NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001.

Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc.
Show detailsSUMMARY
Vitamin A is important for normal vision, gene expression, reproduction, embryonic development, growth, and immune function. There are a variety of foods rich in vitamin A and provitamin A carotenoids that are available to North Americans. Thus, current dietary patterns appear to provide sufficient vitamin A to prevent deficiency symptoms such as night blindness. The Estimated Average Requirement (EAR) is based on the assurance of adequate stores of vitamin A. The Recommended Dietary Allowance (RDA) for men and women is 900 and 700 μg retinol activity equivalents (RAE)/day, respectively. The Tolerable Upper Intake Level (UL) for adults is set at 3,000 μg/day of preformed vitamin A.
There are a number of sources of dietary vitamin A. Preformed vitamin A is abundant in some animal-derived foods, whereas provitamin A carotenoids are abundant in darkly colored fruits and vegetables, as well as oily fruits and red palm oil.
For dietary provitamin A carotenoids—β-carotene, α-carotene, and β-cryptoxanthin—RAEs have been set at 12, 24, and 24 μg, respectively. Using μg RAE, the vitamin A activity of provitamin A carotenoids is half the vitamin A activity assumed when using μg retinol equivalents (μg RE) (NRC, 1980, 1989). This change in equivalency values is based on data demonstrating that the vitamin A activity of purified β-carotene in oil is half the activity of vitamin A, and based on recent data demonstrating that the vitamin A activity of dietary β-carotene is one-sixth, rather than one-third, the vitamin activity of purified β-carotene in oil. This change in bioconversion means that a larger amount of provitamin A carotenoids, and therefore darkly colored, carotene-rich fruits and vegetables, is needed to meet the vitamin A requirement. It also means that in the past, vitamin A intake has been overestimated.
The median intake of vitamin A ranges from 744 to 811 μg RAE/ day for men and 530 to 716 μg RAE/day for women. Using μg RAE, approximately 26 and 34 percent of vitamin A activity consumed by men and women, respectively, is provided from provitamin A carotenoids. Ripe, colored fruits and cooked, yellow tubers are more efficiently converted to vitamin A than equal amounts of dark green, leafy vegetables.
Although a large body of observational epidemiological evidence suggests that higher blood concentrations of β-carotenes and other carotenoids obtained from foods are associated with a lower risk of several chronic diseases, there is currently not sufficient evidence to support a recommendation that requires a certain percentage of dietary vitamin A to come from provitamin A carotenoids in meeting the vitamin A requirement. However, the existing recommendations for increased consumption of carotenoid-rich fruits and vegetables for their health-promoting benefits are strongly supported (see Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids [IOM, 2000]).
BACKGROUND INFORMATION
Vitamin A is a fat-soluble vitamin that is essential for humans and other vertebrates. Vitamin A comprises a family of molecules containing a 20 carbon structure with a methyl substituted cyclohexenyl ring (beta-ionone ring) (Figure 4-1) and a tetraene side chain with a hydroxyl group (retinol), aldehyde group (retinal), carboxylic acid group (retinoic acid), or ester group (retinyl ester) at carbon-15. The term vitamin A includes provitamin A carotenoids that are dietary precursors of retinol. The term retinoids refers to retinol, its metabolites, and synthetic analogues that have a similar structure. Carotenoids are polyisoprenoids, of which more than 600 forms exist. Of the many carotenoids in nature, several have provitamin A nutritional activity, but food composition data are available for only three (α-carotene, β-carotene, and β-cryptoxanthin) (Figure 4-1). The all-trans isomer is the most common and stable form of each carotenoid; however, many cis isomers also exist. Carotenoids usually contain 40 carbon atoms, have an extensive system of conjugated double bonds, and contain one or two cyclic structures at the end of their conjugated chain. An exception is lycopene, which has no ring structure and does not have vitamin A activity. Preformed vitamin A is found only in animal-derived food products, whereas dietary carotenoids are present primarily in oils, fruits, and vegetables.
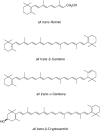
FIGURE 4-1
Structure of retinol and provitamin A carotenoids.
Function
The 11-cis-retinaldehyde (retinal) form of vitamin A is required by the eye for the transduction of light into neural signals necessary for vision (Saari, 1994). The retinoic acid form is required to maintain normal differentiation of the cornea and conjunctival membranes, thus preventing xerophthalmia (Sommer and West, 1996), as well as for the photoreceptor rod and cone cells of the retina. Rods contain the visual pigment rhodopsin (opsin protein bound to 11-cis-retinal). The absorption of light catalyzes the photoisomerization of rhodopsin's 11-cis-retinal to all-trans-retinal in thousands of rods, which triggers the signaling to neuronal cells associated with the brain's visual cortex. After photoisomerization, all-trans-retinal is released, and for vision to continue, 11-cis-retinal must be regenerated. Regeneration of 11-cis-retinal requires the reduction of all-trans retinal to retinol, transport of retinol from the photoreceptor cells (rods) to the retinal pigment epithelium, and esterification of all-trans-retinol, thereby providing a local storage pool of retinyl esters. When needed, retinyl esters are hydrolyzed and isomerized to form 11-cis-retinol, which is oxidized to 11-cis-retinal and transported back to the photoreceptor cells for recombination with opsin to begin another photo cycle.
Vitamin A is required for the integrity of epithelial cells throughout the body (Gudas et al., 1994). Retinoic acid, through the activation of retinoic acid (RAR) and retinoid X (RXR) receptors in the nucleus, regulates the expression of various genes that encode for structural proteins (e.g., skin keratins), enzymes (e.g., alcohol dehydrogenase), extracellular matrix proteins (e.g., laminin), and retinol binding proteins and receptors.
Retinoic acid plays an important role in embryonic development. Retinoic acid, as well as RAR, RXR, cellular retinol-binding protein (CRBP), and cellular retinoic acid-binding proteins (CRABP-I and CRABP-II), is present in temporally specific patterns in the embryonic regions known to be involved in the development of structures posterior to the hindbrain (e.g., the vertebrae and spinal cord) (Morriss-Kay and Sokolova, 1996). Retinoic acid is also involved in the development of the limbs, heart, eyes, and ears (Dickman and Smith, 1996; Hofmann and Eichele, 1994; McCaffery and Drager, 1995).
Retinoids are necessary for the maintenance of immune function, which depends on cell differentiation and proliferation in response to immune stimuli. Retinoic acid is important in maintaining an adequate level of circulating natural killer cells that have antiviral and anti-tumor activity (Zhao and Ross, 1995). Retinoic acid has been shown to increase phagocytic activity in murine macrophages (Katz et al., 1987) and to increase the production of interleukin 1 and other cytokines, which serve as important mediators of inflammation and stimulators of T and B lymphocyte production (Trechsel et al., 1985). Furthermore, the growth, differentiation, and activation of B lymphocytes requires retinol (Blomhoff et al., 1992).
Proposed functions of provitamin A carotenoids are described in Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (IOM, 2000).
Physiology of Absorption, Metabolism, and Excretion
Absorption and Bioconversion
Absorption of Vitamin A. Intestinal absorption of preformed vitamin A occurs following the processing of retinyl esters in the lumen of the small intestine. Within the water-miscible micelles formed from bile salts, solubilized retinyl esters as well as triglycerides are hydrolyzed to retinol and products of lipolysis by various hydrolases (Harrison, 1993). A small percentage of dietary retinoids is converted to retinoic acid in the intestinal cell. In addition, the intestine actively synthesizes retinoyl β-glucuronide that is hydrolyzed to retinoic acid by β-glucuronidases (Barua and Olson, 1989). The efficiency of absorption of preformed vitamin A is generally high, in the range of 70 to 90 percent (Sivakumar and Reddy, 1972). A specific retinol transport protein within the brush border of the enterocyte facilitates retinol uptake by the mucosal cells (Dew and Ong, 1994). At physiological concentrations, retinol absorption is carrier mediated and saturable, whereas at high pharmacological doses, the absorption of retinol is nonsaturable (Hollander and Muralidhara, 1977). As the amount of ingested preformed vitamin A increases, its absorbability remains high (Olson, 1972). Vitamin A absorption and intestinal retinol esterification are not markedly different in the elderly compared to young adults, although hepatic uptake of newly absorbed vitamin A in the form of retinyl ester is slower in the elderly (Borel et al., 1998).
Absorption and Bioconversion of Provitamin A Carotenoids. Carotenoids are also solubilized into micelles in the intestinal lumen from which they are absorbed into duodenal mucosal cells by a passive diffusion mechanism. Percent absorption of a single dose of 45 μg to 39 mg β-carotene, measured by means of isotopic methods, has been reported to range from 9 to 22 percent (Blomstrand and Werner, 1967; Goodman et al., 1966; Novotny et al., 1995). However, the absorption efficiency decreases as the amount of dietary carotenoids increases (Brubacher and Weiser, 1985; Tang et al., 2000). The relative carotene concentration in micelles can vary in response to the physical state of the carotenoid (e.g., whether it is dissolved in oil or associated with plant matrix materials). A number of factors affect the bioavailability and bioconversion of carotenoids (Castenmiller and West, 1998). Carotene bioavailability can differ with different processing methods of the same foods and among different foods containing similar levels of carotenoids (Boileau et al., 1999; Hume and Krebs, 1949; Rock et al., 1998; Torronen et al., 1996; Van den Berg and van Vliet, 1998) (also see Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids [IOM, 2000]).
Absorbed β-carotene is principally converted to vitamin A by the enzyme β-carotene-15, 15′-dioxygenase within intestinal absorptive cells. The central cleavage of β-carotene by this enzyme will, in theory, result in two molecules of retinal. β-Carotene can also be cleaved eccentrically to yield β-apocarotenals that can be further degraded to retinal or retinoic acid (Krinsky et al., 1993). The predominant form of vitamin A in human lymph, whether originating from ingested vitamin A or provitamin A carotenoids, is retinyl ester (retinol esterified with long-chain fatty acids, typically palmitate and stearate) (Blomstrand and Werner, 1967; Goodman et al., 1966). Along with exogenous lipids, the newly synthesized retinyl esters and nonhydrolyzed carotenoids are transported from the intestine to the liver in chylomicrons and chylomicron remnants. Derived from dietary retinoids, retinoic acid is absorbed via the portal system bound to albumin (Blaner and Olson, 1994; Olson, 1991).
Vitamin A Activity of Provitamin A Carotenoids: Rationale for Developing Retinol Activity Equivalents. The carotene:retinol equivalency ratio (μg:μg) of a low dose (less than 2 mg) of purified β-carotene in oil is approximately 2:1 (i.e., 2 μg of β-carotene in oil yields 1 μg of retinol) (Table 4-1). This ratio was derived from the relative amount of β-carotene required to correct abnormal dark adaptation in vitamin A-deficient individuals (Hume and Krebs, 1949; Sauberlich et al., 1974). The data by Sauberlich et al. (1974) were given greater consideration because (1) the actual amount (μg) of vitamin A and β-carotene consumed was cited, (2) varied amounts of vitamin A or β-carotene were consumed by each individual, and (3) a greater sample size was employed (six versus two subjects). In addition to these studies, an earlier study by Wagner (1940) estimated a carotene:retinol equivalency ratio of 4:1; however, the method employed for measuring dark adaptation was not standardized and used an imprecise outcome measure.
TABLE 4-1
Relative Absorption of Vitamin A and Supplemental β-Carotene.
Studies have been performed to compare the efficiency of absorption of β-carotene after feeding physiological amounts of β-carotene in oil, in individual foods, and as part of a mixed vegetable and fruit diet. Many of the earlier studies analyzed the fecal content of β-carotene after the consumption of a supplement, fruit, or vegetable. Data from these studies were not considered because the portion of unabsorbed β-carotene that is degraded by the intestinal microflora is not known. The efficiency of absorption of β-carotene in food is lower than the absorption of β-carotene in oil by a representative factor of a. Assuming that after absorption of β-carotene, whether from oil or food, the metabolism of the molecule is similar and that the retinol equivalency ratio of β-carotene in oil is 2:1, the vitamin A activity of β-carotene from food can be derived by multiplying a by 2:1.
Until recently it was thought that 3 μg of dietary β-carotene was equivalent to 1 μg of purified β-carotene in oil (NRC, 1989) due to a relative absorption efficiency of about 33 percent of β-carotene from food sources. Only one study has compared the relative absorption of β-carotene in oil versus its absorption in a principally mixed vegetable diet in healthy and nutritionally adequate individuals (Van het Hof et al., 1999). This study concluded that the relative absorption of β-carotene from the mixed vegetable diet compared to β-carotene in oil is only 14 percent, as assessed by the increase in plasma β-carotene concentration after dietary intervention. Based on this finding, approximately 7 μg of dietary β-carotene is equivalent to 1 μg of β-carotene in oil. This absorption efficiency value of 14 percent is supported by the relative ranges in β-carotene absorption reported by others using similar methods for mixed green leafy vegetables (4 percent) (de Pee et al., 1995), carrots (18 to 26 percent) (Micozzi et al., 1992; Torronen et al., 1996), broccoli (11 to 12 percent) (Micozzi et al., 1992), and spinach (5 percent) (Castenmiller et al., 1999) (Table 4-2).
TABLE 4-2
Relative Absorption of Supplemental and Dietary β-Carotene.
Only one study has been published to assess the relative bioconversion of β-carotene from fruits versus vegetables by measuring the rise in serum retinol concentration after the provision of a diet high in vegetables, fruits, or retinol (de Pee et al., 1998). This study used methods similar to those employed by other researchers (Castenmiller et al. [1999], de Pee et al. [1995], Micozzi et al. [1992], Torronen et al. [1996], and Van het Hof et al. [1999]), and indicated that the vitamin A activity was approximately half the activity for dark, green leafy vegetables compared to equal amounts of β-carotene from orange fruits and some yellow tubers, such as pumpkin squash (de Pee et al., 1998) (Table 4-2). Because of the low content of fruits contained in the principally mixed vegetable diet of Van het Hof et al. (1999) and the low proportion of dietary β-carotene that is consumed from fruits compared to vegetables in the United States (16 percent from the 14 major dietary contributors of β-carotene which provide a total of 70 percent of dietary β-carotene) (Chug-Ahuja et al., 1993), it is estimated that 6 μg, rather than 7 μg, of β-carotene from a mixed diet is nutritionally equivalent to 1 μg of β-carotene in oil. Therefore, the retinol activity equivalency (μg RAE) ratio for β-carotene from food is estimated to be 12:1 (6 × 2:1) (Figure 4-2). Unfortunately, studies using a positive control group (preformed vitamin A) at a level equivalent to β-carotene from a mixed vegetable and fruit diet using levels similar to the RAE have not been conducted in healthy and nutritionally adequate individuals. An RAE of 12 μg for dietary β-carotene is supported by Parker et al. (1999) who reported that 8 percent of ingested β-carotene from carrots was absorbed and converted to retinyl esters contained in chylomicrons, resulting in a carotene:retinol equivalency ratio of 13:1.

FIGURE 4-2
Absorption and bioconversion of ingested provitamin A carotenoids to retinol based on new equivalency factors (retinol activity equivalency ratio).
One RAE for dietary provitamin A carotenoids other than β-carotene is set at 24 μg on the basis of the observation that the vitamin A activity of β-cryptoxanthin and α-carotene is approximately half of that for β-carotene (Bauernfeind, 1972; Deuel et al., 1949). Therefore, the amount of vitamin A activity of provitamin A carotenoids in μg RAE is half the amount obtained if using μg RE (Table 4-3).
TABLE 4-3
Comparison of the 1989 National Research Council and 2001 Institute of Medicine Interconversion of Vitamin A and Carotenoid Units.
Example: A diet contains 500 μg retinol, 1,800 μg β-carotene and 2,400 μg α-carotene.
500 + (1,800 ÷ 12) + (2,400 ÷ 24) = 750 μg RAE.
Example: A diet contains 1,666 IU of retinol and 3,000 IU of β-carotene.
(1,666 ÷ 3.33) + (3,000 ÷ 20) = 650 μg RAE.
Example: A supplement contains 5,000 IU of vitamin A (20 percent as β-carotene).
5,000 ÷ 3.33 = 1,500 μg RAE.
