NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances: 10th Edition. Washington (DC): National Academies Press (US); 1989.

Recommended Dietary Allowances: 10th Edition.
Show detailsAlthough water and the principal electrolytes (sodium, potassium, and chloride) are often excluded from lists of nutrients, these substances are essential dietary components, in that they must be acquired from the diet either exclusively or—in the case of water—in amounts well in excess of that produced by metabolism in the body. Concerns about possible overconsumption (sodium and chloride) or underconsumption (potassium) of these substances in the United States are comparatively recent (NRC, 1989; Tobian, 1979).
WATER
Water is the most abundant constituent of the human body, accounting for one-half to four-fifths of body weight, depending mainly on body fat content. Accordingly, body water, as a percentage of body mass, is higher in men than in women and tends to fall with age in both.
Figure 11-1 shows the routes and approximate magnitudes of water intake and loss in an environment cool enough to prevent sweating. The normal daily turnover of water via these routes is approximately 4% of total body weight in adults and much higher, 15% of total body weight, in infants. As Figure 11-1 shows, even in the absence of visible perspiration, approximately one-half of the turnover occurs through what is called insensible water loss, i.e., water lost from the lungs and skin. These insensible losses can all be increased under certain conditions, including high temperatures, high altitude, and dry air. Exertion under any of these conditions can cause up to a 10-fold increase in water loss from skin and lungs. Diarrhea can increase intestinal loss dramatically.
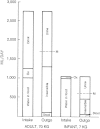
FIGURE 11-1
Routes and approximate magnitude of water intake and outgo without sweating. From NRC, 1980b. M is minimal urine volume at maximal solute concentration. Ox is water of oxidation.
Figure 11-1 includes an estimate of minimal urine volume required when urinary solute concentration is maximal (about 1,400 mosmol/ liter in the healthy adult and 700 mosmol/liter in the infant). Because the kidney must excrete waste products, the solute load—composed of the nitrogen-containing breakdown products of protein metabolism (principally urea), sulfates, phosphates, and other electrolytes—determines the minimal volume of water required for urine formation. Normally functioning kidneys can adjust urine osmolarity from 40 to 1,400 mosmol/liter, depending both on water intake and on dietary solute load. Despite the kidney's ability to compensate, its limitations require the effective use of the thirst sensation to maintain water balance. If the sensation of thirst is not met by water consumption, or if the thirst mechanism is inoperative because of intense, sustained exertion, especially at a high altitude (Buskirk and Mendez, 1967), dehydration will eventually result. This can become life threatening when more than 10% of body weight is lost.
Sources
Although water, consumed as water, is a major source of liquid in some parts of the world, much of the water consumed in the United States is taken in the form of other beverages. Median daily intake of water as such among respondents in the 1977–1978 Nationwide Food Consumption Survey was 2.8 cups (USDA, 1984). In 1981, daily per capita milk consumption was approximately one and one-third cups, per capita coffee and tea consumption was about one and one-half cups, and soft drink consumption was one and three-fourths cups per capita. In addition, many solid foods, especially fruits and vegetables, contain from 85 to 95% water.
Estimate of Requirements
The primary determinant of maintenance water requirement appears to be metabolic (Holliday and Segar, 1957), but the actual estimation of water requirement is highly variable and quite complex. Because the water requirement is the amount necessary to balance the insensible losses (which can vary markedly) and maintain a tolerable solute load for the kidneys (which may vary with dietary composition and other factors), it is impossible to set a general water requirement.
Adults For practical purposes, 1 ml/kcal of energy expenditure can be recommended as the water requirement for adults under average conditions of energy expenditure and environmental exposure. However, there is so seldom a risk of water intoxication that the specified requirement for water is often increased to 1.5 ml/kcal to cover variations in activity level, sweating, and solute load.
Special attention must be given to the water needs of the elderly whose thirst sensation may be blunted. Even though these people may be less physically active, they may still have a high water requirement, especially during the summer. If uncorrected, water depletion with heat exhaustion, resulting from inadequate replacement of fluid losses, can eventually cause a loss of consciousness and heat stroke (NRC, 1980b).
