NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Committee on the Learning Health Care System in America; Institute of Medicine; Smith M, Saunders R, Stuckhardt L, et al., editors. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press (US); 2013 May 10.

Best Care at Lower Cost: The Path to Continuously Learning Health Care in America.
Show detailsDr. Charles Bennett, an academic oncologist whose clinical practice has been devoted solely to prostate cancer for 25 years, was diagnosed with prostate cancer in 2006. Upon examining his own biopsy results under the microscope, he was confronted with the same decision so many of his patients had faced before: surgery, radiation, or active surveillance? In an effort to be an informed patient, Dr. Bennett pursued opinions from medical, surgical, and radiation oncologists, and eventually chose to undergo a radical prostatectomy, convinced that his risks were small and the benefits would be great. Five years later, he remains cancer-free, but his right arm and leg are permanently weak, a dysfunction that appeared immediately after the surgery. Looking back, Dr. Bennett would have made a different decision. Prostatectomy provides the benefit of high prostate cancer–specific 20-year survival rates; even when performed by skilled surgeons, however, it carries significant risks of sexual, bladder, and bowel dysfunction, along with less common side effects such as Dr. Bennett's. Active surveillance, coupled with regular screening tests and physical examinations, is associated with much lower rates of these effects and allows for appropriate identification of when to switch from surveillance to treatment. Knowing what he now knows, Dr. Bennett would have opted for active surveillance, proving that even the most informed members of the health care system have difficulty making informed medical decisions as patients (Bennett, 2012).
Over the past century, the health of the U.S. population has improved dramatically. Life expectancy has increased by almost 60 percent, maternal mortality has declined by almost 99 percent, and infant mortality has dropped by more than 90 percent (Guyer et al., 2000). While these increases in survival have been due to many factors, such as public health efforts (CDC, 1999, 2011b), technical improvements in health care have played an increasingly significant role. The health care field today has a better understanding of the causes of individual diseases, as well as new techniques, treatments, and interventions for managing these diseases.
At the same time, the resulting complexity has implications for both patients and providers. The complexity of different health care options—in terms of treatments, diagnostics, and care management—increases the difficulty of the care decisions patients face. When making these decisions, patients often lack clear and understandable information on their options, the risks and benefits of each, and the actions they can take in managing their condition. For those working in the health care enterprise, the current complexity of clinical decision making challenges human cognitive capacity to manage information. One notable example of this complexity is advances in genetics, which offer unprecedented opportunities for personalized treatments but add to the already expansive array of clinical considerations for patients and providers. Moreover, administrative complexities, from complicated workflows to fragmented financing, add inefficiency and waste at the system level and prevent health care from centering its efforts on the patients it serves.
CLINICAL COMPLEXITY
Advances in clinical knowledge have allowed for dramatic improvements in the health of the U.S. population. One area in which these improvements are notable is the treatment of heart attack, or myocardial infarction. During most of the twentieth century, little could be done for a patient who had just suffered a heart attack. The most common intervention was to prescribe weeks of bed rest in the hope that the patient would heal on his or her own. Some patients did heal, but many lost skeletal muscle mass and the ability to care for themselves after the prolonged time in bed (Certo, 1985).
Recent decades have seen a transformation in cardiac care. Today, diagnostics recognize the different types of heart attacks, allowing for customized treatments for patients. Pharmaceutical therapies, such as beta-blockers and thrombolytics, improve survival and reduce the chances of subsequent heart attacks for many groups of patients. Finally, interventions such as percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) can reopen or bypass blockages in blood vessels and restore blood flow to the heart (Antman et al., 2004, 2008; Braunwald et al., 2000, 2002).
As illustrated in Figure 2-1, the research in cardiovascular disease has allowed for better understanding of the disease and new options in cardiac care (Nabel and Braunwald, 2012). These improvements in care, along with improvements in prevention, have contributed to decades-long declines in both acute and long-term mortality from heart attack (Heidenreich and McClellan, 2001; Rogers et al., 2008). For example, one study found that improvements in medications and interventions over the past three decades were associated with better hospital survival rates, which increased from 81 percent in 1975 to 91 percent in 2005 (Floyd et al., 2009). Similarly, another assessment found that in-hospital fatalities for heart attack patients dropped by almost two-thirds from 1979 to 2005 (Fang et al., 2010).

FIGURE 2-1
Timeline of advances in cardiac care, highlighting how improvements in care, prevention, and reduction in risk factors have contributed to declines in cardiovascular mortality over the same time frame. NOTE: ALLHAT = Antihypertensive and Lipid-Lowering (more...)
Comparable advances have been achieved in the treatment of many other diseases. One notable example is in care for HIV/AIDS, as summarized in Figure 2-2. In the three decades since this disease was first documented, 35 medications have been introduced for its treatment, sensitive tests have been developed to diagnose the disease at even earlier stages, and other tests have been developed to allow clinicians to identify specific genetic characteristics of the virus in a given patient (Fauci, 2003; FDA, 2011a; Fischl et al., 1987; Simon et al., 2006). These advances have transformed HIV from an almost entirely fatal disease to a chronic condition. At the same time, this remarkable achievement brings new complexity to clinical care. Clinicians must understand the resistance profiles of patients, tailoring the combination of therapies accordingly. They must monitor the patient's viral load to ensure that the treatment continues to work, assess over the course of treatment whether it is causing any adverse effects, and seek to prevent interactions between the patient's HIV drugs and treatments for other health conditions (from antacids to cardiac medications). Further, the pace of treatment advances, as well as mutations in the virus found in the general population, requires that clinicians who work in this area constantly update the way they practice care (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2011).
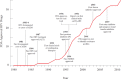
FIGURE 2-2
Timeline of advances in HIV treatment, highlighting increases in Food and Drug Administration (FDA)-approved HIV drugs in the same time frame. NOTE: HAART = highly active antiretroviral therapy; NNRTI = non-nucleotide reverse transcriptase inhibitor. (more...)
Such advances are not limited to these two diseases but are widespread, as illustrated for the example of cancer care in Figure 2-3. As a result of improved scientific understanding, new treatments and interventions, and new diagnostic technologies, the U.S. health care system now is characterized by more to do, more to know, and more to manage than at any time in history. The result is a paradox: advances in science and technology have improved the ability of the health care system to treat diseases, yet the sheer volume of new discoveries stresses the capabilities of the system to effectively generate and manage knowledge and apply it to regular care. As discussed in Chapter 3, these advances have occurred at the same time as, and sometimes have contributed to, challenges of health care quality and value.
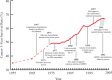
FIGURE 2-3
Timeline of advances in cancer care, highlighting improvements in the 5-year survival rate in the same time frame. NOTE: BRCAI = breast cancer susceptibility gene 1; FDA = Food and Drug Administration; IL-2 = interleukin-2; IMRT = intensity modulated (more...)
