NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Environmental Studies and Toxicology; Committee on Endocrine-Related Low-Dose Toxicity. Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals. Washington (DC): National Academies Press (US); 2017 Jul 18.

Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals.
Show detailsSECTION E-1. PBDE (ANIMAL) SYSTEMATIC REVIEW PROTOCOL
BACKGROUND AND INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are synthetic brominated flame retardants that are ubiquitous environmental contaminants that have been measured in animals and in humans. Because the developing organism has been shown to be particularly vulnerable to endocrine-disrupting chemicals, such as PBDEs, the committee decided to focus on studies of developmental exposure. PBDEs have been linked to effects on neurodevelopment after developmental exposure in animal studies.
OBJECTIVE AND SPECIFIC AIMS
Review Question
The overall objective of this systematic review is to answer the question is developmental exposure to PBDEs in nonhuman mammals associated with alterations in learning, memory, attention, or response inhibition?
The specific aims of the review are to:
- Identify literature reporting the effects of developmental PBDE exposure on learning, memory, attention, or response inhibition in nonhuman mammals.
- Extract data on learning, memory, attention, or response inhibition from relevant studies.
- Assess the internal validity (risk of bias) of individual studies.
- Summarize the extent of evidence available.
- Synthesize the evidence using a narrative approach or meta-analysis (if appropriate) considering limitations on data integration, such as study-design heterogeneity.
- Rate the confidence in the body of evidence for studies in nonhuman mammals according to one of five statements: (1) high; (2) moderate; (3) low; (4) very low/no evidence available; or (5) evidence of lack of neurotoxicity.
PECO Statement
A PECO (Population, Exposure, Comparator, and Outcome) statement was developed by the review team as an aid to identify search terms and inclusion/exclusion criteria as appropriate for addressing the review question for the systematic review.
Population: Nonhuman mammals
Exposure:
- PBDE refers to any single PBDE congener or combination of grouped congeners.
- Any developmental exposure to PBDEs, with no restrictions based on route of exposure or administered dose or concentration. To be considered “developmental,” the exposure occurred during any of the following periods: prior to conception in one or both parents, prenatal in the pregnant female (exposure to offspring in utero), or postnatal until sexual maturation.
Comparator: Nonhuman mammals exposed during development to different doses of PBDEs or vehicle-only treatment.
Outcomes: Measures of learning, memory, attention, or response inhibition. Examples of tests include Morris water maze, radial arm maze, and operant tests of cognition.
METHODS
Problem Formulation and Protocol Development
The review question and specific aims were developed and refined through a series of problem formulation steps. The committee considered review articles on endocrine disruptors in surveying the types of chemicals that might make good case examples and held a workshop to explore potential case examples. The committee sought an example of a chemical for which both the human and the animal evidence on effects appears to be associated with different exposure levels of that chemical and due to perturbation of the estrogen or androgen hormone system. PBDEs appeared to fit this case criterion.
The protocol will be peer reviewed by subject-matter and systematic-review experts in accordance with standard report-review practices of the National Academies of Sciences, Engineering, and Medicine. The protocols will be revised in response to peer review comments and will subsequently be published as appendices to the committee's final report. The identity of the peer reviewers will remain anonymous to the committee until the publication of the final report, when their names and affiliations are disclosed in the Preface.
Committee and Staff
There are 11 committee members, supported by two staff members of the National Academies. The committee members were appointed in accordance with the standard policies and practices of the National Academies on the basis of their expertise in general toxicology, reproductive toxicology, developmental toxicology, endocrinology, neurotoxicology, epidemiology, risk assessment, biostatistics, and systematic-review methods. The membership of the committee and the staff was determined before the topic of the systematic review was selected. It was known, however, that each case study would be on an endocrine-disrupting chemical, so committee members who have relevant expertise were specifically recruited and appointed.
Review Team
The review team for this case study will be a subgroup of the committee (BH, SSc), two National Academies staff members (EM, SM), and an information specialist (JB). If a member of the review team was a coauthor of a study under review, that member will recuse himself or herself from the evaluation of the quality of that study.
The review team will be responsible for performing all aspects of the review, including conducting the literature searches; applying inclusion/exclusion criteria to screen studies; extracting data; assessing risk of bias for included studies; and analyzing and synthesizing data. The roles and responsibilities of the team members will be documented throughout the protocol. Throughout the course of its work, the review team will also engage other members of the committee to provide consultation as needed. The involvement of those individuals will be documented and acknowledged.
Biographical information on the review team is presented in Section E-1a.
Search Methods
Search for Existing Systematic Reviews
The review team will consider using existing systematic reviews to address or help to address its research question. English-language systematic reviews conducted within the last 3 years will be sought. The review team will incorporate prior reviews, update prior reviews, and/or use the reviews as part of its searching, depending on determination of their relevancy and quality (Whitlock et al. 2008). Current guidance on using existing systematic reviews will be used (Robinson et al. 2014, 2015, 2016).
Search
Recent, relevant high-quality systematic reviews addressing the research question about PBDEs and neurodevelopment will be searched. PubMed will be searched by adding the qualifier “systematic review”[ti] OR “meta-analysis”[pt] OR “meta-analysis”[ti] OR (“systematic”[ti] AND “review”[ti]) OR (systematic review [tiab] AND review [pt]) OR “meta synthesis”[ti] OR “meta synthesis”[ti] OR “integrative review”[tw] OR “integrative research review”[tw] OR “cochrane database syst rev”[ta] OR “evidence synthesis”[tiab] to the preliminary search strategy (see Section E-1b). Language and date restrictions will be applied (English language; published 2013 to present). The systematic review protocol registries PROSPERO (CRD) and CAMARADES will also be searched using key terms from the preliminary PubMed strategy.
Study Selection
Two team members (SM, EM) will independently screen search results, applying the following exclusion criteria:
- Not a systematic review.1 The minimum criteria for a study to be considered a systematic review are
- conduct of an explicit and adequate literature search,
- application of predefined eligibility criteria,
- consideration of the quality of included studies or risk of bias assessment, and
- synthesis (or attempt at synthesis) of the findings, either qualitatively or quantitatively.
- Not in English.
- Search date prior to 2013.
- Does not match the research question or PECO elements.
For PubMed results, screening will be conducted first using abstracts and then at the full-text level. Results from PROSPERO and CAMARADES will be conducted at one level, using the information in the registry. Disagreements regarding eligibility will be resolved through discussion or, where necessary, by a third team member.
Assessment for Quality
Two investigators (KR, AR) will independently assess the risk of bias of eligible systematic reviews using ROBIS (Whiting et al. 2016). Disagreements in rating will be resolved through discussion or, where necessary, through consultation with a third team member. Systematic reviews rated as low quality will be excluded from further consideration at this stage.
Use of Existing Reviews
Eligible systematic reviews of high quality will be reviewed, considering date of search, match with the PECO statement, as well as availability of data from the primary studies, how risk of bias was conducted, and other factors. Current reviews considered a good match will be used to address the research question. Reviews that are a good match but with search dates more than a year ago will be updated. If no relevant systematic reviews are found, an independent systematic review will be performed.
Literature Search for Independent Systematic Review
The review team will collaborate with an information specialist (JB) who has training, expertise, and familiarity with developing and performing systematic review literature searches. A variety of methods will be used to identify relevant data (see below). Literature searches will not be limited by publication date.
Online Databases
Electronic searches of the following three online databases will be performed using the search terms outlined in Section E-1b: PubMed, Embase, and Toxline. The search strategy and search terms will be developed by the information specialist (JB), who will implement the search for relevant studies.
Other Resources
Hand searching the reference lists of all the included studies after full-text review will be conducted using the same study selection process as was used for screening records retrieved from the electronic search. Relevant studies identified through these steps will be marked as “provided from other sources” in the study selection flow diagram.
Study Selection
All search results will be imported or manually entered into EndNote (Version x7) reference management software. EndNote will be used to eliminate any duplicate citations before evaluating the eligibility of the citations.
Screening Process
References retrieved from the literature search will be screened for relevance and eligibility against the evidence selection criteria using DistillerSR (Evidence Partners; https://www.evidencepartners.com). Screeners from the review team will be trained with an initial pilot phase on 25 studies undertaken to improve clarity of the evidence selection criteria and to improve accuracy and consistency among screeners. Screening forms are presented in Section E-1c.
Title and Abstract Screening
Each citation will be independently screened by two reviewers (SM, EM) to determine whether it meets the selection criteria for inclusion that reflect the PECO statement with some additional considerations as listed below. Citations included at the title/abstract screening level will be subject to a full-text review by the same two reviewers. Disagreements regarding citation eligibility will be resolved via consensus and, where necessary, by consulting a committee member.
The title/abstract screening form will be used to screen and EXCLUDE references if at least one of the following criteria is met:
- 1.
No original data (e.g., review article, commentary, editorial)
- 2.
Study does not include nonhuman mammals
- 3.
Study does not report PBDE exposure
- 4.
No relevant outcomes
- 5.
Incomplete information (e.g., conference abstract, meeting poster)
- 6.
Not in English and unable to determine eligibility
- 7.
Other (explanation required)
The following types of records will be INCLUDED at the title/abstract level: any English-language study of nonhuman mammals exposed to PBDEs.
Only English-language publications will be included, because of time and resource constraints. There appears to be no indication that foreign-language publications would make a contribution that is distinct from what is found in the English-language literature.
Updated details to instructions and interpretations for title and abstract screening will be added to Section E-1f to document the process of the review team during the screening process.
Full-Text Screening
Citations included at the title/abstract screening level will be subject to a full-text review by the same two reviewers involved in title and abstract screening (SM, EM). Each reference will be screened in duplicate and independently. Disagreements regarding citation eligibility will be resolved via consensus and, where necessary, by consulting a committee member.
Citations will be EXCLUDED at the full-text level if at least one of the following criteria is met:
- 1.
No original data (e.g., review article, commentary, editorial)
- 2.
Study does not include nonhuman mammals
- 3.
Study does not report experimental PBDE exposure
- 4.
Study does not quantify exposure to PBDE
- 5.
Study does not include developmental exposure (prior to conception in one or both parents, prenatal in the pregnant female [exposure to offspring in utero], or postnatal until sexual maturation)
- 6.
Study does not assess or report quantitative measures of learning, memory, attention, or response inhibition
- 7.
No comparator group (animals exposed to different doses of PBDEs or vehicle-only treatment)
- 8.
Not in English
- 9.
Other reason (explanation required)
The reason for exclusion at the full-text-review stage will be annotated and reported in a study selection flow diagram in the final report (following PRISMA [Moher et al. 2009]). The reasons for exclusion will be documented from the list (1-9) above.
Citations will be INCLUDED if they meet the PECO statement criteria:
- Study includes nonhuman mammals
- Study includes developmental exposure
- Study includes comparison with animals exposed to different doses of PBDEs or vehicle-only treatment
- Study measures (1) learning, (2) memory, (3) attention, or (4) response inhibition.
Updated details to instructions and interpretations for full-text screening will be added to Section E-1f to document the process of the review team during the screening process.
Data Extraction
Data will be collected and recorded (i.e., extracted) from included studies by one member of the review team and checked by a second member for completeness and accuracy. Any discrepancies in data extraction will be resolved through discussion. The extracted data will be used to summarize study designs and findings and/or to conduct statistical analyses. Section E-1d presents the data extraction elements that will be used.
The review team will attempt to contact authors of included studies to obtain missing data considered important for evaluating key study findings (e.g., level of data required to conduct a meta-analysis). The study extraction files will note whether an attempt was made to contact study authors by email for missing data considered important for evaluating key study findings (and whether or not a response was received).
Multiple publications with overlapping data for the same study (e.g., publications reporting subgroups, additional outcomes or exposures outside the scope of an evaluation, or longer follow-up) are identified by examining author affiliations, study designs, cohort name, enrollment criteria, and enrollment dates. If necessary, study authors will be contacted to clarify any uncertainty about the independence of two or more articles. The review will include all publications on the study, select one publication to use as the primary publication, and consider the others as secondary publications with the annotation as being related to the primary record during data extraction. The primary study will generally be the publication with the longest follow-up or, for studies with equivalent follow-up periods, the study with the largest number of cases or the most recent publication date. The review will include relevant data from all publications of the study, although if the same outcome is reported in more than one report, the review team will include a single instance of the data (and avoid more than one; that is, duplicate instances of the data).
Data extraction will be completed using the Health Assessment Workspace Collaborative (HAWC) software, an open source and freely available Web-based interface application, for visualization and warehousing.2
Risk of Bias (Quality) Assessment of Individual Studies
Risk of bias is related to the internal validity of a study and reflects study-design characteristics that can introduce a systematic error (or deviation from the true effect) that might affect the magnitude and even the direction of the apparent effect. Internal validity or risk of bias will be assessed for individual studies using a tool developed by the National Toxicology Program's Office of Health Assessment and Translation (OHAT) that outlines an approach to evaluating risk of bias for experimental animal studies. The risk of bias domains and questions for experimental animal studies are based on established guidance for experimental human studies (randomized clinical trials) (Viswanathan et al. 2012, 2013; Sterne et al. 2014; Higgins and Green 2011) and recent tools for animal studies (Hooijmans et al. 2014; Koustas et al. 2014). The risk of bias tool includes a common set of questions (Section E-1e) that are answered based on the specific details of individual studies to develop risk of bias ratings (using the four options: definitely low risk of bias; probably low risk of bias; probably high risk of bias; or definitely high risk of bias). Information or study procedures that were not reported are assumed not to have been conducted, resulting in an assessment of “probably high” risk of bias. Study design determines the subset of questions that should be used to assess risk of bias for an individual study (see Table E1-1).
Studies are independently assessed by two assessors (BH, SSc) who answer all applicable risk of bias questions with one of four options (see Table E1-2) following prespecified criteria detailed in Section E-1e. The criteria describe aspects of study design, conduct, and reporting required to reach risk of bias ratings for each question and specify factors that can distinguish among ratings (e.g., what separates “definitely low” from “probably low” risk of bias). The instructions and detailed criteria are tailored to the specific type of human study designs. Risk of bias will be assessed at the outcome level because study-design or method specifics may increase the risk of bias for some outcomes and not others within the same study. Information or study procedures that were not reported are assumed not to have been conducted, resulting in an assessment of “probably high” risk of bias. Authors will be queried by email to obtain missing information, and responses received will be used to update risk of bias ratings.
Assessors will be trained using the criteria in an initial pilot phase undertaken to improve clarity of criteria that distinguish between adjacent ratings and to improve consistency among assessors. All team members involved in the risk of bias assessment will be trained on the same set of studies and asked to identify potential ambiguities in the criteria used to assign ratings for each question. Any ambiguities and rating conflicts will be discussed relative to opportunities to refine the criteria to more clearly distinguish between adjacent ratings. If major changes to the risk of bias criteria are made based on the pilot phase (i.e., those that would likely result in revision of response), they will be documented in a protocol amendment along with the date modifications were made and the logic for the changes. It is also expected that information about confounding, exposure characterization, outcome assessment, and other important issues may be identified during or after data extraction, which can lead to further refinement of the risk of bias criteria.
After assessors have independently made risk of bias determinations for a study across all risk of bias questions, the two assessors will compare their results to identify discrepancies and attempt to resolve them. Any remaining discrepancies will be considered and resolved with the review team. The final risk of bias rating for each question will be recorded along with a statement of the basis for that rating.