Pregnancy and Lactation Pregnancy is associated with an increased need for water because of the expanded extracellular fluid space, the needs of the fetus, and the amniotic fluid. However, calculations indicate that the increment amounts to only about 30 ml/ day. A lactating woman, on the other hand, requires an increased volume of water to match that secreted in the milk. Since milk is 87% water and average milk secretion is 750 ml/day for the first 6 months, the extra fluid required would be less than 1,000 ml/day.
Infants and Children Infants must be treated as a separate category for several reasons: their large surface area per unit of body weight, their higher percentage of body water and its high rate of turnover, the limited capacity of their kidneys for handling the solute load from high protein intakes required for growth, and their susceptibility to severe dehydration due in part to their inability to express thirst. It is prudent, therefore, to recommend an average water intake of 1.5 ml/kcal of energy expenditure for infants. This figure corresponds to the water-to-energy ratio in human milk and common formulas and has been well established as a satisfactory level for the growing infant.
Excessive Intakes and Toxicity
Toxicity results from the ingestion of water at a rate beyond the capacity of the kidneys to excrete the extra load, resulting in hyposmolarity. Such a condition is rarely observed in a normal healthy adult. The manifestations usually include a gradual mental dulling, confusion, coma, convulsion, and even death.
SODIUM
Sodium, the principal cation of extracellular fluid, is the primary regulator of extracellular fluid volume. Both the body content of sodium and its concentration in body fluids are under homeostatic control, and the volume of extracellular fluid is thus normally determined by its sodium content. In addition to its role in regulating extracellular fluid volume, sodium is important in the regulation of osmolarity, acid-base balance, and the membrane potential of cells. Sodium is also involved in active transport across cell membranes and must be pumped out in exchange for potassium in order to maintain an appropriate intracellular milieu—a process that requires an appreciable fraction of the energy required in the basal metabolic state.
Sodium homeostasis is maintained over a wide range of environmental and dietary circumstances, primarily through the action of the hormone aldosterone on the renal tubules of the kidney. When sodium intake is high, the aldosterone level decreases and urinary sodium increases. When dietary sodium intake is low, the aldosterone level increases and urinary excretion of sodium rapidly falls almost to zero. Although the kidney can thus conserve sodium, there is some obligatory loss via feces and sweat. Sodium deficiency resulting from low dietary intake thus does not normally occur, even among those existing on very low sodium diets (Page, 1976, 1979). Even relatively heavy sweating does not normally create a need to provide salt supplements (Conn, 1949). The body may be depleted of sodium under extreme conditions of heavy and persistent sweating, or where trauma, chronic diarrhea, or renal disease produce an inability to retain sodium (Gothberg et al., 1983). These latter conditions require medical attention.
Dietary Sources and Usual Intakes
Foods and beverages containing sodium chloride (39% sodium by weight) are the primary sources of sodium. Sources other than table salt—e.g., sodium bicarbonate and monosodium glutamate—are believed to account for less than 10% of total dietary sodium intake (Sanchez-Castillo, 1987a). Water from community systems usually contains less than 20 mg of sodium per liter, and it has been estimated that water contributes less than 10% of daily sodium intake (NRC, 1977). By using a lithium chloride marker to trace the use of salt in cooking and at the table, Sanchez-Castillo et al. (1987a, 1987b) found that only 10% of the salt came from the natural salt content of foods, 15% from salt added during cooking and at the table, and fully 75% from salt added during processing and manufacturing.
Because of the high proportion of dietary sodium accounted for by processing, the highest salt intakes are normally associated with a diet high in processed foods and the lowest intakes are associated with diets emphasizing fresh fruits, vegetables, and legumes. In the first National Health and Nutrition Examination Survey (Abraham and Carroll, 1981), 32% of the sodium chloride consumed came from baked goods and cereals, approximately 21% came from meats, and 14% from dairy products. The FDA's Total Diet Study, in which a very different methodology was used, showed similar results (Pennington et al., 1984).
Usual levels of sodium consumption have been estimated in dietary surveys by assessing salt intake and by measuring urinary sodium. Reported dietary intakes of sodium range from 1.8 g/day to 5 g/day in various studies, depending on the methods of assessment used (Abraham and Carroll, 1981; Dahl, 1960; Pennington et al., 1984) and on whether or not discretionary sodium use is assessed. The discretionary intake of sodium is quite variable and can be quite large. In one 28-day study, males were found to add about 5.5 g of sodium chloride (2.2 g of sodium) to their food per day (Mickelson et al., 1977).
