NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse; Phillips JK, Ford MA, Bonnie RJ, editors. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington (DC): National Academies Press (US); 2017 Jul 13.

Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use.
Show detailsOver the past 25 years, the United States has experienced an unprecedented increase in opioid use disorder (OUD), opioid overdose, and other opioid-related harms. As of 2015, 2 million Americans aged 12 years or older had an OUD involving prescription opioids, and about 600,000 had an OUD involving heroin, an illicit opioid (HHS, 2016a). Drug overdose, driven primarily by opioids, is now the leading cause of unintentional injury death in the United States (more than 60 percent of overdose deaths in 2015 involved a prescription or illicit opioid) (Rudd et al., 2016). This increase in opioid-related deaths has occurred in tandem with an equally unprecedented increase in prescribing of opioid medications for purposes of pain management.
Millions of Americans experience acute and/or chronic painful conditions each year, and many of them are prescribed opioids. The vast majority of these patients do not misuse these drugs. Yet the pain-relieving and other effects of opioids (e.g., the feelings of pleasure, relaxation, and contentment opioids can produce) (NIDA, 2017) may lead to an overreliance on these drugs in many patients and to misuse and OUD in others. Moreover, many lawfully dispensed opioids make their way into the hands of people for whom they were not intended, including participants in illicit markets. As a result, the harms associated with use of prescription opioids (including OUD, overdose, and death) affect not only the patients with pain themselves but also their families, their communities, and society at large. The purpose of this report is to assess the nation's response to what is, by any measure, a grievous public health problem.
STUDY CHARGE
When the U.S. Food and Drug Administration (FDA) approved the opioid analgesic OxyContin in 1995, the drug had not been shown to be more efficacious or safe than short-acting oxycodone, which was already on the market. The idea promoted by OxyContin's manufacturer was that it was less likely to lead to addiction and misuse because of its time-release formulation. Yet, as discussed below, OxyContin was widely diverted, and many people became addicted to it. In 2013, the FDA approved Zohydro ER (extended-release) (hydrocodone bitartrate), an opioid without abuse-deterrent properties, although several abuse-deterrent formulations (ADFs) were by then available. The approval of this drug exacerbated frustration among some stakeholders that the societal impacts of opioids were not being sufficiently accounted for. In 2014, the FDA approved an ADF version of Zohydro to replace the original version.
In the wake of these decisions and in light of concerns about the growing opioid problem, the FDA launched an Opioids Action Plan in early 2016. In this plan, the agency described actions it would take in its role as the federal agency responsible for protecting the public's health by ensuring the efficacy and safety of drugs in the United States (Califf et al., 2016; FDA, 2016a,b). The actions outlined in the FDA plan include the following:
- Expand the use of advisory committees, including by
- convening an expert advisory committee before approving any new drug application for opioids without abuse-deterrent properties;
- consulting an advisory committee on ADFs when they raise novel issues; and
- assembling and consulting with a pediatric advisory committee regarding a framework for pediatric opioid labeling before any new labeling is approved.
- Develop changes to immediate-release (IR) opioid labeling, including additional warnings and safety information incorporating elements similar to the ER/long-acting (LA) opioid labeling, to give providers better information about the risks of opioids and how to prescribe safely.
- Strengthen the requirements for drug companies to generate postmarket data on the long-term impact of ER/LA opioids.
- Expand access to and encourage the development of ADFs of opioid products.
- Support better treatment by making naloxone more accessible and supporting the U.S. Centers for Disease Control and Prevention (CDC) guideline for prescribing opioids for chronic pain (discussed later in this chapter) (Dowell et al., 2016).
- Reassess the risk-benefit approval framework for opioids to incorporate risks of opioids to patients as well as to others who obtain them (FDA, 2016a,b).
As part of efforts to implement its Opioids Action Plan, the FDA asked the National Academies of Sciences, Engineering, and Medicine (the National Academies) to establish an ad hoc committee to advise the agency on the development of “a regulatory framework for opioid review, approval, and monitoring that balances individual need for pain control with considerations of the broader public health consequences of abuse and misuse” (Califf et al., 2016). This specific task was embedded in a broad charge (see Box 1-1). Specifically, the committee was asked to provide an update on the state of the science of pain research, care, and education since publication of the 2011 Institute of Medicine (IOM) report Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (IOM, 2011), including the evolving role of opioids in pain management and practices for reducing their misuse; to characterize the epidemiology of the opioid epidemic; and to review the evidence on approaches for addressing the problem. Based on its review of the evidence, the committee was to identify regulatory actions the FDA can take to address the opioid epidemic, with a focus on the agency's development of a formal method (a regulatory framework) for incorporating the broader public health impacts of opioids into its future opioid approval decisions. The committee also was asked to outline steps that can be taken by other stakeholders (e.g., prescribers; professional societies; federal, state, and local government agencies). In addition, the committee was charged to identify important research questions that need to be addressed to assist the FDA with the development of its regulatory framework.
In spring 2016, the National Academies convened an 18-member committee to carry out this task. Members included individuals with expertise in pain management, basic pain research, epidemiology, medical anthropology, substance use disorder (SUD), nursing, law, drug development, public health, health policy and policy modeling, and decision science. Two consultants with expertise in health care and food and drug law were appointed to contribute to the regulatory components of this report.
BOX 1-1
Statement of Task.
STUDY APPROACH
The committee conducted an extensive review of the scientific literature relevant to its statement of task. This literature review entailed English-language searches of a number of databases, including the Cochrane Database of Systematic Reviews, Embase, Google Scholar, Medline, PubMed, Scopus, and Web of Science. In addition to research published in peer-reviewed journals and books, the committee reviewed reports issued by government agencies and other organizations.
FDA representatives provided the committee with a number of background materials describing the agency's current processes and activities related to regulation of prescription drugs, including opioids. Among these materials were FDA guidance documents, presentations from FDA science board and advisory committee meetings, and research articles.
In addition, the committee held two public workshops to hear from researchers and agency representatives on topics germane to its task. The first workshop featured presentations on and discussion of topics relevant to the first four bullet points in the committee's statement of task (see Box 1-1); these presentations are summarized in a Proceedings of a Workshop—in Brief titled Pain Management and Prescription Opioid-Related Harms: Exploring the State of the Evidence (NASEM, 2016). The second workshop focused on the regulatory aspects of the committee's charge, including how the FDA might incorporate public health considerations into its regulatory framework for evaluation of prescription drugs.
Additional detail on the committee's literature search and workshops can be found in Appendix A.
DEFINITIONS AND TERMINOLOGY
In recent years, several factors have increased attention to the language of SUD. Patient advocacy groups have long advocated for language describing SUD that avoids stigma and negative stereotypes. In 2013, the fifth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-5) replaced the categories of “abuse” and “dependence” with the single term “substance use disorder.” This change led major addiction journals to publish guidelines for clinical, nonstigmatizing language that is viewed as acceptable terminology for manuscripts. On October 4, 2016, the Office of National Drug Control Policy (ONDCP) released a guidance document titled Changing the Language of Addiction (ONDCP, 2017). And in a related effort, the American Society of Addiction Medicine (ASAM) proposed a series of definitions aimed at the development of a vocabulary that is humanizing, nonstigmatizing, medically defined, and precise. This proposed terminology is a partial basis for the definitions presented in Box 1-2, which reviews both acceptable language and language that has been identified as no longer acceptable.
BOX 1-2
Key Definitions.
