NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium; Oria M, Harrison M, Stallings VA, editors. Dietary Reference Intakes for Sodium and Potassium. Washington (DC): National Academies Press (US); 2019 Mar 5.

Dietary Reference Intakes for Sodium and Potassium.
Show detailsThe Dietary Reference Intakes (DRIs) are a set of quantitative reference values developed jointly for the United States and Canada. They are derived through an iterative process that has evolved to account for advancements in their supporting data and evidence, changes in population-based public health concerns, and a widening range of adaptation to various applications and uses. The DRIs recognize the need for adequate intakes of essential nutrients in order to prevent deficiency diseases, and they have been broadened to recognize the need for safe intakes of nutrients and other food substances, as well as the role of nutrients and other food substances in reducing the risk of chronic disease.
The DRIs were built on the concepts that defined their precursor, the Recommended Dietary Allowances (RDAs). The RDAs were conceived as recommended nutrient intake levels “judged on the basis of available scientific evidence to meet the known nutritional needs of practically all healthy persons in the United States” (IOM, 1994, p. 4). These recommended nutrient intakes provided “standards to serve as a goal for good nutrition and as a ‘yardstick' by which to measure progress toward that goal” (NRC, 1941, p. 1). When the RDAs were developed, nutritional deficiency diseases were prevalent across the population. As the public health burden of these conditions diminished with improvements in dietary intake, concerns about the risk of diet-related chronic disease began to emerge. The growing evidence of, and attention to, the relationship between diet and risk of chronic disease (HHS, 1988; NRC, 1989) prompted members of the Food and Nutrition Board to consider whether the RDAs should be revised to better integrate the concept of a health-promoting diet while retaining their foundational concepts (IOM, 1994).
The model that emerged, the DRIs, included reference values to ensure intake adequacy (Estimated Average Requirement [EAR] and RDA) and an upper bound of a safe and adequate intake range (Tolerable Upper Intake Level [UL]). As the DRIs were further developed, the Adequate Intake (AI), Acceptable Macronutrient Distribution Range (AMDR), and Estimated Energy Requirement (EER) were incorporated into the model.
Although it envisioned consideration of evidence for chronic disease risk (IOM, 1994), the DRI model proved to be challenging and insufficient for that purpose (IOM, 2008). For example, whereas threshold models were useful for setting DRIs for adequacy and toxicity, these models did not work well for informing DRI decisions about the role of nutrient intake in reducing chronic disease risk. The relationships of nutrient intakes to adequacy are based on experimental evidence for a deficiency, and for some nutrients there are relationships with toxicity outcomes. This manifests in a curvilinear relationship (between inadequacy on the lower end and toxicity on the upper end) that makes it possible to identify a “cut point” or threshold effect for defining the DRIs. Conversely, relationships between diet and chronic disease risk are dependent on a variety of factors, both nutritional and nonnutritional. These factors involve lengthy exposure times and include an individual's baseline risk for the chronic disease, environmental factors, nutrient–diet or nutrient–nutrient interactions, and lifestyle factors other than diet. The intake–response relationships between nutrient intakes and chronic disease risk often differ from the threshold relationships observed for adequacy and toxicity effects.
With the evolution of both the DRI model and the definition of nutritional health to include not only essential nutrients but also other nutritional substances in foods, the DRI Steering Committee of the U.S. and Canadian governments recognized the need to reexamine the DRI model to consider inclusion of chronic disease endpoints in the process. It asked the National Academies of Sciences, Engineering, and Medicine (the National Academies) to undertake the task. The resulting report, Guiding Principles for Developing Dietary Reference Intakes Based on Chronic Disease (hereafter referred to as the Guiding Principles Report), provides guidance and recommendations for expanding the DRI model to include a new category of values specific to chronic disease risk reduction (NASEM, 2017).
This study represents the first effort to apply the recommendations from the Guiding Principles Report to the process of deriving DRIs for sodium and potassium. Potassium and sodium are physiologically essential nutrients. Their functions are closely intertwined, and each has important roles in maintaining physiological homeostasis. Both nutrients have also been implicated in chronic disease risk, particularly cardiovascular disease, mainly through their effects on blood pressure. Additionally, a possible association of sodium intake with adverse outcomes has been suggested at low levels of intake. For purposes of reviewing intake recommendations for these nutrients, the coexistence of their physiological essentiality with their relationships to adverse health effects, including chronic disease risk, called for an expanded DRI model. Guidance on expanding the DRI model is offered in the Guiding Principles Report.