Data Analysis and Evidence Synthesis
The review team will qualitatively synthesize the body of evidence for each outcome and, where appropriate, a meta-analysis will be performed. If a meta-analysis is performed, summaries of main characteristics for each included study will be compiled and reviewed by two team members to determine comparability between studies, to identify data transformations necessary to ensure comparability, and to determine whether heterogeneity is a concern. The main characteristics considered across all eligible studies include the following:
- Experimental design (e.g., acute, chronic, multigenerational)
- Animal model used (e.g., species, strain, sex, genetic background)
- Age of animals (e.g., at start of treatment, mating, and/or pregnancy status)
- Developmental stage of animals at treatment and outcome assessment
- Dose levels, frequency of treatment, timing, duration, and exposure route
- Health outcome(s) reported
- Type of data (e.g., continuous or dichotomous), statistics presented in paper, access to raw data
- Variation in degree of risk of bias at individual study level
TABLE E1-1
OHAT Risk of Bias Tool.
TABLE E1-2
Answers to the Risk of Bias Questions.
The review team expects to require input from subject-matter experts to help assess the heterogeneity of the studies. Subgroup analyses to examine the extent to which risk of bias contributes to heterogeneity will be performed. If a meta-analysis is considered appropriate, the review team will stratify by species and further consider separate meta-analyses by species. Situations where it may not be appropriate to include a study are when data on exposure or outcome are too different to be combined or other circumstances that may indicate that averaging study results would not produce meaningful results. When considering outcome measures for conducting meta-analyses, benchmark dose (BMD) estimates (and their associated confidence intervals) with a benchmark response (BMR) set to a common percent of control (for continuous outcomes) or extra risk (for dichotomous outcomes) are preferred. A secondary alternative, when there are more than two groups, is the conduct of BMD modeling and the use of the derived BMD estimates. Meta-analyses are not possible with lowest-observed-adverse-effect levels or no-observed-adverse-effect levels, since no confidence interval can be derived for these measures.
If a meta-analysis is conducted, a random effects model will be used for the analysis. Heterogeneity will be assessed using the I-squared statistic. Interpretation of I-squared will be based on the Cochrane Handbook: 0% to 40% (might not be important); 30% to 60% (may represent moderate heterogeneity); 50% to 90% (may represent substantial heterogeneity); and 75% to 100% (considerable heterogeneity). Additionally, as described in the Cochrane Handbook, for the last three categories, the importance of the I-squared will be interpreted considering not only the magnitude of effects but also the strength of the evidence (90% two-tailed confidence interval).
The review team will also perform sensitivity analyses on the exclusion of individual studies in succession.
If sufficient studies are available, subgroup analyses will be performed based on the following characteristics described above: experimental design, animal model used (e.g., species and/or strain), age of animals, and developmental stage of animals at treatment and outcome assessment.
In the event that these proposed methods for data analysis are altered to tailor to the evidence base from included studies, the protocol will be amended accordingly, and the reasons for change will be justified in the documentation.
Confidence Rating: Assessment of the Body of Evidence
The quality of evidence for each outcome will be evaluated using the GRADE system for rating the confidence in the body of evidence (Guyatt et al. 2011; Rooney et al. 2014). More detailed guidance on reaching confidence ratings in the body of evidence as “high,” “moderate,” “low,” or “very low” is provided in NTP (2016, see Step 5). In brief, available studies on a particular outcome are initially grouped by key study-design features, and each grouping of studies is given an initial confidence rating by those features.
The initial rating is downgraded for factors that decrease confidence in the results, including
- risk of bias
- unexplained inconsistency
- indirectness or lack of applicability
- imprecision
- publication bias
The initial rating is upgraded for factors that increase confidence in the results, including
- large magnitude of effect
- dose response
- consistency across study designs/populations/animal models or species
- consideration of residual confounding
- other factors that increase confidence in the association or effect (e.g., particularly rare outcomes)
The reasons for downgrading (or upgrading) confidence may not be due to a single domain of the body of evidence. If a decision to downgrade is borderline for two domains, the body of evidence is downgraded once in a single domain to account for both partial concerns based on considering the key drivers of the strengths or weaknesses. Similarly, the body of evidence is not downgraded twice for what is essentially the same limitation (or upgraded twice for the same asset) that could be considered applicable to more than one domain of the body of evidence. Consideration of consistency across study designs, human populations, or animal species is not included in the GRADE guidance (Guyatt et al. 2011); however, it is considered in the modified version of GRADE used by OHAT (Rooney et al. 2014).
Confidence ratings are independently assessed by members of the review team, and discrepancies will be resolved by consensus and consultation with technical advisors as needed. Confidence ratings will be summarized in evidence profile tables.
REFERENCES
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW Jr., Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schunemann HJ. GRADE guidelines 6. Rating the quality of evidence—imprecision. J. Clin. Epidemiol. 2011;64(12):1283–1293. [PubMed: 21839614]
- Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. [May 6, 2016]. http://handbook
.cocharne.org. - Hooijmans CR, Rovers MM, de Vries RB, Leenars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Method. 2014;14:43. [PMC free article: PMC4230647] [PubMed: 24667063]
- IOM (Institute of Medicine). Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: The National Academies Press; 2011. [PubMed: 24983062]
- Koustas E, Lam J, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. The Navigation Guide—evidence-based medicine meets environmental health: Systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ. Health Perspect. 2104;122(10):1015–1027. [PMC free article: PMC4181920] [PubMed: 24968374]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. [PubMed: 19631508]
- NTP (National Toxicology Program). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Office of Health Assessment and Translation, Division, National Toxicology Program, National Institute of Environmental Health Sciences; Jan 9, 2015. 2015. [September 21, 2015]. http://ntp
.niehs.nih .gov/ntp/ohat/pubs/handbookjan2015_508 .pdf. - Robinson KA, Whitlock EP, O'Neil ME, Anderson JK, Hartling L, Dryden DM, Butler M, Newberry SJ, McPheeters M, Berkman ND, Lin JS, Chang S S. Research white paper. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [May 9, 2016]. Integration of Existing Systematic Reviews. https://www
.ncbi.nlm .nih.gov/books/NBK216379/ AHRQ Publication No. 14-EHC016-EF. [PubMed: 25032273] - Robinson KA, Chou R, Berkman ND, Newberry SJ, Fu R, Hartling L, Dryden D, Butler M, Foisy M, Anderson J, Motu'apuaka M, Relevo R, Guise JM, Chang S. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [May 9, 2016]. Integrating Bodies of Evidence: Existing Systematic Reviews and Primary Studies. https://www
.ncbi.nlm .nih.gov/books/NBK279904/ AHRQ Publication No. 15-EHC007-EF. - Robinson KA, Chou R, Berkman ND, Newberry SJ, Fu R, Hartling L, Dryden D, Butler M, Foisy M, Anderson J, Motu'apuaka M, Relevo R, Guise JM, Chang S. Twelve recommendations for integrating existing systematic reviews into new reviews: EPC guidance. J. Clin. Epidemiol. 2016;70:38–44. [PubMed: 26261004]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health assessments. Environ. Health Perspect. 2014;122(7):711–718. [PMC free article: PMC4080517] [PubMed: 24755067]
- Sterne JAC, Higgins JPT, Reeves BC. ACROBAT-NRSI: A Cochrane risk of Bias Assessment Tool for Non-randomized Studies of Interventions. Version 1.0.0. Sep 24, 2014. 2014. [May 6, 2016]. www
.riskofbias.info. - Viswanathan M, Ansari M, Berkman ND, Chang S, Hartling L, McPheeters LM, Santaguida PL, Shamliyan T, Singh K, Tsertsvadze A, Treadwell JR. Assessing the Risk of Bias of Individual Studies when Comparing Medical Interventions. Rockville, MD: Agency for Healthcare Research and Quantitative Methods Guide for Comparative Effectiveness Reviews; 2012. [May 6, 2016]. www
.effectivehealthcare.ahrq.gov/ AHRQ Publication No. 12-EHC047-EF. - Viswanathan M, Berkman ND, Dryden DM, Hartling L. Methods Research Report. Rockville, MD: Agency for Healthcare Research and Quantitative Methods Guide for Comparative Effectiveness Reviews; 2013. [May 6, 2016]. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank. www
.effectivehealthcare .ahrq.gov/reports/final.cfm. AHRQ Publication No. 13-EHC106-EF. [PubMed: 24006553] - Whiting P, Savovic J, Higgins JB, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016;69:225–234. [PMC free article: PMC4687950] [PubMed: 26092286]
- Whitlock EP, Lin JS, Chou R, Shekelle P, Robinson KA. Using existing systematic reviews in complex systematic reviews. Ann. Intern. Med. 2008;148(10):776–782. [PubMed: 18490690]
Footnotes
- 1
A systematic review “is a scientific investigation that focuses on a specific question and uses explicit, prespecified scientific methods to identify, select, assess, and summarize the findings of similar but separate studies” (IOM 2011, p. 1).
- 2
HAWC (Health Assessment Workspace Collaborative): A Modular Web-based Interface to Facilitate Development of Human Health Assessments of Chemicals (https://hawcproject
.org/portal/).
SECTION E-1a. REVIEW TEAM BIOGRAPHICAL INFORMATION
Jaime F. Blanck is a clinical informationist at the Welch Medical Library at Johns Hopkins University. She creates and implements systematic review search strategies across multiple databases and provides comprehensive reference, research, and information services to multiple departments within the School of Medicine. She received an MLIS from the University of Pittsburgh and an MPA from the University of Baltimore.
Barbara F. Hales is a James McGill Professor in the Department of Pharmacology and Therapeutics at McGill University. Her research interests are in the mechanisms of action of drugs as teratogens. She studies developmental toxicity using a combination of in vivo, in vitro, and molecular approaches with the goal of elucidating how the embryo responds to insult after direct or maternal exposure and the consequences to progeny of paternal drug exposure. Dr. Hales is a past president of the Teratology Society, and is currently co-chair of the Chemicals Management Plan Science Committee of the Government of Canada. She received an MSc in pharmacognosy from the Philadelphia College of Pharmacy and Science and a PhD in pharmacology and therapeutics from McGill University.
Ellen Mantus is a scholar and director of risk assessment on the Board on Environmental Studies and Toxicology at the National Academies of Sciences, Engineering, and Medicine with more than 20 years of experience in the fields of toxicology and risk assessment. She has served as the study director on numerous projects, including ones that have assessed the health implications of various chemical exposures; developed strategies for applying modern scientific approaches in toxicology and risk assessment; provided guidance to federal agencies on risk-based decision making; and evaluated barriers to deployment of electric vehicles and associated charging infrastructure. Before joining the National Academies, Dr. Mantus was a project manager with ICF Consulting where she served as a primary reviewer for numerous toxicological studies and provided risk assessment and regulatory support on a wide array of projects. Dr. Mantus received a PhD in chemistry from Cornell University.
Susan Martel is a senior program officer in the Board on Environmental Studies and Toxicology at the National Academies of Sciences, Engineering, and Medicine. She has 20 years of experience in supporting toxicology and risk assessment projects for the US Environmental Protection Agency, the US Department of Defense, and the National Aeronautics and Space Administration. Recent projects include working with committees evaluating the toxicological effect of arsenic, developing exposure guidelines for use on spacecraft, and assessing pesticide risks-assessment practices. Before joining the National Academies, she was the administrator of the Registry for Toxicology Pathology for Animals at the American Registry of Pathology. She received a BA in biology from Skidmore College.
Susan L. Schantz is a professor of toxicology in the Department of Comparative Biosciences, College of Veterinary Medicine, at the University of Illinois at Urbana-Champaign. She is also director of a National Institute of Environmental Health Sciences (NIEHS) T32 training program in endocrine, developmental, and reproductive toxicology and director of a Children's Environmental Health Research Center jointly funded by the NIEHS and the EPA. In addition, she is currently the interim director of the Neuroscience Program. Dr. Schantz's research interests involve understanding the neurobehavioral effects of chemical exposures during development and aging. She conducts research in both laboratory-based animal studies and parallel epidemiologic studies. She has served as president of the Neurotoxicology Specialty Section of the Society of Toxicology and president of the Neurobehavioral Teratology Society. Dr. Schantz was also a member of the NRC's Committee to Assess the Health Implications of Perchlorate Ingestion. She received a PhD in environmental toxicology from the University of Wisconsin–Madison.
SECTION E-1b. LITERATURE SEARCH STRATEGY
The review team will employ a multi-method process to identify all potentially relevant studies as detailed below.
Electronic Searches
PubMed
A search string employing medical subject heading (MeSH) terms and keyword synonyms will be developed. The PubMed search strategy will be considered the primary search strategy and will provide the basis of the other electronic search strategies. To assist in compiling these terms, the review team will consult an existing systematic review protocol studying PBDEs in humans (J. Lam et al. Applying the navigation guide systematic review methodology. Case study #5: association between developmental exposures to PBDEs and human neurodevelopment. PROSPERO 2015:CRD42015019753 Available from http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42015019753). This protocol was selected because it examines the substances of interests and timing of exposure in a parallel human population. The search strategies will address each of the following concepts:
- Flame retardants (PBDEs)—The review team will use the MeSH database (http://www.ncbi.nlm.nih.gov/mesh) to find all MeSH heading and Supplementary Concept headings that relate to the Flame retardants (PBDEs) concept. The review team will mine the “Entry Terms” list for each of the controlled vocabulary terms identified and include all unique keyword synonyms listed for each. CAS registry numbers for each PBDE substance will also be included in the list of search terms. All MeSH terms, Supplementary Concept terms, keyword synonyms, and CAS registry numbers will be searched together as one concept using the Boolean operator “OR.”
- Exposure—The review team will use the MeSH database (http://www.ncbi.nlm.nih.gov/mesh) to find all MeSH heading and Supplementary Concept headings that relate to the exposure concept. The review team will mine the “Entry Terms” list for each of the controlled vocabulary terms identified and include all unique keyword synonyms listed for each. All MeSH terms and keyword synonyms will be searched together as one concept using the Boolean operator “OR.”
- Animal studies—The review team will adapt the search filter published in Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Laboratory Animals. 2010;44(3):170-175 to eliminate nonmammalian animals. doi:10.1258/la.2010.009117.
- Outcomes—The review team will use the MeSH database (http://www.ncbi.nlm.nih.gov/mesh) to find all MeSH heading and Supplementary Concept headings that relate to measures of learning, memory, attention, and cognition. The review team will mine the “Entry Terms” list for each of the controlled vocabulary terms identified and include all unique keyword synonyms listed for each. All MeSH terms and keyword synonyms will be searched together as one concept using the Boolean operator “OR.”
Each of the above concepts will be searched together using the Boolean operator “AND.” There will not be limitations on date of publication, language, or publication type. All citation records will be exported to EndNote. Additional citations identified through the search processes identified below will also be exported to the project EndNote library. Duplicates will be removed from the citation library using the “Find Duplicates” tool in EndNote as well as a manual review of citations by the project librarian to identify any duplicates not found during the automated process. The number of citations found in each database will be recorded as well as the number of duplicates and final tally of unique citations. The final library of citations will be uploaded to the Health Assessment Workspace Collaboration Web-based tool (www.hawcproject.org) for systematic reviews where they will be reviewed by the team.
Embase
The controlled vocabulary database Emtree is used by Embase. For each MeSH term identified through process above, Emtree will be searched for the appropriate corresponding term. Additional keywords will identified using the list of synonyms from each Emtree record and added to the keywords from the MeSH records. The review team will substitute the animal study search filter used in the PubMed search with the comparable Embase filter published in De Vries RBM, Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. A search filter for increasing the retrieval of animal studies in Embase. Laboratory Animals. 2011; 45(4):268-270. doi:10.1258/la.2011.011056. This version of the animal filter will also be adapted to remove all nonmammalian animals.
Toxline
The review team will develop the Toxline search strategy by removing any database specific formatting from the PubMed search strategy to create a keyword-only search (Toxline does not employ a controlled vocabulary).