STUDY CONTEXT
Historical Context
Opioids have been used for medicinal and recreational purposes for millennia. While the use of opioids for treatment of acute severe pain has generally been accepted, their use for managing chronic noncancer pain has been controversial since the 19th century, with the popular view shifting over the decades between broad acceptance and a more restrictive perspective (Rosenblum et al., 2009). The tension between the desire to make opioids available to those who may benefit from them and the recognition that opioids are addictive drugs with societal consequences began with medical developments that occurred during the 1800s (Booth, 1986; Musto, 1999; Rosenblum et al., 2009). These developments included the extraction of morphine from opium in 1803 and the development of the hypodermic needle (which can be used to inject morphine to relieve neuralgic pain) in the 1850s (Rosenblum et al., 2009). Morphine was used widely for pain management during the American Civil War, and many soldiers developed OUD. With few effective alternatives, moreover, many medical professionals used morphine to treat chronic pain conditions. This and the nonmedical use of opioids were major drivers of an opioid addiction epidemic that took place in the latter 19th century (Courtwright, 2015).
By the late 1800s, scientists were starting to recognize the problem of OUD, and a policy response began to emerge. What is thought to be the first accurate and comprehensive description of addiction to morphine was produced in 1877. In hopes of developing a less addictive alternative to morphine, heroin (diacetylmorphine) was synthesized in 1874 (although it was later found to be more potent than morphine) (Rosenblum et al., 2009). Medical professionals became increasingly critical of the use of opioids to treat pain and lobbied successfully for state and local laws to control the sale of opioids and other narcotics. Consumption of medicinal opioids declined as a result (Courtwright, 2015).
Reform efforts continued in the early 20th century. The Harrison Narcotics Act, enacted by Congress in 1914, required persons who imported, produced, sold, or dispensed opium-based drugs (as well as coca-based drugs) to register, pay a tax, and keep detailed records that officials could use in enforcing laws to restrict opioid transactions to legitimate medical channels. This act had the effect of criminalizing the use of opium for nonmedical purposes (Courtwright, 2015; Hoffman, 2016).3 The use of heroin for medicinal and other purposes was specifically banned by the Heroin Act, enacted by Congress in 1924.
The consensus among medical professionals for most of the 20th century was that opioids should not be used for the management of chronic pain because of the lack of evidence regarding their effectiveness for this type of pain and the risk of OUD (Rosenblum et al., 2009). Research aimed at developing new and potentially less addictive opioids continued, however, and Percocet and Vicodin—which combined semisynthetic opioids with acetaminophen—became available in the 1970s for relief of moderate to moderately severe pain. These and most other prescription opioids are now regulated under the Controlled Substances Act (CSA) of 1970 as Schedule II drugs—those with a “high potential for abuse which may lead to severe psychological or physical dependence” (DEA, 2017b).4
Liberalization of Prescribing in 1990s
Medical practice in the United States began to shift markedly toward more liberal use of opioids for chronic noncancer pain following the development and marketing of new formulations of opioid drugs in the 1990s (Compton and Volkow, 2006; Rosenblum et al., 2009). As noted earlier, in 1995 the FDA approved OxyContin (oxycodone controlled-release), which allowed dosing every 12 instead of every 4 to 6 hours (FDA, 2017c). The drug's manufacturer (Purdue Pharma) marketed it aggressively to providers and patients in the years following its release to the market in 1996. Purdue claimed in some of its promotional materials that the risk of addiction to the drug was small (Van Zee, 2009).
Around the same time, there was growing recognition in the medical community that many individuals with chronic pain were being treated inadequately (Pokrovnichka, 2008). In 1996, the American Academy of Pain Medicine and American Pain Society issued a joint consensus statement titled The Use of Opioids for the Treatment of Chronic Pain, describing potential benefits of using opioids for management of chronic (including noncancer) pain (Haddox et al., 1997; Hoffman, 2016). Advocates representing the interests of pain patients suggested that pain be considered a “fifth vital sign” in an effort to improve pain assessment and treatment (Campbell, 1996), and some health care organizations incorporated this concept into guidelines and clinical practice (Mularski et al., 2006). There were also concerted efforts by pain specialists to persuade state medical boards and state legislatures to remove legal impediments to medically accepted pain treatment (Hoffman, 2016).5 This shift in professional understanding was accompanied by a public campaign to call public and professional attention to the prevalence of pain and its seriousness as a public health problem.
Congress declared 2001–2011 the “Decade of Pain Control and Research” (Brennan, 2015). The 2010 Patient Protection and Affordable Care Act (ACA) directed the U.S. Department of Health and Human Services (HHS) to work with the IOM to increase recognition of pain as a public health problem (IOM, 2011). In response, HHS, through the National Institutes of Health (NIH), commissioned an IOM committee to review the science on pain and recommend actions to advance the field. The resulting report, Relieving Pain in America, provided a blueprint for “transforming the way pain is understood, assessed, treated, and prevented” (IOM, 2011, p. 2).
In the context of Purdue's substantial promotional expenditures and these changing professional attitudes, sales of OxyContin rose from $48 million in 1996 to more than $1 billion by 2000 (Van Zee, 2009). Sales of prescription opioids are estimated to have quadrupled between 1999 and 2010 (CDC, 2011), driven in part by OxyContin during the early portion of this period (GAO, 2003). However, problems began to emerge around 2000, with reports of widespread diversion, tampering, and misuse of OxyContin (Cicero et al., 2005; GAO, 2003; Hoffman, 2016). In response, the FDA changed the OxyContin label in 2001 “to add and strengthen warnings about the drug's potential for abuse and misuse” and in 2003 issued a warning letter to the manufacturer regarding promotional materials that omitted and minimized the drug's safety risks (FDA, 2017c).6 The U.S. Drug Enforcement Administration (DEA) prosecuted many physicians for illegal distribution of OxyContin (Hoffman, 2016).7
Nonetheless, sales of prescription opioids continued to increase (Pan, 2016). Data from the National Prescription Audit show that the number of opioid prescriptions dispensed from U.S. outpatient retail pharmacies for all approved and marketed ER/LA and some of the most common IR opioid analgesics grew from 148 million in 2005 to 206 million by 2011. Opioid dispensing during this period was driven primarily by IR opioids (which work quickly and often are prescribed for short-term, intermittent, or “breakthrough” pain) rather than ER/LA opioids such as OxyContin (see Figure 1-1).8 Sales of OxyContin increased from just over $1 billion in 2000 to $1.84 billion in 2003 and then declined in the wake of the FDA actions described above until 2006, after which there was another increase in sales until 2010 (Public Citizen, 2007).
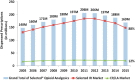
FIGURE 1-1
Nationally estimated number of prescriptions dispensed for extended-release/long-acting (ER/LA) and selected immediate-release (IR) opioid analgesics (oral solids and transdermal products) from U.S. outpatient retail pharmacies, 2005–2015. *ER/LA (more...)
Public Health Consequences
During the years coinciding with the growth in opioid prescribing, the United States experienced an increase in deaths from opioid overdose and in admissions to treatment associated with opioid use. According to CDC data, there was a 1.9-fold increase in the total number of deaths from prescription opioids (excluding nonmethadone synthetics) between 1999 and 2011 (see Figure 1-2). While the number of overdose deaths from prescription opioids remained relatively stable between 2011 and 2015, overdose deaths from illicit opioids (e.g., heroin and synthetic opioids such as fentanyl) continued to increase, related in part to a growing number of people with OUD in connection with prescription opioids. Overdose deaths from illicit opioids increased rather steadily during 1999 to 2015, growing 6.4-fold over that period (see Figure 1-2). Poisoning, driven largely by opioids, became the leading cause of death due to injury in the United States in 2008, surpassing motor vehicle crashes (Warner et al., 2011). The annual incidence of hospitalization for prescription opioid poisoning among children and adolescents aged 1–19 increased 165 percent (from 1.4 to 3.7 per 100,000) between 1997 and 2012 (Gaither et al., 2016). Between 2003 and 2013, the proportion of admissions to treatment associated primarily with nonheroin opioid use and heroin use increased from 3 to 9 percent and 15 to 19 percent, respectively (SAMHSA, 2015).