STUDY OVERVIEW AND STATEMENT OF TASK
In 2013, the DRI Steering Committee implemented a new process by which nutrients and other food substances would be nominated for DRI review (HHS, 2018). The intent of this process was for DRI updates to be determined by the emergence of new and significant evidence with public health relevance, rather than being determined by the amount of time that had elapsed since the last DRI review. Twenty-six submissions nominating 16 different nutrients were received. The federal agencies that make up the DRI Steering Committee prioritized the list of nominated nutrients, and selected omega-3 fatty acids, potassium and sodium, magnesium, and vitamin E for further consideration.
Preparation for new DRI reviews included efforts to determine how evidence on chronic disease could be used in deriving DRI values. Recognizing that a DRI based on chronic disease would not necessarily fit the existing DRI framework, the DRI Steering Committee organized a multidisciplinary working group in 2014 to identify and offer solutions to the challenges that DRI committees would likely encounter. The working group released its report in 2017, Options for Basing Dietary Reference Intakes (DRIs) on Chronic Disease: Report from a Joint U.S.-/Canadian-Sponsored Working Group (Options Report) (Yetley et al., 2017). As noted above, a National Academies consensus committee was charged with reviewing the Options Report and providing guiding principles for developing DRIs based on chronic disease. The resulting report, the Guiding Principles Report (NASEM, 2017), provides guidance and recommendations for expanding the DRI model to include a new category of values specific to chronic disease risk reduction; the intent was for future DRI committees to incorporate this guidance into the existing DRI process.
Since the DRIs for sodium were established in 2005, two Institute of Medicine reports were published with widely varying conclusions about the implications for optimal intake levels of sodium and strategies for reduction in intake (IOM, 2010, 2013). These reports, along with additional emerging evidence, reignited the debate about optimal levels of sodium intake. In response, Congress requested that the Centers for Disease Control and Prevention (CDC) undertake a review of the DRIs for sodium. The inextricable link between potassium and sodium, in both physiology and study designs, make their concurrent review for purposes of the DRIs both scientifically justified and efficient. CDC, together with the Food and Drug Administration, Health Canada, the National Institutes of Health, the Public Health Agency of Canada, and the U.S. Department of Agriculture, asked the National Academies to undertake a review of the DRIs for sodium and potassium.
As set forth in the Statement of Task (see Box 1-1), the committee was asked to review current evidence and update, as appropriate, the DRIs for sodium and potassium that were established in the 2005 report, Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate (hereafter referred to as the 2005 DRI Report) (IOM, 2005). In its review of the evidence, the committee was asked to apply the guidance provided in the Guiding Principles Report (NASEM, 2017), which allows for a new category of DRIs to be established when there is sufficient strength of evidence for the relationship between intake and chronic disease risk.1 To fulfill its task, the committee was provided with an Agency for Healthcare Research and Quality (AHRQ) systematic review, Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks (hereafter referred to as the AHRQ Systematic Review) (Newberry et al., 2018).
BOX 1-1
Statement of Task.
The committee was tasked with reviewing and assessing the evidence on potassium and sodium adequacy and toxicity, as well as each nutrient's relationship with chronic disease risk, to derive the quantitative reference intake values, as appropriate. Translating the DRIs into food-based guidance is an important application, but it is beyond the scope of this study. Thus, the committee focused its evidence review on the independent effects of potassium and sodium intake. To guide its review of the evidence and its derivation of DRIs for potassium and sodium, the committee followed the previously existing DRI model, where applicable, and integrated guidance from the Guiding Principles Report. The Guiding Principles Report expands the model to include a new DRI category that characterizes the relationship between intake and chronic disease risk. This new DRI category, described in detail in Chapter 2 as it applies to potassium and sodium, does not replace the prior DRI model and leaves the other DRI categories largely intact. The following sections provide a brief overview of key concepts of the DRI model that existed before the Guiding Principles Report and provide a summary of two reports central to the committee's task—the 2005 DRI Report and the AHRQ Systematic Review.