Implications of Complexity for Clinical Decision Making
The complexity of the U.S. health care system means that patients and clinicians have more information to consider and more decisions to make than ever before. Often, these decisions are neither easy nor straightforward, and they include varying options, trade-offs, benefits, and risks. Further complicating matters, patients often lack the information they need to make decisions. Fewer than half of patients receive clear information on the benefits and trade-offs of the treatments for their condition (Fagerlin et al., 2010; Sepucha et al., 2010; Zikmund-Fisher et al., 2010).
As the description of Dr. Bennett's case at the beginning of this chapter demonstrates, one condition that entails difficult decisions is prostate cancer. Prostate cancer is common, developed by one in six men during their lifetime. In at least 80 percent of cases, it is diagnosed at a stage when it is still localized to the prostate gland (Howlader et al., 2011). Patients receiving a diagnosis of localized prostate cancer then must decide what course of action to take. They may choose either to wait and monitor the cancer for any changes (watchful waiting) or to treat it immediately. If they choose to treat it, they have a number of options to consider, including surgery to remove the prostate gland (traditional, laparoscopic, and robotic-assisted versions), various forms of radiation treatment (such as intensity modulated radiation therapy [IMRT], brachytherapy, and proton beam therapy), freezing of the prostate (cryotherapy), and hormone treatment (androgen deprivation therapy) (Institute for Clinical and Economic Review, 2010; Wilt et al., 2008b).
The difficulty of this decision is that localized prostate cancer generally is slow-growing and often causes no harm during the patient's lifetime. In addition, there is a distinct lack of evidence on which treatment works best for a given patient with localized cancer. This absence of evidence is acutely felt for emerging technologies, such as IMRT, proton beam therapy, laparoscopic and robotic-assisted prostatectomy, and cryotherapy, which nevertheless are increasingly being used (Hegarty et al., 2010; Institute for Clinical and Economic Review, 2010; Makarov et al., 2011; Wilt et al., 2008a,b). All treatments for this disease have varying, potentially long-lasting side effects, including sexual, urinary, and bowel problems. While it is unknown which treatment option is the right choice for a given patient, the cost of the treatments varies widely. For example, the Medicare reimbursement for traditional surgical removal of the prostate is approximately $10,000, while the first-year costs for proton beam therapy are nearly $40,000 (Institute for Clinical and Economic Review, 2010).
Increasing Occurrence of Multiple Chronic Conditions
Prostate cancer is not a unique case. For many conditions, patients and clinicians are presented with many diagnostic and treatment options but lack the evidence to know which option would be most effective. This situation is particularly prevalent for patients with chronic conditions. The prevalence of chronic conditions has increased over time. In 2000, 125 million people suffered from chronic conditions; by 2020, that number is projected to grow to an estimated 157 million (Anderson et al., 2010). Today one such condition, diabetes, affects almost 10 percent of the U.S. population (CDC, 2011a). Furthermore, approximately 75 million people in the United States have multiple, concurrent chronic conditions (Parekh and Barton, 2010). The costs of treating chronic conditions are high, with one study estimating that the care of patients with these conditions constitutes almost 80 percent of health care costs (Anderson and Horvath, 2004). A related finding illustrates the importance of caring for patients with serious health needs. An analysis of health care expenditures found that while patients with the highest health care costs represent just 5 percent of the total U.S. population, their care consumes 50 percent of total health care resources (Cohen and Yu, 2011).
The role of chronic conditions has changed as the demographics of the population have shifted. In general, the population has gotten older, with the portion of the population over the age of 65 having increased at 1.5 times the rate of the rest of the population in the past decade (Howden and Meyer, 2011). Almost half of the individuals in this population receive treatment for at least one chronic condition (Schneider et al., 2009); one-quarter are affected by just one of those conditions, diabetes (CDC, 2011a; Schneider et al., 2009). Furthermore, more than 20 percent of the elderly are receiving treatment for multiple chronic conditions (Schneider et al., 2009).
The complexity of care is particularly acute for patients with multiple chronic conditions. Treating these patients requires a holistic approach, because the use of multiple clinical practice guidelines developed for single diseases may have adverse effects (Boyd et al., 2005; Parekh and Barton, 2010; Tinetti et al., 2004). For example, various existing clinical practice guidelines would suggest that a hypothetical 79-year-old woman with osteoporosis, osteoarthritis, type 2 diabetes, hypertension, and chronic obstructive pulmonary disease should take as many as 19 doses of medication per day. Adherence to five separate sets of clinical practice guidelines for the woman's five diseases could result in adverse interactions between her medications, or a medication for one disease could exacerbate the symptoms of another (see Table 2-1 for potential treatment interactions). Such guidelines might also make conflicting recommendations for the woman's care. If she had peripheral neuropathy, guidelines for osteoporosis would recommend that she perform weight-bearing exercise, while guidelines for diabetes would recommend that she avoid such exercise (Boyd et al., 2005). These situations create uncertainty for clinicians and patients as to the best course of action to pursue as they attempt to manage the treatments for multiple conditions.
TABLE 2-1
Potential Treatment Interactions for a Hypothetical 79-Year-Old Woman with Multiple Chronic Diseases.
A Strain on Human Capacity
As clinicians endeavor to provide the best and most appropriate care for their patients, they also struggle with the cognitive complexities inherent in making care decisions. In the clinical setting, providers begin the decision-making process from the moment they set eyes on their patients. For example, an emergency medicine clinician must make decisions on clinical factors such as the patient's medical history, test ordering, interpretation of laboratory results, diagnosis, treatment, and patient preferences, as well as nonclinical factors such as cost, allocation of resources, and administrative considerations (Croskerry, 2002).
Like the emergency department, the intensive care unit (ICU) is a particularly difficult environment for clinicians. These specialized units help the sickest and most fragile patients, who could not survive without the support of specialized technologies and equipment. The price of these new capabilities is extraordinary complexity that stresses the capabilities of individual clinicians. One observational study found that clinicians in ICUs perform in the range of 180 activities per patient per day, from replacing intravenous fluids, to calibrating a transducer, to administering drugs (Donchin et al., 2003). With new monitoring technologies, clinicians are able to observe the patient's health status precisely. For example, a patient who enters the ICU with acute respiratory distress is monitored with more than 20 vital sign parameters. With 6 to 12 patients in a typical ICU, a provider must monitor and act on up to 240 vital sign inputs, which stresses any individual provider's cognitive capabilities (Donchin and Seagull, 2002).