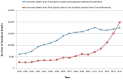
FIGURE 1-2
Number of overdose deaths from prescription and illicit opioids, United States, 1999–2015. SOURCE: NCHS, 2016.
Policy Responses
By the end of the first decade of the 21st century, alarm about the opioid epidemic was growing in public health circles. An increasing number of medical organizations were urging greater caution in prescribing opioids in light of the growing opioid problem and the lack of evidence that the drugs are effective for long-term pain management (VonKorff et al., 2011). At the federal level, in 2009, the FDA held public and stakeholder meetings to discuss opioid-related harms; partnered with the Substance Abuse and Mental Health Services Administration (SAMHSA), the DEA, and others on efforts to improve the safe use and disposal of opioids; and launched a Safe Use Initiative to reduce preventable harms from opioids and other drugs. In 2010 the agency approved an ADF of OxyContin (FDA, 2017c). During approximately 2013–2015, ONDCP and HHS ramped up efforts to reduce OUD and opioid overdose, including the creation of an HHS opioid initiative in 2015 (HHS, 2015). CDC's 2016 Guideline for Prescribing Opioids for Chronic Pain explicitly declares that nonpharmacologic and nonopioid therapies are preferred for treating chronic pain (Dowell et al., 2016). And in December 2016, the U.S. Congress passed the 21st Century Cures Act, which included $1 billion in funding over 2 years for grants to states targeting opioid prevention and treatment activities.
State and local governments also have scaled up efforts to identify problematic prescribing (e.g., via prescription drug monitoring programs [PDMPs], discussed in Chapter 5), prevent diversion of prescription opioids, and increase access to naloxone and to treatment for OUD. Some jurisdictions have declared public health emergencies (e.g., Massachusetts Department of Public Health, 2014; Virginia Department of Health, 2016).
In the context of these federal and state policy initiatives, the total number of prescriptions for opioid analgesics dispensed from outpatient retail pharmacies decreased between 2012 and 2015.9 Large health care providers and professional associations also have recently suggested that pain no longer be considered a vital sign (Frieden, 2016; Lowes, 2016). Some have suggested that routine pain assessment is not in the best interest of providers and may contribute to overprescribing (Lowes, 2016).
International Context
Historically, the United States has consumed a large majority of the world's supply of opioid drugs. An older figure that continues to be cited is that approximately 80 percent of the world's supply of opioid drugs is consumed in the United States (Manchikanti and Singh, 2008). According to another estimate, 90 percent of the world's supply of morphine, fentanyl, and oxycodone was used in the United States, Canada, Australia, and New Zealand in 2009, and in that same year, the United States consumed 83 and 99 percent of the world's oxycodone and hydrocodone, respectively (Hauser et al., 2016). Based on available data (UNODC, 2017), other countries, including Mexico and countries in Central and South America, Africa, and Asia, appear to have a considerably lower prevalence of past-year use of both prescription and illicit opioids, although this does not necessarily mean that these countries are free of problems related to opioids.
Consumption of opioid drugs has increased globally since the 1980s. Data indicate that in more recent decades, increases in consumption have been highest in the United States and to a lesser extent in other industrialized nations. For example, during 2000–2010, opioid consumption increased 400 percent in the United States, compared with 65 percent in Great Britain and 37 percent in Germany (Hauser et al., 2014). In Australia, where the prevalence of opioid use also is high, opioid dispensing increased nearly four-fold between 1990 and 2014 (from 4.6 to 17.4 defined daily doses/1,000 population/day) (Karanges et al., 2016). Spain saw a 14-fold increase in opioid daily doses between 1992 and 2006 (Garcia del Pozo et al., 2008).
The responses in countries experiencing high rates of opioid misuse, OUD, and opioid overdose have varied. Some are noteworthy for their public health orientation. In the Canadian province of British Columbia (Canada has the second highest rate of opioid consumption after the United States), harm reduction strategies implemented to reduce opioid overdose included making the opioid overdose reversal drug naloxone available outside of pharmacies without a prescription and opening supervised injection facilities (SIFs) (British Columbia was the first region in North America to open a SIF, in 2003) (Voon, 2016). The British Columbia Ministry of Health also issued guidelines for the clinical management of OUD to foster improved linkage to medically supervised treatment (Dunlap and Cifu, 2016). SIFs, which have been found to be associated with reductions in syringe sharing and overdose fatality (Kerr et al., 2005; Marshall et al., 2011), are operating as well in several other countries that have experienced significant opioid misuse problems, including Spain, Australia, and Germany, and are now being considered in the United States.
Some countries have reduced criminalization of drug use, with positive results. Portugal, while not having opioid-related problems at the levels seen in other countries, became the first country to decriminalize the possession and use of drugs in 2001, making these violations administrative as opposed to criminal offenses (Greenwald, 2009). Individuals who are addicted to heroin or other drugs are offered access to treatment, which is widely available through health centers, hospitals, and pharmacies, as well as to needle exchange and other services. Since these changes were implemented, the country has seen more people enter treatment, and HIV transmission rates have declined among injection drug users (EMCDDA, 2016).
The United States' response to the opioid epidemic also has taken on an increasingly public health focus. Examples include efforts to make OUD treatment, naloxone, syringe exchange, and other services more widely available, and the promulgation of guidelines for prescribers that emphasize greater caution in opioid prescribing and recommend referral to evidence-based treatment for patients with OUD. As discussed in this report, these strategies are at various stages of implementation and evaluation.
Statutory Context
Opioid regulation lies at the intersection of two federal statutes, each with its roots in the early 20th century. The first is the Food, Drug, and Cosmetic Act (FDCA), a successor to the groundbreaking Pure Food and Drug Act of 1906, which now requires manufacturers of medical drugs and devices to prove that they are safe and effective for their intended uses before they may be marketed to consumers. The second applicable statute is the CSA, enacted in 1970 as a successor to the Harrison Narcotics Act of 1914, mentioned above. The CSA was designed to provide an overarching framework for tight federal regulation, including both public health oversight and aggressive enforcement, for all drugs with “potential for abuse,” whether or not intended for medical use. Previously, those functions had operated relatively autonomously, with drug development and prescription control under the FDA, and enforcement responsibility originally lodged in the U.S. Department of the Treasury and later transferred to the U.S. Department of Justice (Spillane, 2004). Enforcement duties under the CSA are now exercised by the DEA, but the CSA also retains a significant role for HHS, usually acting through the FDA, in the regulation of controlled substances with medical uses.
The CSA created tiered levels of control and reporting responsibilities based on the potential danger posed by a given drug, and established a structure for coordinating regulatory and enforcement action (Spillane, 2004). The act also was designed to create a “big tent” for all drugs that might be subject to misuse and to explicitly subject such drugs as barbiturates and amphetamines to the same control as narcotics. Each controlled substance is assigned to a specific schedule. Schedule I substances are strictly limited and may be used only in some highly controlled research contexts, if at all. Schedule II substances are subject to production quotas and registry requirements for importers and exporters. Drugs assigned to the lower schedules are subject to progressively diminished levels of control. A controlled substance may be prescribed only for a “legitimate medical purpose” by a practitioner licensed by the DEA “acting in the usual course of his professional practice.” The CSA gives the DEA the power to revoke licensure when a physician is determined to have violated that standard, and offending practitioners may be subject to criminal prosecution.