Search Strategies
PubMed
(“Flame Retardants”[Mesh] OR”Flame Retardants” [Pharmacological Action] OR “Halogenated Diphenyl Ethers”[Mesh] OR (“Phenyl Ethers”[Mesh:NoExp] AND (“1974/01/01”[PDAT]: “2008/12/31”[PDAT])) OR “pentabromodiphenyl ether” [Supplementary Concept] OR “2,2',3,3',4,4',6,6'‐octabromodiphenyl ether” [Supplementary Concept] OR “decabromobiphenyl ether” [Supplementary Concept] OR “tribromodiphenyl ether 28”[Supplementary Concept] OR “2,2',4,4'‐tetrabromodiphenyl ether” [Supplementary Concept] OR “2,2',4,5'‐tetrabromodiphenyl ether” [Supplementary Concept] OR “hexabromodiphenyl ether 154” [Supplementary Concept] OR “2,2',4,4',5,6'hexabromodiphenyl ether” [Supplementary Concept] OR “2,2',3,4,4',5',6heptabromodiphenyl ether” [Supplementary Concept] OR “2,2',3,3',4,5,5',6,6'‐nonabromo-diphenyl ether” [Supplementary Concept] OR “2,2',3,3',4,4',5,6,6'‐nonabromodiphenyl ether” [Supplementary Concept] OR “2,2',3,3',4,4',5,5',6‐nonabromodiphenyl ether” [Supplementary Concept] OR “2,2',4,4',5,5'‐hexabrominated diphenyl ether” [Supplementary Concept] OR “hexabrominated diphenyl ether 153” [Supplementary Concept] OR “pentabrominated diphenyl ether 100” [Supplementary Concept] OR “5‐OH‐BDE‐47” [Supplementary Concept] OR “6‐OH‐BDE‐47” [Supplementary Concept] OR flame retard*[tw] OR fire retard*[tw] OR fireproofing agent*[tw] OR “FireMaster”[tw] OR “Bromkal”[tw] OR diphenyl ether deriv*[tw] OR halogenated diphenyl*[tw] OR brominated diphenyl*[tw] OR PBDE*[tw] OR polybrominated diphenyl*[tw] OR polybromodiphenyl*[tw] OR PBDP*[tw] OR BDE*[tw] OR pentabromodiphenyl*[tw] OR cpentaBDE*[tw] OR PentaBDE*[tw] OR “PeBDE”[tw] OR “DE 71”[tw] OR “DE71”[tw] OR “pentabrominated diphenyl”[tw] OR “pentabrominated diphenyls”[tw] OR “PBDPO”[tw] OR “Planelon PB 501”[tw] OR pentabromo deriv*[tw] OR pentabromophenyl*[tw] OR octabromodiphenyl*[tw] OR c‐octaBDE*[tw] OR OctaBDE*[tw] OR “OcBDE”[tw] OR “Octabrom”[tw] OR octabromo deriv*[tw] OR “OBDE”[tw] OR “OBDPO”[tw] OR “octabrominated diphenyl”[tw] OR “octabrominated diphenyls”[tw] OR decabromodiphenyl*[tw] OR cdecaBDE*[tw] OR DecaBDE*[tw] OR “DeBDE”[tw] OR “DBDPO”[tw] OR “decabrominated diphenyl”[tw] OR “decabrominated diphenyls”[tw] OR decabromo deriv*[tw] OR “Decabrom”[tw] OR “Berkflam B 10E”[tw] OR “FR 300BA”[tw] OR “FR 300 BA”[tw] OR tribromodiphenyl*[tw] OR “tribrominated diphenyl”[tw] OR “tribrominated diphenyls”[tw] OR “TrBDE”[tw] OR tribromo deriv*[tw] OR tetrabromodiphenyl*[tw] OR TetraBDE*[tw] OR “TeBDE”[tw] OR “TBDE”[tw] OR “BPDE”[tw] OR tetrabromo deriv*[tw] OR “TBDP”[tw] OR “tetrabrominated diphenyl”[tw] OR “tetrabrominated diphenyls”[tw] OR hexabromodiphenyl*[tw] OR HexaBDE*[tw] OR “HxBDE”[tw] OR “hexabrominated diphenyl”[tw] OR “hexabrominated diphenyls”[tw] OR hexabromo deriv*[tw] OR heptabromodiphenyl*[tw] OR HeptaBDE*[tw] OR “HeBDE”[tw] OR “heptabrominated diphenyl”[tw] OR “heptabrominated diphenyls”[tw] OR heptabromo deriv*[tw] OR nonabromodiphenyl*[tw] OR NonaBDE*[tw] OR “NoBDE”[tw] OR “nonabrominated diphenyl”[tw] OR “nonabrominated diphenyls”[tw] OR nonabromo deriv*[tw] OR “7025‐06‐1” OR “6876‐00‐2” OR “101‐55‐3” OR “51452-870” OR “446254‐14‐4” OR “147217‐72‐9” OR “171977‐449” OR “147217‐71‐8” OR “33513‐66‐3” OR “51930‐04‐2” OR “6903‐63‐5” OR “189084‐59‐1” OR “83694‐71‐7” OR “46438‐88‐4” OR “2050‐47‐7” OR “147217‐74‐1” OR “147217‐75‐2” OR “407606‐55‐7” OR “147217‐73‐0” OR “147217‐76‐3” OR “337513‐67‐4” OR “446254‐15‐5” OR “446254‐16‐6” OR “147217‐77‐4” OR “337513‐75‐4” OR “337513‐53‐8” OR “41318‐75‐6” OR “337513‐56‐1” OR “155999‐95‐4” OR “65075‐08‐3” OR “189084-60‐4” OR “147217‐78‐5” OR “446254‐17‐7” OR “147217‐80‐9” OR “147217‐79‐6” OR “147217‐81‐0” OR “337513‐54‐9” OR “337513‐68‐5” OR “446254‐18‐8” OR “446254‐19‐9” OR “446254‐20‐2” OR “446254‐22‐4” OR “5436‐43‐1” OR “337513‐55‐0” OR “243982‐82‐3” OR “446254‐23‐5” OR “189084-57‐9” OR “446254‐24‐6” OR “446254‐25‐7” OR “446254‐31‐5” OR “446254‐32‐6” OR “446254‐33‐7” OR “446254‐34‐8” OR “189084‐61‐5” OR “446254‐37‐1” OR “446254‐38‐2” OR “327185‐09‐1” OR “446254‐39‐3” OR “189084‐62‐6” OR “446254‐40‐6” OR “446254‐41‐7” OR “446254‐42‐8” OR “189084‐63‐7” OR “446254‐43‐9” OR “93703‐48‐1” OR “446254‐45‐1” OR “446254‐48‐4” OR “103173-66‐6” OR “446254‐50‐8” OR “446254‐51‐9” OR “182346‐21‐0” OR “446254‐53‐1” OR “446254‐54‐2” OR “446254‐55‐3” OR “446254‐55‐3” OR “446254‐57‐5” OR “446254‐59‐7” OR “446254‐61‐1” OR “446254‐64‐4” OR “38463‐82‐0” OR “60348‐60‐9” OR “189084‐64‐8” OR “446254‐65‐5” OR “446254-66‐6” OR “446254‐67‐7” OR “446254‐68‐8” OR “373594‐78‐6” OR “446254‐69‐9” OR “446254‐71‐3” OR “446254‐72‐4” OR “446254‐74‐6” OR “446254‐77‐9” OR “446254‐78‐0” OR “189084‐65‐9” OR “446254‐80‐4” OR “189084‐66‐0” OR “182677‐30‐1” OR “243982‐83‐4” OR “68631‐49‐2” OR “207122-15‐4” OR “35854‐94‐5” OR “189084‐58‐0” OR “189084‐67‐1” OR “207122‐16‐5” OR “189084‐68‐2” OR “1163‐19‐5” OR “109945‐70‐2” OR “113152‐37‐7” OR “113172‐79‐5” OR “139598‐16‐6” OR “139749-52‐3” OR “145538‐74‐5” OR “32534‐81‐9” OR “32536‐52‐0” OR “40088‐47‐9” OR “446254‐27‐9” OR “446255‐20‐5” OR “446255‐22‐7” OR “49690‐94‐0” OR “63936‐56‐1” OR “64589‐00‐0” OR “68928‐80-3” OR “85446‐17‐9” OR “36483‐60‐0” OR “437701‐79‐6” OR “446255‐26‐1” OR “117948‐63‐7” OR “446255‐30‐7” OR “61262‐53‐1” OR “405237‐85‐6” OR “39275‐89‐3” OR “13654‐09‐6” OR “61288‐13-9” OR “446255‐39‐6” OR “337513‐72‐1” OR “366791‐32‐4” OR “2050‐47‐7”) AND (“Occupational Exposure”[Mesh:NoExp] OR “Maternal Exposure”[Mesh] OR “Environmental Exposure”[Mesh] OR “Prenatal Exposure Delayed Effects”[Mesh] OR “Exposure”[tw] OR “Exposed”[tw] OR “exposures”[tw] OR “exposing”[tw]) AND (“animal experimentation”[MeSH Terms] OR “models, animal”[MeSH Terms] OR “invertebrates”[MeSH Terms] OR “Animals”[Mesh:noexp] OR “animal population groups”[MeSH Terms] OR “mammals”[MeSH Terms:noexp] OR “primates”[MeSH Terms:noexp] OR “artiodactyla”[MeSH Terms] OR “carnivora”[MeSH Terms] OR “cetacea”[MeSH Terms] OR “chiroptera”[MeSH Terms] OR “elephants”[MeSH Terms] OR “hyraxes”[MeSH Terms] OR “insectivora”[MeSH Terms] OR “lagomorpha”[MeSH Terms] OR “marsupialia”[MeSH Terms] OR “monotremata”[MeSH Terms] OR “perissodactyla”[MeSH Terms] OR “rodentia”[MeSH Terms] OR “scandentia”[MeSH Terms] OR “sirenia”[MeSH Terms] OR “xenarthra”[MeSH Terms] OR “haplorhini”[MeSH Terms:noexp] OR “strepsirhini”[MeSH Terms] OR “platyrrhini”[MeSH Terms] OR “tarsii”[MeSH Terms] OR “catarrhini”[MeSH Terms:noexp] OR “cercopithecidae”[MeSH Terms] OR “hylobatidae”[MeSH Terms] OR “hominidae”[MeSH Terms:noexp] OR “gorilla gorilla”[MeSH Terms] OR “pan paniscus”[MeSH Terms] OR “pan troglodytes”[MeSH Terms] OR “pongo pygmaeus”[MeSH Terms] animals[tiab] OR animal[tiab] OR mice[Tiab] OR mus[Tiab] OR mouse[Tiab] OR murine[Tiab] OR woodmouse[tiab] OR rats[Tiab] OR rat[Tiab] OR murinae[Tiab] OR muridae[Tiab] OR cottonrat[tiab] OR cottonrats[tiab] OR hamster[tiab] OR hamsters[tiab] OR cricetinae[tiab] OR rodentia[Tiab] OR rodent[Tiab] OR rodents[Tiab] OR pigs[Tiab] OR pig[Tiab] OR swine[tiab] OR swines[tiab] OR piglets[tiab] OR piglet[tiab] OR boar[tiab] OR boars[tiab] OR “sus scrofa”[tiab] OR ferrets[tiab] OR ferret[tiab] OR polecat[tiab] OR polecats[tiab] OR “mustela putorius”[tiab] OR “guinea pigs”[Tiab] OR “guinea pig”[Tiab] OR cavia[Tiab] OR callithrix[Tiab] OR marmoset[Tiab] OR marmosets[Tiab] OR cebuella[Tiab] OR hapale[Tiab] OR octodon[Tiab] OR chinchilla[Tiab] OR chinchillas[Tiab] OR gerbillinae[Tiab] OR gerbil[Tiab] OR gerbils[Tiab] OR jird[Tiab] OR jirds[Tiab] OR merione[Tiab] OR meriones[Tiab] OR rabbits[Tiab] OR rabbit[Tiab] OR hares[Tiab] OR hare[Tiab] OR cats[Tiab] OR cat[Tiab] OR felis[Tiab] OR dogs[Tiab] OR dog[Tiab] OR canine[Tiab] OR canines[Tiab] OR canis[Tiab] OR sheep[Tiab] OR sheeps[Tiab] OR mouflon[Tiab] OR mouflons[Tiab] OR ovis[Tiab] OR goats[Tiab] OR goat[Tiab] OR capra[Tiab] OR capras[Tiab] OR rupicapra[Tiab] OR chamois[Tiab] OR haplorhini[Tiab] OR monkey[Tiab] OR monkeys[Tiab] OR anthropoidea[Tiab] OR anthropoids[Tiab] OR saguinus[Tiab] OR tamarin[Tiab] OR tamarins[Tiab] OR leontopithecus[Tiab] OR hominidae[Tiab] OR ape[Tiab] OR apes[Tiab] OR pan[Tiab] OR paniscus[Tiab] OR “pan paniscus”[Tiab] OR bonobo[Tiab] OR bonobos[Tiab] OR “pan troglodytes”[Tiab] OR gibbon[Tiab] OR gibbons[Tiab] OR siamang[Tiab] OR siamangs[Tiab] OR nomascus[Tiab] OR symphalangus[Tiab] OR chimpanzee[Tiab] OR chimpanzees[Tiab] OR prosimians[Tiab] OR “bush baby”[Tiab] OR prosimian[Tiab] OR bush babies[Tiab] OR galagos[Tiab] OR galago[Tiab] OR pongidae[Tiab] OR gorilla[Tiab] OR gorillas[Tiab] OR pongo[Tiab] OR “pongo pygmaeus”[Tiab] OR orangutans[Tiab] OR lemur[Tiab] OR lemurs[Tiab] OR lemuridae[Tiab] OR horse[Tiab] OR horses[Tiab] OR pongo[Tiab] OR equus[Tiab] OR cow[Tiab] OR calf[Tiab] OR bull[Tiab] OR chicken[Tiab] OR chickens[Tiab] OR squirrel[Tiab] OR squirrels[Tiab] OR chipmunk[Tiab] OR chipmunks[Tiab] OR suslik[Tiab] OR susliks[Tiab] OR vole[Tiab] OR voles[Tiab] OR lemming[Tiab] OR lemmings[Tiab] OR muskrat[Tiab] OR muskrats[Tiab] OR lemmus[Tiab] OR otter[Tiab] OR otters[Tiab] OR marten[Tiab] OR martens[Tiab] OR martes[Tiab] OR weasel[Tiab] OR badger[Tiab] OR badgers[Tiab] OR ermine[Tiab] OR mink[Tiab] OR minks[Tiab] OR sable[Tiab] OR sables[Tiab] OR gulo[Tiab] OR gulos[Tiab] OR wolverine[Tiab] OR wolverines[Tiab] OR minks[Tiab] OR mustela[Tiab] OR llama[Tiab] OR llamas[Tiab] OR alpaca[Tiab] OR alpacas[Tiab] OR camelid[Tiab] OR camelids[Tiab] OR guanaco[Tiab] OR guanacos[Tiab] OR chiroptera[Tiab] OR chiropteras[Tiab] OR bat[Tiab] OR bats[Tiab] OR fox[Tiab] OR foxes[Tiab] OR donkey[Tiab] OR donkeys[Tiab] OR mule[Tiab] OR mules[Tiab] OR zebra[Tiab] OR zebras[Tiab] OR shrew[Tiab] OR shrews[Tiab] OR bison[Tiab] OR bisons[Tiab] OR buffalo[Tiab] OR buffaloes[Tiab] OR deer[Tiab] OR deers[Tiab] OR bear[Tiab] OR bears[Tiab] OR panda[Tiab] OR pandas[Tiab] OR “wild hog”[Tiab] OR “wild boar”[Tiab] OR fitchew[Tiab] OR fitch[Tiab] OR beaver[Tiab] OR beavers[Tiab] OR jerboa[Tiab] OR jerboas[Tiab] OR capybara[Tiab] OR capybaras[Tiab]) AND (“Attention”[Mesh] OR “attention”[tiab] OR “concentration”[tiab] OR “attentiveness”[tiab] OR “Behavior”[Mesh] OR “behavior”[tiab] OR “behaviour”[tiab] OR “behavioral”[tiab] OR “behavioural”[tiab] OR “behaviors”[tiab] OR “behaviours”[tiab] OR “Cognition”[Mesh] OR “Cognition Disorders”[Mesh] OR “cognition”[tiab] OR “cognitive”[tiab] OR “Developmental Disabilities”[Mesh] OR “developmental”[tiab] OR “Neurodevelopmental Disorders”[Mesh] OR “neurodevelopmental”[tiab] OR “neurodevelopment”[tiab] OR “neuropsychological”[tiab] OR “Executive Function”[Mesh] OR “executive function”[tiab] OR “executive functioning”[tiab] OR “Motor Activity”[Mesh] OR “locomotor”[tiab] OR “motor”[tiab] OR “Memory”[Mesh] OR “memory”[tiab] OR “Metacognition”[Mesh] OR “metacognition”[tiab] OR “metacognitive”[tiab] OR “Neurobehavioral Manifestations”[Mesh] OR “neurobehavioural”[tiab] OR “neurobehavioral”[tiab] OR “Neurotoxicity Syndromes”[Mesh] OR “neurotoxic”[tiab] OR “neurotoxicity” OR “neurotoxicant”[tiab] OR “neurotoxicants”[tiab] OR “neurotoxia”[tiab] OR “neurotoxicosis”[tiab] OR “processing speed”[tiab] OR “Spatial Learning”[Mesh] OR “spatial learning”[tiab] OR “Maze Learning”[Mesh] OR “maze”[tiab])