The use of μg RAE rather than μg RE or international units (IU) is preferred when calculating and reporting the amount of the total vitamin A in mixed foods or assessing the amount of dietary and supplemental vitamin A consumed. Given the need to be able to calculate the intake of carotenoids, food composition data tables should report food content in amounts of each carotenoid whenever possible.
Metabolism, Transport, and Excretion
Retinyl esters and carotenoids are transported to the liver in chylomicron remnants. Apoprotein E is required for the uptake of chylomicron remnants by the liver. Some retinyl esters can also be taken up directly by peripheral tissues (Goodman et al., 1965). Several specific hepatic membrane receptors (low density lipoprotein [LDL] receptor, LDL receptor-related protein, lipolysis-stimulated receptor) have been proposed to also be involved with the uptake of chylomicron remnants (Cooper, 1997). The hydrolysis of retinyl ester to retinol is catalyzed by retinyl ester hydrolase following endocytosis. To meet tissue needs for retinoids, retinol binds to retinol-binding protein (RBP) for release into the circulation. In the blood, holo-RBP associates with transthyretin (a transport protein) to form a trimolecular complex with retinol in a 1:1:1 molar ratio. Retinol is transported in this trimolecular complex to various tissues, including the eye. The mechanism through which retinol is taken up from the circulation by peripheral cells has not been conclusively established. Retinol that is not immediately released into circulation by the liver is reesterified and stored in the lipid-containing stellate (Ito) cells of the liver until needed to maintain normal blood retinol concentrations.
Carotenoids are incorporated into very low density lipoproteins (VLDL) and exported from the liver into the blood. VLDL are converted to LDL by lipoprotein lipase on the surface of blood vessels. Plasma membrane-associated receptors of peripheral tissue cells bind apolipoprotein B100 on the surface of LDL, initiating receptor-mediated uptake of LDL and their lipid contents. The liver, lung, adipose, and other tissues possess carotene 15, 15′-dioxygenase activity (Goodman and Blaner, 1984; Olson and Hayaishi, 1965), and thus it is presumed that carotenes may be converted to vitamin A as they are delivered to tissues. The major end products of the enzyme's activity are retinol and retinoic acid (Napoli and Race, 1988). It is unclear, however, whether carotenoids stored in tissues other than the intestinal mucosa cells are cleaved to yield retinol. Thatcher et al. (1998) demonstrated that β-carotene stored in liver is not utilized for vitamin A needs in gerbils.
Typically, the majority of vitamin A metabolites are excreted in the urine. Sauberlich et al. (1974) reported that the percentage of a radioactive dose of vitamin A recovered in breath, feces, and urine ranged from 18 to 30 percent, 18 to 37 percent, and 38 to 60 percent, respectively, after 400 days on a vitamin A-deficient diet. Almost all of the excreted metabolites are biologically inactive.
Retinol is metabolized in the liver to numerous products, some of which are conjugated with glucuronic acid or taurine for excretion in bile (Sporn et al., 1984). The portion of excreted vitamin A metabolites in bile increases as the liver vitamin A exceeds a critical concentration. This increased excretion has been suggested to serve as a protective mechanism for reducing the risk of excess storage of vitamin A (Hicks et al., 1984).
Body Stores
The hepatic vitamin A concentration can vary markedly depending on dietary intake. When vitamin A intake is adequate, over 90 percent of total body vitamin A is located in the liver (Raica et al., 1972) as retinyl ester (Schindler et al., 1988), where it is concentrated in the lipid droplets of perisinusoidal stellate cells (Hendriks et al., 1985). The average concentration of vitamin A in postmortem livers of American and Canadian adults is reported to range from 10 to as high as 1,400 μg/g liver (Furr et al., 1989; Hoppner et al., 1969; Mitchell et al., 1973; Raica et al., 1972; Schindler et al., 1988; Underwood et al., 1970). In developing countries where vitamin A deficiency is prevalent, the vitamin A concentration in liver biopsy samples is much lower (17 to 141 μg/g) (Abedin et al., 1976; Flores and de Araujo, 1984; Haskell et al., 1997; Olson, 1979; Suthutvoravoot and Olson, 1974). A concentration of at least 20 μg retinol/g of liver in adults is suggested to be the minimal acceptable reserve (Loerch et al., 1979; Olson, 1982). The mean liver stores of vitamin A in children (1 to 10 years of age) have been reported to range from 171 to 723 μg/g (Flores and de Araujo, 1984; Mitchell et al., 1973; Money, 1978; Raica et al., 1972; Underwood et al., 1970), whereas the mean liver vitamin A stores in apparently healthy infants is lower, ranging from 0 to 320 μg/g of liver (Flores and de Araujo, 1984; Huque, 1982; Olson et al., 1979; Raica et al., 1972; Schindler et al., 1988).
With use of radio-isotopic methods, the efficiency of storage (retention) of vitamin A in liver has been estimated to be approximately 50 percent (Bausch and Rietz, 1977; Kusin et al., 1974; Sauberlich et al., 1974). More recently, stable-isotopic methods have shown an efficiency of storage of 42 percent for individuals with concentrations greater than or equal to 20 μg retinol/g of liver (Haskell et al., 1997). The efficiency of storage was lower in those with lower vitamin A status. The percentage of total body vitamin A stores lost per day was approximately 0.5 percent in adults consuming a vitamin A-free diet (Sauberlich et al., 1974).
Clinical Effects of Inadequate Intake
The most specific clinical effect of inadequate vitamin A intake is xerophthalmia. It is estimated that 3 to 10 million children, mostly in developing countries, become xerophthalmic, and 250,000 to 500,000 go blind annually (Sommer and West, 1996; WHO, 1995). The World Health Organization (WHO, 1982) classified various stages of xerophthalmia to include night blindness (impaired dark adaptation due to slowed regeneration of rhodopsin), conjunctival xerosis, Bitot's spots, corneal xerosis, corneal ulceration, and scarring, all related to vitamin A deficiency. Night blindness is the first ocular symptom to be observed with vitamin A deficiency (Dowling and Gibbons, 1961), and it responds rapidly to treatment with vitamin A (Sommer, 1982). High-dose (60 mg) vitamin A supplementation reduced the incidence of night blindness by 63 percent in Nepalese children (Katz et al., 1995). Similarly, night blindness was reduced by 50 percent in women after weekly supplementation with either 7,500 μg RE of vitamin A or β-carotene (Christian et al., 1998b).
An association of vitamin A deficiency and impaired embryonic development is well documented in animals (Morriss-Kay and Sokolova, 1996; Wilson et al., 1953). In laboratory animals, fetal resorption is common in severe vitamin A deficiency, while fetuses that survive have characteristic malformations of the eye, lungs, urogenital tract, and cardiovascular system. Similar abnormalities are observed in rat embryos lacking nuclear retinoid receptors (Wendling et al., 1999). Morphological abnormalities associated with vitamin A deficiency are not commonly found in humans; however, functional defects of the lungs have been observed (Chytil, 1996).
Because of the role of vitamin A in maintaining the structural integrity of epithelial cells, follicular hyperkeratosis has been observed with inadequate vitamin A intake (Chase et al., 1971; Sauberlich et al., 1974). Men who were made vitamin A deficient under controlled conditions were then supplemented with either retinol or β-carotene, which caused the hyperkeratosis to gradually clear (Sauberlich et al., 1974).
Vitamin A deficiency has been associated with a reduction in lymphocyte numbers, natural killer cells, and antigen-specific immunoglobulin responses (Cantorna et al., 1995; Nauss and Newberne, 1985). A decrease in leukocytes and lymphoid organ weights, impaired T cell function, and decreased resistance to immunogenic tumors have been observed with inadequate vitamin A intake (Dawson and Ross, 1999; Wiedermann et al., 1993). A generalized dysfunction of humoral and cell-mediated immunity is common in experimental animals and is likely to exist in humans.
In addition to xerophthalmia, vitamin A deficiency has been associated with increased risk of infectious morbidity and mortality in experimental animals and humans, especially in developing countries. A higher risk of respiratory infection and diarrhea has been reported among children with mild to moderate vitamin A deficiency (Sommer et al., 1984). Mortality rates were about four times greater among children with mild xerophthalmia than those without it (Sommer et al., 1983). The risk of severe morbidity and mortality decreases with vitamin A repletion. In children hospitalized with measles, case fatality (Barclay et al., 1987; Hussey and Klein, 1990) and the severity of complications on admission were reduced when they received high doses (60 to 120 mg) of vitamin A (Coutsoudis et al., 1991; Hussey and Klein, 1990). In some studies, vitamin A supplementation (30 to 60 mg) has been shown to reduce the severity of diarrhea (Barreto et al., 1994; Donnen et al., 1998) and Plasmodium falciparum malaria (Shankar et al., 1999) in young children, but vitamin A supplementation has had little effect on the risk or severity of respiratory infections, except when associated with measles (Humphrey et al., 1996).
In developing countries, vitamin A supplementation has been shown to reduce the risk of mortality among young children (Ghana VAST Study Team, 1993; Muhilal et al., 1988; Rahmathullah et al., 1990; Sommer et al., 1986; West et al., 1991), infants (Humphrey et al., 1996), and pregnant and postpartum women (West et al., 1999). Meta-analyses of the results from these and other community-based trials are consistent with a 23 to 30 percent reduction in mortality of young children beyond 6 months of age after vitamin A supplementation (Beaton et al., 1993; Fawzi et al., 1993, Glasziou and Mackerras, 1993). WHO recommends broad-based prophylaxis in vitamin A-deficient populations. It also recommends treating children who suffer from xerophthalmia, measles, prolonged diarrhea, wasting malnutrition, and other acute infections with vitamin A (WHO, 1997). Furthermore, the American Academy of Pediatrics (AAP, 1993) recommends vitamin A supplementation for children in the United States who are hospitalized with measles.
SELECTION OF INDICATORS FOR ESTIMATING THE REQUIREMENT FOR VITAMIN A
Dark Adaptation
The ability of the retina to adapt to dim light depends upon an adequate supply of vitamin A, because 11-cis retinal is an integral part of the rhodopsin molecule of the rods. Without adequate levels of vitamin A in the retina, the function of the rods in dim light situations becomes compromised, resulting in abnormal dark adaptation (night blindness). Before clinically apparent night blindness occurs, abnormal rod function may be detected by dark adaptation testing. In addition to vitamin A deficiency, zinc deficiency and severe protein deficiency also may affect dark adaptation responses (Bankson et al., 1989; Morrison et al., 1978).
Dark Adaptation Test
To perform a dark adaptation test, the eye is first dilated and the subject fixates on a point located approximately 15 degrees above the center of the test light. The test stimulus consists of light flashes of approximately 1-second duration separated by 1-second intervals of darkness. A tracking method is used with the luminance of the test light being increased or decreased depending upon the response of the subject. The ascending threshold is the intensity at which the subject first sees the test light as its luminance is increased. The descending threshold is the intensity at which the subject ceases to see the test light as its luminance is lowered. Each threshold intensity is plotted versus time and the values are read from the graph at the end of a test session. Testing is continued until the final threshold is stabilized. The final dark-adapted threshold is defined as the average of three ascending and three descending thresholds and is obtained after 35 to 40 minutes in darkness.
When the logarithm of the light perception is plotted as a function of time in darkness, the change in threshold follows a characteristic course. There is an initial rapid fall in threshold attributed to cones, followed by a plateau. A steeper descent, referred to as the rod-cone break, usually occurs at 3 to 9 minutes followed by a slower descent attributed to adaptation of the rods. The final threshold attained at about 35 to 40 minutes is the most constant indicator of dark adaptation. Among stable subjects, test results are reproducible over a 1- to 6-month interval with final threshold differences ranging from 0 to 0.1 log candela/meter2. In one series, the dark adapted final threshold among 50 normal subjects (aged 20 to 60 years) was –5.0 ± 0.3 candela/ meter2 (Carney and Russell, 1980).
Similar information on retinal function may be obtained by an electroretinogram or an electrooculorgram. However, these tests are more invasive than dark adaptation and there are not as many data relating these functional tests to dietary vitamin A levels.
There is literature relating dark adaptation test results to dietary levels of vitamin A under controlled experimental conditions (Table 4-4). Under controlled feeding conditions, dark adaptation, objectively measured by dark adaptometry, is one of the most sensitive indicators of a change in vitamin A deficiency status (Figure 4-3). Epidemiological evidence suggests that host resistance to infection is impaired at lesser stages of vitamin A deficiency, prior to clinical onset of night blindness (Arroyave et al., 1979; Arthur et al., 1992; Barreto et al., 1994; Bloem et al., 1990; Ghana VAST Study Team, 1993; Loyd-Puryear et al., 1991; Salazar-Lindo et al., 1993). Moreover, laboratory animals fed a vitamin A-deficient diet maintain ocular levels of vitamin A despite a significant reduction in hepatic vitamin A levels (Bankson et al., 1989; Wallingford and Underwood, 1987). Nevertheless, this approach can be used to estimate the average requirement for vitamin A but without assurance of adequate tissue levels to meet nonvisual needs for vitamin A.
TABLE 4-4
Correction of Abnormal Dark Adaptation with Vitamin A.
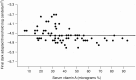
FIGURE 4-3
Serum vitamin A concentrations and dark adaptation final thresholds. Upper limit of normal final threshold = –4.6 log candela/m2. Adapted from Carney and Russell (1980).
Pupillary Response Test
Another test of ability to dark adapt, one that avoids reliance on psychophysical responses, is the pupillary response test that measures the threshold of light at which a pupillary reflex (contraction) first occurs under dark-adapted conditions (Stewart and Young, 1989). The retina of one eye is briefly exposed to incremental pulses of light while a trained observer monitors the consensual response of the other pupil under dark conditions. A high scotopic (vision in dim light) threshold indicates low retinal sensitivity, a pathophysiological response to vitamin A deficiency. An early report of pupillary nonresponse to candlelight among night blind Confederate soldiers in the Civil War (Hicks, 1867) led to the development and validation of instrumentation for this test as a reliable, functional measure of vitamin A deficiency in Indonesian (Congdon et al., 1995) and Indian (Sanchez et al., 1997) children. However, data do not currently exist relating pupillary threshold sensitivity as determined by this test to usual vitamin A intakes, and so measures of pupillary response cannot be used at the present to establish dietary vitamin A requirements.
Plasma Retinol Concentration
The concentration of plasma retinol is under tight homeostatic control in individuals and therefore is insensitive to liver vitamin A stores. The relationship is not linear and over a wide range of adequate hepatic vitamin A reserves there is little change in plasma retinol or retinol binding protein (RBP) concentrations (Underwood, 1984). When liver vitamin A reserves fall below a critical concentration, thought to be approximately 20 μg/g of liver (Olson, 1987), plasma retinol concentration declines. When dietary vitamin A is provided to vitamin A-deficient children, plasma retinol concentration increases rapidly, even before liver stores are restored (Devadas et al., 1978; Jayarajan et al., 1980). Thus, a low concentration of plasma retinol may indicate inadequacy of vitamin A status, although median or mean concentrations for plasma retinol may not be well correlated with valid indicators of vitamin A status.
In malnourished populations, often 25 percent or more individuals exhibit a plasma retinol concentration below 0.70 μmol/L (20 μg/dL), a level considered to reflect vitamin A inadequacy in a population (Flores, 1993; Underwood, 1994). However, a low plasma retinol concentration also may result from an inadequate supply of dietary protein, energy, or zinc, all of which are required for a normal rate of synthesis of RBP (Smith et al., 1974). Plasma retinol concentration may also be low during infection as a result of transient decreases in the concentrations of the negative acute phase proteins, RBP, and transthyretin, even when liver retinol is adequate (Christian et al., 1998a; Filteau et al., 1995; Golner et al., 1987; Rosales et al., 1996). The presence of one or more of these factors could lead to an overestimation of the prevalence of vitamin A deficiency when serum retinol concentration is used as an indicator. According to an analysis of the Third National Health and Nutrition Examination Survey, individuals in the highest quartile for vitamin A intake had only slightly higher serum retinol concentrations than those in the lowest quartile for vitamin A intake (Appendix Tables H-1 and H-2).