The primary focus of the CSA was ambiguous from the outset: the Nixon administration saw it principally as a way to control street use of illicit drugs, while its congressional sponsors saw it as a vehicle for limiting overproduction and overprescription of legally marketed drugs based on balancing the dangers of abuse against the health benefits of legitimate medical use (Spillane and McAllister, 2003, p. S8). To its congressional sponsors, the CSA represented a key step in the direction of a national public health approach to drug abuse and addiction. The second step, taken in the Drug Abuse Office and Treatment Act of 1972, established a Special Action Office for Drug Abuse Prevention in the White House and enacted sweeping federal protection of the confidentiality of SUD treatment records that continues to serve as a centerpiece of national policy.
The DEA was created in 1973 to carry out the U.S. Department of Justice's responsibility for enforcing the CSA (Senate Committee on Government Operations, 1973, pp. 5–6). It was believed that making one agency accountable would “maximize coordination between Federal investigation and prosecution efforts.” The new agency was to draw on Federal Bureau of Investigation expertise with organized crime, and to provide a single focal point for enforcement with state, local, and international authorities (Senate Committee on Government Operations, 1973, pp. 5–6). The DEA enforces both the criminal and noncriminal regulatory requirements of the CSA, but it does so as a law enforcement agency; it is not designed to function as a public health agency, nor does it pretend to be one (DEA, 2017a).
Over the four and a half decades since its passage, the CSA has been amended many times, usually to increase law enforcement authority. The Comprehensive Crime Control Act of 1984 and the Anti-Drug Abuse Acts of 1986 and 1988 added provisions to deal with synthetic compounds and new enforcement mechanisms, such as forfeiture provisions, and introduced mandatory minimum sentences. The Illicit Drug Anti-Proliferation Act of 2003 amended the CSA to deal with MDMA (3,4-methylenedioxy-methamphetamine, or ecstasy) and other club drugs. The Ryan-Haight Act of 2008 amended the CSA to regulate online pharmacy distribution. The Secure and Responsible Drug Disposal Act of 2010 requires the DEA to establish programs for voluntary disposal of controlled substances that are no longer required by patients. And the Synthetic Drug Abuse Prevention Act of 2012 not only mandated restrictive scheduling for various synthetic drugs but also streamlined the scheduling process so that newly approved drugs could enter the market more quickly.
Among the many important issues that have surfaced during the opioid crisis are whether the public health goals of the CSA envisioned by its architects have been achieved, and whether regulatory activities carried out by the FDA and the DEA under the FDCA and the CSA have been suitably coordinated and harmonized. One issue of particular interest in the context of this report is surveillance. As a key component of its public health aims, the CSA mandated the collection of epidemiologic data on use and abuse of the drugs controlled by the act and on other substances that might warrant control. The first such effort, the Drug Abuse Warning Network (DAWN), created in 1972 and discontinued in 2011, revealed a problem that continues to this day: it is difficult to break down the data by specific drug products (Mansbach et al., 2010; Spillane, 2004), which is essential to determining the nature and level of misuse for specific substances. The discontinuation of DAWN in 2011 left a substantial gap in the nation's capacity to monitor, anticipate, and respond to the opioid epidemic as it unfolded.
Recent Federal Policy Initiatives
As noted above, the IOM's 2011 report Relieving Pain in America highlighted the public health significance of pain and the need for fundamental changes in pain policy and practice (IOM, 2011). The report details the landscape of pain in the United States of that time, including such key factors as its overall prevalence; its personal, economic, and social consequences; and the significant shortcomings of prevailing treatment approaches. The report also describes the status of some of the available pain treatment approaches, including pharmacologic options, injection-based interventions, surgery, rehabilitative strategies, psychological therapies, and complementary modalities. The report presents highlights of then-current knowledge about pain mechanisms and the impact of interacting comorbid conditions such as depression, anxiety, and SUD, as well as areas in which knowledge was critically lacking. While the report ably describes the contemporary state of the art, however, important advances have since occurred on many fronts.
One element of this committee's charge was to “provide an update on the state of the science of pain research, care, and education since the 2011 report and characterize the evolving role of opioid analgesics in pain management,” a task that the committee carries out in several chapters of this report. The subsections below summarize three major federal policy activities related to pain management and opioids that have taken place since the 2011 report was published and that provide additional context for the present study: the ongoing formulation of a National Pain Strategy, promulgation of a guideline for opioid prescribing under the auspices of the CDC, and ONDCP's development of a comprehensive plan for managing the opioid crisis.
National Pain Strategy
One of the principal recommendations of the 2011 IOM report was that HHS develop “a comprehensive population health-level strategy for pain prevention, treatment, management, and research” (IOM, 2011, p. 102). In response, the HHS assistant secretary requested that the Interagency Pain Research Coordinating Committee (IPRCC) develop a National Pain Strategy to provide a blueprint for transforming pain prevention, care, education, and research. After several years of work, the National Pain Strategy was published in 2016 (HHS, 2016b). The document's findings and recommendations fall into six primary areas: population research, prevention and care, disparities, service delivery and reimbursement, professional education and training, and public awareness and communication.
The National Pain Strategy highlights difficulties surrounding the use of opioids in pain management. Its recommendations include augmenting the use of population-level data to inform national policy on opioid use, including regulatory actions undertaken by the FDA and the DEA. Perhaps more significant, the Strategy lists as an objective, “Develop and implement a national educational campaign to promote safer use of all medications, especially opioid use, among patients with pain” (HHS, 2016b, p. 48). The document, however, makes no specific recommendations to the FDA.
The work of the IPRCC is far from complete. The committee, composed of 7 federal and 12 nonfederal members, is engaged in several ongoing tasks, including summarizing advances in pain research, identifying critical gaps in the research, and advising NIH and other federal agencies on how best to streamline research efforts and improve the collection and dissemination of information on pain research and treatment.
U.S. Centers for Disease Control and Prevention's Guideline for Prescribing Opioids for Chronic Pain
In parallel with the efforts of the IPRCC, the CDC issued its Guideline for Prescribing Opioids for Chronic Pain in 2016, offering a detailed set of recommendations for prescribing opioids to adults for chronic pain (Dowell et al., 2016). Specific issues addressed by the guideline include (1) when to consider opioids for chronic pain; (2) what types and doses of opioids to use, as well as when to consider tapering off the drugs; and (3) how to assess patient-specific risks. The CDC developed the guideline using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, and its recommendations are based on a systematic review of the scientific evidence, as well as consideration of benefits and harms, values and preferences, and resource allocation. The guideline was specifically developed for primary care clinicians, including physicians, nurse practitioners, and physician assistants, prescribing opioids to patients with chronic pain (>3 months' duration) in outpatient settings. It acknowledges the existence of other sets of opioid prescribing guidelines, such as those issued by the American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel and the U.S. Department of Veterans Affairs (Chou et al., 2009; VA and DoD, 2010). The CDC guideline, however, has the advantage of reflecting more recent data on the effectiveness and risks of prescription opioids. In addition to review of the direct clinical evidence and complementary contextual evidence, the CDC process engaged federal partners and other stakeholders, and entailed subjecting the guideline to peer review and publishing it for public comment prior to dissemination.
The guideline ultimately published provides 12 recommendations concerning the use of opioids for the management of chronic pain (see Box 5-3 in Chapter 5) (Dowell et al., 2016). The guideline generally can be regarded as more conservative than many previous sets of recommendations on this topic. Some of its specific provisions should be noted. First, the guideline stresses the general approach of using nonopioid and nonpharmacologic therapy for chronic pain. In fact, it stresses that opioids are not first-line medications for the treatment of chronic pain. This recommendation is based on the finding that nonpharmacologic therapies appear to have efficacy similar to that of pharmacologic therapies, at least for the first several months of treatment, as well as a superior long-term risk profile. Second, the guideline recommends that when opioid therapy is used, IR rather than ER/LA opioids be prescribed and at relatively low doses. The guideline generally recommends doses below 50 morphine milligram equivalents (MME)/day and suggests careful justification of doses above 90 MME/day. Finally, the guideline stresses the evaluation of risks prior to opioid initiation, careful ongoing evaluation of those risks, and regular assessment of response to the therapy. The guideline specifically mentions the potential for adverse interactions between opioids and such sedatives as benzodiazepines as it is now clear that such interactions contribute to many opioid-related deaths (Park et al., 2015).