The growth in complexity is not limited to hospital environments. Physicians, nurses, physician assistants, and other health care professionals in outpatient settings are managing a great number of conditions and interventions. Quantifying the range of conditions managed by clinicians, a 2008 study of a large multispecialty practice in Massachusetts found that the practice managed more than 5,600 unique primary diagnoses and 6,400 unique secondary diagnoses, or almost half of all known identified diagnoses. Each clinician managed a median of approximately 250 unique primary diagnoses, 280 unique medications, and 130 unique laboratory tests. These figures were even higher for those clinicians in primary care fields, such as internal medicine, who managed a median of 370 unique primary diagnoses, 600 unique medications, and approximately 150 unique laboratory tests (Semel et al., 2010). These findings highlight the variety of needs clinicians now address, along with the variety of interventions and diagnostic tests they must manage.
Further, physicians often feel as though they do not have enough time to meet their patients' care needs (Burdi and Baker, 1999; Trude, 2003). Among primary care physicians responding to one survey, 30 percent reported not having adequate time to spend with their patients during a typical visit (Center for Studying Health System Change, 2004–2005), and a similar percentage of patients reported concerns about the amount of time their providers have to spend with them (AHRQ, 2010)—this despite evidence that the average length of a primary care visit has actually increased in recent years (Mechanic et al., 2001). Evidence suggests, however, that clinicians' perceptions are warranted. One study found that meeting a standard patient panel's acute, preventive, and chronic disease management needs would require more than 21 hours a day, as shown in Figure 2-4 (Yarnall et al., 2009).
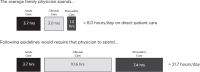
FIGURE 2-4
Time requirements for a primary care physician to treat a standard patient panel. SOURCE: Data derived from Yarnall et al., 2009.
As outlined above, the complexity of modern health care is reaching levels that challenge human cognitive capacity. Research in several areas has found that complexity can have negative effects on people's ability to make decisions (Simon, 1979, 1990; Weick and Sutcliffe, 2001). Complexity can cause people to defer making a decision, choose the default option, make no decision at all, or make an incorrect decision (Dhar, 1997; Shafir and Tversky, 1992; Shafir et al., 1993). As one example, when confronted with highly complex situations, people tend to use mental shortcuts, or heuristics, to manage the volume of evidence (Berner and Graber, 2008; Bullen and Sacks, 2003; Kampmann and Sterman, 1998; Payne et al., 1993; Timmermans, 1993; Tversky and Kahneman, 1973, 1974). These mental shortcuts range from overrelying on memorable past experiences to accepting data that confirm preexisting expectations and ignoring data that do not (see Table 2-2 for a summary of five of the most common cognitive errors). Several studies suggest that heuristics are used in health care settings and can have real impacts on patient care (Gandhi et al., 2006; Graber et al., 2005).
TABLE 2-2
Common Cognitive Errors in Clinical Decision Making.
In most cases, the shortcut works well to solve the problem at hand (Redelmeier, 2005). Precisely because these shortcuts usually produce the desired outcome, however, most people are unaware of their own susceptibility to cognitive errors. While strategies to overcome cognitive errors in clinical decision making are beginning to be identified (Croskerry, 2002, 2003; Redelmeier, 2005), time and resource constraints, increasing stress among providers, and growing complexity are all barriers to overcoming the risks of these errors.
The volume of biomedical and clinical knowledge being produced has increased steadily over the past few decades. The number of journal articles in biomedical and clinical research fields has quadrupled since 1970, rising from more than 200,000 a year in 1970 to more than 750,000 in 2010 (see Figure 2-5).1 The pace of research now averages 75 trials and 11 systematic reviews of trials per day (Bastian et al., 2010). The pace at which new knowledge is produced outstrips the ability of any individual clinician to read, remember, and manage information that could inform clinical practice. A survey of faculty at one academic medical center found that they each read up to 322 papers per year (Tenopir et al., 2004). Given the almost 450,000 papers published in 2000, this amounts to less than 0.1 percent of the medical literature produced during the initial year in which the survey was conducted. Even within a narrow specialty, it is impossible for a clinician to keep pace with the published medical literature. If a clinician training in cardiac imaging read 40 papers a day for 5 days a week, then it would take more than 11 years for that clinician to become up to date in the field. By that time, however, another 82,000 potentially relevant papers would have been published, which would require another 8 years of reading. These figures assume that the clinician needs to know only about cardiac imaging and need not remain current in any other area of medical knowledge (Fraser and Dunstan, 2010).

FIGURE 2-5
Number of journal articles published on health care topics per year from 1970 to 2010. Publications have increased steadily over 40 years, with the rate of increase becoming more pronounced starting approximately in 2000. SOURCE: Data obtained from online (more...)
The ever-increasing volume of evidence makes it difficult for clinicians to maintain a working knowledge of new clinical information. Even after identifying relevant information for a given condition, clinicians and their patients still must ensure that the information is of high quality. Clinicians must consider the quality of a study to minimize the possibility that the evidence will be contradicted by later studies, and ensure that the research is free of conflicts of interest and applies to their particular patient's clinical circumstances (Ioannidis, 2005; Prasad et al., 2011).
Uneven Diffusion of Knowledge
Although the supply of knowledge is increasing, there are lags in the time it takes to translate promising evidence into clinical practice. It is estimated that the results of a landmark study will take 15–16 years to be widely implemented following the study's publication (Balas and Boren, 2000). For example, it took 13 years for most experts to recommend thrombolytic drugs for heart attack treatment after their first positive clinical trial (Antman et al., 1992). Similarly, the results of major clinical trials often are not implemented in regular clinical practice, as was the case for the Occluded Artery Trial (OAT) on the timing of coronary angioplasty after heart attack (Deyell et al., 2011; Redberg, 2011), the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) on effective treatments for high blood pressure (Avorn, 2010; Stafford et al., 2010), and the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) study on coronary angioplasty versus medical therapy (Borden et al., 2011). As a result of this slow diffusion of knowledge and other factors, Americans receive only half of the preventive, acute, and chronic care recommended by clinical guidelines (McGlynn et al., 2003) and approximately 60 percent of recommended pharmaceutical treatments (Shrank et al., 2006).
Implications of Advances in Genetics
The accelerating pace of research has led to striking prospects for individualized diagnoses and treatments. Although the potential is still largely unrealized, ongoing developments offer promise to accelerate this progress. For example, the cost of sequencing the whole genome has decreased from $2.7 billion, the cost when the first human genome was sequenced, to $10,000 in 2010 and may fall to as little as $1,000 in the foreseeable future (Samani et al., 2010). Between 2005 and 2008, more than 100 genetic variants associated with nearly 40 complex diseases and traits were identified and replicated using genomewide scans, and in 2008, genetic tests for more than 1,200 clinical conditions were available (Genetics and Public Policy Center, 2008; Manolio, 2010; Pearson and Manolio, 2008). The genetic factors associated with a variety of diseases are now known and can be used in diagnosis and treatment (see Table 2-3) (IOM, 2011). These new discoveries highlight the magnitude of individual variation, adding numerous factors that clinicians may have to consider when evaluating the utility of different treatments and interventions.