Because of the difficulty of assessing sodium use from dietary recall, dietary surveys probably underestimate total sodium intake, even when contributions of water and other marginal sources are included. From data on daily urinary sodium excretion over 24 hours, Dahl and Love (1957) calculated the average daily adult intake of salt to be 10 g/day (4 g of sodium per day). Dahl subsequently reported a mean sodium chloride intake of 10.3 g (range, 4 to 24 g) for 71 working men in New York. Coatney et al. (1958) reported that a 5-month sodium excretion in a military population corresponded to an intake of 11 g of salt per day. Sanchez-Castillo et al. (1987a, b) found sodium chloride excretion over a 12-day period to be 10.6 ± 0.55 g in men and 7.4 ± 2.9 g in women.
Estimate of Requirements
Calculations of sodium requirements (shown in Table 11-1) are based on estimates of what is needed for growth and for replacement of obligatory losses. The amount needed to support growth depends on the rate at which extracellular fluid volume is expanded, a rate that varies with age and reproductive status.
Adults In a temperate climate, the healthy adult can maintain sodium balance with a very low intake of sodium (Kempner, 1948). Dole et al. (1950) have estimated obligatory urinary and fecal losses by adults to be 23 mg (1 mEq) a per day. The other source of loss is sweat, which normally averages a sodium concentration of 25 mEq/ liter (Consolazio et al., 1963). Sanchez-Castillo et al. (1987a) found that sweat and fecal excretion contributed only 2 to 5% of the sodium lost by British men and women. Obligatory dermal losses have been assumed to range from 46 to 92 mg (2 to 4 mEq) per day (Fregley, 1984). Thus, a minimum average requirement for adults can be estimated under conditions of maximal adaptation and without active sweating as no more than 5 mEq/day, which corresponds to 115 mg of sodium or approximately 300 mg of sodium chloride per day. In consideration of the wide variation of patterns of physical activity and climatic exposure, a safe minimum intake might be set at 500 mg/day. Such an intake is substantially exceeded by usual diets in the United States, even in the absence of added sodium chloride. Although no optimal range of salt intake has been established, there is no known advantage in consuming large amounts of sodium, and clear disadvantages for those susceptible to hypertension. From this and other considerations, a Food and Nutrition Board committee recently recommended that daily intakes of sodium chloride be limited to 6 g (2.4 g of sodium) or less (NRC, 1989).
Pregnancy and Lactation During pregnancy, there is an increased need for sodium because of the increased extracellular fluid volume in the mother, the requirements of the fetus, and the level of sodium in the amniotic fluid. This need is normally met in part by physiological responses of the renin-angiotensin-aldosterone systems (Pike and Smiciklas, 1972). Given a pregnancy weight gain of 11 kg (70% of which is extracellular water containing 150 mEq of sodium per liter), the average total sodium requirement for the duration of pregnancy is 3 mEq (69 mg) per day in addition to the normal requirement. Since the average intake is, as has been noted, considerably above that, the sodium requirement for pregnancy is met by usual salt intake.
Lactation increases sodium requirements considerably. Since human milk contains about 7.8 mEq of sodium (180 mg) per liter (AAP, 1985), and the average milk secretion when established is about 750 ml, lactation would add about 6 mEq (135 mg) per day to the usual adult requirement. This increase is easily met by the usual dietary sodium intake.
Infants and Children The sodium requirement is obviously highest in infants and young children in whom extracellular fluid volume is rapidly expanding. Forbes (1952) calculated that from birth to 3 months of age, 0.5 mEq/kg (11.5 mg/kg) daily is needed for growth, or approximately 2 mEq (46 mg) per day for the reference infant. At 6 months of age, the daily requirement for growth is approximately 0.2 mEq (4.6 mg)/kg. According to calculations by Cooke et al. (1950), daily losses of sodium from the skin range from 0.4 to 0.7 mEq/kg (9 to 16 mg/kg). Because sodium losses from the kidney can be regulated precisely when intakes are not excessive, the convenient value of 1 mEq/kg (23 mg/kg) daily is considered more than satisfactory for the healthy infant and young child residing in a temperate climate. Human milk contains 7 mEq of sodium per liter (range, 3 to 19 mEq/liter) (Gross, 1983; Macy, 1949). Consumed at a rate of 750 ml/day, this provides the reference infant with an average of 120 mg/day, which corresponds to 1.16 mEq/kg (27 mg/kg) daily from birth through 2 months of age and 0.8 mEq/kg (18 mg/kg) daily from 3 through 5 months of age. Except for the premature infant, in whom hyponatremia can occur (Roy et al., 1976), human milk certainly provides adequate sodium for the growing infant.