Some have cautioned that the CDC guideline may have unintended consequences in terms of unduly limiting access to opioid medications (e.g., Guerriero and Reid, 2016; Pergolizzi et al., 2016). It should be noted, however, that additional publications providing separate analyses of the use of opioids for low back pain, a common indication, have become available since the CDC guideline was published (Abdel Shaheed et al., 2016; Qaseem et al., 2017). Consistent with the CDC findings and recommendations, these more recent analyses also find little evidence of meaningful pain relief provided by opioids for low back pain.
Office of National Drug Control Policy's Comprehensive Plan
ONDCP was created in 1989 by the Anti-Drug Abuse Act of 1988 to coordinate activities of the DEA, the FDA, the CDC, the National Institute on Drug Abuse (NIDA), and SAMHSA. In 2011, ONDCP issued a four-pronged comprehensive plan for managing the opioid crisis aimed at balancing the need to curb opioid-related harms with the needs of individuals for adequate pain treatment (ONDCP, 2011, p. 2).
The first prong entailed educating the public and health care providers. Practitioners seeking DEA registration for prescribing controlled substances would have been required to receive training on responsible opioid prescribing practices. Opioid REMS would have been required to include effective educational materials, and efforts would have been made to enhance education in health professional schools as well as continuing education through state and federal agencies. Second, the plan called for improved monitoring through state-authorized PDMPs. The plan noted that standardized monitoring programs with enhanced interoperability (with each other and with national monitoring systems) and access were needed in all 50 states. The plan also encouraged legal changes to allow more sharing of clinical data and innovative use of electronic health records. Third, the plan recommended new actions to increase environmentally responsible disposal of prescription drugs to prevent misuse and diversion. Finally, the plan recommended methods for improving enforcement, including a Model Pain Clinic Regulation Law and improved coordination among federal, state, and local agencies for investigation of illicit trafficking and illegitimate prescribing and prosecution of offenders (ONDCP, 2011).
In 2014, the DEA issued a new rule that largely addressed the goals of the 2011 ONDCP plan's drug disposal requirements. The DEA also has created a DEA 360 program, developed “Tactical Diversion Squads,” and formulated the HIDTA (High Intensity Drug Trafficking Areas) Heroin Response Strategy, all of which are designed to improve enforcement while taking a “balanced public health and public safety approach” (White House, 2016, p. 68). However, the ONDCP plan's education goals, which would have linked DEA registration and training requirements, have not been implemented, and the REMS education goals have been underutilized. ONDCP has pointed to the new CDC practice guideline as evidence of progress in education (White House, 2016, p. 66), but adherence to those recommendations is voluntary. Similarly, while progress has been made in expanding PDMPs—now in 49 states—and new federal monitoring plans have been developed, a lack of standardization and interoperability and poor access impede the effectiveness of these systems.
Ethical Context
The statement of task for this study (see Box 1-1) directed the committee to recommend policy actions by the FDA and other policy makers that would properly “balance the needs of pain patients and the [societal] need to address opioid misuse.” This deceptively simple statement entails many technical challenges related to measurement quantification that are explored in this report. However, it also exposes a genuine ethical quandary that is fundamental to this entire report: How exactly does a regulator (or this committee) weigh and balance, for any particular regulatory action limiting access to opioids, the otherwise avoidable suffering that patients with pain would experience against the harms, not only to those individuals and their families but also to society, that would be prevented by the restriction? The “societal need to reduce opioid misuse” is particularly challenging in ethical terms because much of the harm to society arising from opioid misuse is attributable to diversion of the prescribed drugs from lawful markets and to the operation of black markets. Are these two sets of needs morally commensurate? Are they convertible to a common metric?
The task is made somewhat easier if one recognizes that the point of contention regarding the use of opioids in serving the “needs of pain patients” focuses almost entirely on treatment of chronic noncancer pain. As long as the quantity prescribed, dispensed, and administered is suitably limited, there is little disagreement about the need for opioids for treatment of patients with acute pain within controlled settings such as hospitals (e.g., the perioperative use of opioids for many types of surgeries), or for treatment of patients with cancer or terminal conditions. The area of dispute concerns long-term use of take-home doses for chronic noncancer pain by people who are not terminally ill.
It is instructive to attempt to operationalize the balancing task at the policy level. On the one hand, the policy maker must quantify or otherwise characterize the aggregate reduction in pain experienced by patients if opioids are prescribed and used for these chronic indications. As discussed in Chapter 2, this is a difficult task because of a lack of data on the effectiveness of opioid therapy for long-term (>1 year) outcomes related to pain, function, and quality of life (Chou et al., 2015; Dowell et al., 2016)—notwithstanding the reported experience of many patients and their providers who believe the drugs are beneficial. On the other hand, policy makers must quantify or otherwise characterize the harms that would not have occurred had prescribing of opioids been more restricted. These harms include death from overdose and other harms to patients who become addicted to opioids in the course of treatment, and importantly, it also includes harms due to the misuse of drugs that have been diverted from lawful channels to people other than the patients to whom the drugs are prescribed.
This policy balance between benefits and harms inevitably involves many uncertain parameters requiring considerable speculation: the numbers of patients with pain who will be affected, the nature and intensity of the pain that will be experienced or mitigated under different sets of assumptions about access to the drugs, and the effect of more or less restrictive regulatory approaches on access to the drugs by persons other than the patients to whom they have been prescribed and the harms that might subsequently occur. Converting all these postulated impacts to a common metric, such as quality-adjusted life years (QALYs), would be one way to proceed, although this approach would require overcoming many technical challenges. Moreover, other outcomes at the societal level might be difficult to quantify, such as the impact of one or another policy on public trust in the medical profession and the health care system. Loss of confidence can arise from perceived overprescribing or perceived underprescribing.
This analytic approach of identifying, quantifying, and balancing relevant outcomes at the societal level is the only way policy makers can think clearly about such a complex issue and make their arguments transparent and open to critical review by others. However, one of the confounding features of the policy discourse on the regulation of opioids and opioid prescribing is that many physicians and patient advocates ground their arguments not in an aggregated balance of benefits and harms at the population level but in the patient-centered ethics of clinical medicine (ethics “at the bedside,” so to speak). When viewed from the perspective of an individual physician and an individual patient seeking treatment for chronic pain, regulations restricting access to opioids may be objectionable because they are perceived as unduly constraining the options available to physicians seeking to alleviate the suffering of each patient under their care. This ethical duty entails making an individualized judgment about each patient's needs, recognizing that the needs of a particular patient may differ from those of the “average” patient experiencing a particular type of pain; that the patient's response to treatment may differ from the “typical” response in relation to both specific risks and potential benefits; and that these effects in any particular case are difficult to quantify, especially when there is so little evidence about long-term use of opioids for chronic noncancer pain. From this perspective, the duty to exercise individualized clinical judgment lies at the heart of the physician–patient relationship. Individualized decision making is all the more important in the context of pain, given its inherently subjective nature, and in the context of the ethical paradigm of shared decision making.