The DRI Organizing Framework
The DRI organizing framework provides a structured process for establishing DRIs (see Box 1-2). Based on a risk assessment framework, the DRI organizing framework outlines four steps for the scientific assessment conducted by DRI committees and provides an objective and flexible scheme for transparent decision making. Although it predates the Guiding Principles Report, and therefore describes the approach in the context of identifying adequate and excessive intakes, the DRI framework generally applies to the expanded DRI model as well.
BOX 1-2
Dietary Reference Intakes Organizing Framework.
Indicators for Developing DRIs
A critical step in the DRI organizing framework is identifying and selecting indicators of adequate and excessive intakes. In this context, an indicator broadly refers to clinical endpoints, biomarkers, surrogate markers, and chronic disease risk factors. As quantitative reference intake values, the DRIs are intended to be derived using evidence based on indicators that are “feasible, valid, reproducible, sensitive, and specific” (WHO, 2006, p. 24). The scientific literature includes evaluation of relationships between potassium and sodium intakes and a variety of indicators (see Appendix D), but not all indicators are relevant for establishing a DRI. Final selection of an indicator is guided by consideration of the strength of the evidence and its public health significance.
Dietary Reference Intake Categories
The DRIs include several categories of reference values that serve different purposes and convey different information. The DRI categories described below are those that are relevant to the committee's task of reviewing DRIs for potassium and sodium that existed in the DRI model prior to the Guiding Principles Report. Other reference values not relevant to this task—the EER and the AMDR—have been detailed elsewhere (IOM, 2006). Chapter 2 provides additional context and information regarding the new DRI category based on chronic disease.
Estimated Average Requirement The EAR is “the average daily nutrient intake level that is estimated to meet the nutrient needs of half of the healthy individuals in a life-stage or gender group” (IOM, 2006, p. 10). Because nutrient needs in a population are variable, the EAR is based on the statistical concept of distribution and is an estimate of the median requirement for a nutrient (see Figure 1-1). As such, the EAR is expected to exceed the needs of half of the population and fall below the needs of the other half. The EAR is used in the planning and assessment of adequate dietary intake of groups.
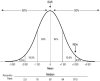
FIGURE 1-1
Normal requirement distribution of hypothetical nutrient showing percentile rank and placement of the EAR and the RDA on the distribution. NOTE: EAR = Estimated Average Requirement; RDA = Recommended Dietary Allowance; SD = standard deviation. SOURCE: (more...)
Recommended Dietary Allowance The RDA is “an estimate of the daily average dietary intake that meets the nutrient needs of nearly all (97–98 percent) healthy members of a particular life-stage and gender group” (IOM, 2006, p. 10). As shown in Figure 1-1, an RDA is generally established as the intake level that is two standard deviations above the EAR. An RDA cannot be established without an EAR. Because an RDA exceeds the nutrient requirements for nearly all individuals in the group, it is not intended to be used for assessing or planning intakes for groups; instead, the RDA has been used for individuals. A usual intake at or above the RDA is characterized as having low probability of being inadequate; the probability of inadequacy increases as intakes fall further away from the RDA (IOM, 2006).
Adequate Intake The AI is “a recommended average daily nutrient intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people who are assumed to be maintaining an adequate nutritional state” (IOM, 2006, p. 11). An AI is established when there is insufficient evidence to establish an EAR and an RDA and is expected to meet or exceed the needs of nearly all members of a given sex and life-stage group. Because it cannot characterize risk of inadequacy, an AI is limited in its applications.
Tolerable Upper Intake Level The UL is “the highest average daily nutrient intake level likely to pose no risk of adverse health effects for nearly all people in a particular group” (IOM, 2006, p. 11). The potential for risk increases as intake increases above the UL. The absence of a UL for a nutrient likely reflects a lack of evidence rather than a lack of adverse effects, and therefore does not necessarily mean that excessive intakes pose no risks. As discussed in Chapter 2, the Guiding Principles Report recommended that in the expanded DRI model, the UL should characterize toxicological risk.