TABLE 2-3
Genetic Variants Used for Disease Diagnosis and Treatment.
One area in which advances in genetics have led to a more sophisticated understanding of disease is the ability to distinguish among different types of lung cancers. Traditionally, lung cancers were divided into types—small-cell and non-small-cell—based on the tumor's histological appearance. However, genetic discoveries have allowed histological classification to be replaced by classification based on the cancer cells' genetic profile, and more specifically, the genetic mutations that are the molecular drivers of cancer cell proliferation (see Figure 2-6). In 1987, one driver mutation, KRAS, was discovered, and another, EGFR, was discovered in 2004. Since then, knowledge of the molecular drivers of non-small-cell lung cancer has increased dramatically; by 2009, nine different driver mutations had been identified (IOM, 2011). While the development of therapies targeting specific driver mutations is just beginning, genetic classification of diseases holds great promise for tailoring care to patients' genetic variations.

FIGURE 2-6
Evolution in knowledge of the genetic driver mutations associated with non-small-cell lung cancer. SOURCE: Reprinted from Pao and Girard, 2011. Copyright (2011), with permission from Elsevier.
An example of a case in which genetics are beginning to have a substantial impact on care is maturity-onset diabetes of the young (MODY). This rare form of diabetes, generally diagnosed in later adolescence or early adulthood, often is undiagnosed and is easily confused with other forms of diabetes. Treating patients with this condition used to be difficult because different patients would respond very differently to various treatments (O'Rahilly, 2009). With improved genetic understanding, however, MODY was found to be a cluster of diseases, each entailing a specific genetic abnormality. To date, six different varieties of this disease have been identified, each with a specific genetic component (Chromosome 12, HNF-1α; Chromosome 7, glucokinase; Chromosome 20, HNF-4α; Chromosome 13, insulin promoter factor-1 (IPF-1); Chromosome 17, HNF-1β; Chromosome 2, NeuroD1) (American Diabetes Association, 2011). This progress in genetics has allowed clinicians to personalize treatments based on which form of the disease a patient has. Some patients will respond well to low doses of oral hyperglycemia medications and will not need insulin therapy; others will require insulin injections; and others may have a stable condition and may not need aggressive glucose reduction therapies (Gill-Carey and Hattersley, 2007; Hattersley and Pearson, 2006; O'Rahilly, 2009).
Figure 2-7 illustrates how such advances in genetics and related fields have increased over time, adding to the complexity of clinical decision making. Indeed, as noted earlier, this growth in knowledge may be expanding beyond the limits of what human cognitive capacity can handle.
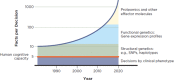
FIGURE 2-7
Schematic describing the sources of complexity in clinical decision making, highlighting their increase over time. NOTE: SNP = single nucleotide polymorphism. SOURCE: Adapted with permission from IOM, 2008.
Conclusion 2-1: Diagnostic and treatment options are expanding and changing at an accelerating rate, placing new stresses on clinicians and patients, as well as potentially impacting the effectiveness and efficiency of care delivery.
Related findings:
Conclusion 2-2: Chronic diseases and comorbid conditions are increasing, exacerbating the clinical, logistical, decision-making, and economic challenges faced by patients and clinicians.
Related findings:
ADMINISTRATIVE COMPLEXITY
Administrative complexity, including complicated workflows and fragmented financing, exacerbate the challenges posed by the clinical complexity described above.
Complicated Workflows
The health care system is characterized by administrative complexity that can waste clinicians' time and interfere with their caring for their patients, as well as increase costs and adversely impact patient outcomes. For example, a study investigating waste in the activities of front-line health care workers found that 35 percent of the workers' time was wasted (Wallace and Savitz, 2008).
Even accomplishing a seemingly straightforward activity such as filling a medication order is marked by unexpected intricacies. As illustrated in Figure 2-8, a medication order at one academic medical center can be filled in 786 different ways, involving a number of different health care professionals and technological channels (Thompson et al., 2003). Another study found that inefficient medication administration practices at one hospital caused nurses to waste 50 minutes per shift looking for the keys to the narcotics cabinet (Spear and Schmidhofer, 2005; Thompson et al., 2003). The results of this administrative complexity and inefficiency are delayed medications, potential errors, waste, and higher costs. Inefficient workflows also restrict the amount of time nurses can spend directly caring for patients; indeed, it has been found that hospital nurses spend only about 30 percent of their time in direct patient care (Hendrich et al., 2008; Hendrickson et al., 1990; Tucker and Spear, 2006).
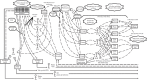
FIGURE 2-8
Diagram of processes for filling a medication order at one academic medical center. SOURCE: Adapted and reprinted with permission from Thompson et al., 2003.
Studies also have revealed the effects of system complexity on hospital staffing, and in turn on patient outcomes. Despite an average bed occupancy rate of 65 percent, hospitals frequently are overcrowded (Litvak, 2005; Litvak and Bisognano, 2011). Hospital admissions generally come from two sources: emergency departments (EDs), which are unpredictable as a source of admissions, and scheduled elective procedures, which are a seemingly predictable source (Litvak et al., 2005). Because hospitals staff for average occupancy and not for peaks, an unexpected influx of patients creates demands for resources and staff time that are impossible to meet, which can cause problems such as emergency room overcrowding, ICU readmissions, and ICU workload and safety problems (Baker et al., 2009; Carayon and Gurses, 2005; IOM, 2007). Studies have found associations between overcrowding and increased mortality (Needleman et al., 2011), as well as decreased adherence to safety practices, such as reconciling of medications, prevention practices for central-line-associated bloodstream infection, and handwashing (Jayawardhana et al., 2011).
Fragmented Financing
Approximately 60 percent of Americans under age 65 obtain health insurance from more than 1.5 million different employers that purchase insurance plans from more than 1,200 insurers (Cebul et al., 2008). In a typical year, moreover, roughly 20 percent of health insurance policyholders change their plans (Cebul et al., 2008; Cunningham and Kohn, 2000). Switches in health plans can occur because of transitions in job status, changes in eligibility for public programs (such as Medicaid or the Children's Health Insurance Program [CHIP]), or decisions to enroll in another employer-sponsored or individual plan. This frequent turnover in insurance relationships has implications for health care costs and outcomes. While many payers support preventive care and chronic care management, frequent changes in insurance enrollment lessen the incentives for investing in early interventions that can reduce long-term health care costs (Cebul et al., 2008).