In the United States (Looker et al., 1988; Pilch, 1987) (Appendix Table G-4), serum retinol concentration is rarely low (< 0.7 μmol/ L) in more than 5 percent of preschool children, although 20 to 60 percent may exhibit concentrations between 0.70 and 1.05 μmol/L, a range that may be marginal for some individuals (Underwood, 1994). Excluding pregnant women, less than 5 percent of adults had a serum retinol concentration less than 1.05 μmol/L (Appendix Table G-4). The median concentration of serum retinol in adults was 1.7 to 2.2 μmol/L (48 to 63 μg/dL).
At the usual U.S. range of plasma retinol concentration, the concentration is neither related to observed levels of usual vitamin A intake, from either dietary preformed vitamin A or provitamin A carotenoid sources (Hallfrisch et al., 1994), nor responsive to supplement use (Krasinski et al., 1989; Nierenberg et al., 1997; Stauber et al., 1991). Because of the relatively insensitive relationship between plasma retinol concentration and liver vitamin A in the adequate range, and because of the potential for confounding factors to affect the level and interpretation of the concentration, it was not chosen as a primary status indicator for a population for estimating an average requirement for vitamin A.
Total Liver Reserves by Isotope Dilution
Body stores of vitamin A can be estimated directly by liver biopsy, but this is not an appropriate indicator of status, except at autopsy, for a population. Vitamin A stores can also be estimated by an indirect approach using an isotope dilution technique. This technique involves administering an oral dose of stable-isotopically labeled vitamin A and, after a period of equilibration, drawing blood for measurement of the isotopic ratio in plasma. The Bausch and Rietz (1977) equation used to calculate liver reserves is: TLR = F × dose × [(H:D) – 1] where TLR is the pretreatment total liver reserve of vitamin A in millimoles of retinol, F is a factor that expresses the efficacy of storage of an early administered dose, dose is the oral dose of labeled retinol in millimoles, H:D is the ratio of hydrogen to deuterated retinol in the plasma after an equilibration period, and –1 corrects TLR for the contribution of the administered dose to the total body pool. Furr et al. (1989) have suggested modification of this formula to: TLR = F × dose × (S × a × [H:D) –1]) where S is the ratio of the specific activities of retinol in serum to that in liver and a is the fraction of the absorbed dose of deuterated retinol remaining in the liver at the time of blood sampling. Liver reserves of vitamin A can be correlated with known dietary intake levels of vitamin A. An Estimated Average Requirement (EAR) could be derived by knowing the population median intake of vitamin A at which half the population has hepatic stores above a certain desired level (e.g., 20 μg/g) and half has stores below it. Although theoretically such an approach could be used to establish an EAR, no studies have been conducted in which detailed and long-term dietary data have been obtained in the tested subjects.
Relative Dose Response and Modified Relative Dose Response
In healthy individuals, approximately 90 percent of vitamin A in the body is stored in the liver and this percentage decreases to 50 percent or less in severely deficient individuals (Olson, 1987). Hepatic vitamin A stores can thus be interpreted to reflect nutrient adequacy to meet total body needs, barring factors that impede their release into circulation (e.g., liver disease and severe protein malnutrition). The relative dose response (RDR) is a method that permits indirect assessment of the relative adequacy of hepatic vitamin A stores. The RDR test was first demonstrated in rats where the release of RBP from liver was shown to depend on the availability of retinol (Loerch et al., 1979). In experimental vitamin A deficiency in rats, RBP accumulated in liver but was rapidly released after vitamin A (retinol) was administered (Carney et al., 1976; Keilson et al., 1979). This observation led Loerch et al. (1979) to propose that a positive plasma retinol response to a small test dose of vitamin A could be used as an indicator of inadequate liver vitamin A reserves.
The test was subsequently validated against measured liver retinol stores in humans (Amedee-Manesme et al., 1984, 1987; Mobarhan et al., 1981). For the test, a blood sample is drawn before retinol administration (zero time), and then a small dose of vitamin A is administered; a second blood sample is taken after an interval, generally 5 hours. The concentration of retinol in each sample is determined and the difference (response) in plasma retinol concentration (5 hours minus zero hours) is calculated and expressed as a percentage of the 5-hour concentration.
Although various cutoff levels have been used, a plasma retinol response greater than or equal to 20 percent is generally considered to indicate that liver vitamin A is inadequate (Tanumihardjo, 1993). The synthesis of RBP depends on the adequacy of other nutrients, and other deficiencies, such as zinc deficiency and protein energy malnutrition, can confound the results of the RDR test, particularly when a repeat test is conducted within a week or less after the first or baseline test. With proper controls the RDR test is considered a valid test to determine inadequate vitamin A status. However, just as plasma retinol concentration is insensitive across a wide range of “adequate” liver vitamin A reserves, the RDR test does not distinguish among different levels of adequate vitamin A reserves (Solomons et al., 1990).
The modified relative dose response (MRDR) test is a variation of the RDR test (Tanumihardjo and Olson, 1991). The MRDR requires a single blood sample and uses as the test dose vitamin A2 (dehydroretinol), which combines with RBP in the same manner as retinol but is not found endogenously in human plasma (with the possible exception of populations consuming high levels of fresh water fish). The test is subject to the same limitations as the RDR test. Neither the RDR nor the MRDR was chosen for estimating an EAR because little data exist relating usual dietary intakes of individuals or populations to RDR or MRDR test value distributions.
Conjunctival Impression Cytology
Before the clinical onset of xerophthalmia, mild vitamin A deficiency leads to early keratinizing metaplasia and losses of mucinsecreting goblet cells on the bulbar surface of the conjunctiva of the eye. These functional changes on the ocular surface can be detected by microscopic examination of PAS-hematoxylin stained epithelial cells obtained by briefly applying a cellulose acetate filter paper strip (Hatchell and Sommer, 1984; Natadisastra et al., 1987; Wittpenn et al., 1986) or disc (Keenum et al., 1990) against the temporal conjunctivum. An alternative approach involves transferring cell specimens from the filter paper to a glass slide before staining and examination (Carlier et al., 1991). Specimens are classified as normal or into degrees of abnormality, depending on the density and distribution of stained normal epithelial cells, goblet cells, and mucin “spots” (contents of goblet cells). Vitamin A status is defined by target tissue cellularity, integrity, and function, which, unlike biochemical measures, if compromised may take several weeks to normalize following vitamin A repletion (Keenum, 1993). In spite of that, there is an association between the prevalence of conjunctival impression cytology (CIC) abnormality and serum retinol and RDR test results (Sommer and West, 1996). Although CIC is used for assessment, there are few data that relate CIC status to dietary vitamin A intake in the United States, other well-nourished populations, or malnourished populations. As a result, CIC was not selected as the functional indicator for the EAR for vitamin A.
Immune Function
There is sound evidence for a role of vitamin A in the maintenance of both humoral antibody responses and cell-mediated immunity. In experimental animals, both nonspecific immunity (Butera and Krakowka, 1986; Cohen and Elin, 1974) and antigen-specific responses, including delayed-type hypersensitivity (Smith et al., 1987), blastogenesis (Butera and Krakowka, 1986; Friedman and Sklan, 1989), and antibody production (Carman et al., 1989, 1992; Pasatiempo et al., 1990; Ross, 1996; Stephensen et al., 1993), have been shown to be altered by a deficiency of vitamin A or enhanced by vitamin A supplementation. The number and cytotoxic activity of natural killer cells (Dawson et al., 1999; Zhao et al., 1994) is reduced in vitamin A deficiency, although responsiveness to activation is maintained.
Several human studies have linked impairment in immunity to low plasma or serum vitamin A concentrations (Coutsoudis et al., 1992; Semba et al., 1992, 1996). However, there are no human studies using controlled diets that have evaluated immune function tests as a means to assess the adequacy of different levels of dietary vitamin A. In addition to a lack of relevant dietary studies, there are some inherent limitations to using immune functions as indicators to establish dietary recommendations. Most changes in immune functions that have been associated with a nutrient deficiency are not specific to the nutrient under study (e.g., low T cell-mediated immunity may be caused by a lack of vitamin A, but also by a deficiency of protein or energy, zinc, or other specific nutrient deficiencies or imbalances). Thus, human dietary studies would have to be highly controlled with respect to the contents of potentially confounding nutrients. Another limitation of many immune function tests is related to difficulties encountered in standardizing tests of immunity (e.g., proliferative responses to antigen or mitogen challenge which are often used within studies to assess T and B cell responses). These tests are affected by many factors, such as the type and quality of mitogen used, cell culture conditions, and how subjects' cells have been collected, that cannot be readily controlled among laboratories or over time. Thus, for these reasons, immune function tests could not be used as an indicator for establishing the EAR for vitamin A.
FACTORS AFFECTING THE VITAMIN A REQUIREMENT
Intestinal Absorption
Dietary Fat
Dietary vitamin A is digested in mixed micelles and absorbed with fat. In some studies, increasing the level of fat in a low fat diet has been shown to improve retinol and carotene absorption (Reddy and Srikantia, 1966) and vitamin A nutriture (Jalal et al., 1998; Roels et al., 1963). Other studies, however, have not demonstrated a beneficial effect of fat on vitamin A absorption (Borel et al., 1997; Figueira et al., 1969).
For optimal carotenoid absorption, a number of research groups have demonstrated that dietary fat must be consumed along with carotenoids. Roels and coworkers (1958) reported that the addition of 18 g/day of olive oil improved carotene absorption from 5 to 25 percent. Jayarajan and coworkers (1980) reported that the addition 5 g of fat to the diet significantly improved serum vitamin A concentrations among children after the consumption of a low fat vegetable diet. The addition of 10 g of fat did not improve serum vitamin A concentrations any more than did 5 g of fat.
Infections
Malabsorption of vitamin A can occur with diarrhea and intestinal infections and infestations. Sivakumar and Reddy (1972) demonstrated depressed absorption of labeled vitamin A in children with gastroenteritis and respiratory infections. Malabsorption of vitamin A is also associated with intestinal parasitism (Mahalanabis et al., 1979; Sivakumar and Reddy, 1975).
The malabsorption of vitamin A that is observed in children with Ascaris lumbricoides infection was associated with an altered mucosal morphology that was reversed with deworming (Jalal et al., 1998; Maxwell et al., 1968).
Food Matrix
The matrix of foods affects the ability of carotenoids to be released from food and therefore affects intestinal absorption. The rise in serum β-carotene concentration was significantly less when individuals consumed β-carotene from carrots than when they received a similar amount of β-carotene supplement (Micozzi et al., 1992; Tang et al., 2000; Torronen et al., 1996). This observation was similar for broccoli (Micozzi et al., 1992) and mixed green leafy vegetables (de Pee et al., 1995; Tang et al., 2000) as compared with a β-carotene supplement. The food matrix effect on β-carotene bioavailability has been reviewed (Boileau et al., 1999).
Food Processing
The processing of foods greatly affects the absorption of carotenoids (Van het Hof et al., 1998). The absorption of carotene was 24 percent from sliced carrots, whereas the absorption of carotene from homogenized carrots was 56 percent (Hume and Krebs, 1949). Rock et al. (1998) reported that the rise in serum β-carotene concentration was significantly greater in subjects consuming cooked carrots and spinach as compared with those consuming an equal amount of raw carrots and spinach. Similarly, the rise in serum β-carotene concentration was greater after the consumption of carrot juice than after the same amount of raw carrots (Torronen et al., 1996).
Nutrient-Nutrient Interactions
Iron
A direct correlation between hemoglobin and serum retinol concentrations has been observed (Suharno et al., 1993; Wolde-Gebriel et al., 1993). Anemic rats have been shown to have reduced plasma retinol concentrations when fed a vitamin A-rich diet (Amine et al., 1970), although normal hepatic stores of vitamin A were observed (Staab et al., 1984). Rosales and coworkers (1999) reported that iron deficiency in young rats alters the distribution of vitamin A concentration between plasma and liver. In a cross-sectional study of children in Thailand, serum retinol concentration was positively associated with serum iron and ferritin concentrations (Bloem et al., 1989). Intervention studies among Indonesian girls demonstrated that combining vitamin A with iron supplementation was more effective in increasing hemoglobin concentrations than was giving iron alone (Suharno et al., 1993). As discussed in further detail in Chapter 9, various studies suggest that vitamin A deficiency impairs iron mobilization from stores and therefore vitamin A supplementation improves hemoglobin concentrations (Lynch, 1997).
Zinc
Zinc is required for protein synthesis, including the hepatic synthesis and secretion of retinol binding protein (RBP) and transthyretin; therefore, zinc deficiency influences the mobilization of vitamin A from the liver and its transport into the circulation (Smith et al., 1974; Terhune and Sandstead, 1972). In animal models, circulating and hepatic concentrations of retinol decline and rise with experimental zinc deficiency and repletion, respectively (Baly et al., 1984; Duncan and Hurley, 1978). In humans, cross-sectional studies and supplementation trials have failed to establish a consistent relationship between zinc and vitamin A status (Christian and West, 1998). Because zinc is important in the biosynthesis of RBP, it has been suggested that zinc intake may positively affect vitamin A status only when individuals are moderately to severely protein-energy deficient (Shingwekar et al., 1979).
Although the alcohol dehydrogenase enzymes involved in the formation of retinal from retinol in the eye are not zinc dependent (Duester, 1996; Persson et al., 1995), zinc-deficient rats had a significant reduction in the synthesis of rhodopsin (Dorea and Olson, 1986), which was postulated to be due to impaired protein (opsin and alcohol dehydrogenase) synthesis. Morrison and coworkers (1978) reported that dark adaptation improved after the provision of 220 mg/day of zinc to zinc-deficient patients.
Carotenoids
Competitive interactions among different carotenoids have been observed. When subjects were given purified β-carotene and lutein in a combined dose, β-carotene significantly reduced lutein absorption, and therefore serum lutein concentration, compared to when lutein was given alone (Kostic et al., 1995). However, lutein given in combination with β-carotene significantly increased β-carotene serum concentrations compared to when β-carotene was given alone. Johnson et al. (1997) reported that lycopene does not affect the absorption of β-carotene, and β-carotene improved the absorption of lycopene.
Alcohol
Because both retinol and ethanol are alcohols, there is potential for overlap in the metabolic pathways of these two compounds. Competition with each other for similar enzymatic pathways has been reported (Leo and Lieber, 1999), while other retinol and alcohol dehydrogenases show greater substrate specificity (Napoli et al., 1995). Ethanol consumption results in a depletion of hepatic vitamin A concentrations in animals (Sato and Lieber, 1981) and in humans (Leo and Lieber, 1985). Although the effect on vitamin A is due, in part, to hepatic damage (Leo and Lieber, 1982) and malnutrition, the reduction in hepatic stores is also a direct effect of alcohol consumption. Patients with low vitamin A stores, in the study by Leo and Lieber (1982), were otherwise well nourished. Furthermore, the reduction in hepatic vitamin A stores was reduced before the onset of fibrosis or cirrhosis of the liver (Sato and Lieber, 1981). Results suggest that vitamin A is mobilized from the liver to other organs (Mobarhan et al., 1991) with ethanol consumption. Chronic ethanol intake resulted in increased destruction of retinoic acid through the induction of P450 enzymes, resulting in reduced hepatic retinoic acid concentrations (Wang, 1999).
FINDINGS BY LIFE STAGE AND GENDER GROUP
Infants Ages 0 through 12 Months
Method Used to Set the Adequate Intake
No functional criteria of vitamin A status have been demonstrated that reflect response to dietary intake in infants. Thus, recommended intakes of vitamin A are based on an Adequate Intake (AI) that reflects a calculated mean vitamin A intake of infants principally fed human milk.
Ages 0 through 6 Months. Using the method described in Chapter 2, the AI of vitamin A for infants ages 0 though 6 months is based on the average amount of vitamin A in human milk that is consumed. After rounding, an AI of 400 μg retinol activity equivalents (RAE)/day is set based on the average volume of milk intake of 0.78 L/day (see Chapter 2) and an average concentration of vitamin A in human milk of 1.70 μmol/L (485 μg/L) during the first 6 months of lactation (Canfield et al., 1997, 1998) (see Table 4-5). Because the bioconversion of carotenoids in milk and in infants is not known, the contribution of carotenoids in human milk to meeting the vitamin A requirement of infants was not considered.
TABLE 4-5
Vitamin A in Human Milk.
Ages 7 through 12 Months. Using the method described in Chapter 2 to extrapolate from the AI for infants ages 0 through 6 months fed human milk, the intake from human milk for the older infants is 483 μg RAE/day of vitamin A.