In thinking about the task of balancing the aggregated needs of patients in pain at the societal level and the need to prevent harms associated with misuse of opioid analgesics, the committee was sensitive to the ethical tension between the population perspective of public health and the patient-centered perspective of clinical ethics. The bottom line is that these two perspectives address two different questions. The committee's charge was to answer the societal question: What should the FDA and other government entities do when acting to further society's collective interest? The committee was not charged with asking what physicians and other prescribers should do or what options they should have available for particular clinical indications. This does not imply, however, that the ethics of clinical medicine are irrelevant: the framework used by policy makers in balancing the aggregated needs of patients with pain against society's collective interest in preventing opioid-related harms must be sensitive to the impact of alternative policies on public confidence in the health care system, including trust in the physician–patient relationship.
STUDY SCOPE AND EMPHASIS AND REPORT ORGANIZATION
Study Scope and Emphasis
The breadth of the committee's charge posed several challenges. First, the charge envisioned two fairly distinct tasks—an update of the science of pain research, care, and education since the IOM's 2011 report, including the evolving role of opioids in pain management, and a “new” report summarizing the “state of the science” on the use and misuse of prescription opioids and on approaches for addressing the problem. The committee interpreted its charge as focusing primarily on the misuse of prescribed opioids, the occurrence of OUD, and the associated public health harms, with updates to the 2011 report being limited to those bearing on indications for opioid prescribing, alternatives to opioids for pain management, physician education, and priorities for research.
A second challenge was the multiple audiences for this report. The charge requested that the committee provide advice not only to the FDA but also to other policy makers and stakeholders. The committee understood that the FDA's primary reason for requesting this report was its desire for an expanded framework for review, approval, and monitoring of opioids that would encompass the societal harms resulting from opioid prescribing, and accordingly attempted to develop such a framework. However, the FDA knows it cannot address the opioid problem on its own, and its charge to the committee clearly invited a broader view of the report's intended audience. The committee chose to take this broader view because it was convinced that successful efforts to prevent, ameliorate, and minimize the public health harms associated with use and misuse of prescription opioids will require coordinated action at all levels of government and by a diverse array of stakeholder organizations.
A third challenge was that the committee was charged with addressing a complex, multifaceted problem that can be viewed through many lenses. The approach the committee took to carrying out this charge was shaped by the expertise of the its members and its interpretation of the charge. Accordingly, the committee focused on improving the treatment of pain and on responding to the policy challenges presented by the opioid epidemic. Many other relevant topics could have been included, such as why this epidemic has occurred. However, the committee was not directed to investigate the causes of the prescription opioid problem or to judge how it could have been avoided or ameliorated. Indeed, in its initial conversations with FDA officials, the committee was specifically advised that the purpose of this report was not to place blame for the current state of affairs.
Not surprisingly, however, questions about who bears responsibility for the current situation surfaced repeatedly in the committee's public workshops. Some observers, for example, suggested that the 2011 IOM report underemphasized then-emerging opioid-related harms as it highlighted the prevalence and cost of inadequately treated pain. Other speakers argued that the FDA has not been aggressive enough in its regulatory decisions, while still others directed attention to the systemic failures of the nation's health care system.
Nonetheless, the committee did not aim to assign responsibility for past mistakes. Its task was to review and assess approaches and actions that the FDA and others have taken, and could take, to resolve the problem and prevent such problems from arising in the future. To this end, the committee naturally posits a predictive model concerning what interventions might work. In so doing, it relies on a traditional multifactorial causal model commonly used in public health, encompassing considerations ranging from structural factors to individual susceptibility. Using this approach, certain hypotheses about causes of the epidemic are inescapable. For example, the data presented earlier in this chapter make a prima facie case that heavy promotion of opioid prescribing by drug manufacturers (including misleading claims by some) and substantially increased prescribing by physicians were key contributors to the increase in misuse, OUD, and accompanying harms.
It is also clear, however, that overprescribing was not the sole cause of the problem. While increased opioid prescribing for chronic pain has been a vector of the opioid epidemic, researchers agree that such structural factors as lack of economic opportunity, poor working conditions, and eroded social capital in depressed communities, accompanied by hopelessness and despair, are root causes of the misuse of opioids and other substances and SUD (Carpenter et al., 2016; Compton et al., 2014; Nagelhout et al., 2017). It was beyond the scope of the committee's task to review and offer recommendations for mitigating the effects of these underlying structural determinants of opioid misuse and OUD. Nonetheless, the committee believes it is extremely important to keep these determinants in mind while reading this report, which focuses largely, although not entirely, on the supply side of the equation (increased prescribing of opioids) rather than on the more complex structural and environment factors that contribute to the demand side of the equation.
Report Organization
This report is divided into six chapters. Part I, consisting of Chapters 2 and 3, updates the 2011 IOM report. Chapter 2 describes the scope of the problem of pain in the United States and the state of the science on pain management, with an emphasis on the evolving role of prescription opioids and other forms of treatment in pain management. Areas for future research on pain and its management and on OUD to assist the FDA with the development of a framework for opioid approval and monitoring are discussed in Chapter 3. Part II, consisting of Chapters 4, 5 and 6, characterizes the opioid epidemic and the nation's response to it. Chapter 4 describes the epidemiology of opioid use and misuse, OUD, overdose, and other harms from both prescription and illicit opioids (e.g., heroin). Chapter 5 reviews the evidence regarding the effectiveness of strategies being used to address the opioid epidemic and makes recommendations where indicated. Specific topics covered include regulating the types of products approved for use (e.g., ADFs); restricting legal access to approved drugs; modifying prescribing practices; providing patient education; increasing access to treatment for OUD; and reducing harms from opioid use, such as by providing naloxone to prevent opioid overdose and making clean needles available for injection drug users to reduce transmission of HIV and hepatitis C virus. Finally, based on content presented in earlier chapters, Chapter 6 outlines steps the FDA can take to improve its regulation of opioids, including an approach for improving incorporation of individual and public health risks and benefits into future FDA approval and monitoring of these drugs.
REFERENCES
- Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: A systematic review and meta-analysis. JAMA Internal Medicine. 2016;2016;176(7):958–968. [PubMed: 27213267]
- ASAM (American Society of Addiction Medicine). Public policy statement: Definition of addiction. Short definition of addiction. 2011. [May 1, 2017]. http://www
.asam.org/quality-practice /definition-of-addiction. - Booth M. Opium: A history. New York: St. Martin's Press; 1986.
- Brennan F. The U.S. Congressional “Decade on Pain Control and Research” 2001-2011: A review. Journal of Pain and Palliative Care Pharmacotherapy. 2015;29(3):212–227. [PubMed: 26458017]
- Califf RM, Woodcock J, Ostroff S. A proactive response to prescription opioid abuse. Special Report. New England Journal of Medicine. 2016;374:1480–1485. [PubMed: 26845291]
- Campbell JN. APS 1995 Presidential address. The Journal of Pain. 1996;5(1):85–88.