Managing the requirements of many different health benefit plans places a heavy administrative burden on clinicians. A recent study found that physicians reported spending an average of 43 minutes a day on interactions with health plans—adding up to almost 3 weeks per year on such activities. Nursing staff spent 9 hours per physician per week interacting with health plans, and clerical staff 30 hours per physician per week. In monetary terms, in 2006 practices spent an average of $68,274 per physician per year, the equivalent of roughly $31 billion, interacting with health plans (Casalino et al., 2009).
Continuity of care is compromised as a result of fragmented financing. A study of the overlap among health maintenance organizations (HMOs) in the same cities found that a person switching from one HMO to another had a 50 percent chance of having to change his or her primary care physician (Chernew et al., 2004). This finding is significantly problematic, as continuity of care is associated with a reduced likelihood of future hospitalizations and emergency visits (Gill and Mainous, 1998; Mainous and Gill, 1998; Menec et al., 2006; van Walraven et al., 2010). A recent study of low-income veterans found that as financing become more fragmented, patients were more likely to be hospitalized; the effect of fragmented financing on hospitalizations was similar to that of being diagnosed with a major chronic disease (Pizer and Gardner, 2011).
Finally, it is important to recognize that health care delivery did not begin this way. Rather, it has evolved into a fragmented, disorganized amalgamation characterized by increasingly unmanageable complexity. Prevailing incentives—economic and cultural—allowed for and facilitated this development, and because many health care stakeholders contributed to this evolutionary process, all will need to be engaged in the transition to a continuously learning health care system.
Conclusion 2-3: Care delivery has become increasingly fragmented, leading to coordination and communication challenges for patients and clinicians.
Related findings:
REFERENCES
- AHRQ (Agency for Healthcare Research and Quality). The CAHPS database: Preliminary comparative data for the CAHPS clinician & group survey (12-month version). Rockville, MD: AHRQ; 2010.
- American Diabetes Association. Diabetes Care. Suppl. 1. Vol. 34. 2011. Diagnosis and classification of diabetes mellitus; pp. S62–S69. [PMC free article: PMC3006051] [PubMed: 21193628]
- Anderson GF. Chronic care: Making the case for ongoing care. Princeton, NJ: Robert Wood Johnson Foundation; 2010.
- Anderson GF, Horvath J. Public Health Reports. 3. Vol. 119. 2004. The growing burden of chronic disease in America; p. 263. [PMC free article: PMC1497638] [PubMed: 15158105]
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. Journal of the American Medical Association. 2. Vol. 268. 1992. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction; pp. 240–248. [PubMed: 1535110]
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK., American College of Cardiology, American Heart Association Task Force on Practice Guidelines, and Canadian Cardiovascular Society. Circulation. 9. Vol. 110. 2004. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) pp. e82–e292. [PubMed: 15339869]
- Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW., Canadian Cardiovascular Society, American Academy of Family Physicians, American College of Cardiology, and American Heart Association. Journal of the American College of Cardiology. 2. Vol. 51. 2008. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines; pp. 210–247. [PubMed: 18191746]
- Avorn J. Archives of Internal Medicine. 10. Vol. 170. 2010. Transforming trial results into practice change: The final translational hurdle: Comment on “impact of the ALLHAT/JNC7 dissemination project on thiazide-type diuretic use.” pp. 858–860. [PubMed: 20498412]
- Baker DR, Pronovost PJ, Morlock LL, Geocadin RG, Holzmueller CG. Critical Care Medicine. 11. Vol. 37. 2009. Patient flow variability and unplanned readmissions to an intensive care unit; pp. 2882–2887. [PubMed: 19866504]
- Balas E, Boren S. Yearbook of Medical Informatics. 2000. Managing clinical knowledge for health care improvement; pp. 65–70. [PubMed: 27699347]
- Bastian H, Glasziou P, Chalmers I. PLoS Medicine. 9. Vol. 7. 2010. Seventy-five trials and eleven systematic reviews a day: How will we ever keep up; p. e1000326. [PMC free article: PMC2943439] [PubMed: 20877712]
- Bennett CL. Archives of Internal Medicine. 4. Vol. 172. 2012. A 56-year-old physician who underwent a PSA test; p. 311. [PubMed: 22371918]
- Berner ES, Graber ML. American Journal of Medicine. Suppl. 5. Vol. 121. 2008. Overconfidence as a cause of diagnostic error in medicine; pp. S2–S23. [PubMed: 18440350]
- Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Journal of the American Medical Association. 18. Vol. 305. 2011. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention; pp. 1882–1889. [PubMed: 21558519]
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Journal of the American Medical Association. 6. Vol. 294. 2005. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases; p. 716. [PubMed: 16091574]
- Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, Steward DE, Theroux P, Alpert JS, Eagle KA, Faxon DP, Fuster V, Gardner TJ, Gregoratos G, Russell RO, Smith SC. Journal of the American College of Cardiology. 3. Vol. 36. 2000. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina) pp. 970–1062. [PubMed: 10987629]
- Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC., American College of Cardiology, American Heart Association Committee on the Management of Patients with Unstable Angina. Journal of the American College of Cardiology. 7. Vol. 40. 2002. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction—summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina) pp. 1366–1374. [PubMed: 12383588]
- Bullen G, Sacks L. Version. 1: 2003. Towards new modes of decision making—complexity and human factors; pp. 1–5.
- Burdi MD, Baker LC. Health Affairs. 4. Vol. 18. 1999. Physicians' perceptions of autonomy and satisfaction in California; p. 134. [PubMed: 10425851]
- Carayon P, Gurses AP. Intensive & Critical Care Nursing: The Official Journal of the British Association of Critical Care Nurses. 5. Vol. 21. 2005. A human factors engineering conceptual framework of nursing workload and patient safety in intensive care units; pp. 284–301. [PubMed: 16182125]
- Casalino LP, Nicholson S, Gans DN, Hammons T, Morra D, Karrison T, Levinson W. Health Affairs. 4. Vol. 28. 2009. What does it cost physician practices to interact with health insurance plans; pp. W533–W543. [PubMed: 19443477]
- CDC (Centers for Disease Control and Prevention). Morbidity and Mortality Weekly Report. 12. Vol. 48. 1999. Ten great public health achievements—United States, 1900–1999; pp. 241–243. [PubMed: 10220250]
- CDC. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2011a.