The vitamin A intake for older infants can also be determined by estimating the intake from human milk (concentration × 0.6 L/ day) and complementary foods (Chapter 2). Vitamin A intake data (n = 45) from complementary foods was estimated to be 244 μg/day based on data from the Third National Health and Nutrition Examination Survey. The average intake from human milk is approximately 291 μg/day (485 μg/L × 0.6 L/day). Thus, the total vitamin A intake is estimated to be 535 μg RAE/day (244 μg/day + 291 μg/day).
On the basis of these two approaches and rounding, the AI was set at 500 μg RAE/day. The AI for infants is greater than the Recommended Dietary Allowance (RDA) for young children because the RDA is based on extrapolation of adult data (see “Children and Adolescents Ages 1 through 18 Years”).
Vitamin A AI Summary, Ages 0 through 12 Months
| AI for Infants | |
| 0–6 months | 400 μg RAE/day of vitamin A |
| 7–12 months | 500 μg RAE/day of vitamin A |
Special Considerations
Concentrations of 520 to 590 μg/L of vitamin A in milk from Holstein cows have been reported (Tomlinson et al., 1976), which is significantly less than the levels observed in human milk (Table 4-5). The majority of vitamin A and carotenes are located in the fat globule and fat globule membrane in cow milk (Patton et al., 1980; Zahar et al., 1995). The concentrations of retinol and β-carotene in cow milk averaged 18 to 27 μg/g of milk fat in one study (Jensen and Nielsen, 1996). Retinol in cow milk is bound to β-lactoglobulin, which has a structure very similar to retinol binding protein (Papiz et al., 1986). There is minimal isomerization of trans-retinol to cis-retinol in unheated cow milk (Panfili et al., 1998), the latter being less well absorbed. Cow milk submitted to pasteurization resulted in 3 to 6 percent isomerization to cis-retinol. Greater isomerization was observed with severe heat treatments (16 percent in ultra high temperature milk and 34 percent in sterilized milk).
Children and Adolescents Ages 1 through 18 Years
Method Used to Estimate the Average Requirement
No data are available to estimate an average requirement for children and adolescents. A computational method is used that includes an allowance for adequate liver vitamin A stores to set the Estimated Average Requirement (EAR) (see “Adults Ages 19 Years and Older”). The EAR for children and adolescents is extrapolated from adults by using metabolic body weight and the method described in Chapter 2. If total body weight is used, the RDA for children 1 through 3 years would be 200 μg RAE/day. If metabolic weight (kg0.75) is used, the RDA would be 300 μg RAE/day. Studies conducted in developing countries indicate that xerophthalmia and serum retinol concentrations of less than 20 μg/dL exist among preschool children with daily intakes of up to 200 μg of vitamin A, whereas 300 μg/day of vitamin A is associated with serum retinol concentrations greater than 30 μg/dL (Reddy, 1985). Although similar data are lacking in developed countries, to ensure that the RDA will meet the requirement of almost all North American preschool children, metabolic weight was used to extrapolate from adults.
Vitamin A EAR and RDA Summary, Ages 1 through 18 Years
The RDA for vitamin A is set by using a coefficient of variation (CV) of 20 percent based on the calculated half-life values for liver vitamin A (see “Adults Ages 19 Years and Older”). The RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of individuals in the group (therefore, for vitamin A the RDA is 140 percent of the EAR). The calculated values for the RDAs have been rounded to the nearest 100 μg.
Adults Ages 19 Years and Older
Evidence Considered in Estimating the Average Requirement
The calculation described below can be used for estimating the vitamin A requirement and is calculated on the basis of the amount of dietary vitamin A required to maintain a given body-pool size in well-nourished subjects. Olson (1987) determined the average requirement of vitamin A by this approach using the calculation:
A × B × C × D × E × F
A = Percent of body vitamin A stores lost per day when ingesting a vitamin A-free diet
B = Minimum acceptable liver vitamin A reserve
C = The liver weight:body weight ratio
D = Reference weight for a specific age group and gender
E = Ratio of total body:liver vitamin A reserves
F = Efficiency of storage of ingested vitamin A.
By using this approach, a daily vitamin A intake can be determined that will assure vitamin A reserves to cover increased needs during periods of stress and low vitamin A intake. That value can be used for estimating the average requirement for vitamin A.
The portion of body vitamin A stores lost per day has been estimated to be 0.5 percent based on the rate of excretion of radio-activity from radiolabeled vitamin A and by the calculation of the half-life of vitamin A. The minimal acceptable liver reserve is estimated to be 20 μg/g and is based on the concentration at which (1) no clinical signs of a deficiency are observed, (2) adequate plasma retinol concentrations are maintained (Loerch et al., 1979), (3) induced biliary excretion of vitamin A is observed (Hicks et al., 1984), and (4) there is a protection against a vitamin A deficiency for approximately 4 months while the person consumes a vitamin A-deficient diet. The liver weight:body weight ratio is 1:33 (0.03) and is an average of ratios for infants and adults. The reference weights for adult women and men are 61 and 76 kg, respectively (see Chapter 1). The ratio of total body:liver vitamin A reserves is 10:9 (1.1) and is based on individuals with adequate vitamin A status. Finally, the efficiency of storage can be determined by isotope dilution methods following the administration of either radioactive or stable-isotopically labeled vitamin A to subjects adequate in vitamin A (Bausch and Reitz, 1977; Haskell et al., 1997). Recent studies by Haskell and coworkers (1997) suggest that the efficiency of storage is approximately 40 percent, rather than the 50 percent that was previously reported (Olson, 1987). Based on these current estimations, the EAR of preformed vitamin A required to assure an adequate body reserve in an adult man is 0.005 × 20 μg/g × 0.03 × 76 kg × 1.1 × 2.5, or 627 μg RAE/day. With a reference weight of 61 kg for women, the EAR would be 503 μg RAE/day.
Based on the study of Sauberlich and coworkers (1974), Olson (1987) estimated that the liver vitamin A concentration was less than 10 μg/g at the time the first clinical signs of vitamin A deficiency appeared. From this assumption, it was estimated that the half-life of vitamin A is approximately 128 days, and the CV is 21 percent. Because the portion of this variability that is due to experimental error is not known, a CV of 20 percent is used for setting the RDA.
Vitamin A EAR and RDA Summary, Ages 19 Years and Older
| EAR for Men | |
| 19–30 years | 625 μg RAE/day of vitamin A |
| 31–50 years | 625 μg RAE/day of vitamin A |
| 51–70 years | 625 μg RAE/day of vitamin A |
| > 70 years | 625 μg RAE/day of vitamin A |
| EAR for Women | |
| 19–30 years | 500 μg RAE/day of vitamin A |
| 31–50 years | 500 μg RAE/day of vitamin A |
| 51–70 years | 500 μg RAE/day of vitamin A |
| > 70 years | 500 μg RAE/day of vitamin A |
The RDA for vitamin A is set by using a CV of 20 percent (see Chapter 1) using the EAR for adequate body stores of vitamin A. The RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for vitamin A the RDA is 140 percent of the EAR). The calculated values for the RDAs have been rounded to the nearest 100 μg.
| RDA for Men | |
| 19–30 years | 900 μg RAE/day of vitamin A |
| 31–50 years | 900 μg RAE/day of vitamin A |
| 51–70 years | 900 μg RAE/day of vitamin A |
| > 70 years | 900 μg RAE/day of vitamin A |
| RDA for Women | |
| 19–30 years | 700 μg RAE/day of vitamin A |
| 31–50 years | 700 μg RAE/day of vitamin A |
| 51–70 years | 700 μg RAE/day of vitamin A |
| > 70 years | 700 μg RAE/day of vitamin A |
Pregnancy
Evidence Considered in Estimating the Average Requirement
Direct studies of the requirement for vitamin A during pregnancy are lacking. The model used to establish the EAR is based on the accumulation of vitamin A in the liver of the fetus during gestation and an assumption that liver contains approximately half of the body's vitamin A when liver stores are low, as in the case of newborns. Liver vitamin A concentrations for full-term stillborn infants (Dorea et al., 1984; Hoppner et al., 1968; Montreewasuwat and Olson, 1979; Olson, 1979) have ranged from less than 10 to greater than 100 μg/g liver, with values tending to be skewed towards the lower range (Olson, 1979). A vitamin A concentration of 1,800 μg per liver for 37 to 40 week gestation age (Montreewasuwat and Olson, 1979) was used to calculate a concentration of 3,600 μg per fetus. Assuming the efficiency of maternal vitamin A absorption to average 70 percent and vitamin A to be accumulated mostly in the last 90 days of pregnancy, the mother's requirement would be increased by approximately 50 μg/day during the last trimester. Because vitamin A in the mother's diet may be stored and mobilized later as needed and some vitamin A may be retained in the placenta, the EAR is estimated to be ~50 μg/day in addition to the EAR for nonpregnant adolescent girls and women for the entire pregnancy period.
Vitamin A EAR and RDA Summary, Pregnancy
| EAR for Pregnancy | |
| 14–18 years | 530 μg RAE/day of vitamin A |
| 19–30 years | 550 μg RAE/day of vitamin A |
| 31–50 years | 550 μg RAE/day of vitamin A |
The RDA for vitamin A is set by using a CV of 20 percent based on the calculated half-life values for liver vitamin A (see “Adults Ages 19 Years and Older”). The RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of individuals in the group (therefore, for vitamin A the RDA is 140 percent of the EAR). The calculated values for the RDAs have been rounded up to the nearest 10 μg.
| RDA for Pregnancy | |
| 14–18 years | 750 μg RAE/day of vitamin A |
| 19–30 years | 770 μg RAE/day of vitamin A |
| 31–50 years | 770 μg RAE/day of vitamin A |
Lactation
Evidence Considered in Estimating the Average Requirement
As indicated earlier in the section on infants, human milk-fed infants consume on average 400 μg/day of vitamin A in the first 6 months of life. The carotenoid content of human milk has been summarized in Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (IOM, 2000). Because the bioconversion of carotenoids in milk and in infants is not known, the contribution of carotenoids in human milk to meeting the vitamin A requirement of infants was not considered. To set an EAR during pregnancy, 400 μg RAE/day is added to the EAR for nonpregnant adolescent girls and women to assure adequate body stores of vitamin A.
Vitamin A EAR and RDA Summary, Lactation
| EAR for Lactation | |
| 14–18 years | 885 μg RAE/day of vitamin A |
| 19–30 years | 900 μg RAE/day of vitamin A |
| 31–50 years | 900 μg RAE/day of vitamin A |
The RDA for vitamin A is set by using a CV of 20 percent based on the calculated half-life values for liver vitamin A (see “Adults Ages 19 Years and Older”). The RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of individuals in the group (therefore, for vitamin A the RDA is 140 percent of the EAR). The calculated values for the RDAs have been rounded to the nearest 100 μg.
| RDA for Lactation | |
| 14–18 years | 1,200 μg RAE/day of vitamin A |
| 19–30 years | 1,300 μg RAE/day of vitamin A |
| 31–50 years | 1,300 μg RAE/day of vitamin A |
Requirement for Provitamin A Carotenoids
Although a large body of observational epidemiological evidence suggests that higher blood concentrations of β-carotene and other carotenoids obtained from foods are associated with a lower risk of several chronic diseases (see Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids [IOM, 2000]), no evidence pointed to the need for a certain percentage of dietary vitamin A to come from provitamin A carotenoids to meet the vitamin A requirement. In view of the health benefits associated with consumption of fruits and vegetables, existing recommendations for increased consumption of carotenoid-rich fruits and vegetables are strongly supported (see IOM, 2000). Consumption of five servings of fruits and vegetables per day could provide 5.2 to 6 mg/day of provitamin A carotenoids (Lachance, 1997), which would contribute approximately 50 to 65 percent of the men's RDA for vitamin A.
Special Considerations
Alcohol Consumption
Excessive alcohol consumption results in a depletion of liver vitamin A stores (Leo and Lieber, 1985). Depletion is partly due to the reduced consumption of foods. Furthermore, mobilization of vitamin A out of the liver may be increased with excessive alcohol consumption (Lieber and Leo, 1986). Because alcohol intake has been shown to enhance the toxicity of vitamin A (Leo and Lieber, 1999) (see “Tolerable Upper Intake Levels”), individuals who consume alcohol may be distinctly susceptible to the adverse effects of vitamin A and any increased intake to meet one's needs should be in the context of maintaining health.
Developing Countries and Vegetarian Diets
A number of factors can influence the requirement for vitamin A, including iron status, the presence and severity of infection and parasites, the level of dietary fat, protein energy malnutrition, and the available sources for preformed vitamin A and provitamin A carotenoids.
Parasites and Infection. Malabsorption of vitamin A can occur with diarrhea and intestinal infestations (Jalal et al., 1998; Sivakumar and Reddy, 1972). Furthermore, the urinary excretion of vitamin A is increased with infection, and especially with fever (Alvarez et al., 1995; Stephensen et al., 1994). For these reasons, with parasitic infestation and during infection, the requirement for vitamin A may be greater than the requirements set in this report, which are based on generally healthy individuals.
Protein Energy Malnutrition. Protein synthesis generally, and specifically retinol binding protein synthesis, is reduced with severe protein energy malnutrition (PEM) (marasmus and kwashiorkor), and therefore release of retinol from the liver (assuming stores are present) is also reduced (Large et al., 1980). With successful dietary treatment of PEM, growth and tissue weight gain will be stimulated, and the relative requirement of vitamin A will increase during the recovery period.
Vegetarianism. Preformed vitamin A is found only in animal-derived food products. A clinical sign of vitamin A deficiency, night blindness, is prevalent in developing countries where animal and vitamin A-fortified products are not commonly available. Although carotenoids such as β-carotene are abundant in green leafy vegetables and certain fruits, because it takes 12 μg of dietary β-carotene to provide 1 retinol activity equivalent (RAE) (as compared to previous recommendations where 1 μg of retinol was thought to be provided by 6 μg of β-carotene [NRC, 1989 and Table 4-3]), a greater amount of fruits and vegetables than previously recommended are required to meet the daily vitamin A requirement for vegetarians and those whose primary source of vitamin A is green leafy vegetables.
Analyzing intakes of vitamin A and β-carotene and using an RAE of 12 μg for dietary β-carotene indicate that the RDA for vitamin A can be met by those consuming a strict vegetarian diet containing the deeply colored fruits and vegetables (1,262 μg RAE) that are major sources of β-carotene in the United States (Chug-Ahuja et al., 1993) (Table 4-6). The United States has several vitamin A-fortified foods, including milk, cereals, and infant formula. Furthermore, certain food products, such as sugar, are being fortified with vitamin A in some developing countries. If menus are restricted in the amounts of provitamin A carotenoids consumed and such fortified products are not part of routine diets, then vitamin A supplements may be required.
TABLE 4-6
Vitamin A Intake from a Vegan Diet High in Carotene-Rich Fruits and Vegetables.
Populations Where Consumption of Vitamin A-Rich Foods is Limited. Three major intervention trials have been conducted in developing countries to evaluate the efficacy of provitamin A carotenoids in maintaining or improving vitamin A status in lactating women (de Pee et al., 1995), preschool children (Jalal et al., 1998), and young children (Takyi, 1999). Vitamin A status, as determined by serum retinol concentration, was not improved in Indonesian lactating women after the consumption of dark green leafy vegetables (de Pee et al., 1995). These women had hemoglobin concentrations less than 13 mg/dL. There is evidence that iron deficiency impairs the metabolism of vitamin A in laboratory animals (Jang et al., 2000; Rosales et al., 1999). In some, but not all, studies (Suharno et al., 1993), iron supplementation improved vitamin A status in humans (Munoz et al., 2000). Therefore the presence of iron deficiency, which is prevalent in developing countries, may impair the efficacy of dark green leafy vegetables. Jalal and coworkers (1998) reported that the addition of β-carotene-rich foods to the diets of preschool children improved vitamin A status, however, vitamin A status improved almost as well when fat was added to the diet and an anthelmintic drug to destroy parasitic worms was provided. This finding demonstrates the importance of dietary fat, which is often low in the diets of developing countries and the importance of intestinal parasites on vitamin A status. Takyi (1999) reported that the vitamin A status of young children improved similarly when fed either a pureed β-carotene-rich diet or provided a similar amount of β-carotene as a supplement. Here, in contrast to the findings of Jalal et al. (1998), dietary fat and anthelmentic drugs did not appear to have a beneficial effect on vitamin A status, possibly because the carotenoid was already provided in a highly absorbable, pureed form.