- Carpenter CS, McClellan CB, Rees DI. Economic conditions, illicit drug use, and substance use disorders in the United States. Journal of Health Economics. 2016;52:63–73. [PubMed: 28235697]
- CDC (U.S. Centers for Disease Control and Prevention). Vital Signs: Overdoses of prescription opioid pain relievers—United States, 1999-2008. Morbidity and Mortality Weekly Report. 2011;60(43):1487–1492. [PubMed: 22048730]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelster A, Mauskop A, O'Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miakowski C., American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal of Pain. 2009;10(2):113–130. [PMC free article: PMC4043401] [PubMed: 19187889]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Annals of Internal Medicine. 2015;162(4):276–286. [PubMed: 25581257]
- Cicero TJ, Inciardi JA, Muñoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002-2004. The Journal of Pain. 2005;6(10):662–672. [PubMed: 16202959]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug and Alcohol Dependence. 2006;81(2):103–107. [PubMed: 16023304]
- Compton WM, Gfoerer J, Conway KP, Finger MS. Unemployment and substance outcomes in the United States, 2002-2010. Drug and Alcohol Dependence. 2014;142:350–353. [PMC free article: PMC4127107] [PubMed: 25042761]
- Courtwright D. Preventing and treating narcotic addiction—A century of federal drug control. New England Journal of Medicine. 2015;373(22):2095–2097. [PubMed: 26605925]
- DEA (U.S. Drug Enforcement Administration). DEA mission statement. 2017a. [April 23, 2017]. https://www
.dea.gov/about/mission.shtml. - DEA. List of controlled substances. 2017b. [February 7, 2017]. https://www
.deadiversion .usdoj.gov/schedules. - Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. Morbidity and Mortality Weekly Report. 2016;65(No. RR-1):1–49. [PubMed: 26987082]
- Dunlap B, Cifu AS. Clinical management of opioid use disorder. Journal of the American Medical Association. 2016;316(3):338–339. [PubMed: 27434445]
- EMCDDA (European Monitoring Centre for Drugs and Drug Addiction). Portugal country overview. 2016. [March 17, 2017]. http://www
.emcdda.europa .eu/countries/portugal. - FDA (U.S. Food and Drug Administration). Fact sheet—FDA opioids action plan. 2016a. [January 5, 2017]. http://www
.fda.gov/NewsEvents /Newsroom/FactSheets/ucm484714 .htm. - FDA. FDA News Release. Califf, FDA top officials call for sweeping review of agency opioids policies. 2016b [January 5, 2017]; http://www
.fda.gov/NewsEvents /Newsroom/PressAnnouncements /ucm484765.htm. - FDA. A brief overview of Risk Evaluation and Mitigation Strategies (REMS). 2017a. [January 11, 2017]. FDA Basics Webinar. http://www
.fda.gov/AboutFDA /Transparency/Basics/ucm325201 .htm. - FDA. Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting opioid analgesics. 2017b. [June 4, 2017]. https://www
.fda.gov/Drugs /DrugSafety/InformationbyDrugClass /ucm163647.htm. - FDA. Timeline of selected FDA activities & significant events addressing opioid misuse & abuse. 2017c. [January 10, 2017]. http://www
.fda.gov/downloads /drugs/drugsafety /informationbydrugclass/ucm332288 .pdf. - Frieden J. Remove pain as 5th vital sign, AMA urged. 2016. [May 25, 2017]. https://www
.medpagetoday .com/meetingcoverage/ama/58486. - Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatrics. 2016;170(12):1195–1201. [PMC free article: PMC7245935] [PubMed: 27802492]
- GAO (U.S. General Accounting Office). Prescription drugs. OxyContin abuse and diversion and efforts to address the problem. 2003. [February 21, 2017]. http://www
.gao.gov/new.items/d04110.pdf. - Garcia del Pozo J, Carvajal A, Viloria JM, Velasco A, Garcia del Pozo V. Trends in the consumption of opioid analgesics in Spain. Higher increases as fentanyl replaces morphine. European Journal of Clinical Pharmacology. 2008;64(4):411–415. [PubMed: 18157671]
- Greenwald G. Drug decriminalization in Portugal: Lessons for creating fair and successful drug policies. 2009. [March 17, 2017]. https://www
.cato.org /publications/white-paper /drug-decriminalization-portugal-lessons-creating-fair-successful-drug-policies. - Guerriero F, Reid MC. New opioid prescribing guidelines released in the U.S.: What impact will they have in the care of older patients with persistent pain? Current Medical Research and Opinion. 2016;33(2):275–278. [PMC free article: PMC5472202] [PubMed: 27786538]
- Haddox JD, Joranson D, Angarola RT, Brady A, Carr DB, Blonsky ER, Burchiel K, Gitlin M, Midcap M, Payne R, Simon D, Vasudeyan S, Wilson P. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clinical Journal of Pain. 1997;13(1):6–8. [PubMed: 9084947]
- Hauser W, Petzke F, Radbruch L, Tolle TR. The opioid epidemic and the long-term opioid therapy for chronic noncancer pain revisited: A transatlantic perspective. Pain Management. 2016;6(3):249–263. [PubMed: 26988312]
- HHS (U.S. Department of Health and Human Services). HHS takes strong steps to address opioid-drug related overdose, death and dependence. 2015. [January 10, 2017]. https://www
.hhs.gov/about /news/2015/03/26 /hhs-takes-strong-steps-to-address-opioid-drug-related-overdose-death-and-dependence.html. - HHS. Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016a. (NSDUH Series H-51). HHS Publication SMA 16-4984.
- HHS. National Pain Strategy: A comprehensive population health-level strategy for pain. Washington, DC: HHS; 2016b. [June 27, 2017]. https://iprcc
.nih.gov /docs/HHSNational_Pain_Strategy.pdf. - Hoffman DE. Treating pain V. Reducing drug diversion and abuse: Recalibrating the balance in our drug control laws and policies. Saint Louis University Journal of Health Law & Policy. 2016;1:231–310.
- IOM (Institute of Medicine). Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011. [PubMed: 22553896]
- Karanges EA, Blanch B, Buckley NA, Pearson SA. Twenty-five years of prescription opioid use in Australia: A whole-of-population analysis using pharmaceutical claims. British Journal of Clinical Pharmacology. 2016;82(1):255–267. [PMC free article: PMC4917790] [PubMed: 26991673]
- Kerr T, Tyndall M, Li K, Montaner J, Wood E. Safer injection facility use and syringe sharing in injection drug users. Lancet. 2005;366(9482):316–318. [PubMed: 16039335]
- Lowes R. Drop pain as the fifth vital sign, AAFP says. 2016. [May 25, 2017]. http://www
.medscape.com /viewarticle/869169. - Manchikanti L, Singh A. Therapeutic opioids: A ten-year perspective on the complexities and complications of escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(2 Suppl.):S63–S88. [PubMed: 18443641]
- Mansbach RS, Schoedel KA, Kittrelle JP, Sellers EM. The role of adverse events and related safety data in the pre-market evaluation of drug abuse potential. Drug and Alcohol Dependence. 2010;112(3):173–177. [PubMed: 20817417]
- Marshall BD, Milloy MJ, Wood E, Montaner JS, Kerr T. Reduction in overdose mortality after the opening of North America's first medically supervised safer injecting facility: A retrospective population-based study. Lancet. 2011;377(9775):1429–1437. [PubMed: 21497898]
- Massachusetts Department of Public Health. Findings of the Opioid Task Force and Department of Public Health recommendations on priorities for investments in prevention, intervention, treatment and recovery. 2014. [February 21, 2017]. http://www
.mass.gov/eohhs /docs/dph/substance-abuse /opioid/report-of-the-opioid-task-force-6-10-14 .pdf. - Mularksi RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. Journal of General Internal Medicine. 2006;21(6):607–612. [PMC free article: PMC1924634] [PubMed: 16808744]
- Musto DF. The American disease: Origins of narcotic control. 3rd ed. New York: Oxford University Press; 1999.
- Nagelhout GE, Hummel K, de Goeij MCM, de Vries H, Kaner E, Lemmens P. How economic recessions and unemployment affect illegal drug use: A systematic realist literature review. International Journal of Drug Policy. 2017;44:69–83. [PubMed: 28454010]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Pain management and prescription opioid-related harms: Exploring the state of the evidence: Proceedings of a workshop—in brief. Washington, DC: The National Academies Press; 2016.