Life-Stage Groups
The DRIs are expressed as reference values for groups defined by age, sex, and life stage (e.g., infants, 0–6 months old; males, 14–18 years old; lactating women, 31–50 years old). The DRI age, sex, and life-stage groups allow for the variation in nutrient recommendations to be reflected within a given DRI category (e.g., the AI for an infant can be set lower than the AI for an adult for a given nutrient, as appropriate). The defining characteristics and rationale for such divisions have been detailed in previous reports (IOM, 2006). In context of the expanded DRI model, one of the recommendations in the Guiding Principles Report is that extrapolation of DRIs based on chronic disease is appropriate “only to populations that are similar to studied populations in the underlying factors related to the chronic disease of interest” (NASEM, 2017, p. 214).
Applicable Population
The DRIs are intended to provide recommendations for an apparently healthy population, defined as individuals who do not have medical diagnoses or conditions or require medications, medical nutrition therapy, or dietary management with medical foods. Such conditions or diagnoses include but are not limited to malabsorption, malnutrition, or disability requiring decreased energy intakes. Furthermore, the DRIs for adequacy (i.e., EARs and RDAs or AIs) are intended to reflect intakes that meet the needs of apparently healthy age, sex, and life-stage groups.
The Guiding Principles Report acknowledged that an apparently healthy population may include individuals with chronic conditions such as obesity, diabetes, or hypertension (whether under medical management or not), but also highlighted the need for DRI committees to characterize which subpopulations are included or excluded in terms of health status for each DRI (NASEM, 2017).
The 2005 DRI Report
In the 2005 DRI Report, there was insufficient intake–response data to establish EARs and RDAs for potassium. The potassium AI, established at 4,700 mg/d (120 mmol/d) for adults 19 years of age and older, was “based on blunting the severe salt sensitivity prevalent in African-American men and decreasing the risk of kidney stones, as demonstrated in a 3-year double-blind controlled study” (IOM, 2005, p. 235). The selected intake level was further supported by blood pressure evidence in nonhypertensive individuals and epidemiological studies on the relationship between potassium intake and bone loss. The potassium AI for adults was extrapolated to the other DRI age, sex, and life-stage groups. No potassium UL was established, as generally healthy individuals with normal kidney function excrete excess potassium. Individuals with impaired kidney function caused by a medical condition or some medications were identified as groups that should not exceed the potassium AI.
Like potassium, insufficient data on sodium requirements prevented the derivation of EARs and RDAs. The sodium AI, 1,500 mg/d (65 mmol/d) for adults 19–50 years of age, was described as ensuring adequate intake of other important nutrients and covering sodium losses from sweat from physical activity or high temperatures in unacclimatized individuals. The sodium AI was also described as being above the intake level that some studies had reported to have a detrimental effect on blood lipids and insulin resistance. The sodium AI for adults was extrapolated to other DRI age, sex, and life-stage groups. The relationship between sodium intake and blood pressure informed the sodium UL, which was established as 2,300 mg/d (100 mmol/d) for adults 19 years of age and older and extrapolated to other DRI age, sex, and life-stage groups.
The AHRQ Systematic Review
Provision of a systematic review to the DRI committee is a recent addition to the process. Only one previous DRI committee—the Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (IOM, 2011)—was provided with systematic reviews to inform its work. AHRQ was responsible for the systematic reviews that informed the 2011 DRI review of calcium and vitamin D, as well as the systematic review that informs this study.
The AHRQ Systematic Review sought to answer 8 key questions and 22 subquestions (Newberry et al., 2018) (see Box 1-3). Sodium and potassium each had two key questions designed to explore effects of intake on select indicators and outcomes, based on evidence from randomized controlled trials; each nutrient also had two key questions designed to explore associations between intake and selected indicators and outcomes, based on evidence from observational studies. The AHRQ Systematic Review provided detailed syntheses of the available evidence, including meta-analyses of trial data when sufficient evidence was available.2 The AHRQ Systematic Review contains several appendixes that provide in-depth methodological details, including the search strategy, evidence tables, quality assessment, summary of the strength of evidence, and sensitivity analyses.
BOX 1-3
Key Questions Excerpted from the AHRQ Systematic Review.