The EARs that have been set for the North American population are achievable through diet because of the abundance of vitamin A-rich foods. Populations of less developed countries may have difficulty in meeting the EAR that ensures adequate vitamin A stores. Therefore, an EAR that does not assure adequate vitamin A stores has been determined on the basis of the level of vitamin A for correction of abnormal dark adaptation in adults. This approach does not assure adequate stores of vitamin A because animal studies indicate that vitamin A depletion of the eye occurs after the depletion of hepatic vitamin A reserves (Bankson et al., 1989; Lewis et al., 1942). Furthermore, epidemiological studies in children suggest impaired host resistance to infection, presumably reflecting compromised immunity and represented by increased risk of morbidity and mortality at lesser stages of depletion (Arroyave et al., 1979; Arthur et al., 1992; Barreto et al., 1994; Bloem et al., 1990; Ghana VAST Study Team, 1993; Loyd-Puryear et al., 1991; Rahmathullah et al., 1990; Salazar-Lindo et al., 1993; West et al., 1991).
An EAR of 300 μg RAE/day can be calculated based on the dark adaptation data obtained from 13 individuals from four studies on adults (Table 4-4). The duration of depletion and repletion varied among these four studies and the majority of the studies were conducted on men. Interpolation of the level of vitamin A at which dark adaptation of each individual was corrected in these four studies results in a median intake of 300 μg RAE/day, which can be used to set an EAR based on dark adaptation for adults. Using this method, there was insufficient evidence to support setting a different EAR for men and for women, as there were too few women studied. EARs using dark adaptation as the indicator for children (1–3 years, 112 μg RAE/day; 4–8 years, 150 μg RAE/day; 9–13 years, 230 μg RAE/day) and adolescents (14–18 years, 300 μg RAE/day) are based on extrapolation from the adult EAR as described in Chapter 2.
INTAKE OF VITAMIN A
Food Sources
Common dietary sources of preformed vitamin A in the United States and Canada include liver, dairy products, and fish. Chug-Ahuja et al. (1993) reported that carrots were the major contributor of β-carotene (25 percent). Other major contributors to β-carotene intakes included cantaloupe, broccoli, squash, peas, and spinach. Carrots were also the major contributor (51 percent) of α-carotene. Fruits were the sole contributors of β-cryptoxanthin. According to data collected from the 1994–1996 Continuing Survey of Food Intakes by Individuals (CFSII), the major contributors of vitamin A from foods were grains and vegetables (approximately 55 percent), followed by dairy and meat products (approximately 30 percent).
Dietary Intake
The Third National Health and Nutrition Examination Survey (NHANES III) (Appendix Table C-8) estimated that the median dietary intake of vitamin A is 744 to 811 μg/day for men and 530 to 716 μg/day for women using the new provitamin A carotenoid conversion factors for calculating retinol activity equivalents (RAE) (see Table 4-3). When one examines Appendix Table C-8 to determine the proportion of individuals with intakes that were less than the EAR (500 μg RAE/day for women and 625 μg RAE/day for men), it is apparent that for most age groups between 25 and 50 percent of adults fell in this category. The EAR for vitamin A is based on a criterion of adequate liver stores; thus, these data suggest that considerable proportions of adults have liver vitamin A stores that are less than desirable. It should be recognized that this does not represent a clinical deficiency state, such as abnormal dark adaptation.
Because the level of vitamin A intake varies greatly (Beaton et al., 1983), it is very important that the daily intake distribution be adjusted for day-to-day variability in intakes when assessing intake distributions of groups to determine the proportion with intakes below the EAR. This adjustment can be carried out using the methods of Nusser et al. (1986) and the National Research Council (NRC, 1986).
When reporting as RAE, the vitamin A activity of provitamin A carotenoids is half the activity given as retinol equivalents (RE) (Table 4-3). Therefore, vitamin A intakes calculated using RAE are less than intakes determined using RE (compare Appendix Tables C-7 and C-8) resulting in a higher percentage of certain groups who consume levels of vitamin A less than the EAR. Thus, a greater amount of provitamin A carotenoids, and therefore darkly colored, carotene-rich fruits and vegetables, is needed to meet the vitamin A requirement.
Data from NHANES III indicate that for men 31 to 50 years of age, the median intakes of the provitamin A carotenoids α-carotene, β-carotene, and β-cryptoxanthin, were 51 (2 μg RAE), 1,942 (162 μg RAE), and 39 (1.6 μg RAE) μg/day, respectively (Appendix Tables C-1, C-2 and C-3). Using RAE, dietary β-carotene contributes approximately 21 percent of the total vitamin A intake. All provitamin A carotenoids contributed 26 and 34 percent of vitamin A consumed by men and women, respectively.
The median intake of other carotenoids, lutein and zeaxanthin, ranged from 1,353 μg/day to 1,966 μg/day for men and women (Appendix Table C-4). For men and women, the median intake of lycopene ranged from 842 to 5,079 μg/day (Appendix Table C-5).
The menus in Table 4-7 show that the total day's vitamin A intake (1,168 μg RAE/day) exceeds the Recommended Dietary Allowance (RDA) when consuming an omnivorous diet and choosing five fruits and vegetables that are major contributors of β-carotene in the United States. The RDA can also be achieved for individuals consuming vegetarian diets high in carotene-rich fruits and vegetables (Table 4-6).
TABLE 4-7
Vitamin A Intake from an Omnivorous Diet High in Carotene-Rich Fruits and Vegetables.
Intake from Supplements
Information from NHANES III on Americans' use of supplements containing vitamin A is given in Appendix Table C-9. The median intake of vitamin A from supplements was approximately 1,430 μg RAE/day for men and women. In 1986, approximately 26 percent of adults in the United States took supplements that contained vitamin A (Moss et al., 1989; see Table 2-2).
TOLERABLE UPPER INTAKE LEVELS
The Tolerable Upper Intake Level (UL) is the highest level of daily vitamin A intake that is likely to pose no risk of adverse health effects in almost all individuals. Although members of the general population should be advised not to routinely exceed the UL, intake above the UL may be appropriate for investigation within well-controlled clinical trials. Clinical trials of doses above the UL should not be discouraged as long as subjects participating in these trials have signed informed consent documents regarding possible toxicity and as long as these trials employ appropriate safety monitoring of trial subjects. In addition, the UL is not meant to apply to individuals who are receiving vitamin A under medical supervision. The UL for provitamin A carotenoids has been addressed in the report Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (IOM, 2000).
Hazard Identification
There are substantial data on the adverse effects of high vitamin A intakes. Acute toxicity is characterized by nausea, vomiting, headache, increased cerebrospinal fluid pressure, vertigo, blurred vision, muscular incoordination (Olson, 1983), and bulging fontanel in infants (Persson et al., 1965). These are usually transient effects involving single or short-term large doses of greater than or equal to 150,000 μg in adults and proportionately less in children (Bendich and Langseth, 1989). The clinical picture for chronic hypervitaminosis A is varied and nonspecific and may include central nervous system effects, liver abnormalities, bone and skin changes, and other adverse effects. Chronic toxicity is usually associated with ingestion of large doses greater than or equal to 30,000 μg/day for months or years. Both acute and chronic vitamin A toxicity are associated with increased plasma retinyl ester concentrations (Krasinski et al., 1989; Ross, 1999).
For the purpose of deriving a UL, three primary adverse effects of chronic vitamin A intake are discussed below: (1) reduced bone mineral density, (2) teratogenicity, and (3) liver abnormalities. High β-carotene intake has not been shown to cause hypervitaminosis A. Therefore, this review is limited to the adverse effects of preformed vitamin A or retinol. The terms vitamin A and retinol will be used interchangeably in the following sections. Because provitamin A carotenoids were not included in vitamin A supplements until the late 1980s, it is assumed that studies and case reports published before 1990 used preformed vitamin A in supplements. The UL derived here applies to chronic intake of preformed vitamin A from food, fortified food, and/or supplements.
Adverse Effects in Adults
Bone Mineral Density. Chronic, excessive vitamin A intake has been shown to lead to bone mineral loss in animals (Rohde et al., 1999), making such a consequence in humans biologically plausible. Most human case reports are not well described and epidemiological studies are inadequate in design. However, four studies provide interpretable evidence relating changes in bone mineral density (BMD) and risk of hip fracture with variation in dietary intake of preformed vitamin A (Freudenheim et al., 1986; Houtkooper et al., 1995; Melhus et al., 1998). The studies are distinguished by their well-described study designs and populations, adequate dietary intake estimates, and accurate methods for measuring BMD at multiple sites.
One two-part study (Melhus et al., 1998) suggests that a chronic intake of 1.5 mg/day of preformed vitamin A is associated with osteoporosis and increased risk of hip fracture. The first part, a cross-sectional multivariate regression analysis in 175 Swedish women 28 to 74 years of age, showed a consistent loss in BMD at four sites and in total BMD with increased preformed vitamin A intake. Numerous nutritional and non-nutritional exposures were concurrently assessed, allowing substantial control of potential confounders. With the use of stratified estimates of retinol intake in a univariate regression analysis, BMD was shown to increase with each 0.5 mg/day increment in intake above a reference intake of less than 0.5 mg/day, until intakes exceeded 1.5 mg/day. Above this level, mean BMD decreased markedly at each site. In a multivariate model, adjusting for effects of 14 other covariates, similar results were found. It is not clear whether the findings are equally applicable to pre- and postmenopausal women.
The second part was a nested case-control study on the risk factors for hip fracture. Cases were mostly postmenopausal women with first hip fracture within 2 to 64 months after entry into the large cohort study, or 5 to 67 months after the mid-point of the recalled dietary assessment. Four matched control subjects were selected for each case. A total of 247 cases and 873 control subjects completed the study. Univariate and multivariate conditional logistic regression analysis showed a dose-dependent increase in the risk of hip fracture with each 0.5 mg/day increment in reported retinol intake above 0.5 mg/day (baseline). The odds ratio was 2.05 (95 percent confidence interval, 1.05–3.98) at intakes above 1.5 mg/day.
In contrast to the results of Melhus and coworkers (1998), which suggest that risk of bone mineral loss and hip fracture occurs at estimated intakes above 1.5 mg/day, two U.S. studies provide no evidence of increased bone mineral loss in women with intakes of preformed vitamin A up to 1.5 to 2.0 mg/day (Freudenheim et al., 1986; Houtkooper et al., 1995). Freudenheim and coworkers (1986) evaluated the correlation between mean 3-year vitamin A intakes ranging from approximately 2 to 3 mg/day and rates of change in BMD in 84 women, 35 to 65 years of age (17 pre- and 67 postmenopausal). No consistent relationship was reported between vitamin A intake and the rate of bone mineral content loss in pre- and postmenopausal women. The single subject who showed rapid bone mineral loss with very high vitamin A intake also appeared to have consumed large amounts of other micronutrients as well, obscuring the significance of this relationship. Further, this study suffers from a small sample size in each of the four key groups (i.e., pre- and postmenopausal women by calcium supplement status), making correlations of potential nutritional or pathological importance indeterminate.
Houtkooper and coworkers (1995), in a longitudinal study of 66 women 28 to 39 years of age, showed that vitamin A intake was significantly associated with the increased annual rate of change in total body BMD. The mean rate of change in total body BMD over the 18-month study was negative, although several sites (lumbar spine, trochanter, and Ward's triangle) showed small positive slopes. The estimated mean intake of preformed vitamin A from the diet was 1,220 ± 472 (standard deviation [SD]) μg/day. The estimated vitamin A intake from provitamin A carotenoids was 595 ± 352 (SD) μg/day. In multivariable regression models that included covariables for body composition and treatment (exercise versus sedentary) status, the slopes for vitamin A and carotene (two separate models) were both positive [b = 0.007 and 0.008 mg/(cm2-year)] with r2 values of ~0.30 for each model. While the positive association between vitamin A and carotene intake and change in BMD may not be causal, the data provide evidence that vitamin A does not adversely affect premenopausal bone health within this range of intake.
The findings from these studies are provocative but conflicting, and therefore, they are not useful for setting a UL for vitamin A. More research is needed to clarify whether chronic vitamin A intake, at levels that characterize upper-usual intake ranges for many American and European populations, may lead to loss in BMD and consequent increased risk of hip fracture in certain population groups, particularly among pre- and postmenopausal women.
Teratogenicity. Concern for the possible teratogenicity of high vitamin A intake in humans is based on the unequivocal demonstration of human teratogenicity of 13-cis-retinoic acid (Lammer et al., 1985) after supplementation with high doses of vitamin A (Eckhoff and Nau, 1990; Eckhoff et al., 1991). Numerous studies in experimental animals clearly establish the teratogenic potential of excessive intakes of vitamin A (Cohlan, 1953, 1954; Geelen, 1979; Hutchings and Gaston, 1974; Hutchings et al., 1973; Kalter and Warkany, 1961; Pinnock and Alderman, 1992).
Epidemiological data show the possibility of teratogenic effects with high intakes of preformed vitamin A (Table 4-8). The critical period for susceptibility appears to be the first trimester of pregnancy and the primary birth defects associated with excess vitamin A intake are those derived from cranial neural crest (CNC) cells such as craniofacial malformations and abnormalities of the central nervous system (except neural tube defects), thymus, and heart. Examination of the data suggests a likely dose-dependent association between vitamin A intake at excessive levels and the risk of birth defects. One case-control report showed a statistically nonsignificant association between a reported maternal intake of greater than 12,000 μg/day and malformations, but not below that level (Martinez-Frias and Salvador, 1990). Two other large case-control studies showed no relationship between risk of malformation and likely supplemental daily doses of 2,400 to 3,000 μg by mothers (Khoury et al., 1996; Shaw et al., 1996). An observational study by Rothman and coworkers (1995) involving 22,748 pregnant women found that those who ingested greater than 4,500 μg/day of preformed vitamin A from food and supplements were at greater risk of delivering infants with malformations of CNC cell origin (e.g., cleft lip or palate) than were women consuming less than 1,500 μg/day. But questions have been raised about the accuracy of intake estimates and birth defects diagnosed. It has been argued further that the limited number of excess cases used to identify a toxicity threshold of 4,500 μg/day of preformed vitamin A (or 3,000 μg from supplements) permits the study's findings to be consistent with a larger threshold than other studies would suggest (Brent et al., 1996; Mastroiacovo et al., 1999; Watkins et al., 1996; Werler et al., 1996). Thus, while few dispute a causal association between excessive periconceptual vitamin A intake and risk of malformation, the threshold at which risk increases remains a matter of debate. However, in the context of the totality of data on vitamin A and birth defects, the data of Rothman and coworkers (1995) provide supportive evidence of a causal association. Case reports of malformations exist to support an increased risk of birth defects above a maternal intake of 7,800 μg/day of vitamin A (Bauernfeind, 1980; Bernhardt and Dorsey, 1974). Human case reports support a temporal association between maternal exposure to elevated vitamin A intakes and birth defects (Bernhardt and Dorsey, 1974; Von Lennep et al., 1985).
Liver Abnormalities. There is a strong causal association shown by human and animal data between excess vitamin A intake and liver abnormalities because the liver is the main storage site and target organ for vitamin A toxicity. The wide spectrum of vitamin A-induced liver abnormalities ranges from reversibly elevated liver enzymes to widespread fibrosis, cirrhosis, and sometimes death. Table 4-9 shows consistency and specificity of the following effects in liver pathology: spontaneous green fluorescence of sinusoidal cells, perisinusoidal fibrosis, hyperplasia, and hypertrophy of Ito cells. Human data are potentially confounded by other factors related to liver damage such as alcohol intake, hepatitis A, B, and C, hepatotoxic medications, or preexisting liver disease. A thorough evaluation of the liver data is provided in the later section, “Dose-Response Assessment”.
Adverse Interactions. Alcohol intake has been shown to enhance the toxicity of vitamin A (Leo and Lieber, 1999). In particular, the hepatotoxicity of vitamin A may be potentiated by alcohol use. Therefore, alcohol drinkers may be distinctly susceptible to the adverse effects of vitamin A.