Formula-fed infants consuming 750 ml/day now receive a minimum of 100 mg/day and a maximum of 300 mg/day (AAP, 1985). The American Academy of Pediatrics has estimated that there is a threefold increase in dietary sodium between 2 and 12 months of age (AAP, 1981).
Excessive Intakes and Toxicity
Acute excessive intake of sodium chloride leads to an increase in the extracellular space as water is pulled from cells to maintain sodium concentration. The end result is edema and hypertension. Such acute toxicity from dietary sodium is not a concern, however, since as long as water needs can be met, the kidney can excrete the excess sodium. Sustained overconsumption of sodium, particularly as salt, has been related to development of hypertension in sensitive individuals (NRC, 1989; Tobian, 1979).
POTASSIUM
Potassium is the principal intracellular cation, occurring in cell water at a concentration of 145 mEq/liter, b more than 30 times the concentration at which it is found in plasma and interstitial fluid (3.8 to 5.0 mEq/liter). This small percentage of extracellular potassium is, however, of great physiological importance, contributing to the transmission of nerve impulses, to the control of skeletal muscle contractility, and to the maintenance of normal blood pressure.
More than 90% of ingested potassium is absorbed from the gastrointestinal tract, but higher or lower intakes are not reflected in fluctuations in plasma potassium concentrations because the kidney can regulate potassium balance. Potassium is lost from the body in the urine and, to a lesser extent, in gastrointestinal secretions, whereas only minimal amounts are excreted in sweat.
Under normal circumstances, dietary deficiency of potassium does not occur. The most important cause of potassium deficiency is excessive losses, usually through the alimentary tract or the kidneys. Large alimentary potassium losses may occur through prolonged vomiting, chronic diarrhea, or laxative abuse. The most common cause of excessive renal loss is the use of diuretic agents, especially for the treatment of hypertension. Some forms of chronic renal disease and metabolic disturbances (e.g., diabetic acidosis) can also lead to severe potassium loss. Deficiency symptoms include weakness, anorexia, nausea, listlessness, apprehension, drowsiness, and irrational behavior. Severe hypokalemia may result in cardiac dysrhythmias that can be fatal.
Dietary Sources and Usual Intakes
Potassium is widely distributed in foods, since it is an essential constituent of all living cells. Animal tissue concentration of potassium is fairly constant, but varies inversely with the amount of fat. Some potassium is also added in food processing, but the overall effect of processing on the food supply has been to increase the sodium and decrease the potassium (NRC, 1989). Thus, the richest dietary sources are unprocessed foods, especially fruits, many vegetables, and fresh meats. The contribution of drinking water to potassium intake is negligible. The mean concentration in household tap water was reported to be 2.15 mg/liter (range, 0.72 to 8.3 mg/liter) (Greathouse and Crown, 1979; NRC, 1980a).
Potassium intakes vary considerably, depending on food selection. People who eat large amounts of fruits and vegetables have a high potassium intake, on the order of 8 to 11 g/day (NRC, 1989). In the FDA's Total Diet Study, mean potassium intake in the United States during 1981–1982 was found to be 1,500 mg/day for 6-month-old infants, 1,800 mg/day for 2-year-old children, and 3,400 mg/day for 15- to 20-year olds (Pennington et al., 1984). Urban whites eat about 2,500 mg/day (Khaw and Barrett-Connor, 1987); low intakes of about 1,000 mg/day have been reported in blacks (Grim et al., 1980; Langford, 1985).