Embase
(‘flame retardant'/de OR ‘2,2',4,4',5,5' hexabromodiphenyl ether'/exp OR ‘polybrominated diphenyl ether'/exp OR ‘diphenyl ether derivative'/exp OR ((flame NEXT/1 retard*) OR (fire NEXT/1 retard*) OR (fireproofing NEXT/1 agent*) OR “FireMaster” OR “Bromkal” OR (‘diphenyl ether' NEXT/1 deriv*) OR (Halogenated NEXT/1 Diphenyl*) OR (Brominated NEXT/1 Diphenyl*) OR PBDE* OR (Polybrominated NEXT/1 Diphenyl*) OR polybromodiphenyl* OR PBDP* OR BDE* OR pentabromodiphenyl* OR PentaBDE* OR “PeBDE” OR “DE 71” OR “DE71” OR “pentabrominated diphenyl” OR “pentabrominated diphenyls” OR “PBDPO” OR “Planelon PB 501” OR (pentabromo NEXT/1 deriv*) OR Pentabromophenyl* OR octabromodiphenyl* OR OctaBDE* OR “OcBDE” OR “Octabrom” OR “OBDE” OR “OBDPO” OR (octabromo NEXT/1 deriv*) OR “octabrominated diphenyl” OR “octabrominated diphenyls” OR decabromodiphenyl* OR DecaBDE* OR “DeBDE” OR “DBDPO” OR “decabrominated diphenyl” OR “decabrominated diphenyls” OR (decabromo NEXT/1 deriv*) OR “Decabrom” OR “Berkflam B 10E” OR “FR 300BA” OR “FR 300 BA” OR tribromodiphenyl* OR “tribrominated diphenyl” OR “tribrominated diphenyls” OR “TrBDE” OR (tribromo NEXT/1 deriv*) OR tetrabromodiphenyl* OR TetraBDE* OR “TeBDE” OR “TBDE” OR “BPDE” OR (tetrabromo NEXT/1 deriv*) OR “TBDP” OR “tetrabrominated diphenyl” OR “tetrabrominated diphenyls” OR hexabromodiphenyl* OR HexaBDE* OR “HxBDE” OR “hexabrominated diphenyl” OR “hexabrominated diphenyls” OR (hexabromo NEXT/1 deriv*) OR heptabromodiphenyl* OR HeptaBDE* OR “HeBDE” OR “heptabrominated diphenyl” OR “heptabrominated diphenyls” OR (heptabromo NEXT/1 deriv*) OR nonabromodiphenyl* OR NonaBDE* OR “NoBDE” OR “nonabrominated diphenyl” OR “nonabrominated diphenyls” OR (nonabromo NEXT/1 deriv*)):ti,ab,tn,rn OR (“7025‐06‐1” OR “6876‐00‐2” OR “101‐55‐3” OR “51452‐87‐0” OR “44625414‐4” OR “147217‐72‐9” OR “171977‐44‐9” OR “147217‐71‐8” OR “33513‐663” OR “51930‐04‐2” OR “6903‐63‐5” OR “189084‐59‐1” OR “83694‐71‐7” OR “46438‐88‐4” OR “2050‐47‐7” OR “147217‐74‐1” OR “147217‐75‐2” OR “407606‐55‐7” OR “147217‐73‐0” OR “147217‐76‐3” OR “337513‐67‐4” OR “446254‐15‐5” OR “446254‐16‐6” OR “147217‐77‐4” OR “337513‐75‐4” OR “337513‐53‐8” OR “41318‐75‐6” OR “337513‐56‐1” OR “155999‐95‐4” OR “65075-08‐3” OR “189084‐60‐4” OR “147217‐78‐5” OR “446254‐17‐7” OR “147217‐80‐9” OR “147217‐79‐6” OR “147217‐81‐0” OR “337513‐54‐9” OR “337513‐68‐5” OR “446254‐18‐8” OR “446254‐19‐9” OR “446254‐20‐2” OR “446254‐22‐4” OR “5436‐43‐1” OR “337513‐55‐0” OR “243982‐82‐3” OR “446254-23‐5” OR “189084‐57‐9” OR “446254‐24‐6” OR “446254‐25‐7” OR “446254‐31‐5” OR “446254‐32‐6” OR “446254‐33‐7” OR “446254‐34‐8” OR “189084‐61‐5” OR “446254‐37‐1” OR “446254‐38‐2” OR “327185‐09‐1” OR “446254‐39‐3” OR “189084‐62‐6” OR “446254‐40‐6” OR “446254‐417” OR “446254‐42‐8” OR “189084‐63‐7” OR “446254‐43‐9” OR “93703‐481” OR “446254‐45‐1” OR “446254‐48‐4” OR “103173‐66‐6” OR “446254‐508” OR “446254‐51‐9” OR “182346‐21‐0” OR “446254‐53‐1” OR “446254‐542” OR “446254‐55‐3” OR “446254‐55‐3” OR “446254‐57‐5” OR “44625459‐7” OR “446254‐61‐1” OR “446254‐64‐4” OR “38463‐82‐0” OR “6034860‐9” OR “189084-64‐8” OR “446254‐65‐5” OR “446254‐66‐6” OR “446254‐67‐7” OR “446254‐68‐8” OR “373594‐78‐6” OR “446254‐69‐9” OR “446254‐71‐3” OR “446254‐72‐4” OR “446254‐74‐6” OR “446254‐779” OR “446254‐78‐0” OR “189084‐65‐9” OR “446254‐80‐4” OR “18908466‐0” OR “182677‐30‐1” OR “243982‐83‐4” OR “68631‐49‐2” OR “20712215‐4” OR “35854‐94‐5” OR “189084‐58‐0” OR “189084-67‐1” OR “20712216‐5” OR “189084‐68‐2” OR “1163‐19‐5” OR “109945‐70‐2” OR “113152‐37‐7” OR “113172‐79‐5” OR “139598‐16‐6” OR “139749‐52‐3” OR “145538‐74‐5” OR “32534‐81‐9” OR “32536-52‐0” OR “40088‐47‐9” OR “446254‐27‐9” OR “446255‐20‐5” OR “446255‐22‐7” OR “49690‐94‐0” OR “63936‐56‐1” OR “64589‐00‐0” OR “68928‐80‐3” OR “85446‐17‐9” OR “36483‐60‐0” OR “437701‐79‐6” OR “446255‐26‐1” OR “117948‐63‐7” OR “446255‐30‐7” OR “61262‐53‐1” OR “405237‐85‐6” OR “39275‐89‐3” OR “13654‐09‐6” OR “61288‐13‐9” OR “446255‐39‐6” OR “337513-72‐1” OR “366791‐32‐4” OR “2050‐47‐7”):ti,ab,rn) AND (‘ape'/de OR ‘bat'/exp OR ‘carnivora'/exp OR ‘catarrhini'/de OR ‘cercopithecidae'/exp OR ‘cetacea'/exp OR ‘chimpanzee'/exp OR ‘chordata'/de OR ‘elephant'/exp OR ‘gorilla'/exp OR ‘haplorhini'/de OR ‘hominid'/de OR ‘hylobatidae'/exp OR ‘hyrax'/exp OR ‘lagomorph'/exp OR ‘mammal'/de OR ‘marsupial'/exp OR ‘monotremate'/exp OR ‘orangutan'/exp OR ‘placental mammals'/de OR ‘platyrrhini'/exp OR ‘primate'/de OR ‘prosimian'/exp OR ‘rodent'/exp OR ‘scandentia'/exp OR ‘simian'/de OR ‘sirenia'/exp OR ‘tarsiiform'/exp OR ‘ungulate'/exp OR ‘vertebrate'/de OR ‘xenarthra'/exp OR animals:ti,ab OR animal:ti,ab OR mice:ti,ab OR mus:ti,ab OR mouse:ti,ab OR murine:ti,ab OR woodmouse:ti,ab OR rats:ti,ab OR rat:ti,ab OR murinae:ti,ab OR muridae:ti,ab OR cottonrat:ti,ab OR cottonrats:ti,ab OR hamster:ti,ab OR hamsters:ti,ab OR cricetinae:ti,ab OR rodentia:ti,ab OR rodent:ti,ab OR rodents:ti,ab OR pigs:ti,ab OR pig:ti,ab OR swine:ti,ab OR swines:ti,ab OR piglets:ti,ab OR piglet:ti,ab OR boar:ti,ab OR boars:ti,ab OR “sus scrofa”:ti,ab OR ferrets:ti,ab OR ferret:ti,ab OR polecat:ti,ab OR polecats:ti,ab OR “mustela putorius”:ti,ab OR “guinea pigs”:ti,ab OR “guinea pig”:ti,ab OR cavia:ti,ab OR callithrix:ti,ab OR marmoset:ti,ab OR marmosets:ti,ab OR cebuella:ti,ab OR hapale:ti,ab OR octodon:ti,ab OR chinchilla:ti,ab OR chinchillas:ti,ab OR gerbillinae:ti,ab OR gerbil:ti,ab OR gerbils:ti,ab OR jird:ti,ab OR jirds:ti,ab OR merione:ti,ab OR meriones:ti,ab OR rabbits:ti,ab OR rabbit:ti,ab OR hares:ti,ab OR hare:ti,ab OR cats:ti,ab OR cat:ti,ab OR felis:ti,ab OR dogs:ti,ab OR dog:ti,ab OR canine:ti,ab OR canines:ti,ab OR canis:ti,ab OR sheep:ti,ab OR sheeps:ti,ab OR mouflon:ti,ab OR mouflons:ti,ab OR ovis:ti,ab OR goats:ti,ab OR goat:ti,ab OR capra:ti,ab OR capras:ti,ab OR rupicapra:ti,ab OR chamois:ti,ab OR haplorhini:ti,ab OR monkey:ti,ab OR monkeys:ti,ab OR anthropoidea:ti,ab OR anthropoids:ti,ab OR saguinus:ti,ab OR tamarin:ti,ab OR tamarins:ti,ab OR leontopithecus:ti,ab OR hominidae:ti,ab OR ape:ti,ab OR apes:ti,ab OR pan:ti,ab OR paniscus:ti,ab OR “pan paniscus”:ti,ab OR bonobo:ti,ab OR bonobos:ti,ab OR “pan troglodytes”:ti,ab OR gibbon:ti,ab OR gibbons:ti,ab OR siamang:ti,ab OR siamangs:ti,ab OR nomascus:ti,ab OR symphalangus:ti,ab OR chimpanzee:ti,ab OR chimpanzees:ti,ab OR prosimians:ti,ab OR “bush baby”:ti,ab OR prosimian:ti,ab OR bush babies:ti,ab OR galagos:ti,ab OR galago:ti,ab OR pongidae:ti,ab OR gorilla:ti,ab OR gorillas:ti,ab OR pongo:ti,ab OR “pongo pygmaeus”:ti,ab OR orangutans:ti,ab OR lemur:ti,ab OR lemurs:ti,ab OR lemuridae:ti,ab OR horse:ti,ab OR horses:ti,ab OR pongo:ti,ab OR equus:ti,ab OR cow:ti,ab OR calf:ti,ab OR bull:ti,ab OR chicken:ti,ab OR chickens:ti,ab OR squirrel:ti,ab OR squirrels:ti,ab OR chipmunk:ti,ab OR chipmunks:ti,ab OR suslik:ti,ab OR susliks:ti,ab OR vole:ti,ab OR voles:ti,ab OR lemming:ti,ab OR lemmings:ti,ab OR muskrat:ti,ab OR muskrats:ti,ab OR lemmus:ti,ab OR otter:ti,ab OR otters:ti,ab OR marten:ti,ab OR martens:ti,ab OR martes:ti,ab OR weasel:ti,ab OR badger:ti,ab OR badgers:ti,ab OR ermine:ti,ab OR mink:ti,ab OR minks:ti,ab OR sable:ti,ab OR sables:ti,ab OR gulo:ti,ab OR gulos:ti,ab OR wolverine:ti,ab OR wolverines:ti,ab OR minks:ti,ab OR mustela:ti,ab OR llama:ti,ab OR llamas:ti,ab OR alpaca:ti,ab OR alpacas:ti,ab OR camelid:ti,ab OR camelids:ti,ab OR guanaco:ti,ab OR guanacos:ti,ab OR chiroptera:ti,ab OR chiropteras:ti,ab OR bat:ti,ab OR bats:ti,ab OR fox:ti,ab OR foxes:ti,ab OR donkey:ti,ab OR donkeys:ti,ab OR mule:ti,ab OR mules:ti,ab OR zebra:ti,ab OR zebras:ti,ab OR shrew:ti,ab OR shrews:ti,ab OR bison:ti,ab OR bisons:ti,ab OR buffalo:ti,ab OR buffaloes:ti,ab OR deer:ti,ab OR deers:ti,ab OR bear:ti,ab OR bears:ti,ab OR panda:ti,ab OR pandas:ti,ab OR “wild hog”:ti,ab OR “wild boar”:ti,ab OR fitchew:ti,ab OR fitch:ti,ab OR beaver:ti,ab OR beavers:ti,ab OR jerboa:ti,ab OR jerboas:ti,ab OR capybara:ti,ab OR capybaras:ti,ab) AND (“attention”/exp OR “attention”:ti,ab OR “concentration”:ti,ab OR “attentiveness”:ti,ab OR “behavior”/exp OR “behavior”:ti,ab OR “behaviour”:ti,ab OR “behavioral”:ti,ab OR “behavioural”:ti,ab OR “behaviors”:ti,ab OR “behaviours”:ti,ab OR “cognition”/exp OR “cognition”:ti,ab OR “cognitive”:ti,ab OR “cognition assessment”/exp OR “developmental disorder”/exp OR “developmental”:ti,ab OR “executive function”/exp OR “executive function”:ti,ab OR “executive functioning”:ti,ab OR “motor activity”/exp OR “locomotor”:ti,ab OR “motor”:ti,ab OR “memory”/exp OR “memory”:ti,ab OR “metacognition”/exp OR “metacognition”:ti,ab OR “metacognitive”:ti,ab OR “neurobehavioural”:ti,ab OR “neurobehavrioral”:ti,ab OR “neurotoxicity”/exp OR “neurotoxic”:ti,ab OR “neurotoxicity” OR “neurotoxicant”:ti,ab OR “neurotoxicants”:ti,ab OR “neurotoxia”:ti,ab OR “neurotoxicosis”:ti,ab OR “processing speed”:ti,ab OR “spatial learning”/exp OR “spatial learning”:ti,ab OR “maze test”/exp OR “maze”:ti,ab)
Toxline
(“flame retard*” OR “fire retard*” OR “fireproofing agent*” OR “FireMaster” OR “Bromkal” OR “diphenyl ether deriv*” OR “Halogenated Diphenyl*” OR “Brominated Diphenyl*” OR PBDE* OR “Polybrominated Diphenyl*” OR polybromodiphenyl* OR PBDP* OR BDE* OR pentabromodiphenyl* OR “c‐pentaBDE*” OR PentaBDE* OR “PeBDE” OR “DE 71” OR “DE71” OR “pentabrominated diphenyl*” OR “PBDPO” OR “Planelon PB 501” OR “pentabromo deriv*” OR Pentabromophenyl* OR octabromodiphenyl* OR “c‐octaBDE*” OR OctaBDE* OR “OcBDE” OR “Octabrom” OR “octabromo deriv*” OR “OBDE” OR “OBDPO” OR “octabrominated diphenyl*” OR decabromodiphenyl* OR “cdecaBDE*” OR DecaBDE* OR “DeBDE” OR “DBDPO” OR “decabrominated diphenyl*” OR “decabromo deriv*” OR “Decabrom” OR “Berkflam B 10E” OR “FR 300BA” OR “FR 300 BA” OR tribromodiphenyl* OR “tribrominated diphenyl*” OR “TrBDE” OR “tribromo deriv*” OR tetrabromodiphenyl* OR TetraBDE* OR “TeBDE” OR “TBDE” OR “BPDE” OR “tetrabromo deriv*” OR “TBDP” OR “tetrabrominated diphenyl*” OR hexabromodiphenyl* OR HexaBDE* OR “HxBDE” OR “hexabrominated diphenyl*” OR “hexabromo deriv*” OR heptabromodiphenyl* OR HeptaBDE* OR “HeBDE” OR “heptabrominated diphenyl*” OR “heptabromo deriv*” OR nonabromodiphenyl* OR NonaBDE* OR “NoBDE” OR “nonabrominated diphenyl*” OR “nonabromo deriv*” OR “7025‐06‐1” OR “6876‐00‐2” OR “101‐55‐3” OR “51452‐87‐0” OR “446254‐14‐4” OR “147217‐72‐9” OR “171977‐44‐9” OR “147217‐71‐8” OR “33513‐66‐3” OR “51930‐04‐2” OR “6903‐63‐5” OR “189084‐59‐1” OR “83694‐71-7” OR “46438‐88‐4” OR “2050‐47‐7” OR “147217‐74‐1” OR “147217‐75‐2” OR “407606‐55‐7” OR “147217‐73‐0” OR “147217‐763” OR “337513‐67‐4” OR “446254‐15‐5” OR “446254‐16‐6” OR “14721777‐4” OR “337513‐75‐4” OR “337513‐53‐8” OR “41318‐75‐6” OR “337513‐56‐1” OR “155999‐95‐4” OR “65075‐08‐3” OR “189084‐60‐4” OR “147217‐78‐5” OR “446254‐17‐7” OR “147217‐80‐9” OR “147217‐796” OR “147217‐81‐0” OR “337513‐54‐9” OR “337513‐68‐5” OR “44625418‐8” OR “446254‐19‐9” OR “446254‐20‐2” OR “446254‐22‐4” OR “5436‐43‐1” OR “337513-55‐0” OR “243982‐82‐3” OR “446254‐23‐5” OR “189084‐57‐9” OR “446254‐24‐6” OR “446254‐25‐7” OR “446254‐31‐5” OR “446254‐32‐6” OR “446254‐33‐7” OR “446254‐348” OR “189084‐61‐5” OR “446254‐37‐1” OR “446254‐38‐2” OR “327185‐09‐1” OR “446254‐39‐3” OR “189084‐62‐6” OR “446254‐406” OR “446254‐41‐7” OR “446254‐42‐8” OR “189084‐63‐7” OR “446254‐43‐9” OR “93703‐48‐1” OR “446254‐45‐1” OR “446254‐48‐4” OR “103173‐66‐6” OR “446254‐50‐8” OR “446254‐51‐9” OR “18234621‐0” OR “446254‐53‐1” OR “446254‐54‐2” OR “446254‐55‐3” OR “446254‐55‐3” OR “446254‐57‐5” OR “446254‐59‐7” OR “446254‐611” OR “446254‐64‐4” OR “38463‐82‐0” OR “60348‐60‐9” OR “189084‐64‐8” OR “446254‐65‐5” OR “446254‐66‐6” OR “446254-677” OR “446254‐68‐8” OR “373594‐78‐6” OR “446254‐69‐9” OR “446254‐71‐3” OR “446254‐72‐4” OR “446254‐74‐6” OR “446254‐779” OR “446254‐78‐0” OR “189084‐65‐9” OR “446254‐80‐4” OR “189084‐66‐0” OR “182677‐30‐1” OR “243982‐83‐4” OR “68631‐49‐2” OR “207122‐15‐4” OR “35854-94‐5” OR “189084‐58‐0” OR “18908467‐1” OR “207122‐16‐5” OR “189084‐68‐2” OR “1163‐19‐5” OR “109945‐70‐2” OR “113152‐37‐7” OR “113172‐79‐5” OR “139598‐16‐6” OR “139749‐52‐3” OR “145538‐74‐5” OR “32534‐81‐9” OR “32536‐520” OR “40088‐47‐9” OR “446254‐27‐9” OR “446255-20‐5” OR “446255‐22‐7” OR “49690‐94‐0” OR “63936‐56‐1” OR “64589‐00‐0” OR “68928‐80‐3” OR “85446‐17‐9” OR “36483‐60‐0” OR “437701‐796” OR “446255‐26‐1” OR “117948‐63‐7” OR “446255-30‐7” OR “6126253‐1” OR “405237‐85‐6” OR “39275‐89‐3” OR “13654‐09‐6” OR “61288‐13‐9” OR “446255‐39‐6” OR “337513‐72‐1” OR “366791‐32‐4” OR “2050‐47‐7”) AND (animals OR animal OR mice OR mus OR mouse OR murine OR woodmouse OR rats OR rat OR murinae OR muridae OR cottonrat OR cottonrats OR hamster OR hamsters OR cricetinae OR rodentia OR rodent OR rodents OR pigs OR pig OR swine OR swines OR piglets OR piglet OR boar OR boars OR “sus scrofa” OR ferrets OR ferret OR polecat OR polecats OR “mustela putorius” OR “guinea pigs” OR “guinea pig” OR cavia OR callithrix OR marmoset OR marmosets OR cebuella OR hapale OR octodon OR chinchilla OR chinchillas OR gerbillinae OR gerbil OR gerbils OR jird OR jirds OR merione OR meriones OR rabbits OR rabbit OR hares OR hare OR cats OR cat OR felis OR dogs OR dog OR canine OR canines OR canis OR sheep OR sheeps OR mouflon OR mouflons OR ovis OR goats OR goat OR capra OR capras OR rupicapra OR chamois OR haplorhini OR monkey OR monkeys OR anthropoidea OR anthropoids OR saguinus OR tamarin OR tamarins OR leontopithecus OR hominidae OR ape OR apes OR pan OR paniscus OR “pan paniscus” OR bonobo OR bonobos OR “pan troglodytes” OR gibbon OR gibbons OR siamang OR siamangs OR nomascus OR symphalangus OR chimpanzee OR chimpanzees OR prosimians OR “bush baby” OR prosimian OR bush babies OR galagos OR galago OR pongidae OR gorilla OR gorillas OR pongo OR “pongo pygmaeus” OR orangutans OR lemur OR lemurs OR lemuridae OR horse OR horses OR pongo OR equus OR cow OR calf OR bull OR chicken OR chickens OR squirrel OR squirrels OR chipmunk OR chipmunks OR suslik OR susliks OR vole OR voles OR lemming OR lemmings OR muskrat OR muskrats OR lemmus OR otter OR otters OR marten OR martens OR martes OR weasel OR badger OR badgers OR ermine OR mink OR minks OR sable OR sables OR gulo OR gulos OR wolverine OR wolverines OR minks OR mustela OR llama OR llamas OR alpaca OR alpacas OR camelid OR camelids OR guanaco OR guanacos OR chiroptera OR chiropteras OR bat OR bats OR fox OR foxes OR donkey OR donkeys OR mule OR mules OR zebra OR zebras OR shrew OR shrews OR bison OR bisons OR buffalo OR buffaloes OR deer OR deers OR bear OR bears OR panda OR pandas OR “wild hog” OR “wild boar” OR fitchew OR fitch OR beaver OR beavers OR jerboa OR jerboas OR capybara OR capybaras) AND (“Exposure” OR “Exposed” OR “exposures” OR “exposing”) AND (“attention” OR “concentration” OR “attentiveness” OR “behavior” OR “behaviour” OR “behavioral” OR “behavioural” OR “behaviors” OR “behaviours” OR “Cognition Disorders”[Mesh] OR “cognition” OR “cognitive” OR “developmental” OR “executive function” OR “executive functioning” OR “locomotor” OR “motor” OR “memory” OR “metacognition” OR “metacognitive” OR “neurobehavioural” OR “neurobehavrioral” OR “neurotoxic” OR “neurotoxicity” OR “neurotoxicant” OR “neurotoxicants” OR “neurotoxia” OR “neurotoxicosis” OR “processing speed” OR “spatial learning” OR “maze”)
SECTION E-1c. SCREENING FORMS
Title and Abstract Screening Form
Instructions: When a citation is excluded, reason should be specified.
| Exclusion Reasons | |
| No original data (e.g., review article, commentary, editorial) | |
| Study does not include nonhuman mammals | |
| Study does not report PBDE exposure | |
| No relevant outcomes | |
| Incomplete information (e.g., conference abstract, meeting poster) | |
| Not in English and unable to determine eligibility | |
| Other (explanation required) |
Full-Text Screening Form
Instructions: When a citation is excluded, reason should be specified.
| Exclusion Reasons | |
| No original data (e.g., review article, commentary, editorial) | |
| Study does not include nonhuman mammals | |
| Study does not report PBDE exposure | |
| Study does not quantify exposure to PBDE | |
| Study does not include developmental exposure | |
| Study does not assess or report quantitative measures of learning, memory, attention, or response inhibition | |
| No comparator group (different doses or vehicle-only treatment) | |
| Not in English and unable to determine eligibility | |
| Other (explanation required) |
SECTION E-1d. DATA EXTRACTION ELEMENTS FOR ANIMAL STUDIES
| Funding | Funding source(s) |
| Reporting of COI by authors (*reporting bias) | |
| Animal Model | Sex |
| Species | |
| Strain | |
| Source of animals | |
| Age or life stage at start of dosing and at health outcome assessment | |
| Diet and husbandry information (e.g., diet name/source) | |
| Treatment | Chemical name and CAS number |
| Source of chemical | |
| Purity of chemical (*information bias) | |
| Dose levels or concentration (as presented and converted to mg/kg bw/d when possible) | |
| Other dose-related details, such as whether administered dose level was verified by measurement, information on internal dosimetry (*information bias) | |
| Vehicle used for exposed animals | |
| Route of administration (e.g., oral, inhalation, dermal, injection) | |
| Duration and frequency of dosing (e.g., hours, days, weeks when administration was ended, days per week) | |
| Methods | Study design (e.g., single treatment, acute, subchronic (e.g., 90 days in a rodent), chronic, multigenerational, developmental, other) |
| Guideline compliance (i.e., use of EPA, OECD, NTP or another guideline for study design, conducted under GLP guideline conditions, non-GLP but consistent with guideline study, non-guideline peer-reviewed publication) | |
| Number of animals per group (and dams per group in developmental studies) (*missing data bias) | |
| Randomization procedure, allocation concealment, blinding during outcome assessment (*selection bias) | |
| Method to control for litter effects in developmental studies (*information bias) | |
| Use of negative controls and whether controls were untreated, vehicle-treated, or both | |
| Report on data from positive controls—was expected response observed? (*information bias) | |
| End point health category (e.g., reproductive) | |
| End point (e.g., infertility) | |
| Diagnostic or method to measure end point (*information bias) | |
| Statistical methods (*information bias) | |
| Results | Measures of effect at each dose or concentration level (e.g., mean, median, frequency, and measures of precision or variance) or description of qualitative results. When possible, measures of effect will be converted to a common metric with associated 95% confidence intervals (CI). Most often, measures of effect for continuous data will be expressed as mean difference, standardized mean difference, and percent control response. Categorical data will be expressed as relative risk (RR, also called risk ratio). |
| No-observed-effect level (NOEL), lowest-observed-effect level (LOEL), benchmark dose (BMD) analysis, statistical significance of other dose levels, or other estimates of effect presented in paper. Note: The NOEL and LOEL are highly influenced by study design do not give any quantitative information about the relationship between dose and response; and can be subject to author's interpretation (e.g., a statistically significant effect may not be considered biologically important). Also, a NOEL does not necessarily mean zero response. Ideally, the response rate at specific dose levels is used as the primary measure to characterize the response. | |
| Observations on dose response (e.g., trend analysis, description of whether dose-response shape appears to be monotonic, nonmonotonic) | |
| Data on internal concentration, toxicokinetics, or toxicodynamics (when reported) | |
| Other | Documentation of author queries, use of digital rulers to estimate data values from figures, exposure unit, and statistical result conversions, etc. |
Items marked with an asterisk (*) are examples of items that can be used to assess internal validity/risk of bias.
SECTION E-1e. RISK OF BIAS QUESTIONS FOR ANIMAL STUDIES
1. Was administered dose or exposure level adequately randomized?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
2. Was allocation to study groups adequately concealed?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
3. Did selection of study participants result in the appropriate comparison groups? [NA]
4. Did study design or analysis account for important confounding and modifying variables? [NA]
5. Were experimental conditions identical across study groups?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
6. Were the research personnel blinded to the study group during the study?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
7. Were outcome data complete without attrition or exclusion from analysis?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
8. Can we be confident in the exposure characterization?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
9. Can we be confident in the outcome assessment?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
10. Were all measured outcomes reported?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
11. Was litter or litter effects considered appropriately in the statistical analyses and were there no other potential threats to internal validity?
Because this evaluation is focused on developmental exposure, this question was added to address litter effects in data analysis. This question will be used to examine individual studies for appropriate statistical methods (e.g., confirmation of homogeneity of variance for ANOVA and other statistical tests that require normally distributed data). It will also be used for risk of bias considerations that do not fit under the other questions.