Adverse Effects in Infants and Children
There are numerous case reports of infants (Table 4-10), toddlers, and children who have demonstrated toxic effects due to ex cess vitamin A intakes for months to years. Of particular concern are intracranial (bulging fontanel) and skeletal abnormalities that can result in infants given vitamin A doses of 5,500 to 6,750 μg/day (Persson et al., 1965). The clinical presentation of vitamin A toxicity in infants and young children varies widely. The more commonly recognized signs and symptoms include skeletal abnormalities, bone tenderness and pain, increased intracranial pressure, desquamation, brittle nails, mouth fissures, alopecia, fever, headache, lethargy, irritability, weight loss, vomiting, and hepatomegaly (Bush and Dahms, 1984). Furthermore, tolerance to excess vitamin A intake also appears to vary (Carpenter et al., 1987). Carpenter and coworkers (1987) described two boys who developed hypervitaminosis A by age 2 years for one and by age 6 years for the other. Both were given chicken liver that supplied about 690 μg/day of vitamin A and various supplements that supplied another 135 to 750 μg/day. An older sister who had been treated similarly remained completely healthy.
TABLE 4-8
The Relationship Between Reproductive Risk and Excess Preformed Vitamin A in Humans.
Summary
Based on considerations of causality, quality, and completeness of the database, teratogenicity was selected as the critical adverse effect on which to base a UL for women of childbearing age. For all other adults, liver abnormalities were the critical adverse effect. Abnormal liver pathology, characteristic of vitamin A intoxication (or grossly elevated hepatic vitamin A levels), was selected rather than elevated liver enzymes because of the uncertainties regarding other possible causes such as concurrent use of hepatotoxic drugs, alcohol intake, and hepatitis B and C. Bone changes were not used because of the conflicting findings and the lack of other data confirming the findings of Melhus et al. (1998).
Dose-Response Assessment
Women of Reproductive Age
Data Selection. Epidemiological studies evaluating the teratogenicity of vitamin A intake shortly before or during pregnancy (Table 4-8) were used to derive a UL for women of reproductive age. Because adequate human data were available, animal data were not used to derive a UL.
Identification of a No-Observed-Adverse-Effect Level (NOAEL). A NOAEL of approximately 4,500 μg/day of preformed vitamin A from food and supplements was based on a critical evaluation of the data in Table 4-8. There are numerous reports showing no adverse effects at doses below 3,000 μg/day of vitamin A from supplements (Czeizel and Rockenbauer, 1998; Dudas and Czeizel, 1992; Khoury et al., 1996; Rothman et al., 1995) or 4,500 μg/day of preformed vitamin A from food and supplements (Rothman et al., 1995). Rothman and coworkers (1995) showed a significantly increased risk of birth defects at the cranial neural crest sites among women who consumed greater than 4,500 μg of preformed vitamin A/day from food and supplements during the first trimester compared to those who took 1,500 μg/day or less. Most of the human data on teratogenicity of vitamin A involve doses equal to or greater than 7,800 μg/day. There are limited epidemiological data to clearly define a dose-response relationship in the dose range of 3,000 to 7,800 μg/day. Nevertheless, 4,500 μg/day represents a conservative value for a NOAEL in light of the evidence of no adverse effects at or below that level.
TABLE 4-9
Evidence of Liver Abnormalities After Excess Preformed Vitamin A Intakes (< 30,000 μg/day), Based on Increasing Dose.
Uncertainty Assessment. An uncertainty factor (UF) of 1.5 was selected on the basis of inter-individual variability in susceptibility. Because there are substantial data (Table 4-8) showing no adverse effects at doses up to 3,000 μg/day of vitamin A supplements, a higher UF was not justified.
Derivation of a UL. The NOAEL of 4,500 μg/day was divided by the UF of 1.5 to obtain a UL value of 3,000 μg/day for women of reproductive age.
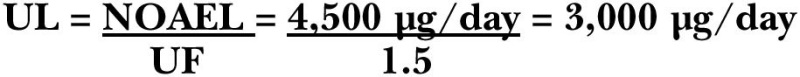
TABLE 4-10
Cases of Subchronic and Chronic, Low-Dose Vitamin A Toxicity in Infants, Based on Increasing Dose.
The UL for adolescent girls was adjusted on the basis of relative body weight as described in Chapter 3 with the use of reference weights from Chapter 1 (Table 1-1).
Vitamin A UL Summary, Adolescent Girls and Women Ages 14 through 50 Years, Pregnancy, Lactation
| UL for Women | |
| 14–18 years | 2,800 μg/day of preformed vitamin A |
| 19–50 years | 3,000 μg/day of preformed vitamin A |
| UL for Pregnancy | |
| 14–18 years | 2,800 μg/day of preformed vitamin A |
| 19–50 years | 3,000 μg/day of preformed vitamin A |
| UL for Lactation | |
| 14–18 years | 2,800 μg/day of preformed vitamin A |
| 19–50 years | 3,000 μg/day of preformed vitamin A |
All Other Adults Ages 19 Years and Older, Excluding Women of Childbearing Age
Data Selection. Data on liver abnormalities in humans were used to derive a UL. Because clear toxicity has been demonstrated in numerous studies at doses above 15,000 μg/day, only data involving doses less than 30,000 μg/day of vitamin A were included in Table 4-9. Data were thoroughly evaluated for other potential causes of liver abnormalities. The following criteria for selecting the data sets were used: (1) data must show grossly elevated liver vitamin A levels or hypertrophy of Ito cells, (2) no alcoholism, (3) no concomitant liver hepatitis, and (4) no hepatotoxic drug use. While hepatitis A and B status are known in most cases, testing for hepatitis C did not begin until the early 1990s and is unknown in most cases. Therefore, hepatitis C was not used as a criterion for exclusion.
Two case studies reported hypertrophy of Ito cells in a 63-year-old woman after vitamin A intake of 14,000 μg/day for 10 years (Minuk et al., 1988) and in a 36-year-old man who took about 15,000 μg/ day for 12 years (Zafrani et al., 1984). Neither of these reports appear to be confounded by hepatitis A or B viral infections or concomitant exposure to other hepatotoxic agents including alcohol. Reports of vitamin A-induced hepatotoxicity at doses less than 14,000 μg/day were found (Eaton, 1978; Hatoff et al., 1982; Kowalski et al., 1994; Oren and Ilan, 1992). However, as Table 4-9 shows, these studies fail to provide information on other predisposing or confounding factors such as alcohol intake, drugs and medications used, and history of viral hepatitis infection.
Uncertainty Assessment. A UF of 5.0 was selected to account for the severe, irreversible nature of the adverse effect, extrapolation from a lowest-observed-adverse-effect level (LOAEL) to a NOAEL, and interindividual variation in sensitivity.
Derivation of a UL. Hepatotoxicity was reported at vitamin A supplement doses of 14,000 μg/day. A LOAEL of 14,000 μg/day was divided by a UF of 5 to obtain a UL after rounding of 3,000 μg/day for adults other than women of reproductive age. This UL is the same as that set for women of reproductive age, given that the UL is defined as the highest level of daily nutrient intake likely to pose no risk of adverse health effects to almost all of the general population.
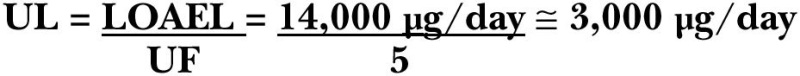
Vitamin A UL Summary, Ages 19 Years and Older, Excluding Women of Childbearing Age
Infants, Children, and Adolescent Boys
Data Selection. Case reports of hypervitaminosis A in infants were used to identify a LOAEL and derive a UL. Data were not available to identify a NOAEL.
Identification of a LOAEL. A LOAEL of 6,460 μg/day of vitamin A (which was rounded to 6,000 μg/day) was identified by averaging the lowest doses of four case reports (Persson et al., 1965). Four cases of hypervitaminosis A occurred after doses of 5,500 to 6,750 μg/day of vitamin A for 1 to 3 months (Table 4-10). The age of onset of symptoms ranged from 2.5 to 5.5 months and included anorexia, hyperirritability, occipital edema, pronounced craniotabes, bulging fontanels, increased intracranial pressure, and skin lesions and desquamation. The lowest dose associated with a bulging fontanel involved a 4-month-old girl given a daily dose of 24 drops of AD-vimin (about 5,500 μg of vitamin A) for 3 months. Her fontanels bulged 0.5 centimeters above the plane of the skull. The other three cases involved a dose of 6,750 μg/day of vitamin A for 1 to 2.5 months. Increased intracranial pressure and bulging fontanels were observed in these cases as well. Other effects observed at the higher dose included anorexia, hyperirritability, occipital edema, pronounced craniotabes, skin lesions, skin desquamation, epiphyseal line changes, and cortical hyperostosis on x-rays.
Uncertainty Assessment. A UF of 10 was selected to account for the uncertainty of extrapolating a LOAEL to a NOAEL for a nonsevere and reversible effect (i.e., bulging fontanel) and the interindividual variability in sensitivity.
Derivation of a UL. The LOAEL of 6,000 μg/day was divided by a UF of 10 to calculate a UL of 600 μg/day of preformed vitamin A for infants.
Children and Adolescent Boys. There are limited case report data of hypervitaminosis A (e.g., bulging anterior fontanels, increased intracranial pressure, hair loss, increased suture markings on the skull, and periosteal new bone formation) in children and adolescents after doses ranging from 7,000 μg/day in young children to 15,000 μg/day in older children and adolescents (Farris and Erdman, 1982; Siegel and Spackman, 1972; Smith and Goodman, 1976). Given the dearth of information and the need for conservativism, the UL values for children and adolescents are extrapolated from those established for adults. Thus, the adult UL of 3,000 μg/day of preformed vitamin A was adjusted for children and adolescents on the basis of relative body weight as described in Chapter 2 with use of reference weights from Chapter 1 (Table 1-1). Values have been rounded.
Vitamin A UL Summary, Infants, Children, and Adolescent Boys
Special Considerations
A review of the literature revealed that individuals with high alcohol intake, pre-existing liver disease, hyperlipidemia, or severe protein malnutrition may be distinctly susceptible to the adverse effects of excess preformed vitamin A intake (Ellis et al., 1986; Hathcock et al., 1990; Leo and Lieber, 1999). These individuals may not be protected by the UL for vitamin A for the general population.
Intake Assessment
Based on data from the Third National Health and Nutrition Survey (NHANES III), the highest median intake of preformed vitamin A for any gender and life stage group was 895 μg/day (Appendix Table C-6). This intake was being consumed by lactating women. The highest reported intake at the ninety-fifth percentile was 1,503 μg/day in lactating women. For adult Americans who take supplements containing vitamin A, intakes at the ninety-fifth percentile ranged from approximately 1,500 to 3,000 μg/day (Appendix Table C-9). Less than 5 percent of pregnant women had dietary and supplemental intake levels exceeding the UL.
Risk Characterization
The risk of exceeding the UL for vitamin A appears to be small based on the intakes cited above. There is not a large difference between the UL for infants (600 μg/day) and the Adequate Intake for older infants (500 μg/day). There is a body of evidence supporting the reversibility of bulging fontanels following the elimination of intermittent supplementation (de Francisco et al., 1993) or chronic ingestion (Naz and Edwards, 1952; Persson et al., 1965; Woodard et al., 1961) of high doses of vitamin A.
The UL is based on healthy populations in developed countries. Supplemental doses exceeding the UL for vitamin A (60 to 120 mg) are currently used in fortification and supplementation programs for the prevention and treatment of vitamin A deficiency, especially in developing countries. The UL is not meant to apply to communities of malnourished individuals receiving vitamin A prophylactically, either periodically or through fortification, as a means to prevent vitamin A deficiency or for individuals being treated for diseases, such as retinitis pigmentosa, with vitamin A.
RESEARCH RECOMMENDATIONS FOR VITAMIN A
- Effects of food matrices (e.g., carotenoids in milk and supplements) on the bioavailability of provitamin A carotenoids.
- Age-related differences in the bioavailability of vitamin A.
- Defined critical endpoints for population assessment for vitamin A and evaluation of their association with liver vitamin A stores.
- Effect of dietary vitamin A and vitamin A status on turnover and utilization of vitamin A. Is there significant adaptation to low vitamin A intakes? Is vitamin A absorption increased in response to low vitamin A intake? Is catabolism upregulated as body stores increase?
- Relationship of bioactive vitamin A indicators (e.g., retinoic acid) to dietary vitamin A intake.
- Effects of pregnancy and lactation on maternal vitamin A turnover.
- Effect of the interaction of vitamin A with other nutrients and food processing on the bioavailability of vitamin A.
REFERENCES
- AAP (American Academy of Pediatrics Committee on Infectious Diseases). 1993. Vitamin A treatment of measles. Pediatrics 91:1014–1015. [PubMed: 8474793]
- Abedin Z, Hussain MA, Ahmad K. 1976. Liver reserve of vitamin A from medico-legal cases in Bangladesh. Bangladesh Med Res Counc Bull 2:43–51. [PubMed: 1037196]
- Alvarez JO, Salazar-Lindo E, Kohatsu J, Miranda P, Stephensen CB. 1995. Urinary excretion of retinol in children with acute diarrhea. Am J Clin Nutr 61:1273–1276. [PubMed: 7762530]
- Amedee-Manesme O, Anderson D, Olson JA. 1984. Relation of the relative dose response to liver concentrations of vitamin A in generally well-nourished surgical patients. Am J Clin Nutr 39:898–902. [PubMed: 6539063]
- Amedee-Manesme O, Mourey MS, Hanck A, Therasse J. 1987. Vitamin A relative dose response test: Validation by intravenous injection in children with liver disease. Am J Clin Nutr 46:286–289. [PubMed: 3618532]
- Amine EK, Corey J, Hegsted DM, Hayes KC. 1970. Comparative hematology during deficiencies of iron and vitamin A in the rat. J Nutr 100:1033–1040. [PubMed: 5456548]
- Arena JM, Sarazen P, Baylin GJ. 1951. Hypervitaminosis A: Report of an unusual case with marked craniotabes. Pediatrics 8:788–794. [PubMed: 14911249]
- Arroyave G, Aguilar JR, Flores M, Guzman MA. 1979. Evaluation of Sugar Fortification with Vitamin A at the National Level . Scientific Publication No. 384. Washington, DC: Pan American Health Organization.
- Arthur P, Kirkwood B, Ross D, Morris S, Gyapong J, Tomkins A, Addy H. 1992. Impact of vitamin A supplementation on childhood morbidity in northern Ghana. Lancet 339:361–362. [PubMed: 1346430]
- Baly DL, Golub MS, Gershwin ME, Hurley LS. 1984. Studies of marginal zinc deprivation in rhesus monkeys. III. Effects on vitamin A metabolism. Am J Clin Nutr 40:199–207. [PubMed: 6540515]
- Bankson DD, Ellis JK, Russell RM. 1989. Effects of a vitamin-A-free diet on tissue vitamin A concentration and dark adaptation of aging rats. Exp Gerontol 24:127–136. [PubMed: 2721601]
- Barclay AJ, Foster A, Sommer A. 1987. Vitamin A supplements and mortality related to measles: A randomised clinical trial. Br Med J 294:294–296. [PMC free article: PMC1245303] [PubMed: 3101849]
- Barreto ML, Santos LM, Assis AM, Araujo MP, Farenzena GG, Santos PA, Fiaccone RL. 1994. Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet 344:228–231. [PubMed: 7913157]
- Barua AB, Olson JA. 1989. Chemical synthesis of all-trans [11-3H]-retinoyl β-glucuronide in its metabolism in rats in vivo. Biochem J 263:403–409. [PMC free article: PMC1133443] [PubMed: 2597112]
- Batchelder EL, Ebbs JC. 1943. Some observations of dark adaptation in man and their bearing on the problem of human requirement for vitamin A. Rhode Island Agricultural Experiment Station Bulletin , No. 645.
- Bauernfeind JC. 1972. Carotenoid vitamin A precursors and analogs in foods and feeds. J Agric Food Chem 20:456–473. [PubMed: 4562921]
- Bauernfeind JC. 1980. The Safe Use of Vitamin A. A report of the International Vitamin A Consultative Group. Washington, DC: The Nutrition Foundation.