Human milk contains about 500 mg (12.8 mEq) of potassium per liter, and therefore provides the reference infant consuming 750 ml daily with 375 mg/day. Infant formulas contain slightly more potassium than human milk on the average, and cow's milk contains almost 3 times as much, 1,365 mg (35 mEq) per liter.
Estimate of Requirements
Adults Potassium requirements have been evaluated in only a few studies. Although losses on a low or “minimum” potassium diet are small, potassium is less well conserved than sodium (see Table 11-1). Fecal losses are less than 400 mg (10 mEq) per day, and renal losses may approach 200 to 400 mg (5 to 10 mEq) per day (Squires and Huth, 1959). Other losses (e.g., in sweat) are negligible. On intakes of about 20 mEq/day, metabolic balance is achieved at the expense of reduced body potassium stores (up to 250 mEq) and in some cases with reduced plasma levels (<4 mEq/liter). To maintain normal body stores and a normal concentration in plasma and interstitial fluid, an intake of about 40 mEq/day may be needed (Sebastian et al., 1971). Therefore, it would appear that the minimum requirement is approximately 1,600 to 2,000 mg (40 to 50 mEq) per day. There is considerable evidence that dietary potassium exerts a beneficial effect in hypertension, and recommendations for increased intake of fruits and vegetables (NRC, 1989) would raise potassium intake of adults to about 3,500 mg (90 mEq) per day.
TABLE 11-1
Estimated Sodium, Chloride, and Potassium Minimum Requirements of Healthy Persons.
Pregnancy and Lactation There is no evidence that potassium requirements are appreciably increased during pregnancy, except for the increment needed to build new tissue, which is easily satisfied by the usual ingestion of potassium. Since maternal milk contains about 500 mg (12.8 mEq) per liter, this increased loss must be considered during lactation, but is supplied by usual intakes.
Infants and Children Since potassium is a necessary constituent of each body cell, an increase in lean body mass is a major determinant of potassium needs. From 60 to 80 mEq are required for each kilogram of weight gained. Using growth rates for infants and children calculated from the reference weight data reported by Hammill et al. (1979), and assuming that 70 mEq of potassium are required for each kg of body weight, one may estimate that the potassium requirement for growth averages 65 mg/day for infants, 15 to 20 mg/ day for 1- to 10-year-old children, and 35 mg/day for adolescents.
To allow for obligatory urinary, cutaneous, and fecal losses, dietary intake must, of course, be higher than the amount required at the tissue level. Holliday and Segar (1957) have estimated that in general, 78 mg (2 mEq) per 100 kcal should maintain potassium balance in children of all ages as long as there is no preexisting potassium deficit or ongoing excessive loss. This is in keeping with data on potassium intake in infants and children showing that average potassium intake (from milk and solid foods) ranges from about 780 mg/day at 2 months of age to about 1,600 mg/day at the end of the first year of life (AAP, 1981).
Excessive Intakes and Toxicity
In the absence of markedly increased losses of potassium from the body, acute intoxication (hyperkalemia) will result from sudden enteral or parenteral increases in potassium intake to levels about 12.0 g/m2 (250 to 300 mEq/m2) of surface area per day—about 18 g for an adult (NRC, 1980b). Although urinary excretion provides some protection, acute hyperkalemia can prove fatal because it can cause cardiac arrest.
CHLORIDE
Chloride, the principal inorganic anion in the extracellular fluid compartment, is essential in maintaining fluid and electrolyte balance, and is a necessary component of gastric juice. It occurs in plasma in concentrations of 96 to 106 mEq/liter, c and in a more concentrated form in cerebrospinal fluid and gastrointestinal secretions. Its concentration in most cells is low.
Under normal circumstances, dietary deficiency of chloride does not occur. The only known instance of diet-related chloride depletion occurred in healthy infants inadvertently fed diets containing 1 to 2 mEq/liter (Grossman et al., 1980; Rodriguez-Soriano et al., 1983; Roy and Arant, 1981) rather than the minimum of 10.4 mEq/liter now recommended (AAP, 1985). Chloride loss tends to parallel losses of sodium; hence, conditions associated with sodium depletion (e.g., heavy, persistent sweating, chronic diarrhea or vomiting, trauma, or renal disease) will also cause chloride loss, resulting in hypochloremic metabolic alkalosis.