- NCHS (National Center on Health Statistics). National overdose deaths from select prescription and illicit drugs. 2016. [June 26, 2017]. https://www
.drugabuse .gov/related-topics /trends-statistics/overdose-death-rates. - NIDA (National Institute on Drug Abuse). How do opioids work? 2017. [May 25, 2017]. https://teens
.drugabuse .gov/teachers/mind-over-matter /opioids /how-do-opioids-work. - ONDCP (Office of National Drug Control Policy). Epidemic: Responding to America's prescription drug abuse crisis. 2011. [April 23, 2017]. https://www
.ncjrs.gov /pdffiles1/ondcp/rx_abuse_plan.pdf. - ONDCP. Changing the language of addiction. 2017. [April 23, 2017]. https://www
.whitehouse .gov/sites/whitehouse .gov/files/images /Memo%20-%20Changing %20Federal%20Terminology %20Regrading%20Substance %20Use%20and %20Substance%20Use%20Disorders.pdf. - Pan G. Challenges in assessing real world use and abuse of pain medicines. Mar, 2016. [January 11, 2017]. PowerPoint presentation. FDA Science Board Meeting. http://www
.fda.gov/downloads /AdvisoryCommittees /CommitteesMeetingMaterials /ScienceBoardtotheFoodandDrugAdministration /UCM489209.pdf. - Park TW, Saitz R, Ganoczy D, Illgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among U.S. veterans receiving opioid analgesics: Case-cohort study. British Medical Journal. 2015;350:h2698. [PMC free article: PMC4462713] [PubMed: 26063215]
- Pergolizzi JV Jr., Raffa RB, LeQuang JA. The Centers for Disease Control and Prevention opioid guidelines: Potential for unintended consequences and will they be abused? Journal of Clinical Pharmacy and Therapeutics. 2016;41(6):592–593. [PubMed: 27576604]
- Pokrovnichka A. History of OxyContin: Labeling and Risk Management Program. Anesthetic and Life Support Drugs and Drug Safety and Risk Management Advisory Committees; Nov 13-14, 2008. [January 11, 2017]. PowerPoint presentation. http://www
.fda.gov/downloads /AdvisoryCommittees /CommitteesMeetingMaterials /Drugs /AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM248776 .pdf. - Public Citizen. Congressional testimony on OxyContin and the prosecution of Purdue. 2017. [August 2, 2017]. https://www
.citizen.org /our-work/health-and-safety /congressional-testimony-oxycontin-and-prosecution-purdue. - Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine. 2017 10.7326/M16-2367. [PubMed: 28192789]
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Experimental and Clinical Psychopharmacology. 2009;16(5):405–416. [PMC free article: PMC2711509] [PubMed: 18837637]
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. Morbidity and Mortality Weekly Report. 2016;65(50-51):1445–1452. [PubMed: 28033313]
- SAMHSA (Substance Abuse and Mental Health Services Administration). National admissions to substance abuse treatment services. 2015. [January 10, 2017]. Treatment Episode Data Set (TEDS) 2003–2013. https://www
.samhsa.gov /data/sites/default /files/2003_2013_TEDS_National /2003_2013 _Treatment_Episode_Data_Set_National .pdf. - Senate Committee on Government Operations. Reorganization plan no. 2 of 1973, establishing a Drug Enforcement Administration in the Department of Justice. Washington, DC: U.S. Government Printing Office; 1973. [June 11, 2017]. https://www
.gpo.gov/fdsys /pkg/USCODE-2010-title5 /pdf/USCODE-2010-title5-app-reorganiz-other-dup96 .pdf. - Spillane JF. Debating the Controlled Substances Act. Drug and Alcohol Dependence. 2004;76(1):17–29. [PubMed: 15380285]
- Spillane J, McAllister WB. Keeping the lid on: A century of drug regulation and control. Drug and Alcohol Dependence. 2003;70(3 Suppl.):S5–S12. [PubMed: 12759194]
- Staffa J. Overview of the prescription opioid epidemic and the FDA activities to address it. Apr 24, 2017. Presentation at DIA/FDA statistics forum.
- UNODC (United Nations Office on Drugs and Crime). UNODC statistics. 2017. [May 26, 2017]. https://data
.unodc.org. - VA (U.S. Department of Veterans Affairs) and DoD (U.S. Department of Defense). VA/DoD clinical practice guidelines for management of opioid therapy for chronic pain. 2010. [February 21, 2017]. https://www
.va.gov/painmanagement /docs/cpg _opioidtherapy_summary.pdf. - VanZee A. The promotion and marketing of OxyContin: Commercial triumph, public health tragedy. American Journal of Public Health. 2009;99(2):221–227. [PMC free article: PMC2622774] [PubMed: 18799767]
- Virginia Department of Health. The opioid crisis is a public health emergency in Virginia. 2016. [February 21, 2017]. http://www
.vdh.virginia .gov/home/resources-for-health-care-professionals /the-opioid-addiction-crisis-is-a-public-health-emergency-in-virginia. - VonKorff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Annals of Internal Medicine. 2011;155(5):325–328. [PMC free article: PMC3280085] [PubMed: 21893626]
- Voon P. Prevalence, correlates, and regulatory strategies related to pain, opioid misuse, and overdose: The experience in Vancouver, Canada. Washington, DC: Nov 4, 2016. Presentation to the Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse.
- Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug poisoning deaths in the United States, 1980–2008. 2011. [February 21, 2017]. https://www
.cdc.gov/nchs /products/databriefs/db81.htm. - White House. National drug control strategy. 2016. [April 23, 2017]. https:
//obamawhitehouse .archives.gov/sites /default/files/ondcp /policy-and-research /2016_ndcs_final_report.pdf.
Footnotes
- 1
A REMS is a safety strategy used by the FDA “to manage a known or potential serious risk associated with a medicine to enable patients to have continued access to such medicines by managing their safe use” (FDA, 2017a).
- 2
ER/LA opioids are currently subject to a REMS program that requires sponsors to fund continuing medical education for providers on the appropriate use of these products at low or no cost. The FDA has stated that it is expanding the REMS requirements to include IR opioids as well (FDA, 2017b).
- 3
The Harrison Narcotics Act has since been replaced by the Controlled Substances Act, enacted in 1970.
- 4
Some opioids are not classified in Schedule II. These include opioids containing less than 90 milligrams of codeine per dosage unit (e.g., Tylenol with Codeine®) and buprenorphine (used in the treatment of OUD), which are Schedule III drugs—those that have “a potential for abuse less than substances in Schedules I or II” and whose “abuse may lead to moderate or low physical dependence or high psychological dependence” (DEA, 2017b).
- 5
Liberalization of prescribing was resisted in some quarters, and worries about possible discipline by state medical boards or even prosecution by the U.S. Drug Enforcement Administration (DEA) continued to affect professional practice during this period.
- 6
Purdue Pharma was eventually prosecuted and, in 2007, paid a $600 million settlement after pleading guilty for its misrepresentation of OxyContin's addiction and abuse potential.
- 7
The DEA reported investigating 247 OxyContin diversion cases between October 1999 and March 2002, which led to 328 arrests. Between May 2001 and January 2004, the DEA arrested approximately 600 people for violation of laws related to distribution, dispensing, or possession of OxyContin. Of these, 60 percent were doctors, pharmacists, or other professionals (Hoffman, 2016).
- 8
The preponderance of IR opioid prescribing may be the result of many factors, including but not limited to the effect of hydrocodone IR combination products being Schedule III drugs/refillable until 2014 (when they were reclassified as Schedule II drugs), the number of prescriptions for acute pain after injuries/surgeries/procedures, the comfort of many providers with short-acting drugs, an overall practice of using relatively low doses of drugs, and the preferences of patients to have control over when they take their drugs.
- 9
It is important to note, however, that opioid prescribing practices, and therefore trends in dispensing, vary widely among states and other localities.
- Introduction - Pain Management and the Opioid EpidemicIntroduction - Pain Management and the Opioid Epidemic
Your browsing activity is empty.
Activity recording is turned off.
See more...