DESIGN AND APPROACH TO THE STUDY
An ad hoc committee of 14 experts was appointed to respond to the charge set forth in the Statement of Task (see Box 1-1). Committee member expertise included human nutrition across the lifespan, intake assessment methodology, biostatistics, epidemiology, systematic review methodology, cardiovascular disease, hypertension, renal disease, health policy, and risk assessment. Two consultants also provided assistance to the committee in literature search methodologies and sodium and potassium physiology.
The committee undertook several activities to inform its work. It hosted three public sessions over the course of the study, one of which included an opportunity for stakeholders to provide public comment directly to the committee (for public session agendas, see Appendix B). The committee also requested public input to help identify published material that would not be found through a peer-review literature search (e.g., academic, business, government, and industry reports). This request was posted on the study website and also circulated via its electronic mailing list. The call for public input was in addition to the study's feedback mechanism through which stakeholders or interested members of the public could submit comments or materials to the committee throughout the study.
The AHRQ Systematic Review (Newberry et al., 2018), provided to the committee by the sponsors, was the primary source of evidence for the relationship between each of the nutrients and chronic disease outcomes, as well as evidence on population subgroups that may be disparately affected by potassium and sodium intake. Prior to using the AHRQ Systematic Review, the committee assessed its quality and methodology (see Appendix C) and identified two aspects that could be strengthened: the need to explore unexplained heterogeneity in meta-analyses and the need for clear explanations of the process used to grade the strength of the evidence. The committee refined these aspects for key analyses central to its decision making, as further described in Chapters 6 and 10.
To be comprehensive in its review of indicators that could potentially inform a potassium or sodium DRI, the committee also conducted a series of literature scoping searches (see Appendix D). The scoping searches informed the committee's selection of additional indicators that were not included in the AHRQ Systematic Review but merited further consideration. Supplemental literature searches were performed for select indicators (see Appendix E).
The Statement of Task directed the committee to provide summary tables of studies used to assess the DRIs (see Box 1-1, numbered item 2). The AHRQ Systematic Review provided extensive documentation and tables summarizing all included studies, which the committee used in its review of the evidence. Thus, it would be duplicative for the committee to provide summary tables for every indicator it reviewed. Accordingly, the committee interpreted this component of its charge as including summary tables only for studies that were part of its supplemental literature search.
The committee took similar approaches to reviewing the evidence on potassium and sodium in support of the DRIs for adequacy, for toxicity, and based on chronic disease. However, each nutrient differed in collection of data, had different strengths of evidence, and had different challenges, meriting separate considerations. Throughout this report, the committee offers its synthesis and interpretation of the evidence. For decisions that relied on expert judgment, the committee describes the alternatives it considered and explains why these decisions were made.
ORGANIZATION OF THIS REPORT
This report is divided into four parts. Part I (Chapters 1–3) provides background information about the DRIs, the milestones in DRI history that led to this committee's work, and the sources of evidence the committee used to fulfill its task. Because this is the first DRI committee to apply the guidance in the Guiding Principles Report to derive DRIs based on chronic disease, Chapter 2 includes a description of how the guidance was applied and a detailed discussion of concepts related to this new DRI category in the context of the committee's review on potassium and sodium. Chapter 3 discusses methodological considerations related to assessing evidence on potassium and sodium intake and their implications for establishing the DRIs for these nutrients. Part II (Chapters 4–7) provides the committee's evaluation of the evidence and its determination of DRIs for potassium. Chapter 4 presents the potassium DRIs for adequacy, Chapter 5 presents the potassium DRIs for toxicity, and Chapter 6 summarizes evidence on the relationship between potassium intake and chronic disease risk and explains the committee's determination regarding potassium DRIs based on chronic disease. Chapter 7 compares the potassium DRIs established in this report with current intake levels in the U.S. and Canadian populations, characterizes risk, and describes public health implications and special considerations of the potassium DRI values. Part III (Chapters 8–11) provides the committee's evaluation of the evidence and its derivation of the DRIs for sodium, following the same structure as Part II. Chapter 8 presents the sodium DRIs for adequacy, Chapter 9 presents the sodium DRIs for toxicity, Chapter 10 presents the sodium DRIs based on chronic disease, and Chapter 11 compares the sodium DRIs established in this report with current intake levels in the U.S. and Canadian populations, characterizes risk, and describes public health implications and special considerations of the sodium DRI values. Part IV (Chapter 12) outlines knowledge gaps and research needs to advance understanding of the role of potassium and sodium intake on health and chronic disease, and offers the committee's suggestions for enhancing the DRI process.