Dietary Sources and Usual Intakes
Dietary chloride comes almost entirely from sodium chloride. Much smaller amounts are supplied from potassium chloride. Therefore, dietary sources of chloride are essentially the same as those described for sodium, and processed foods are the major source. Although chloride is also found in almost all natural waters, estimates by the Environmental Protection Agency (EPA, 1975) suggest a daily contribution of 42 mg/day. This is insignificant compared to the roughly 6 g of chloride a day contributed by added salt.
Estimate of Requirements
Because both the intake of chloride from food and its losses from the body under normal conditions parallel those of sodium, the requirements specified for all age and sex groups except infants parallel those of sodium on a mEq basis (see Table 11-1).
Human milk contains 11 mEq of chloride per liter, which makes the chloride level higher than the sodium level on a mEq basis. The American Academy of Pediatrics has suggested a similar level (10.4 mEq/liter) for infant formulas on the grounds that a 1.5–2.0 ratio of sodium plus potassium to chloride maintained good acid-base regulation in infants (AAP, 1985).
Excessive Intakes and Toxicity
The toxicity of salts containing the chloride ion depends mainly on the characteristics of the cation. The only known dietary cause of hyperchloremia is water-deficiency dehydration. Sustained ingestion of high levels of chloride (as salt) has been associated with elevated blood pressure in sensitive individuals and animal models (Kurtz et al., 1987; Whitescarver et al., 1986).
REFERENCES
- AAP (American Academy of Pediatrics). 1981. Sodium intake of infants in the United States. Pediatrics 68: 444–445. [PubMed: 7279479]
- AAP (American Academy of Pediatrics). 1985. Pediatric Nutrition Handbook, 2nd Ed. American Academy of Pediatrics, Elk Grove Village, Ill.
- Abraham, S., and M.D. Carroll. 1981. Fats, Cholesterol and Sodium Intake in the Diet of Persons 1–74 Years: United States. Advance Data No. 54. U.S. Department of Health, Education, and Welfare, Washington, D.C.
- Buskirk, E.R., and J. Mendez. 1967. Nutrition, environment and work performance with special reference to altitude. Fed. Proc. 26: 1760–1767. [PubMed: 6075911]
- Coatney, G.R., O. Nickelson, R.W. Burgess, M.D. Young, and C.I. Pirkle. 1958. Chloroquin or pyrimethamine in salt as a suppressive against sporozoite-induced vivax malaria (Chesson strain). Bull. W.H.O. 19: 56–67. [PMC free article: PMC2537705] [PubMed: 13585060]
- Conn, J.W. 1949. The mechanisms of acclimitization to heat. Adv. Intern. Med. 3: 373–393.
- Consolazio, C.F., L.O. Matoush, R.A. Nelson, R.S. Harding, and J.E. Canham. 1963. Excretion of sodium, potassium, magnesium and iron in human sweat and the relation of each to balance and requirements. J. Nutr. 79: 407–415. [PubMed: 14022653]
- Cooke, R.E., E.L. Pratt, and D.C. Darrow. 1950. Metabolic response to heat stress. Yale J. Biol. Med. 22: 227. [PMC free article: PMC2598872] [PubMed: 15399987]
- Dahl, L.K. 1960. Possible role of salt intake in the development of essential hypertension. Pp. 53–65 in P. Cottier, editor; and K.D. Bock, editor. , eds. Essential Hypertension: An International Symposium. Springer-Verlag, Heidelberg, Federal Republic of Germany.
- Dahl, L.K., and R.A. Love. 1957. Etiological role of sodium chloride intake in essential hypertension in humans. J. Am. Med. Assoc. 164: 397. [PubMed: 13415996]
- Dole, V.P., L.K. Dahl, G.C. Cotzias, H.A. Eder, and M.E. Krebs. 1950. Dietary treatment of hypertension. Clinical metabolic studies of patients on the rice-fruit diet. J. Clin. Invest. 39: 1189. [PMC free article: PMC436162] [PubMed: 14774466]
- EPA (U.S. Environmental Protection Agency). 1975. Region V. Federal/State Survey of Organics and Inorganics in Selected Drinking Water Supplies. U.S. Environmental Protection Agency, Chicago. 317 pp.