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
SECTION E-1f. AMENDMENTS TO THE PROTOCOL
Original review team members Barbara Hales and Susan Schantz were replaced by the following committee members who have more experience conducting risk of bias evaluations and data extraction:
- David C. Dorman (Chair) is a professor of toxicology in the Department of Molecular Biosciences of North Carolina State University. The primary objective of his research is to provide a refined understanding of chemically induced neurotoxicity in laboratory animals that will lead to improved assessment of potential toxicity in humans. Dr. Dorman's research interests include neurotoxicology, nasal toxicology, pharmacokinetics, and cognition and olfaction in animals. He has chaired or served on several NRC committees, including the Committee on Design and Evaluation of Safer Chemical Substitutions: A Framework to Inform Government and Industry Decisions, the Committee to Review EPA's Draft IRIS Assessment of Formaldehyde, and the Committee to Review the IRIS Process. He has served on other advisory boards for the US Navy, NASA, and USDA, and is currently a member of NTP's Board of Scientific Counselors. Dr. Dorman is an elected fellow of the Academy of Toxicological Sciences and a fellow of the American Association for the Advancement of Sciences. He received a DVM from Colorado State University. He completed a combined PhD and residency program in toxicology at the University of Illinois at Urbana-Champaign, and he is a diplomate of the American Board of Veterinary Toxicology and the American Board of Toxicology.
- Andrew A. Rooney is deputy director of the Office of Health Assessment and Translation (OHAT) in the National Toxicology Program at the National Institute of Environmental Health Sciences. He has been developing risk assessment methods and guidance throughout his professional career and is a principal author of the 2012 WHO/IPCS Guidance for Immunotoxicity Risk Assessment for Chemicals. Most recently, he has been working on emerging issues in toxicology and environmental health, including methods to address study quality in terms of risk of bias for human, animal, and mechanistic studies and adaptation of systematic review methods for addressing environmental health questions. He led the team that developed the OHAT approach to systematic review. Dr. Rooney has an MS and a PhD in zoology from the University of Florida.
SECTION E-2. Results of Literature Searches for Animal Studies on the Effects of Developmental Exposure to PBDEs on Learning, Memory, Attention, or Response Inhibition
Literature searches were performed on August 15, 2016, using the search strategy presented in the PBDE (Animal) Systematic Review Protocol (Section E-1). A summary of the results is presented below.
| Embase: | 1,326 |
| PubMed: | 1,173 |
| Toxline: | 489 |
| Total citations found: | 2,988 |
| Duplicates removed: | 1,137 |
| Total unique citations: | 1,851 |
SECTION E-3. Funding Sources of the Animal Studies on PBDEs and Learning, Memory, or Attention
Sources of funding were used to evaluate publication bias in terms of whether a particular sector funded more studies than another.
| Reference | Governmental NIH/Federal | Industry | Other | Unknown |
|---|---|---|---|---|
| Biesemeier et al. 2011 | X | |||
| Blanco et al. 2013 | X (Spain) | |||
| Bowers et al. 2015 | X (Canada) | |||
| Buratovic et al. 2014 | X (Sweden) | |||
| Chen et al. 2014 | X (China) | |||
| Cheng et al. 2009 | X (China) | |||
| de-Miranda et al. 2016 | X (Brazil) | |||
| Driscoll et al. 2009 | X (Colorado) | |||
| Driscoll et al. 2012 | X | |||
| Dufault et al. 2005 | X (Colorado) | |||
| Eriksson et al. 2001 | X (Sweden) | |||
| Fischer et al. 2008 | X (Sweden) | |||
| He et al. 2009 | X (China) | |||
| He et al. 2011 | X (China) | |||
| Koenig et al. 2012 | X (NIEHS) | JB Johnson Foundation | ||
| Llansola et al. 2009 | X (EU) | |||
| Reverte et al. 2013 | X (FEDR; EU) | |||
| Reverte et al. 2014 | X (FEDR; EU) | |||
| Rice et al. 2009 | X (Maine) | |||
| Ta et al. 2011 | X (NIEHS; EPA) | |||
| Verma et al. 2013 | X (India) | |||
| Verma et al. 2014 | X (India) | |||
| Viberg et al. 2003 | X (Sweden; EU) | |||
| Viberg et al. 2006 | X (EU) | |||
| Woods et al. 2012 | X (NIH; NIEHS; EPA) | |||
| Zhang et al. 2013 | X (China) | |||
| Zhao et al. 2014 | X (China) |
SECTION E-4. Confidence Ratings for the Body of Evidence from Animal Studies of PBDEs
The body of evidence from animal studies of the PBDEs and learning, memory, and attention, were rated in accordance with the guidance presented in Section E-1. No studies of response inhibition were found.
BDE-47
Studies of BDE-47 and effects on learning (see Table E4-1) and memory (see Table E4-2) were available.
Learning: There is moderate confidence in the body of evidence on developmental exposure to BDE-47 and effects on learning in rodents. Six studies in mice and rats were available. The two studies in rats found several indications of decreased learning in the Morris water maze (e.g., prolonged latency periods) after treatment with BDE-47 at doses of 1, 5, or 10 mg/kg-day on PND 10. Both studies were from the same laboratory (He et al. 2009, 2011). Three of the four mouse studies reported decreased learning in at least one test, strain, or sex and were conducted by different research groups (Eriksson et al. 2001; Ta et al. 2011; Koenigs et al. 2012; Woods et al. 2012). Nevertheless, the mouse results were variable across all the tests administered and a clear pattern was not identified to explain the heterogeneity in response relative to a susceptible strain, sex, or dose.
- Risk of bias: Downgraded because all studies had at least one rating of probably high or definitely high risk of bias in one of the key issues (e.g., lack of randomization of treatment), and most of the studies had multiple risk of bias issues, including not controlling for litter effects in the study design or analysis (see Figure E4-1).
- Unexplained inconsistencies: A qualitative evaluation of the evidence suggested a possible downgrade because of the heterogeneity in the evidence. However, a meta-analysis of studies of several PBDEs (see Chapter 4 and Appendix E, Section E-5), including BDE-47, and latency in the last trial of the Morris water maze showed consistent evidence of an effect on this measure of learning, so confidence was not downgraded.
- Indirectness: No downgrade because tests used are considered direct measures of learning.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade. Only two studies in rats were available, and the studies were from the same laboratory. The evidence base was judged to be inadequate for making judgments about cross-species consistency.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
TABLE E4-1
Studies of BDE-47 and Learning in Rodents.
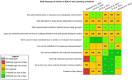
FIGURE E4-1
Risk of bias heatmap of studies of BDE-47 and learning in rodents. In HAWC: https://hawcproject.org/summary/visual/353/.
Memory: There is low confidence in the body of evidence on developmental exposure to BDE-47 and effects on memory in rodents. Five studies in rats and mice were available. The one study in rats (He et al. 2011) reported decreased memory in the Morris water maze (e.g., prolonged latency periods) after exposure at 1, 5, and 10 mg/kg-day on PND 10. Three of the mouse studies (Eriksson et al. 2001; Ta et al. 2011; Koenig et al. 2012) reported no effects on memory at doses up to 12 mg/kg-day; however, Woods et al. (2012) reported decrements in memory in female Mecp2 308+/− mice with no effects on males or in C57BL6 mice of either sex. The body of evidence contained only a single study in rats and inconsistent results in mice. The dataset is similar and contains some of the same studies discussed above with respect to effects of BDE-47 on learning; fewer studies reported an effect, however, and one less study overall.
- Risk of bias: Downgraded because all the studies had a probably high risk of bias rating for at least one major issue (e.g., researchers were not blinded to the study groups during outcome assessment), and most of the studies had multiple risk of bias issues, including not controlling for litter effects in the study design or analysis (Eriksson et al. 2001; Woods et al. 2012) (see Figure E4-2).
- Unexplained inconsistencies: Downgraded because of the heterogeneity of the evidence.
- Indirectness: No downgrade because tests used are considered direct measures of memory.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade. Only one study in rats was available, so the evidence base was judged to be inadequate to make judgments about cross-species consistency.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
TABLE E4-2
Studies of BDE-47 and Memory in Rodents.
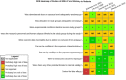
FIGURE E4-2
Risk of bias heatmap of studies of BDE-47 and memory in rodents. In HAWC: https://hawcproject.org/summary/visual/354/.
BDE-99
Studies of BDE-99 and effects on learning (see Table E4-3) and memory (see Table E4-4) were available.
TABLE E4-3
Studies of BDE-99 and Learning in Rodents.
Learning: There is moderate confidence in the body of evidence on developmental exposure to BDE-99 and learning in mice and rats based on five studies. Two of the three studies in rats reported longer latency during the acquisition period of tests using the Morris water maze at a dose of 2 mg/kg-day (Cheng et al. 2009; Blanco et al. 2013). Zhao et al. (2014) reported no effects at a lower dose (0.2 mg/kg-day) under similar exposure and testing conditions. In contrast, developmental exposure of Wistar rats at doses up to 30 mg/kg-day had no effect on learning tested with a Y maze (Llansola et al. 2009). A single study (Fischer et al. 2008) in NMRI mice also reported decrements in learning during the acquisition period in tests using either a radial maze or a Morris water maze at a dose of 0.8 mg/kg-day.
- Risk of bias: Downgraded because of serious concerns about several risk of bias issues. All of the studies were rated as having probably high risk of bias for at least one key risk of bias issue (e.g., researchers were not blinded to the study groups during outcome assessment), two of the studies had a definitely high risk of bias rating for not controlling for litter effects in the study design or analysis, and most of the studies had multiple risk of bias issues (see Figure E4-3).
- Unexplained inconsistencies: A qualitative evaluation of the evidence suggested a possible downgrade because of the heterogeneity in the evidence. Nevertheless, a meta-analysis of studies of several PBDEs (see Chapter 4 and Appendix E, Section E-5), including BDE-99, and latency in the last trial of the Morris water maze showed consistent evidence of an effect on this measure of learning, so confidence was not downgraded.
- Indirectness: No downgrade because tests used were considered direct measures of learning.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
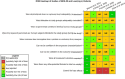
FIGURE E4-3
Risk of bias heatmap of studies of BDE-99 and learning in rodents. In HAWC: https://hawcproject.org/summary/visual/355/.
TABLE E4-4
Studies of BDE-99 and Memory in Rodents.
Memory: There is moderate confidence in the data to evaluate whether developmental exposure to BDE-99 affects memory in rodents. The three available studies found no effects in several memory tests at doses of 0.2-2 mg/kg-day.
- Risk of bias: Downgraded because of serious concerns about several risk of bias issues. All the studies had a rating of probably high risk of bias for at least one key risk of bias issue (e.g., researchers were not blinded to the study groups during outcome assessment) and one study had a definitely high risk of bias rating for not controlling for litter effects in the study design or analysis (see Figure E4-4).
- Unexplained inconsistencies: No downgrade for inconsistency.
- Indirectness: No downgrade because tests used are considered direct measures of memory.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade because one study in mice and two in rats is insufficient to upgrade for evidence of consistency across species for a no-effect finding.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
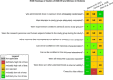
FIGURE E4-4
Risk of bias heatmap of studies of BDE-99 and memory in rodents. In HAWC: https://hawcproject.org/summary/visual/356/.
BDE-153
Studies of BDE-153 and effects on learning (see Table E4-5) and memory (see Table E4-6) were available.
TABLE E4-5
Studies of BDE-153 and Learning in Rodents.
Learning: There is low confidence in the body of evidence to evaluate whether developmental exposure to BDE-153 affects learning in mice or rats. Two studies were available, one in mice and one in rats. Viberg et al. (2003) reported longer latencies in the acquisition period in the Morris water maze when mice were exposed to BDE-153 at 0.9 or 9 mg/kg-day on PND 10. The rat study (Zhang et al. 2013) reported no effect of BDE-153 at 10 mg/kg-day in performance in either the Morris water maze or the passive avoidance test when evaluated at PND 40 or PND 70.
- Risk of bias: Downgraded twice because of serious concerns about multiple risk of bias issues, including lack of randomization of treatment; reduced confidence in outcome assessment due to lack of blinding of outcome assessors; and definitely high risk of bias ratings for not controlling for litter effects in the study design or analysis (see Figure E4-5).
- Unexplained inconsistencies: A qualitative evaluation of the evidence suggested a possible downgrade because of the heterogeneity in the evidence. Nevertheless, a meta-analysis of studies of several PBDEs (see Chapter 4 and Appendix E, Section E-5), including BDE-153, and of latency in the last trial of the Morris water maze showed consistent evidence of an effect on this measure of learning, so confidence was not downgraded.
- Indirectness: No downgrade because the tests used are considered direct measures of learning.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade because no evidence of consistency across species.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
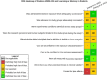
FIGURE E4-5
Risk of bias heatmap of studies of BDE-153 and learning or memory in rodents. In HAWC: https://hawcproject.org/summary/visual/357/.
TABLE E4-6
Studies of BDE-153 and Memory in Rodents.
Memory: There is low confidence in the body of evidence on developmental exposure to BDE-153 and effect on memory in rodents. One study in mice and one in rats reported effects. (The two studies are the same ones that tested the effects of BDE-153 on learning.) The mouse study (Viberg et al. 2003) reported longer latencies in the relearning period of mice tested at 6 months of age in the Morris water maze after exposure to BDE-153 at 0.9 or 9 mg/kg-day. The rat study (Zhang et al. 2013) reported increased swimming time in the Morris water maze 1 month after treatment with BDE-153 at 5 and 10 mg/kg-day evaluated at PND 40 or PND 70 and memory impairment in the passive avoidance test at 10 mg/kg-day at PND 70. The two studies report results that were consistent in direction (both decreased performance on a memory test) across species.
- Risk of bias: Downgraded twice because of serious concerns about multiple risk of bias issues, including lack of randomization of treatment; reduced confidence in outcome assessment due to lack of blinding of outcome assessors; and definitely high risk of bias ratings for not controlling for litter effects in the study design or analysis. (See heatmap in Figure E4-5.)
- Unexplained inconsistencies: Confidence is usually downgraded if only one study in each tested species is available because the database is insufficient to establish or evaluate consistency for a particular species. Consistency across species (see below), however, would be a reason to upgrade confidence. Considering both factors, there was no downgrade for unexplained inconsistency.
- Indirectness: No upgrade because tests were considered direct measures of memory.
- Imprecision: No upgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No change, no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: Confidence is usually upgraded if consistent results are observed across species. As noted above, however, it was not possible to evaluate consistency for each species because of the small data set. Considering both factors, there was no upgrade for consistency.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
BDE-203
One study of BDE-203 and effects on learning (see Table E4-7) or memory (see Table E4-8) was found.
TABLE E4-7
Studies of BDE-203 and Learning in Mice.
Learning: There is very low confidence in the data to evaluate whether developmental exposure to BDE-203 affects learning in mice from the single study available. The results suggest that timing of exposure might have an influence on effects because exposure to BDE-203 at 16.8 mg/kg-day on PND 10 affected learning during the acquisition period, whereas exposure on PND 3 had no effect on learning (Viberg et al. 2006).
- Risk of bias: Downgraded twice because of serious concerns about multiple risk of bias issues, including lack of randomization of treatment; reduced confidence in outcome assessment due to lack of blinding of outcome assessors and a definitely high risk of bias rating for not controlling for litter effects in the study design or analysis (see Figure E4-6).
- Unexplained inconsistencies: Downgraded because unable to establish or evaluate consistency because the study did not have elements that would strengthen conclusions from a single study, such as multiple species, strains, or particularly large sample sizes (n = 50-100).
- Indirectness: No downgrade because test used is considered a direct measure of learning.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because only one dose was tested.
- Cross-species consistency: No upgrade because only mice were tested.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
Memory: There is very low confidence in the data to evaluate whether developmental exposure to BDE-203 affects memory in mice because the single study found no effect at a single dose (16.8 mg/kg-day). The study is the same one that also assessed learning.