- CDC. Morbidity and Mortality Weekly Report. 19. Vol. 60. 2011b. Ten great public health achievements—United States, 2001–2010; pp. 619–623. [PubMed: 21597455]
- Cebul R, Rebitzer J, Taylor L, Votruba M. Organizational fragmentation and care quality in the U.S. health care system. Cambridge, MA: National Bureau of Economic Research; 2008. [PubMed: 19791306]
- Center for Studying Health System Change. Physician survey. 2004–2005. [November 11, 2011]. hscdataonline
.s-3.com/psurvey.asp. - Certo CM. Physical Therapy. 12. Vol. 65. 1985. History of cardiac rehabilitation; pp. 1793–1795. [PubMed: 3906683]
- Chernew ME, Wodchis WP, Scanlon DP, McLaughlin CG. Health Affairs. 2. Vol. 23. 2004. Overlap in HMO physician networks; p. 91. [PubMed: 15046134]
- Cohen SB, Yu W. The concentration and persistence in the level of health expenditures over time: Estimates for the U.S. population, 2008–2009. Rockville, MD: AHRQ; 2011.
- Croskerry P. Academic Emergency Medicine. 11. Vol. 9. 2002. Achieving quality in clinical decision making: Cognitive strategies and detection of bias; pp. 1184–1204. [PubMed: 12414468]
- Croskerry P. Academic Medicine. 8. Vol. 78. 2003. The importance of cognitive errors in diagnosis and strategies to minimize them; p. 775. [PubMed: 12915363]
- Cunningham PJ, Kohn L. Health Affairs. 3. Vol. 19. 2000. Health plan switching: Choice or circumstance; p. 158. [PubMed: 10812794]
- DeVita VT Jr, Chu E. Cancer Research. 21. Vol. 68. 2008. A history of cancer chemotherapy; pp. 8643–8653. [PubMed: 18974103]
- DeVita VT Jr, Rosenberg SA. New England Journal of Medicine. 23. Vol. 366. 2012. Two hundred years of cancer research; pp. 2207–2214. [PMC free article: PMC6293471] [PubMed: 22646510]
- Deyell MW, Buller CE, Miller LH, Wang TY, Dai D, Lamas GA, Srinivas VS, Hochman JS. Archives of Internal Medicine. 18. Vol. 171. 2011. Impact of national clinical guideline recommendations for revascularization of persistently occluded infarct-related arteries on clinical practice in the United States; pp. 1636–1643. [PMC free article: PMC3738051] [PubMed: 21747002]
- Dhar R. Journal of Consumer Research. 2. Vol. 24. 1997. Consumer preference for a no-choice option; pp. 215–231.
- Donchin Y, Seagull FJ. Current Opinion in Critical Care. 4. Vol. 8. 2002. The hostile environment of the intensive care unit; pp. 316–320. [PubMed: 12386492]
- Donchin Y, Gopher D, Olin M, Badihi Y, Biesky M, Sprung CL, Pizov R, Cotev S. Quality & Safety in Health Care. 2. Vol. 12. 2003. A look into the nature and causes of human errors in the intensive care unit; pp. 143–147. [PMC free article: PMC1743697] [PubMed: 12679512]
- Fagerlin A, Sepucha KR, Couper MP, Levin CA, Singer E, Zikmund-Fisher BJ. Medical Decision Making. Suppl 5. Vol. 30. 2010. Patients' knowledge about 9 common health conditions: The decisions survey; pp. S35–S52. [PubMed: 20881153]
- Fang J, Alderman MH, Keenan NL, Ayala C. American Journal of Medicine. 3. Vol. 123. 2010. Acute myocardial infarction hospitalization in the United States, 1979 to 2005; pp. 259–266. [PubMed: 20193835]
- Fauci AS. Nature Medicine. 7. Vol. 9. 2003. HIV and AIDS: 20 years of science; pp. 839–843. [PubMed: 12835701]
- FDA (Food and Drug Administration). Antiretroviral drugs used in the treatment of HIV infection. 2011a. [February 1, 2012]. http://www
.fda.gov/ForConsumers /ByAudience /ForPatientAdvocates /HIVandAIDSActivities/ucm118915.htm. - FDA. Timeline/history. 2011b. [February 2, 2012]. http://www
.fda.gov/ForConsumers /ByAudience /ForPatientAdvocates /HIVandAIDSActivities/ucm117935.htm. - Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Schooley RT. New England Journal of Medicine. 4. Vol. 317. 1987. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex; pp. 185–191. [PubMed: 3299089]
- Floyd KC, Yarzebski J, Spencer FA, Lessard D, Dalen JE, Alpert JS, Gore JM, Goldberg RJ. Circulation. Cardiovascular Quality and Outcomes. 2. Vol. 2. 2009. A 30-year perspective (1975–2005) into the changing landscape of patients hospitalized with initial acute myocardial infarction: Worcester Heart Attack Study; pp. 88–95. [PMC free article: PMC2776766] [PubMed: 20031820]
- Fraser AG, Dunstan FD. British Medical Journal. Vol. 341. 2010. On the impossibility of being expert; p. c6815. [PubMed: 21156739]
- Gandhi TK, Kachalia A, Thomas EJ, Puopolo AL, Yoon C, Brennan TA, Studdert DM. Annals of Internal Medicine. 7. Vol. 145. 2006. Missed and delayed diagnoses in the ambulatory setting: A study of closed malpractice claims; p. 488. [PubMed: 17015866]
- Genetics and Public Policy Center. FDA regulation of genetic tests. 2008. [January 4, 2010]. http://www
.dnapolicy .org/policy.issue.php?action =detail&issuebrief_id =11&print=1. - Gill JM, Mainous AG III. Archives of Family Medicine. 4. Vol. 7. 1998. The role of provider continuity in preventing hospitalizations; p. 352. [PubMed: 9682689]
- Gill-Carey O, Hattersley AT. Pediatric Diabetes. Suppl. 9. Vol. 8. 2007. Genetics and type 2 diabetes in youth; pp. 42–47. [PubMed: 17991132]
- Graber ML, Franklin N, Gordon R. Archives of Internal Medicine. 13. Vol. 165. 2005. Diagnostic error in internal medicine; p. 1493. [PubMed: 16009864]
- Guyer B, Freedman MA, Strobino DM, Sondik EJ. Pediatrics. 6. Vol. 106. 2000. Annual summary of vital statistics: Trends in the health of Americans during the 20th century; pp. 1307–1317. [PubMed: 11099582]
- Hattersley AT, Pearson ER. Endocrinology. 6. Vol. 147. 2006. Minireview: Pharmacogenetics and beyond: The interaction of therapeutic response, beta-cell physiology, and genetics in diabetes; pp. 2657–2663. [PubMed: 16556760]
- Hegarty J, Beirne PV, Walsh E, Comber H, Fitzgerald T, Kazer MWallace. Cochrane Database System Reviews. 11. 2010. Radical prostatectomy versus watchful waiting for prostate cancer; p. CD006590. [PubMed: 21069689]
- Heidenreich PA, McClellan M. American Journal of Medicine. 3. Vol. 110. 2001. Trends in treatment and outcomes for acute myocardial infarction: 1975–1995; pp. 165–174. [PubMed: 11182101]
- Hendrich A, Chow MP, Skierczynski BA, Lu Z. Permanente Journal. 3. Vol. 12. 2008. A 36-hospital time and motion study: How do medical-surgical nurses spend their time; pp. 25–34. [PMC free article: PMC3037121] [PubMed: 21331207]
- Hendrickson G, Doddato TM, Kovner CT. Journal of Nursing Administration. 3. Vol. 20. 1990. How do nurses use their time; pp. 31–37. [PubMed: 2313373]
- Howden LM, Meyer JA. Age and sex composition: 2010. Washington, DC: U.S. Census Bureau, U.S. Department of Commerce; 2011.
- Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse S, Kosary C, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Chen H, Feuer E, Cronin K, Edwards BE. SEER cancer statistics review, 1975–2008. 2011. [December 13, 2011]. http://seer
.cancer.gov /statfacts/html/prost.html. - Institute for Clinical and Economic Review. Management options for low-risk prostate cancer: A report on comparative effectiveness and value. Boston, MA: Institute for Clinical and Economic Review; 2010.
- Ioannidis JPA. PLoS Medicine. 8. Vol. 2. 2005. Why most published research findings are false; pp. 696–701.
- IOM (Institute of Medicine). Hospital-based emergency care: At the breaking point. Washington, DC: The National Academies Press; 2007.
- IOM. Evidence-based medicine and the changing nature of health care: 2007 IOM annual meeting summary. Washington, DC: The National Academies Press; 2008. [PubMed: 21391346]
- IOM. Toward precision medicine: Building a knowlege network for biomedical research and a new taxonomy of disease. Washington, DC: The National Academies Press; 2011. [PubMed: 22536618]
- Jayawardhana J, Welton JM, Lindrooth R. Journal of Nursing Administration. 9. Vol. 41. 2011. Adoption of National Quality Forum safe practices by Magnet® hospitals; pp. 350–356. [PubMed: 21881440]
- Kampmann C, Sterman JD. Feedback complexity, bounded rationality, and market dynamics. Cambridge, MA: Massachusetts Institute of Technology; 1998.
- Litvak E. Front Office to Front Line: Essential Issues for Health Care Leaders. 2005. Optimizing patient flow by managing its variability; pp. 91–111.
- Litvak E, Bisognano M. Health Affairs (Millwood). 1. Vol. 30. 2011. More patients, less payment: Increasing hospital efficiency in the aftermath of health reform; pp. 76–80. [PubMed: 21209441]
- Litvak E, Buerhaus PI, Davidoff F, Long MC, McManus ML, Berwick DM. Joint Commission Journal on Quality and Patient Safety. 6. Vol. 31. 2005. Managing unnecessary variability in patient demand to reduce nursing stress and improve patient safety; pp. 330–338. [PubMed: 15999963]
- Mainous A III, Gill J. American Journal of Public Health. 10. Vol. 88. 1998. The importance of continuity of care in the likelihood of future hospitalization: Is site of care equivalent to a primary clinician; p. 1539. [PMC free article: PMC1508474] [PubMed: 9772859]
- Makarov DV, Yu JB, Desai RA, Penson DF, Gross CP. Medical Care. 4. Vol. 49. 2011. The association between diffusion of the surgical robot and radical prostatectomy rates; pp. 333–339. [PubMed: 21368677]
- Manolio TA. Redesigning the clinical effectiveness research paradigm: Innovation and practice-based approaches: Workshop summary. Institute of Medicine. Washington, DC: The National Academies Press; 2010. Emerging genomic information. In; pp. 189–206. [PubMed: 21210561]
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. New England Journal of Medicine. 26. Vol. 348. 2003. The quality of health care delivered to adults in the United States; pp. 2635–2645. [PubMed: 12826639]
- Mechanic D, McAlpine DD, Rosenthal M. New England Journal of Medicine. 3. Vol. 344. 2001. Are patients' office visits with physicians getting shorter; pp. 198–204. [PubMed: 11172143]
- Menec VH, Sirski M, Attawar D, Katz A. Journal of Health Services Research & Policy. 4. Vol. 11. 2006. Does continuity of care with a family physician reduce hospitalizations among older adults; pp. 196–201. [PubMed: 17018192]
- Nabel EG, Braunwald E. New England Journal of Medicine. 1. Vol. 366. 2012. A tale of coronary artery disease and myocardial infarction; pp. 54–63. [PubMed: 22216842]
- Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. New England Journal of Medicine. 11. Vol. 364. 2011. Nurse staffing and inpatient hospital mortality; pp. 1037–1045. [PubMed: 21410372]
- O'Rahilly S. Nature. 7271. Vol. 462. 2009. Human genetics illuminates the paths to metabolic disease; pp. 307–314. [PubMed: 19924209]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Rockville, MD: Department of Health and Human Services; 2011.
- Pao W, Girard N. Lancet Oncology. 2. Vol. 12. 2011. New driver mutations in non-small-cell lung cancer; pp. 175–180. [PubMed: 21277552]
- Parekh AK, Barton MB. Journal of the American Medical Association. 13. Vol. 303. 2010. The challenge of multiple comorbidity for the US health care system; pp. 1303–1304. [PubMed: 20371790]
- Payne JW, Bettman JR, Johnson EJ. The adaptive decision maker. Cambridge, United Kingdom: Cambridge University Press; 1993.