- Fregley, M.J. 1984. Sodium and potassium. Pp. 439–458 in Nutrition Reviews' Present Knowledge in Nutrition, 5th ed. The Nutrition Foundation. Washington, D.C.
- Gothberg, G., S. Lundin, M. Aurell, and B. Folkow. 1983. Responses to slow graded bleeding in salt-depleted rats. J. Hypertens. Suppl. 2: 24–26. [PubMed: 6599490]
- Greathouse, D.G., and G.F. Crown. 1979. Cardiovascular disease study—occurrence of inorganics in household tap water and relationships to cardiovascular mortality rates. Pp. 31–39 in D.D. Hemphill, editor. , ed. Trace Substances in Environmental Health-XII. Proceedings of the 12th Annual Conference, 1978, University of Missouri-Columbia, Columbia, Mo.
- Grim, C.E., F.C. Luft, J.Z. Miller, G.R. Meneely, H.D. Battarbee, C.G. Hames, and L.K. Dahl. 1980. Racial differences in blood pressure in Evans County, Georgia: relationship to sodium and potassium intake and plasma renin activity. J. Chronic Dis. 33: 87–94. [PubMed: 6986391]
- Gross, S.J. 1983. Growth and biochemical response of preterm infants fed milk or modified infant formula. N. Engl. J. Med. 308: 237. [PubMed: 6848932]
- Grossman, H., E. Duggan, S. McCamman, E. Welchert, and S. Hellerstein. 1980. The dietary chloride deficiency syndrome. Pediatrics 66: 366–374. [PubMed: 6932641]
- Hamill, P.V.V., T.A. Drizd, C.L. Johnson, R.B. Reed, A.F. Roche, and W.M. Moore. 1979. Physical growth. National Center for Health Statistics Percentiles. Am. J. Clin. Nutr. 32: 607–629. [PubMed: 420153]
- Holliday, M.A., and W.E. Segar. 1957. The maintenance need for water in parental fluid therapy. Pediatrics 19: 823. [PubMed: 13431307]
- Kempner, W. 1948. Treatment of hypertension vascular disease with rice diet. Am. J. Med. 4: 545. [PubMed: 18909456]
- Khaw, K.T., and E. Barrett-Connor. 1987. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N. Engl. J. Med. 316: 235–240. [PubMed: 3796701]
- Kurtz, T.W., H.A. Al-Bander, and R.C. Morris. 1987. ‘Salt sensitive' essential hypertension in men. N. Engl. J. Med. 317: 1043–1048. [PubMed: 3309653]
- Langford, H.G. 1985. Dietary potassium and hypertension. Pp. 147–153 in M.J. Horan, editor; , M. Blaustein, editor; , J.B. Dunbar, editor; , W. Kachadorian, editor; , N.M. Kaplan, editor; , and A.P. Simopoulos, editor. , eds. NIH Workshop on Nutrition and Hypertension: Proceedings from a Symposium. Biomedical Information Corp., New York.
- Macy, I.C. 1949. Compositon of human colostrum and milk. Am. J. Dis. Child. 78: 589. [PubMed: 18141078]
- Mickelson, O., D. Makdani, J.L. Gill, and R.L. Frank. 1977. Sodium and potassium intakes and excretions of normal men consuming sodium chloride or a 1:1 mixture of sodium and potassium chloride. Am. J. Clin. Nutr. 30: 2033. [PubMed: 930873]
- NRC (National Research Council). 1977. Drinking Water and Health. Report of the Safe Drinking Water Committee, Advisory Center on Toxicology, Assembly of Life Sciences. National Academy of Sciences, Washington, D.C. 939 pp.
- NRC (National Research Council). 1980. a. Drinking Water and Health, Vol. 3. Report of the Safe Drinking Water Committee, Board on Toxicology and Environmental Health Hazards, Assembly of Life Sciences. National Academy Press, Washington, D.C. 415 pp.
- NRC (National Research Council). 1980. b. Recommended Dietary Allowances, 9th Revised Ed. Committeee on Dietary Allowances, Food and Nutrition Board, Division of Biological Sciences, Assembly of Life Sciences. National Academy of Sciences, Washington, D.C. 185 pp.