- Risk of bias: Downgraded twice because of serious concerns about multiple risk of bias issues, including lack of randomization of treatment; reduced confidence in outcome assessment due to lack of blinding of outcome assessors; and a definitely high risk of bias rating for not controlling for litter effects in the study design or analysis. (See heatmap in Figure E4-6.)
- Unexplained inconsistencies: Downgraded because unable to establish or evaluate consistency because the study did not have elements that would strengthen conclusions from a single study, such as multiple species, strains, or particularly large sample sizes (n = 50-100).
- Indirectness: No downgrade because test used is considered a direct measure of memory.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because only one dose was tested.
- Cross-species consistency: No upgrade because only mice were tested.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.

FIGURE E4-6
Risk of bias heatmap of study of BDE-203 and learning and memory and BDE-206 and learning in mice. In HAWC: https://hawcproject.org/summary/visual/358/.
TABLE E4-8
Studies of BDE-203 and Memory in Mice.
BDE-206
One study of BDE-206 and effects on learning was found (see Table E4-9).
Learning: There is very low confidence in the data to evaluate whether developmental exposure to BDE-206 affects learning in mice as the single study found no effect at a single exposure level (16.8 mg/kg-day). The study is the same one that also tested BDE-203 (see above).
- Risk of bias: Downgraded twice because of serious concerns about multiple risk of bias issues, including lack of randomization of treatment; reduced confidence in outcome assessment due to lack of blinding of outcome assessors; and a definitely high risk of bias rating for not controlling for litter effects in the study design or analysis. (see heatmap in Figure E4-6.)
- Unexplained inconsistencies: Downgraded because unable to establish or evaluate consistency because the study did not have elements that would strengthen conclusions from a single study, such as multiple species, strains, or particularly large sample sizes (n = 50-100).
- Indirectness: No downgrade because test used is considered a direct measure of learning.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because only one dose was tested.
- Cross-species consistency: No upgrade because only mice were tested.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
TABLE E4-9
Studies of BDE-206 and Learning in Mice.
BDE-209
Studies of BDE-209 and effects on learning (see Table E4-10) and memory (see Table E4-11) were available.
Learning: There is moderate confidence in the body of evidence to evaluate whether developmental exposure to BDE-209 affects learning in rodents. Multiple studies show effects on learning at doses of 20 mg/kg-day or greater when learning was assessed using a Morris water maze; other studies, however, show no effects in the same dose range using other test methods.
- Risk of bias: Downgraded because of serious concerns about multiple risk of bias issues, including reduced confidence in outcome assessment due to lack of blinding of outcome assessors in all studies and a definitely high risk of bias rating in three studies because of failure to control for litter effects in the study design or analysis (see Figure E4-7).
- Unexplained inconsistencies: A qualitative evaluation of the evidence suggested a possible downgrade because of the heterogeneity in the evidence. Nevertheless, a meta-analysis of studies of several PBDEs (see Chapter 4 and Appendix E, Section E-5), including BDE-209, and of latency in the last trial of the Morris water maze showed consistent evidence of an effect on this measure of learning, so confidence was not downgraded.
- Indirectness: No downgrade because tests used were considered direct measures of learning.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade because of inconsistencies in the results between rat and mouse studies.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
TABLE E4-10
Studies of BDE-209 and Learning in Rodents.

FIGURE E4-7
Risk of bias heatmap of studies of BDE-209 and learning in rodents. In HAWC: https://hawcproject.org/summary/visual/349/.
Memory: There is low confidence in the body of evidence to evaluate whether developmental exposure to BDE-209 affects learning in rodents. Multiple mouse studies show effects on memory at doses of 3.4 mg/kg-day or greater when memory was assessed using a Morris water maze; other studies, however, show no effects on memory at the same dose range using other methods.
- Risk of bias: Downgraded because of serious concerns about multiple risk of bias issues, including reduced confidence in outcome assessment due to lack of blinding of outcome assessors in all studies and a definitely high risk of bias rating in three studies because of failure to control for litter effects in the study design or analysis (see Figure E4-8).
- Unexplained inconsistencies: Downgraded for inconsistency.
- Indirectness: No downgrade because tests used were considered direct measures of memory.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade because of inconsistencies in the results between rat and mouse studies.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
TABLE E4-11
Studies of BDE-209 and Memory in Rodents.
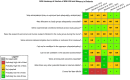
FIGURE E4-8
Risk of bias heatmap of studies of BDE-209 and memory in rodents. In HAWC: https://hawcproject.org/summary/visual/350/.
DE-71
Studies of DE-71 and effects on learning (see Table E4-12), memory (see Table E4-13), and attention (see Table E4-14) were available.
Learning: There is very low confidence in the body of evidence on developmental exposure to DE-71 and effects on learning in rats. The results of the three available studies were inconsistent and used different tests (Morris water maze, radial maze, and visual discrimination) and animals of different ages. One study (Dufault et al. 2005) reported increased errors in the visual discrimination task at the single dose tested (30 mg/kg-day). The other two studies reported no effects of DE-71 on learning at the same dose using longer exposure windows; however, the animals in these studies were evaluated at older ages than were the rats tested in the Dufault et al. (2005) study.
- Risk of bias: Downgraded twice because of serious concerns about multiple risk of bias issues, including reduced confidence in outcome assessment due to lack of blinding of outcome assessors and a definitely high risk of bias rating for exposure characterization in two of the studies (see Figure E4-9).
TABLE E4-12
Studies of DE-71 and Learning in Rats.
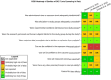
FIGURE E4-9
Risk of bias heatmap of studies of DE-71 and learning in rats. In HAWC: https://hawcproject.org/summary/visual/344/.
TABLE E4-13
Studies of DE-71 and Memory in Rats.
Memory: There is very low confidence in the body of evidence on developmental exposure to DE-71 and effects on memory in rats. The results of the two available studies were inconsistent and were evaluated in animals of different ages and with different tests (Morris water maze and radial maze). One study (de-Miranda et al. 2016) reported a reference memory deficit in female Wistar rats (not males) in the radial maze at the single dose tested (30 mg/kg-day).
- Risk of bias: Downgraded twice because of serious concerns about multiple risk of bias issues, including reduced confidence in outcome assessment due to lack of blinding of outcome assessors and a definitely high risk of bias rating for exposure characterization in one of the studies (see Figure E4-10).
- Unexplained inconsistencies: Downgrade for inconsistency.
- Indirectness: No downgrade because tests used are considered direct measures of memory.
- Imprecision: No downgrade because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade because only rats were tested.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
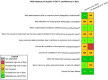
FIGURE E4-10
Risk of bias heatmap of studies of DE-71 and memory in rats. In HAWC: https://hawcproject.org/summary/visual/345/.
TABLE E4-14
Studies of DE-71 and Attention in Rats.
Attention: There is very low confidence in the body of evidence on developmental exposure to DE-71 and effects on attention in rats. All of the data are from a single laboratory (Dufault et al. 2005; Driscoll et al. 2009, 2012) and the majority of the tests reported no effects at doses up to 30 mg/kg-day across multiple tests (various attention tasks and a visual task). In one experiment (Driscoll et al. 2009), rats exposed to DE-71 at 4.5 mg/kg-day demonstrated lower accuracy in Attention Task 1.
- Risk of bias: Downgraded twice because of serious concerns about multiple risk of bias issues, including reduced confidence in outcome assessment due to lack of blinding of outcome assessors and a definitely high risk of bias rating for exposure characterization in one of the studies (see Figure E4-11).
- Unexplained inconsistencies: Downgrade for inconsistency.
- Indirectness: No downgrade because tests used are considered direct measures of attention.
- Imprecision: No downgraded because no or minimal indications of large standard deviations (i.e., SD > mean).
- Dose-response: No upgrade because no clear evidence of dose-response gradient within or across studies.
- Cross-species consistency: No upgrade because only rats were tested.
- Other potential downgrades or upgrades: No evidence of publication bias (see Section E-3), large magnitude of effect, or residual confounding or other related factors that would affect confidence in the estimated effect.
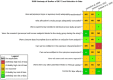
FIGURE E4-11
Risk of bias heatmap of studies of DE-71 and attention in rats. In HAWC: https://hawcproject.org/summary/visual/347/.
SECTION E-5. Supporting Information for the Meta-Analyses of Studies of PBDEs
Meta-Analyses on Combined Data on PBDEs
TABLE E5-1
Overall Analyses and Sensitivity Analyses of Studies of PBDEs and Latency in Last Trial of the Morris Water Maze.

FIGURE E5-1Risk of bias heatmap of studies of PBDEs and latency in last trial of the Morris water maze with standard deviations reported or digitized from figures in the publication
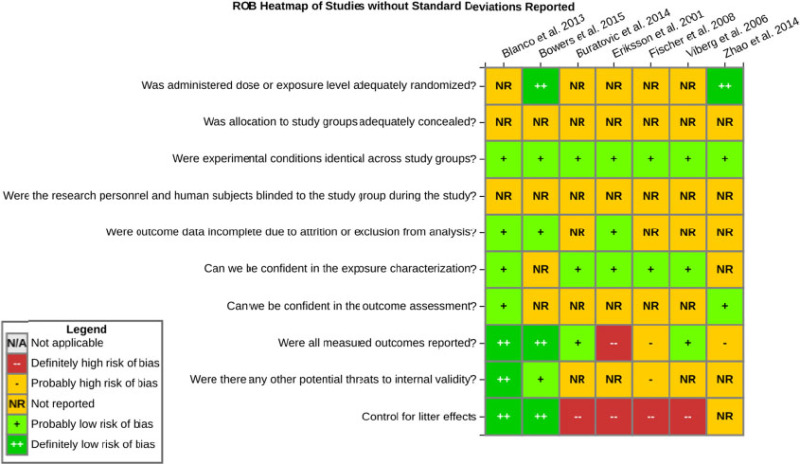
FIGURE E5-2Risk of bias heatmap of studies of PBDEs and latency in last trial of the Morris water maze without standard deviations
FIGURE E5-3
Benchmark dose estimates from studies of PBDEs and latency in last trial of the Morris water maze in rats and mice. Points without error bars are studies for which a standard deviation was not reported or could not be digitized from figures in the publication. (more...)
Meta-Analyses on Individual PBDEs
BDE-47
- Statistically significant overall effect. Heterogeneity (I2 = 44%), but it was not statistically significant. Overall effect was robust to using only highest dose from each study.
- Positive trends in log10(dose) and in dose, but only the latter was statistically significant. Reduced heterogeneity for log10(dose) and linear model. Benchmark dose for a 5% change was estimated to be 1.4 mg/kg-day (95% CI: 1.0, 2.4) from the linear model and 0.83 mg/kg-day (95% CI: 0.34, 5.9) from the linear-quadratic model.
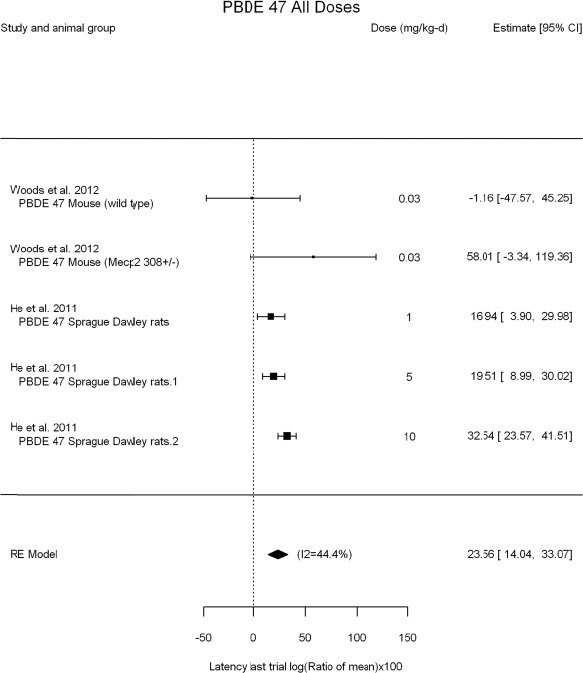
FIGURE E5-4Results of meta-analysis of studies of BDE-47 and latency in last trial of the Morris water maze
TABLE E5-2Overall Analyses and Sensitivity Analyses of Studies BDE-47 and Latency in Last Trial of the Morris Water Maze
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | 23.56 | 14.04 | 33.07 | 0.0000 | 6.72 | 44.43 | 0.1092 | 49.01* |
| Trend in log10(dose) | log10(dose) | 7.19 | -6.23 | 20.61 | 0.2939 | 4.72 | 27.79 | 0.1610 | 54.64 |
| Linear in dose10 | dose10 | 34.55 | 20.13 | 48.97 | 0.0000 | 6.76 | 41.56 | 0.0957 | 50.90 |
| Linear-Quadratic in dose10 | dose10 | 60.96 | -27.12 | 149.04 | 0.1750 | 10.24 | 48.02 | 0.0829 | 58.02 |
| Linear-Quadratic in dose10 | I(dose10^2) | -29.19 | -124.65 | 66.27 | 0.5490 | 10.24 | 48.02 | 0.0829 | 58.02 |
| Sensitivity Analyses | |||||||||
| Highest Doses-Overall | intrcpt | 31.87 | 23.15 | 40.59 | 0.0000 | 0.00 | 0.00 | 0.2638 | 34.52 |
- *
Indicates the lowest AICc.
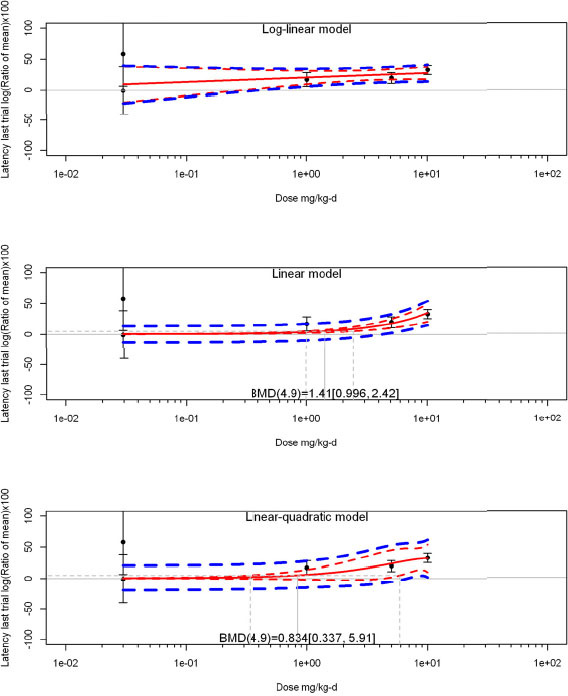
FIGURE E5-5Benchmark dose estimates from studies of BDE-47 and latency in last trial of the Morris water maze
BDE-153
- Statistically significant overall effect; no heterogeneity. Too few data for a sensitivity analysis.
- Positive trend, but not a statistically significant trend. Only central estimate and lower bound could be estimated for a benchmark dose for a 5% change: 1.2 mg/kg-day (95% CI: 0.6, >10).
TABLE E5-3Overall Analyses and Sensitivity Analyses of Studies BDE-153 and Latency in Last Trial of the Morris Water Maze
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | 25.40 | -0.18 | 50.99 | 0.052 | 0 | 0 | 0.82 | 32.56* |
| Trend in log10(dose) | log10(dose) | 14.03 | -30.94 | 58.99 | 0.541 | 0 | 0 | 0.88 | 38.16 |
| Linear in dose10 | dose10 | 41.17 | -4.69 | 87.04 | 0.078 | 0 | 0 | 0.58 | 33.38 |
| Linear-Quadratic in dose10 | dose10 | 304.47 | -193.63 | 802.57 | 0.231 | 0 | 0 | 0.93 | 38.17 |
| Linear-Quadratic in dose10 | I(dose10^2) | -295.30 | -851.58 | 260.97 | 0.298 | 0 | 0 | 0.93 | 38.17 |
- *
Indicates the lowest AICc.