REFERENCES
- HHS (U.S. Department of Health and Human Services). The Surgeon General's report on nutrition and health. Washington, DC: Government Printing Office; 1988.
- HHS. Nutrient assessment for DRI review. 2018. [October 17, 2018]. https://health
.gov/dietaryguidelines /dri /nutrient-assessment.asp. - IOM (Institute of Medicine). How should the Recommended Dietary Allowances be revised? Washington, DC: National Academy Press; 1994. [PubMed: 25101385]
- IOM. Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: The National Academies Press; 2005.
- IOM. Dietary Reference Intakes: The essential guide to nutrient requirements. Washington, DC: The National Academies Press; 2006.
- IOM. The development of DRIs 1994–2004: Lessons learned and new challenges: Workshop summary. Washington, DC: The National Academies Press; 2008.
- IOM. Strategies to reduce sodium intake in the United States. Washington, DC: The National Academies Press; 2010. [PMC free article: PMC3042781] [PubMed: 22043452]
- IOM. Dietary Reference Intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed: 21796828]
- IOM. Sodium intake in populations: Assessment of evidence. Washington, DC: The National Academies Press; 2013. [PMC free article: PMC3884094] [PubMed: 24425717]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Guiding principles for developing Dietary Reference Intakes based on chronic disease. Washington, DC: The National Academies Press; 2017. [PubMed: 29200241]
- Newberry SJ, Chung M, Anderson CAM, Chen C, Fu Z, Tang A, Zhao N, Booth M, Marks J, Hollands S, Motala A, Larkin JK, Shanman R, Hempel S. Sodium and potassium intake: Effects on chronic disease outcomes and risks. Rockville, MD: Agency for Healthcare Research and Quality; 2018. [PubMed: 30125063]
- NRC (National Research Council). Recommended Dietary Allowances. Washington, DC: National Academy Press; 1941.
- NRC. Diet and health: Implications for reducing chronic disease risk. Washington, DC: National Academy Press; 1989. [PubMed: 25032333]
- WHO (World Health Organization). A model for establishing upper levels of intake for nutrients and related substances Report of a joint FAO/WHO Technical Workshop on nutrient risk assessment. Geneva, Switzerland: WHO; 2006. [PubMed: 17310857]
- Yetley EA, MacFarlane AJ, Greene-Finestone LS, Garza C, Ard JD, Atkinson SA, Bier DM, Carriquiry AL, Harlan WR, Hattis D, King JC, Krewski D, O'Connor DL, Prentice RL, Rodricks JV, Wells GA. Options for basing Dietary Reference Intakes (DRIs) on chronic disease endpoints: Report from a joint US-/Canadian-sponsored working group. American Journal of Clinical Nutrition. 2017;105(1):249S–285S. [PMC free article: PMC5183726] [PubMed: 27927637]
Footnotes
- 1
For consistency throughout this report and in alignment with the terminology used in the AHRQ Systematic Review, the committee uses the term strength of the evidence instead of quality of the evidence or certainty of the evidence when describing the grading of the evidence used to derive DRIs based on chronic disease. A description of the guidance provided in the Guiding Principles Report (NASEM, 2017) on using the strength of the evidence and the committee's application of that guidance in its review of the evidence on potassium and sodium is provided in Chapter 2.
- 2
A meta-analysis is a statistical analysis that combines the results of multiple scientific studies. Its interpretation is complicated by the presence of heterogeneity among the studies. Observed differences in the intervention effect between the studies could be attributable to variability in clinical diversity (the participants, interventions, and outcomes studied) and/or methodological diversity (differences in study design and risk of bias).
- Introduction - Dietary Reference Intakes for Sodium and PotassiumIntroduction - Dietary Reference Intakes for Sodium and Potassium
- Sgcd [Arvicanthis niloticus]Sgcd [Arvicanthis niloticus]Gene ID:117710634Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...