- NRC (National Research Council), 1989. Diet and Health: Implications for Reducing Chronic Disease Risk. Report of the Committee on Diet and Health, Fovod and Nutrition Board. National Academy Press, Washington, D.C. 750 pp. [PubMed: 25032333]
- Page, L.B. 1976. Epidemiologic evidence on the etiology of human hypertension and its possible prevention. Am. Heart J. 91: 527–534. [PubMed: 769520]
- Page, L.B. 1979. Hypertension and atherosclerosis in primitive and acculturating societies. Pp. 1–12 in J.C. Hunt, editor. , ed. Hypertension Update, Vol; 1. Health Learning Systems, Lyndhurst, N.J.
- Pennington, J.A.T., D.B. Wilson, R.F. Newell, B.F. Harland, R.D. Johnson, and J.E. Vanderveen. 1984. Selected minerals in food surveys, 1974 to 1981/82. J. Am. Diet. Assoc. 84: 771–780. [PubMed: 6736504]
- Pike, R.L., and H.A. Smiciklas. 1972. A reappraisal of sodium restriction during pregnancy. Int. J. Gynecol. Obstet. 10: 1–8.
- Rodriguez-Soriano, J., A. Vallo, G. Castillo, R. Oiveros, J.M. Cea, and M.J. Balzategui. 1983. Biochemical features of dietary chloride deficiency syndrome: a comparative study of 30 cases. J. Pediatr. 103: 209–214. [PubMed: 6875710]
- Roy, S., and B.S. Arant. 1981. Hypokalemic metabolic alkalosis in normotensive infants with elevated plasma renin activity and hyperaldosteronism: role of dietary chloride deficiency. Pediatrics 67: 423–429. [PubMed: 7017580]
- Roy, R.N., G.W. Chance, I.C. Radde, D.E. Hill, D.M. Willis, and J. Sheepers. 1976. Late hyponatremia in very low birthweight infants less than 1.3 kilograms. Pediatr. Res. 10: 526–531. [PubMed: 934723]
- Sanchez-Castillo, C.P., S. Warrender, T.P. Whitehead, and W.P. James. 1987. a. An assessment of the sources of dietary salt in a British population. Clin. Sci. 72: 95–102. [PubMed: 3802726]
- Sanchez-Castillo, C.P., W.J. Branch, and W.P. James. 1987. b. A test of the validity of the lithium-marker technique for monitoring dietary sources of salt in men. Clin. Sci. 72: 87–94. [PubMed: 3802725]
- Sebastian, A., E. McSherry, and R.C. Morris, Jr. 1971. Renal potassium wasting in renal tubular acidosis (RTA): its occurrence in Types 1 and 2 RTA despite sustained correction of systemic acidosis. J. Clin. Invest. 50: 667–678. [PMC free article: PMC291975] [PubMed: 5101785]
- Squires, R.D., and E.J. Huth. 1959. Experimental potassium depletion in normal human subjects. I. Relation of ionic intakes to the renal conservation of potassium. J. Clin. Invest. 38: 1134–1148. [PMC free article: PMC293261] [PubMed: 13664789]
- Tobian, L., Jr. 1979. The relationship of salt to hypertension. Am. J. Clin. Nutr. 32: 2739–2748. [PubMed: 389029]
- USDA (U.S. Department of Agriculture). 1984. Nationwide Food Consumption Survey. Nutrient Intakes: Individuals in 48 States, Year 1977–78. Report No. 1-2. Consumer Nutrition Division, Human Nutrition Information Service, U.S. Department of Agriculture, Hyattsville, Md. 439 pp.
- Whitescarver, S.A., B.J. Holtzclaw, J.H. Downs, O.H. Co, J.R. Sowers, and T.A. Kotchen. 1986. Effect of dietary chloride on salt-sensitive and renin-dependent hypertension. Hypertension 8: 56–61. [PubMed: 3510973]
Footnotes
- a
1 mEq of sodium is 23 mg, and 1 mmol of sodium chloride is 58.5 mg.
- b
1 mEq of potassium is 39 mg.
- c
1 mEq of chloride is 35.5 mg.
- Water and Electrolytes - Recommended Dietary AllowancesWater and Electrolytes - Recommended Dietary Allowances
Your browsing activity is empty.
Activity recording is turned off.
See more...