- Bausch J, Rietz P. 1977. Method for the assessment of vitamin A liver stores. Acta Vitaminol Enzymol 31:99–112. [PubMed: 580694]
- Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, Harvey B. 1993. Effectiveness of Vitamin A Supplementation in the Control of Young Child Morbidity and Mortality in Developing Countries . Geneva: Subcommittee on Nutrition, Administrative Committee on Coordination, World Health Organization.
- Beaton GH, Milner J, McGuire V, Feather TE, Little JA. 1983. Source of variance in 24-hour dietary recall data: Implications for nutrition study design and interpretation. Carbohydrate source, vitamins and minerals. Am J Clin Nutr 37:986–995. [PubMed: 6846242]
- Bernhardt IB, Dorsey DJ. 1974. Hypervitaminosis A and congenital renal anomalies in a human infant. Obstet Gynecol 43:750–755. [PubMed: 4822659]
- Blanchard EL, Harper HA. 1940. Measurement of vitamin A status of young adults by the dark adaptation technic. Arch Int Med 66:661–669.
- Blaner WS, Olson JA. 1994. Retinol and retinoic acid metabolism. In: Sporn MB, editor; , Roberts AB, editor; , Goodman DS, editor. , eds. The Retinoids: Biology, Chemistry, and Medicine , 2nd ed. New York: Raven Press. Pp.229–255.
- Bloem MW, Wedel M, Egger RJ, Speek AJ, Schrijver J, Saowakontha S, Schreurs WH. 1989. Iron metabolism and vitamin A deficiency in children in northeast Thailand. Am J Clin Nutr 50:332–338. [PubMed: 2756920]
- Bloem MW, Wedel M, Egger RJ, Speek AJ, Schrijver J, Saowakontha S, Schreurs WH. 1990. Mild vitamin A deficiency and risk of respiratory tract diseases and diarrhea in preschool and school children in northeastern Thailand. Am J Epidemiol 131:332–339. [PubMed: 2296985]
- Blomhoff HK, Smeland EB, Erikstein B, Rasmussen AM, Skrede B, Skjonsberg C, Blomhoff R. 1992. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J Biol Chem 267:23988–23992. [PubMed: 1429735]
- Blomstrand RM, Werner B. 1967. Studies on the intestinal absorption of radioactive β-carotene and vitamin A in man. Scand J Clin Lab Invest 19:339–345. [PubMed: 6051928]
- Boileau TW, Moore AC, Erdman JW Jr. 1999. Carotenoids and vitamin A. In: Papas AM, editor. , ed. Antioxidant Status, Diet, Nutrition and Health . Boca Raton, FL: CRC Press. Pp.133–158.
- Borel P, Dubois C, Mekki N, Grolier P, Partier A, Alexandre-Gouabau MC, Lairon D, Azais-Braesco V. 1997. Dietary triglycerides, up to 40 g/meal, do not affect preformed vitamin A bioavailability in humans. Eur J Clin Nutr 51:717–722. [PubMed: 9368804]
- Borel P, Mekki N, Boirie Y, Partier A, Alexandre-Gouabau MC, Grolier P, Beaufrere B, Portugal H, Lairon D, Azais-Braesco V. 1998. Comparison of the postprandial plasma vitamin A response in young and older adults. J Gerontol A Biol Sci Med Sci 53:B133–B140. [PubMed: 9520909]
- Brent RL, Hendrickx AG, Holmes LB, Miller RK. 1996. Teratogenicity of high vitamin A intake. N Engl J Med 334:1196–1197. [PubMed: 8602192]
- Brubacher G, Weiser H. 1985. The vitamin A activity of beta-carotene. Int J Vitam Nutr Res 55:5–15. [PubMed: 3997397]
- Bush ME, Dahms BB. 1984. Fatal hypervitaminosis A in a neonate. Arch Pathol Lab Med 108:838–842. [PubMed: 6548125]
- Butera ST, Krakowka S. 1986. Assessment of lymphocyte function during vitamin A deficiency. Am J Vet Res 47:850–855. [PubMed: 3963587]
- Butte NF, Calloway DH. 1981. Evaluation of lactational performance of Navajo women. Am J Clin Nutr 34:2210–2215. [PubMed: 7293949]
- Canfield LM, Giuliano AR, Neilson EM, Yap HH, Graver EJ, Cui HA, Blashill BM. 1997. Beta-carotene in breast milk and serum is increased after a single beta-carotene dose. Am J Clin Nutr 66:52–61. [PubMed: 9209169]
- Canfield LM, Giuliano AR, Neilson EM, Blashil BM, Graver EJ, Yap HH. 1998. Kinetics of the response of milk and serum beta-carotene to daily beta-carotene supplementation in healthy, lactating women. Am J Clin Nutr 67:276–283. [PubMed: 9459376]
- Cantorna MT, Nashold FE, Hayes CE. 1995. Vitamin A deficiency results in a priming environment conducive for TH1 cell development. Eur J Immunol 25:1673–1679. [PubMed: 7614995]
- Carlier C, Moulia-Pelat J-P, Ceccon J-F, Mourey MS, Fall M, N'Diaye M, Amedee-Manesme. 1991. Prevalence of malnutrition and vitamin A deficiency in the Diourbel, Fatick and Kaolack regions of Senegal: Feasibility of the method of impression cytology with transfer. Am J Clin Nutr 53:66–69. [PubMed: 1898581]
- Carman JA, Smith SM, Hayes CE. 1989. Characterization of a helper T-lymphocyte defect in vitamin A deficient mice. J Immunol 142:388–393. [PubMed: 2463304]
- Carman JA, Pond L, Nashold F, Wassom DL, Hayes CE. 1992. Immunity to Trichinella spiralis infection in vitamin A-deficient mice. J Exp Med 175:111–120. [PMC free article: PMC2119062] [PubMed: 1730911]
- Carney EA, Russell RM. 1980. Correlation of dark adaptation test results with serum vitamin A levels in diseased adults. J Nutr 110:552–557. [PubMed: 7359223]
- Carney SM, Underwood BA, Loerch JD. 1976. Effects of zinc and vitamin A deficient diets on the hepatic mobilization and urinary excretion of vitamin A in rats. J Nutr 106:1773–1781. [PubMed: 993857]
- Carpenter TO, Pettifor JM, Russell RM, Pitha J, Mobarhan S, Ossip MS, Wainer S, Anast CS. 1987. Severe hypervitaminosis A in siblings: Evidence of variable tolerance to retinol intake. J Pediatr 111:507–512. [PubMed: 3655980]
- Castenmiller JJ, West CE. 1998. Bioavailability and bioconversion of carotenoids. Ann Rev Nutr 18:19–38. [PubMed: 9706217]
- Castenmiller JJ, West CE, Linssen JP, Van het Hof KH, Voragen AG. 1999. The food matrix of spinach is a limiting factor in determining the bioavailability of beta-carotene and to a lesser extent of lutein in humans. J Nutr 129:349–355. [PubMed: 10024612]
- Chappell JE, Francis T, Clandinin MT. 1985. Vitamin A and E content of human milk at early stages of lactation. Early Hum Dev 11:157–167. [PubMed: 4029052]
- Chase HP, Kumar V, Dodds JM, Sauberlich HE, Hunter RM, Burton RS, Spalding V. 1971. Nutritional status of preschool Mexican-American migrant farm children. Am J Dis Child 122:316–324. [PubMed: 5115528]
- Christian P, West KP Jr. 1998. Interactions between zinc and vitamin A: An update. Am J Clin Nutr 68:435S–441S. [PubMed: 9701158]
- Christian P, Schulze K, Stoltzfus RJ, West KP Jr. 1998. a. Hyporetinolemia, illness symptoms, and acute phase protein response in pregnant women with and without night blindness. Am J Clin Nutr 67:1237–1243. [PubMed: 9625099]
- Christian P, West KP Jr, Khatry SK, Katz J, LeClerq S, Pradhan EK, Shrestha SR. 1998. b. Vitamin A or beta-carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. J Nutr 128:1458–1463. [PubMed: 9732305]
- Chug-Ahuja JK, Holden JM, Forman MR, Mangels AR, Beecher GR, Lanza E. 1993. The development and application of a carotenoid database for fruits, vegetables, and selected multicomponent foods. J Am Diet Assoc 93:318–323. [PubMed: 8440830]
- Cohen BE, Elin RJ. 1974. Vitamin A-induced nonspecific resistance to infection. J Infect Dis 129:597–600. [PubMed: 4207311]
- Cohlan SQ. 1953. Excessive intake of vitamin A as a cause of congenital anomalies in the rat. Science 117:535–536. [PubMed: 13056604]
- Cohlan SQ. 1954. Congenital anomalies in the rat produced by excessive intake of vitamin A during pregnancy. Pediatrics 13:556–567. [PubMed: 13166454]
- Congdon N, Sommer A, Severns M, Humphrey J, Friedman D, Clement L, Wu LS, Natadisastra G. 1995. Pupillary and visual thresholds in young children as an index of population vitamin A status. Am J Clin Nutr 61:1076–1082. [PubMed: 7733032]
- Cooper AD. 1997. Hepatic uptake of chylomicron remnants. J Lipid Res 38:2173–2192. [PubMed: 9392416]
- Coutsoudis A, Broughton M, Coovadia HM. 1991. Vitamin A supplementation reduces measles morbidity in young African children: A randomized, placebo-controlled, double-blind trial. Am J Clin Nutr 54:890–895. [PubMed: 1951162]
- Coutsoudis A, Kiepiela P, Coovadia HM, Broughton M. 1992. Vitamin A supplementation enhances specific IgG antibody levels and total lymphocyte numbers while improving morbidity in measles. Pediatr Infect Dis J 11:203–209. [PubMed: 1565535]
- Czeizel AE, Rockenbauer M. 1998. Prevention of congenital abnormalities by vitamin A. Int J Vitam Nutr Res 68:219–231. [PubMed: 9706496]
- Dawson HD, Ross AC. 1999. Chronic marginal vitamin A status effects the distribution and function of T cells and natural T cells in aging Lewis rats. J Nutr 129:1782–1790. [PubMed: 10498748]
- Dawson HD, Li NQ, DeCicco KL, Nibert JA, Ross AC. 1999. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J Nutr 129:1510–1517. [PubMed: 10419983]
- de Francisco A, Chakraborty J, Chowdhury HR, Yunus M, Baqui AH, Siddique AK, Sack RB. 1993. Acute toxicty of vitamin A given with vaccines in infancy. Lancet 342:526–527. [PubMed: 8102669]
- de Pee S, West CE, Muhilal, Karyadi D, Hautvast JG. 1995. Lack of improvement in vitamin A status with increased consumption of dark-green leafy vegetables. Lancet 346:75–81. [PubMed: 7603216]
- de Pee S, West CE, Permaesih D, Martuti S, Muhilal, Hautvast JG. 1998. Orange fruit is more effective than dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr 68:1058–1067. [PubMed: 9808223]
- Deuel HJ, Greenberg SM, Straub E, Fukui T, Chatterjee A, Zechmeister L. 1949. Stereochemical configuration and provitamin A activity. VII. Neocryptoxanthin U. Arch Biochem 23:239–240. [PubMed: 18147461]
- Devadas R, Premakumari S, Subramanian G. 1978. Biological availability of β-carotene from fresh and dried green leafy vegetables on preschool children. Ind J Nutr Dietet 15:335–340.
- Dew SE, Ong DE. 1994. Specificity of the retinol transporter of the rat small intestine brush border. Biochemistry 33:12340–12345. [PubMed: 7918456]
- Dickman ED, Smith SM. 1996. Selective regulation of cardiomyocyte gene expression and cardiac morphogenesis by retinoic acid. Dev Dyn 206:39–48. [PubMed: 9019245]
- Donnen P, Dramaix M, Brasseur D, Bitwe R, Vertongen F, Hennart P. 1998. Randomized placebo-controlled clinical trial of the effect of a single high dose or daily low doses of vitamin A on the morbidity of hospitalized, malnourished children. Am J Clin Nutr 68:1254–1260. [PubMed: 9846855]
- Dorea JG, Olson JA. 1986. The rate of rhodopsin regeneration in the bleached eyes of zinc-deficient rats in the dark. J Nutr 116:121–127. [PubMed: 2935599]
- Dorea JG, Souza JA, Galvao MO, Iunes MA. 1984. Concentration of vitamin A in the liver of foetuses and infants dying of various causes in Brasilia, Brazil. Int J Vitam Nutr Res 54:119–123. [PubMed: 6500835]
- Dowling JE, Gibbons IR. 1961. In: Smelser GK, ed, editor. . The Structure of the Eye . New York: Academic Press.
- Dudas I, Czeizel AE. 1992. Use of 6,000 IU vitamin A during early pregnancy without teratogenic effect. Teratology 45:335–336. [PubMed: 1585264]
- Duester G. 1996. Involvement of alcohol dehydrogenase, short-chain dehydrogenase/reductase, aldehyde dehydrogenase, and cytochrome P450 in the control of retinoid signaling by activation of retinoic acid synthesis. Biochemistry 35:12221–12227. [PubMed: 8823154]
- Duncan JR, Hurley LS. 1978. An interaction between zinc and vitamin A in pregnant and fetal rats. J Nutr 108:1431–1438. [PubMed: 682047]
- Eckhoff C, Nau H. 1990. Vitamin A supplementation increases levels of retinoic acid compounds in human plasma: Possible implications for teratogenesis. Arch Toxicol 64:502–503. [PubMed: 2275606]
- Eckhoff C, Bailey JR, Collins MD, Slikker W Jr, Nau H. 1991. Influence of dose and pharmaceutical formulation of vitamin A on plasma levels of retinyl esters and retinol and metabolic generation of retinoic acid compounds and beta-glucuronides in the cynomolgus monkey. Toxicol Appl Pharmacol 111:116–127. [PubMed: 1949028]
- Ellis JK, Russell RM, Makrauer FL, Schaefer EJ. 1986. Increased risk for vitamin A toxicity in severe hypertriglyceridemia. Ann Intern Med 105:877–879. [PubMed: 3777711]
- Farrell GC, Bhathal PS, Powell LW. 1977. Abnormal liver function in chronic hypervitaminosis A. Am J Dig Dis 22:724–728. [PubMed: 879140]
- Farris WA, Erdman JW Jr. 1982. Protracted hypervitaminosis A following long-term, low-level intake. J Am Med Assoc 247:1317. [PubMed: 6460882]
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. 1993. Vitamin A supplementation and child mortality. A meta-analysis. J Am Med Assoc 269:898–903. [PubMed: 8426449]
- Figueira F, Mendonca S, Rocha J, Azevedo M, Bunce GE, Reynolds JW. 1969. Absorption of vitamin A by infants receiving fat-free or fat-containing dried skim milk formulas. Am J Clin Nutr 22:588–593. [PubMed: 5819115]
- Filteau SM, Morris SS, Raynes JG, Arthur P, Ross DA, Kirkwood BR, Tomkins AM, Gyapong JO. 1995. Vitamin A supplementation, morbidity, and serum acute-phase proteins in young Ghanaian children. Am J Clin Nutr 62:434–438. [PubMed: 7542831]
- Flores H. 1993. Frequency distributions of serum vitamin A levels in cross-sectional surveys and in surveys before and after vitamin A supplementation. In: A Brief Guide to Current Methods of Assessing Vitamin A Status . A report of the International Vitamin A Consultative Group (IVACG). Washington, DC: The Nutrition Foundation. Pp.9–11.
