outsourcing facility preparation reports (FDA, 2019a), and online marketing information from compounding pharmacies.
- 23
State of Tennessee v. HRC Medical Centers Inc. 2012. Robert E. Cooper, Jr. (Circuit Court for Davidson County, TN).
- 24
Accusation in Herold v. University Rx Specialist, Case No. 4347 (2015).
- 25
See Drug Products That Present Demonstrable Difficulties for Compounding Under the Federal Food, Drug, and Cosmetic Act; Establishment of a Public Docket. 82 FR 35214 (July 28, 2017) (FDA announces development of criteria to evaluate drugs as demonstrably difficult to compound under 503A and 503B).
- 26
See Section 503B(a)(10) of the Federal Food, Drug, and Cosmetic Act.
- 27
Requirements on content and format of labeling for human prescription drug and biological products. 21 CFR § 201.56 (December 4, 2014).
- 28
Federal Food, Drug, and Cosmetic Act. 21 U.S. Code Chapter 9.
- 29
See Section 503B(a)(10) of the Federal Food, Drug, and Cosmetic Act.
- 30
Accusation in Herold v. University Rx Specialist, Case No. 4347 (2015).
- Compounded Bioidentical Hormone Preparations - The Clinical Utility of Compounde...Compounded Bioidentical Hormone Preparations - The Clinical Utility of Compounded Bioidentical Hormone Therapy
Your browsing activity is empty.
Activity recording is turned off.
See more...