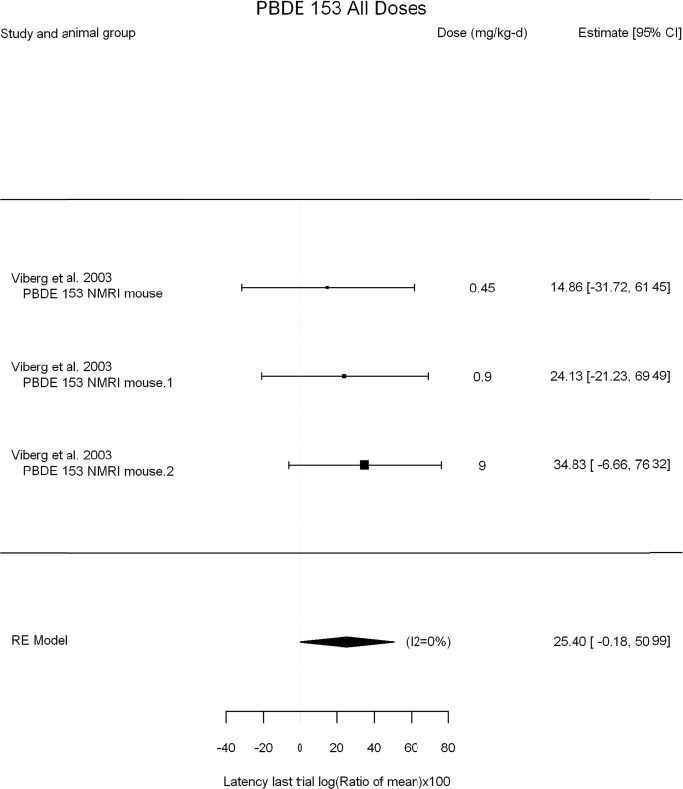
FIGURE E5-6Results of meta-analysis of studies of BDE-153 and latency in last trial of the Morris water maze
BDE-209
- Statistically significant overall effect. Heterogeneity (I2 = 42%), but it was not statistically significant. Overall effect was robust to using only highest dose from each study (only two studies).
- Statistically significant trends in log10(dose) and linear trend in dose, with reduced heterogeneity. BMD estimates for a 5% change were 6.3 mg/kg-day (95% CI: 4.8, 9.2) from the linear model and 3.5 mg/kg-day (95% CI: 2.2, 7.9) from the linear-quadratic model.
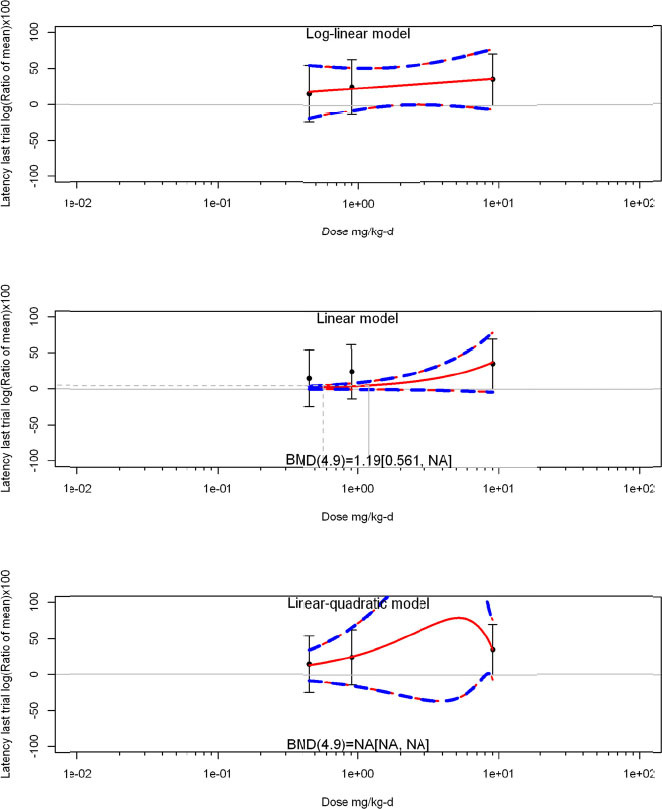
FIGURE E5-7Benchmark dose estimates from studies of BDE-153 and latency in last trial of the Morris water maze in rats and mice
TABLE E5-4Overall Analyses and Sensitivity Analyses of Studies BDE-209 and Latency in Last Trial of the Morris Water Maze
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | 26.69 | 16.79 | 36.60 | 0.00000 | 6.36 | 42.27 | 0.12 | 41.15* |
| Trend in log10(dose) | log10(dose) | 23.92 | 0.01 | 47.83 | 0.04990 | 0.00 | 0.00 | 0.37 | 46.48 |
| Linear in dose10 | dose10 | 7.70 | 5.32 | 10.08 | 0.00000 | 4.01 | 22.14 | 0.16 | 41.33 |
| Linear-Quadratic in dose10 | dose10 | 14.68 | 5.98 | 23.39 | 0.00094 | 0.00 | 0.00 | 0.27 | 46.94 |
| Linear-Quadratic in dose10 | I(dose10^2) | -1.60 | -3.53 | 0.33 | 0.10377 | 0.00 | 0.00 | 0.27 | 46.94 |
| Sensitivity Analyses | |||||||||
| Highest Doses-Overall | intrcpt | 39.61 | 9.96 | 69.26 | 0.00883 | 14.90 | 18.95 | 0.27 | 25.90 |
- *
Indicates the lowest AICc.
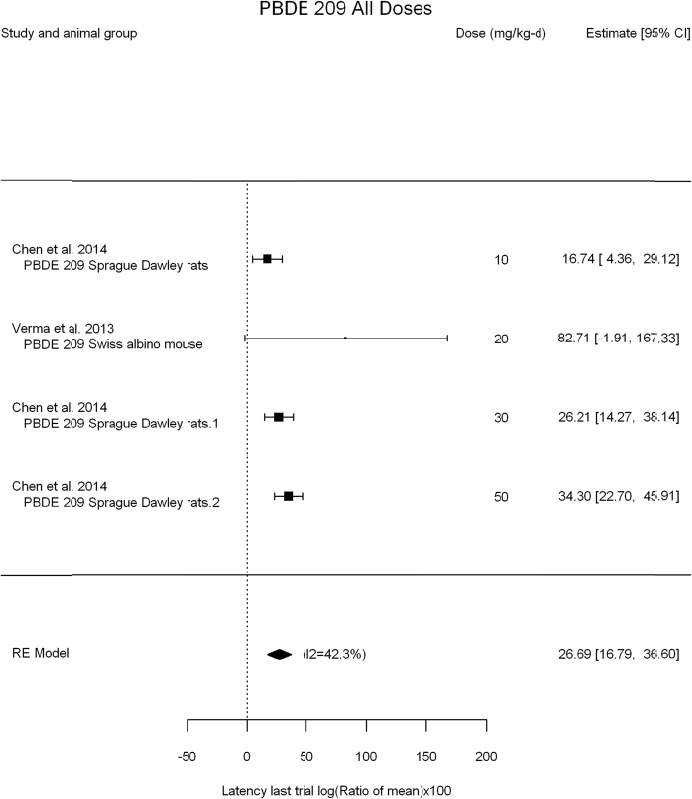
FIGURE E5-8Results of meta-analysis of studies of BDE-209 and latency in last trial of the Morris water maze

FIGURE E5-9Benchmark dose estimates from studies of BDE-209 and latency in last trial of the Morris water maze
REFERENCES
- Biesemeier JA, Beck MJ, Silberberg H, Myers NR, Ariano JM, Radovsky A, Freshwater L, Sved DW, Jacobi S, Stump DG, Hardy ML, Stedeford T. An oral developmental neurotoxicity study of decabromodiphenyl ether (DecaBDE) in rats. Birth Defects Res. B Dev. Reprod. Toxicol. 2011;92(1):17–35. [PubMed: 21284075]
- Blanco J, Mulero M, Heredia L, Pujol A, Domingo JL, Sánchez DJ. Perinatal exposure to BDE-99 causes learning disorders and decreases serum thyroid hormone levels and BDNF gene expression in hippocampus in rat offspring. Toxicology. 2013;308:122–128. [PubMed: 23578391]
- Bowers WJ, Wall PM, Nakai JS, Yagminas A, Wade M, Li N. Behavioral and thyroid effects of in utero and lactational exposure of Sprague-Dawley rats to the polybrominated diphenyl ether mixture DE71. Neurotoxicol. Teratol. 2015;52(Pt. B):127–142. [PubMed: 26271887]
- Buratovic S, Viberg H, Fredriksson A, Eriksson P. Developmental exposure to the polybrominated diphenyl ether PBDE 209: Neurobehavioural and neuroprotein analysis in adult male and female mice. Environ. Toxicol. Pharmacol. 2014;38(2):570–585. [PubMed: 25194327]
- Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjödin A, Dietrich KN, Lanphear BP. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: The HOME study. Environ. Health Perspect. 2014;122(8):856–862. [PMC free article: PMC4123029] [PubMed: 24870060]
- Cheng J, Gu J, Ma J, Chen X, Zhang M, Wang W. Neurobehavioural effects, redox responses and tissue distribution in rat offspring developmental exposure to BDE-99. Chemosphere. 2009;75(7):963–968. [PubMed: 19203780]
- de-Miranda AS, Kuriyama SN, da-Silva CS, do-Nascimento MS, Parente TE, Paumgartten FJ. Thyroid hormone disruption and cognitive impairment in rats exposed to PBDE during postnatal development. Reprod. Toxicol. 2016;63:114–124. [PubMed: 27233481]
- Driscoll LL, Gibson AM, Hieb A. Chronic postnatal DE-71 exposure: Effects on learning, attention and thyroxine levels. Neurotoxicol. Teratol. 2009;31(2):76–84. [PubMed: 19068229]
- Driscoll LL, Kaplan J, Bucuvalas E, Allen H, Kraut J, Fitzpatrick J. Acute postnatal exposure to the pentaBDE commercial mixture DE-71 at 5 or 15mg/kg/day does not produce learning or attention deficits in rats. Neurotoxicol. Teratol. 2012;34(1):20–26. [PubMed: 22024237]
- Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol. Sci. 2005;88(1):172–180. [PubMed: 16107551]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: A novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 2001;109(9):903–908. [PMC free article: PMC1240439] [PubMed: 11673118]
- Fischer C, Fredriksson A, Eriksson P. Coexposure of neonatal mice to a flame retardant PBDE 99 (2, 2',4, 4',5-pentabromodiphenyl ether) and methyl mercury enhances developmental neurotoxic defects. Toxicol. Sci. 2008;101(2):275–285. [PubMed: 17982161]
- He P, Wang AG, Xia T, Gao P, Niu Q, Guo LJ, Chen XM. Mechanisms underlying the developmental neurotoxic effect of PBDE-47 and the enhanced toxicity associated with its combination with PCB153 in rats. NeuroToxicology. 2009;30(6):1088–1095. [PubMed: 19576244]
- He P, Wang A, Niu Q, Guo L, Xia T, Chen X. Toxic effect of PBDE-47 on thyroid development, learning, and memory, and the interaction between PBDE-47 and PCB153 that enhances toxicity in rats. Toxicol. Ind. Health. 2011;27(3):279–288. [PubMed: 20947653]
- Koenig CM, Lango J, Pessah IN, Berman RF. Maternal transfer of BDE-47 to offspring and neurobehavioral development in C57BL/6J mice. Neurotoxicol. Teratol. 2012;34(6):571–580. [PMC free article: PMC3501584] [PubMed: 23022914]
- Llansola M, Hernandez-Viadel M, Erceg S, Montoliu C, Felipo V. Increasing the function of the glutamate-nitric oxide-cyclic guanosine monophosphate pathway increases the ability to learn a Y-maze task. J. Neurosci. Res. 2009;87(10):2351–2355. [PubMed: 19326454]
- Reverte I, Klein AB, Domingo JL, Colomina MT. Long term effects of murine postnatal exposure to decabromodiphenyl ether (BDE-209) on learning and memory are dependent upon APOE polymorphism and age. Neurotoxicol. Teratol. 2013;40:17–27. [PubMed: 23999552]
- Rice DC, Thompson WD, Reeve EA, Onos KD, Assadollahzadeh M, Markowski VP. Behavioral changes in aging but not young mice after neonatal exposure to the polybrominated flame retardant decaBDE. Environ. Health Perspect. 2009;117(12):1903–1911. [PMC free article: PMC2799465] [PubMed: 20049210]
- Ta TA, Koenig CM, Golub MS, Pessah IN, Qi L, Aronov PA, Berman RF. Bioaccumulation and behavioral effects of 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) in perinatally exposed mice. Neurotoxicol. Teratol. 2011;33(3):393–404. [PMC free article: PMC3543834] [PubMed: 21334437]
- Verma P, Singh P, Gandhi BS. Prophylactic efficacy of Bacopa monnieri on decabromodiphenyl ether (PBDE-209)-induced alterations in oxidative status and spatial memory in mice. Asian J. Pharm. Clin. Res. 2013;6(3):242–247.
- Verma P, Singh P, Gandhi BS. Neuromodulatory role of Bacopa monnieri on oxidative stress induced by postnatal exposure to decabromodiphenyl ether (PBDE -209) in neonate and young female mice. Iran. J. Basic Med. Sci. 2014;17(4):307–311. [PMC free article: PMC4046233] [PubMed: 24904725]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 2003;192(2):95–106. [PubMed: 14550744]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol. Sci. 2006;92(1):211–218. [PubMed: 16611620]
- Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, Chi LH, Kostyniak PJ, Pessah IN, Berman RF, LaSalle JM. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum. Mol. Genet. 2012;21(11):2399–2411. [PMC free article: PMC3349420] [PubMed: 22343140]
- Zhang H, Li X, Nie J, Niu Q. Lactation exposure to BDE-153 damages learning and memory, disrupts spontaneous behavior and induces hippocampus neuron death in adult rats. Brain Res. 2013;1517:44–56. [PubMed: 23624224]
- Zhao W, Cheng J, Gu J, Liu Y, Fujimura M, Wang W. Assessment of neurotoxic effects and brain region distribution in rat offspring prenatally co-exposed to low doses of BDE-99 and methylmercury. Chemosphere. 2014;112:170–176. [PubMed: 25048903]
- PBDE (ANIMAL) SYSTEMATIC REVIEW PROTOCOL
- Results of Literature Searches for Animal Studies on the Effects of Developmental Exposure to PBDEs on Learning, Memory, Attention, or Response Inhibition
- Funding Sources of the Animal Studies on PBDEs and Learning, Memory, or Attention
- Confidence Ratings for the Body of Evidence from Animal Studies of PBDEs
- Supporting Information for the Meta-Analyses of Studies of PBDEs
- Supporting Materials for the PBDE (Animal) Systematic Review - Application of Sy...Supporting Materials for the PBDE (Animal) Systematic Review - Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals
- A Longevity Dividend - Global Roadmap for Healthy LongevityA Longevity Dividend - Global Roadmap for Healthy Longevity
- Social Infrastructure for Healthy Longevity - Global Roadmap for Healthy Longevi...Social Infrastructure for Healthy Longevity - Global Roadmap for Healthy Longevity
Your browsing activity is empty.
Activity recording is turned off.
See more...