- Pearson TA, Manolio TA. Journal of the American Medical Association. 11. Vol. 299. 2008. How to interpret a genome-wide association study; pp. 1335–1344. [PubMed: 18349094]
- Pizer S, Gardner J. Inquiry. 2. Vol. 48. 2011. Is fragmented financing bad for your health; pp. 109–122. [PubMed: 21898983]
- Prasad V, Gall V, Cifu A. Archives of Internal Medicine. 18. Vol. 171. 2011. The frequency of medical reversal; pp. 1675–1676. [PubMed: 21747003]
- Redberg RF. Archives of Internal Medicine. 18. Vol. 171. 2011. PCI for late reperfusion after myocardial infarction continues despite negative oat trial: Less is more; p. 1645. [PubMed: 21747000]
- Redelmeier DA. Annals of Internal Medicine. 2. Vol. 142. 2005. The cognitive psychology of missed diagnoses; pp. 115–120. [PubMed: 15657159]
- Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, Pollack CV, Gore JM, Chandra-Strobos N, Peterson ED, French WJ. American Heart Journal. 6. Vol. 156. 2008. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006; pp. 1026–1034. [PubMed: 19032996]
- Samani NJ, Tomaszewski M, Schunkert H. Lancet. 9725. Vol. 375. 2010. The personal genome—the future of personalised medicine; pp. 1497–1498. [PubMed: 20435212]
- Schneider KM, O'Donnell BE, Dean D. Health and Quality of Life Outcomes. Vol. 7. 2009. Prevalence of multiple chronic conditions in the United States' Medicare population; p. 82. [PMC free article: PMC2748070] [PubMed: 19737412]
- Semel ME, Bader AM, Marston A, Lipsitz SR, Marshall RE, Gawande AA. Journal of Evaluation in Clinical Practice. 2. Vol. 18. 2010. Measuring the range of services clinicians are responsible for in ambulatory practice; pp. 404–408. [PubMed: 21114799]
- Sepucha KR, Fagerlin A, Couper MP, Levin CA, Singer E, Zikmund-Fisher BJ. Medical Decision Making. Suppl. 5. Vol. 30. 2010. How does feeling informed relate to being informed? The decisions survey; pp. S77–S84. [PubMed: 20881156]
- Shafir E, Tversky A. Cognitive Psychology. 4. Vol. 24. 1992. Thinking through uncertainty: Nonconsequential reasoning and choice; pp. 449–474. [PubMed: 1473331]
- Shafir E, Simonson I, Tversky A. Cognition. 1–2. Vol. 49. 1993. Reason-based choice; pp. 11–36. [PubMed: 8287671]
- Shrank WH, Asch SM, Adams J, Setodji C, Kerr EA, Keesey J, Malik S, McGlynn EA. Medical Care. 10. Vol. 44. 2006. The quality of pharmacologic care for adults in the United States; pp. 936–945. [PubMed: 17001265]
- Simon HA. American Economic Review. 4. Vol. 69. 1979. Rational decision making in business organizations; pp. 493–513.
- Simon HA. Annual Review of Psychology. 1. Vol. 41. 1990. Invariants of human behavior; pp. 1–20. [PubMed: 18331187]
- Simon V, Ho DD, Karim QAbdool. Lancet. 9534. Vol. 368. 2006. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment; pp. 489–504. [PMC free article: PMC2913538] [PubMed: 16890836]
- Spear SJ, Schmidhofer M. Annals of Internal Medicine. 8. Vol. 142. 2005. Ambiguity and workarounds as contributors to medical error; pp. 627–630. [PubMed: 15838069]
- Stafford RS, Bartholomew LK, Cushman WC, Cutler JA, Davis BR, Dawson G, Einhorn PT, Furberg CD, Piller LB, Pressel SL, Whelton PK., ALLHAT Collaborative Research Group. Archives of Internal Medicine. 10. Vol. 170. 2010. Impact of the ALLHAT/JNC7 dissemination project on thiazide-type diuretic use; pp. 851–858. [PMC free article: PMC2989728] [PubMed: 20498411]
- Tenopir C, King DW, Bush A. Journal of the Medical Library Association. 2. Vol. 92. 2004. Medical faculty's use of print and electronic journals: Changes over time and in comparison with scientists; pp. 233–241. [PMC free article: PMC385305] [PubMed: 15098053]
- Thompson DN, Wolf GA, Spear SJ. Journal of Nursing Administration. 11. Vol. 33. 2003. Driving improvement in patient care: Lessons from Toyota; p. 585. [PubMed: 14608217]
- Timmermans D. Journal of Behavioral Decision Making. 2. Vol. 6. 1993. The impact of task complexity on information use in multi-attribute decision making; pp. 95–111.
- Tinetti ME, Bogardus ST, Agostini JV. New England Journal of Medicine. 27. Vol. 351. 2004. Potential pitfalls of disease-specific guidelines for patients with multiple conditions; pp. 2870–2874. [PubMed: 15625341]
- Trude S. Tracking Report/Center for Studying Health System Change. 8. 2003. So much to do, so little time: Physician capacity constraints, 1997–2001; p. 1. [PubMed: 12744264]
- Tucker AL, Spear SJ. Health Services Research. 3 Pt. 1. Vol. 41. 2006. Operational failures and interruptions in hospital nursing; pp. 643–662. [PMC free article: PMC1713207] [PubMed: 16704505]
- Tversky A, Kahneman D. Cognitive Psychology. 2. Vol. 5. 1973. Availability: A heuristic for judging frequency and probability* 1,* 2; pp. 207–232.
- Tversky A, Kahneman D. Science. 4157. Vol. 185. 1974. Judgment under uncertainty: Heuristics and biases; p. 1124. [PubMed: 17835457]
- vanWalraven C, Oake N, Jennings A, Forster AJ. Journal of Evaluation in Clinical Practice. 5. Vol. 16. 2010. The association between continuity of care and outcomes: A systematic and critical review; pp. 947–956. [PubMed: 20553366]
- Wallace CJ, Savitz L. Journal of Evaluation in Clinical Practice. 1. Vol. 14. 2008. Estimating waste in frontline health care worker activities; pp. 178–180. [PubMed: 18093107]
- Weick KE, Sutcliffe KM. Managing the unexpected: Assuring high performance in an age of complexity. San Francisco, CA: Jossey-Bass; 2001.
- Wilt T, Shamliyan T, Taylor B, MacDonald R, Tacklind J, Rutks I, Koeneman K, Cho C-S, Kane R. Comparative effectiveness of therapies for clinically localized prostate cancer. Comparative effectiveness review no. 13. Rockville, MD: AHRQ; 2008a. [PubMed: 20704036]
- Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Annals of Internal Medicine. 6. Vol. 148. 2008b. Systematic review: Comparative effectiveness and harms of treatments for clinically localized prostate cancer; pp. 435–448. [PubMed: 18252677]
- Yarnall KSH, Krause K, Pollak K, Gradison M, Michener J. Preventing Chronic Disease. 2. Vol. 6. 2009. Family physicians as team leaders: “Time” to share the care; p. A59. [PMC free article: PMC2687865] [PubMed: 19289002]
- Zikmund-Fisher BJ, Couper MP, Singer E, Ubel PA, Ziniel S, Fowler FJ Jr, Levin CA, Fagerlin A. Medical Decision Making. Suppl. 5. Vol. 30. 2010. Deficits and variations in patients' experience with making 9 common medical decisions: The decisions survey; pp. S85–S95. [PubMed: 20881157]
Footnotes
- 1
The number of peer-reviewed journal publications was determined from searches of PubMed for MEDLINE articles published during a given year using the following MeSH terms: Guideline [V02.515], Journal Article [V02.600], Review [V02.912], Technical Report [V02.989] (National Library of Medicine; http://www
.ncbi.nlm.nih.gov/pubmed/).
- Imperative: Managing Rapidly Increasing Complexity - Best Care at Lower CostImperative: Managing Rapidly Increasing Complexity - Best Care at Lower Cost
Your browsing activity is empty.
Activity recording is turned off.
See more...