- Flores H, de Araujo RC. 1984. Liver levels of retinol in unselected necropsy specimens: A prevalence survey of vitamin A deficiency in Recife, Brazil. Am J Clin Nutr 40:146–152. [PubMed: 6741847]
- Freudenheim JL, Johnson NE, Smith EL. 1986. Relationships between usual nutrient intake and bone-mineral content of women 35–65 years of age: Longitudinal and cross-sectional analysis. Am J Clin Nutr 44:863–876. [PubMed: 3491533]
- Friedman A, Sklan D. 1989. Impaired T lymphocyte immune response in vitamin A depleted rats and chicks. Br J Nutr 62:439–449. [PubMed: 2479411]
- Furr HC, Amedee-Manesme O, Clifford AJ, Bergen HR, Jones AD, Anderson LD, Olson JA. 1989. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr 49:713–716. [PubMed: 2648799]
- Geelen JA. 1979. Hypervitaminosis A induced teratogenesis. CRC Crit Rev Toxicol 6:351–375. [PubMed: 389569]
- Geubel AP, De Galocsy C, Alves N, Rahier J, Dive C. 1991. Liver damage caused by therapeutic vitamin A administration: Estimate of dose-related toxicity in 41 cases. Gastroenterology 100:1701–1709. [PubMed: 2019375]
- Ghana VAST Study Team. 1993. Vitamin A supplementation in northern Ghana: Effects on clinic attendances, hospital admissions, and child mortality. Lancet 342:7–12. [PubMed: 8100345]
- Glasziou PP, Mackerras DE. 1993. Vitamin A supplementation and infectious disease: A meta-analysis. Br Med J 306:366–370. [PMC free article: PMC1676417] [PubMed: 8461682]
- Golner BB, Reinhold RB, Jacob RA, Sadowski JA, Russell RM. 1987. The short and long term effect of gastric partitioning surgery on serum protein levels. J Am Coll Nutr 6:279–285. [PubMed: 3598025]
- Goodman DS, Blaner WS. 1984. Biosynthesis, absorption, and hepatic metabolism of retinol. In: Sporn MB, editor; , Roberts AB, editor; , Goodman DS, editor. , eds. The Retinoids , Vol. 2. Orlando: Academic Press. Pp.1–39.
- Goodman DS, Huang HS, Shiratori T. 1965. Tissue distribution and metabolism of newly absorbed vitamin A in the rat. J Lipid Res 6:390–396. [PubMed: 14336210]
- Goodman DS, Blomstrand R, Werner B, Huang HS, Shiratori T. 1966. The intestinal absorption and metabolism of vitamin A and β-carotene in man. J Clin Invest 45:1615–1623. [PMC free article: PMC292843] [PubMed: 5925518]
- Gudas LJ, Sporn MB, Roberts AB. 1994. Cellular biology and biochemistry of the retinoids. In: Sporn MB, editor; , Roberts AB, editor; , Goodman DS, editor. , eds. The Retinoids: Biology, Chemistry, and Medicine , 2nd ed. New York: Raven Press. Pp.443–520.
- Hallfrisch J, Muller DC, Singh VN. 1994. Vitamin A and E intakes and plasma concentrations of retinol, beta-carotene, and alpha-tocopherol in men and women of the Baltimore Longitudinal Study of Aging. Am J Clin Nutr 60:176–182. [PubMed: 8030594]
- Harrison EH. 1993. Enzymes catalyzing the hydrolysis of retinyl esters. Biochim Biophys Acta 1170:99–108. [PubMed: 8399348]
- Haskell MJ, Handelman GJ, Peerson JM, Jones AD, Rabbi MA, Awal MA, Wahed MA, Mahalanabis D, Brown KH. 1997. Assessment of vitamin A status by the deuterated-retinol-dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. Am J Clin Nutr 66:67–74. [PubMed: 9209171]
- Hatchell DL, Sommer A. 1984. Detection of ocular surface abnormalities in experimental vitamin A deficiency. Arch Ophthalmol 102:1389–1393. [PubMed: 6477255]
- Hathcock JN, Hattan DG, Jenkins MY, McDonald JT, Sundaresan PR, Wilkening VL. 1990. Evaluation of vitamin A toxicity. Am J Clin Nutr 52:183–202. [PubMed: 2197848]
- Hatoff DE, Gertler SL, Miyai K, Parker BA, Weiss JB. 1982. Hypervitaminosis A unmasked by acute viral hepatitis. Gastroenterology 82:124–128. [PubMed: 7198070]
- Hendriks HF, Verhoofstad WA, Brouwer A, de Leeuw AM, Knook DL. 1985. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res 160:138–149. [PubMed: 4043241]
- Hicks RJ. 1867. Night-blindness in the Confederate Army. Richmond Med J 3:34–38.
- Hicks VA, Gunning DB, Olson JA. 1984. Metabolism, plasma transport, and biliary excretion of radioactive vitamin A and its metabolites as a function of liver reserves of vitamin A in the rat. J Nutr 114:1327–1333. [PubMed: 6737092]
- Hofmann C, Eichele G. 1994. Retionoids in development. In: Sporn MB, editor; , Roberts AB, editor; , Goodman DS, editor. , eds. The Retinoids: Biology, Chemistry, and Medicine , 2nd ed. New York: Raven Press. Pp.387–441.
- Hollander D, Muralidhara KS. 1977. Vitamin A1 intestinal absorption in vivo: Influence of luminal factors on transport. Am J Physiol 232:E471–E477. [PubMed: 16498]
- Hoppner K, Phillips WE, Murray TK, Campbell JS. 1968. Survey of liver vitamin A stores of Canadians. Can Med Assoc J 99:983–986. [PMC free article: PMC1945441] [PubMed: 5687993]
- Hoppner K, Phillips WE, Erdody P, Murray TK, Perrin DE. 1969. Vitamin A reserves of Canadians. Can Med Assoc J 101:84–86. [PMC free article: PMC1946442] [PubMed: 5364272]
- Houtkooper LB, Ritenbaugh C, Aickin M, Lohman TG, Going SB, Weber JL, Greaves KA, Boyden TW, Pamenter RW, Hall MC. 1995. Nutrients, body composition and exercise are related to change in bone mineral density in premenopausal women. J Nutr 125:1229–1237. [PubMed: 7738683]
- Hume EM, Krebs HA. 1949. Vitamin A Requirement of Human Adults. An Experimental Study of Vitamin A Deprivation in Man . Medical Research Council Special Report Series No. 264. London: His Majesty's Stationery Office.
- Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, Hidayat S, Tielsch J, West KP Jr, Sommer A. 1996. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr 128:489–496. [PubMed: 8618182]
- Huque T. 1982. A survey of human liver reserves of retinol in London. Br J Nutr 47:165–172. [PubMed: 7066284]
- Hussey GD, Klein M. 1990. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med 323:160–164. [PubMed: 2194128]
- Hutchings DE, Gaston J. 1974. The effects of vitamin A excess administered during the mid-fetal period on learning and development in rat offspring. Dev Psychobiol 7:225–233. [PubMed: 4838170]
- Hutchings DE, Gibbon J, Kaufman MA. 1973. Maternal vitamin A excess during the early fetal period: Effects on learning and development in the offspring. Dev Psychobiol 6:445–457. [PubMed: 4796243]
- IOM (Institute of Medicine). 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids . Washington, DC: National Academy Press. [PubMed: 25077263]
- Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP. 1998. Serum retinol concentrations in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment. Am J Clin Nutr 68:623–629. [PubMed: 9734739]
- Jang JT, Green JB, Beard JL, Green MH. 2000. Kinetic analysis shows that iron deficiency decreases liver vitamin A mobilization in rats. J Nutr 130:1291–1296. [PubMed: 10801932]
- Jayarajan P, Reddy V, Mohanram M. 1980. Effect of dietary fat on absorption of β-carotene from green leafy vegetables in children. Indian J Med Res 71:53–56. [PubMed: 7380511]
- Jensen SK, Nielsen KN. 1996. Tocopherols, retinol, beta-carotene and fatty acids in fat globule membrane and fat globule core in cows' milk. J Dairy Sci 63:565–574. [PubMed: 8933307]
- Johnson EJ, Qin J, Krinsky NI, Russell RM. 1997. Ingestion by men of a combined dose of β-carotene and lycopene does not affect the absorption of β-carotene but improves that of lycopene. J Nutr 127:1833–1837. [PubMed: 9278568]
- Kalter H, Warkany J. 1961. Experimental production of congenital malformations in strains of inbred mice by maternal treatment with hypervitaminosis A. Am J Pathol 38:1–14. [PMC free article: PMC1942316] [PubMed: 13750997]
- Katz DR, Drzymala M, Turton JA, Hicks RM, Hunt R, Palmer L, Malkovsky M. 1987. Regulation of accessory cell function by retinoids in murine immune responses. Br J Exp Pathol 68:343–350. [PMC free article: PMC2013255] [PubMed: 3620329]
- Katz J, West KP Jr, Khatry SK, Thapa MD, LeClerq SC, Pradhan EK, Pokhrel RP, Sommer A. 1995. Impact of vitamin A supplementation on prevalence and incidence of xerophthalmia in Nepal. Invest Ophthalmol Vis Sci 36:2577–2583. [PubMed: 7499080]
- Keenum D. 1993. Conjunctival impression cytology. In: A Brief Guide to Current Methods of Assessing Vitamin A Status . A report of the International Vitamin A Consultative Group (IVACG). Washington, DC: The Nutrition Foundation. Pp.19–21.
- Keenum D, Semba RD, Wirasasmita S, Natadisastra G, Muhilal, West KP Jr, Sommer A. 1990. Assessment of vitamin A status by a disk applicator for conjunctival impression cytology. Arch Ophthalmol 108:1436–1441. [PubMed: 2222278]
- Keilson B, Underwood BA, Loerch JD. 1979. Effects of retinoic acid on the mobilization of vitamin A from the liver in rats. J Nutr 109:787–795. [PubMed: 571461]
- Khoury MJ, Moore CA, Mulinare J. 1996. Vitamin A and birth defects. Lancet 347:322. [PubMed: 8569374]
- Kostic D, White WS, Olson JA. 1995. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr 62:604–610. [PubMed: 7661123]
- Kowalski TE, Falestiny M, Furth E, Malet PF. 1994. Vitamin A hepatotoxicity: A cautionary note regarding 25,000 IU supplements. Am J Med 97:523–528. [PubMed: 7985711]
- Krasinski SD, Russell RM, Otradovec CL, Sadowski JA, Hartz SC, Jacob RA, McGandy RB. 1989. Relationship of vitamin A and vitamin E intake to fasting plasma retinol, retinol-binding protein, retinyl ester, carotene, alpha-tocopherol, and cholesterol among elderly people and young adults: Increased plasma retinyl esters among vitamin A-supplement users. Am J Clin Nutr 49:112–120. [PubMed: 2911996]
- Krinsky NI, Wang X-D, Tang G, Russell RM. 1993. Mechanism of carotenoid cleavage to retinoids. Ann NY Acad Sci 691:167–176. [PubMed: 8129286]
- Kusin JA, Reddy V, Sivakumar B. 1974. Vitamin E supplements and the absorption of a massive dose of vitamin A. Am J Clin Nutr 27:774–776. [PubMed: 4846404]
- Lachance PA. 1997. Nutrient addition to foods: The public health impact in countries with rapidly westernizing diets. In: Bendich A, editor; , Deckelbaum RJ, editor. , eds. Preventive Nutrition: The Comprehensive Guide for Health Professionals. Totowa, NJ: Humana Press. Pp.441–454.
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, Curry CJ, Fernhoff PM, Grix AW Jr, Lott IT, Richard JM, Sun SC. 1985. Retinoic acid embryopathy. N Engl J Med 313:837–841. [PubMed: 3162101]
- Large S, Neal G, Glover J, Thanangkul O, Olson RE. 1980. The early changes in retinol-binding protein and prealbumin concentrations in plasma of protein-energy malnourished children after treatment with retinol and an improved diet. Br J Nutr 43:393–402. [PubMed: 6774741]
- Leo MA, Lieber CS. 1982. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med 307:597–601. [PubMed: 7202119]
- Leo MA, Lieber CS. 1985. New pathway for retinol metabolism in liver microsomes. J Biol Chem 260:5228–5231. [PubMed: 3988751]
- Leo MA, Lieber CS. 1999. Alcohol, vitamin A, and beta-carotene: Adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr 69:1071–1085. [PubMed: 10357725]
- Lewis JM, Bodansky O, Falk KG, McGuire G. 1942. Vitamin A requirements in the rat. The relation of vitamin A intake to growth and to concentration of vitamin A in the blood plasma, liver and retina. J Nutr 23:351–363.
- Lieber CS, Leo MA. 1986. Interaction of alcohol and nutritional factors with hepatic fibrosis. Prog Liver Dis 8:253–272. [PubMed: 3520661]
- Loerch JD, Underwood BA, Lewis KC. 1979. Response of plasma levels of vitamin A to a dose of vitamin A as an indicator of hepatic vitamin A reserves in rat. J Nutr 109:778–786. [PubMed: 438896]
- Looker AC, Johnson CL, Woteki CE, Yetley EA, Underwood BA. 1988. Ethnic and racial differences in serum vitamin A levels of children aged 4–11 years. Am J Clin Nutr 47:247–252. [PubMed: 3341255]
- Loyd-Puryear MA, Mahoney J, Humphrey JH, Mahoney F, Siren N, Moorman C, West KP Jr. 1991. Vitamin A deficiency in Micronesia: A statewide survey in Chuuk. Nutr Res 11:1101–1110.
- Mahalanabis D, Simpson TW, Chakraborty ML, Ganguli C, Bhattacharjee AK, Mukherjee KL. 1979. Malabsorption of water miscible vitamin A in children with giardiasis and ascariasis. Am J Clin Nutr 32:313–318. [PubMed: 420128]
- Mahoney CP, Margolis MT, Knauss TA, Labbe RF. 1980. Chronic vitamin A intoxication in infants fed chicken liver. Pediatrics 65:893–897. [PubMed: 7189278]
- Martinez-Frias ML, Salvador J. 1990. Epidemiological aspects of prenatal exposure to high doses of vitamin A in Spain. Eur J Epidemiol 6:118–123. [PubMed: 2361535]
- Mastroiacovo P, Mazzone T, Addis A, Elephant E, Carlier P, Vial T, Garbis H, Robert E, Bonati M, Ornoy A, Finardi A, Schaffer C, Caramelli L, Rodriguez-Pinilla E, Clementi M. 1999. High vitamin A intake in early pregnancy and major malformations: A multicenter prospective controlled study. Teratology 59:7–11. [PubMed: 9988877]
- Maxwell JD, Murray D, Ferguson A, Calder E. 1968. Ascaris lumbricoides infectation associated with jejunal mucosal abnormalities. Scott Med J 13:280–281. [PubMed: 5676286]
- McCaffery P, Drager UC. 1995. Retinoic acid synthesizing enzymes in the embryonic and adult vertebrate. In: Weiner H, editor; , Holmes RS, editor; , Wermuth B, editor. , eds. Enzymology and Molecular Biology of Carbonyl Metabolism 5 . New York: Plenum Press. Pp.173–183.
- Melhus H, Michaelsson K, Kindmark A, Bergstrom R, Holmberg L, Mallmin H, Wolk A, Ljunghall S. 1998. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med 129:770–778. [PubMed: 9841582]
- Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, Beecher GR, Smith JC. 1992. Plasma carotenoid response to chronic intake of selected foods and β-carotene supplements in men. Am J Clin Nutr 55:1120–1125. [PubMed: 1595584]
- Mills JL, Simpson JL, Cunningham GC, Conley MR, Rhoads GG. 1997. Vitamin A and birth defects. Am J Obstet Gynecol 177:31–36. [PubMed: 9240579]
- Minuk GY, Kelly JK, Hwang WS. 1988. Vitamin A hepatotoxicity in multiple family members. Hepatology 8:272–275. [PubMed: 3356407]
- Mitchell GV, Young M, Seward CR. 1973. Vitamin A and carotene levels of a selected population in metropolitan Washington, D.C. Am J Clin Nutr 26:992–997. [PubMed: 4727754]
- Mobarhan S, Russell RM, Underwood BA, Wallingford J, Mathieson RD, Al-Midani H. 1981. Evaluation of the relative dose response test for vitamin A nutriture in cirrhotics. Am J Clin Nutr 34:2264–2270. [PubMed: 7293954]
- Mobarhan S, Seitz HK, Russell RM, Mehta R, Hupert J, Friedman H, Layden TJ, Meydani M, Langenberg P. 1991. Age-related effects of chronic ethanol intake on vitamin A status in Fisher 344 rats. J Nutr 121:510–517. [PubMed: 2007903]
- Money DF. 1978. Vitamin E, selenium, iron, and vitamin A content of livers from Sudden Infant Death Syndrome cases and control children: Interrelationships and possible significance. NZ J Sci 21:41–45.
- Montreewasuwat N, Olson JA. 1979. Serum and liver concentrations of vitamin A in Thai fetuses as a function of gestational age. Am J Clin Nutr 32:601–606. [PubMed: 420152]
- Morrison SA, Russell RM, Carney EA, Oaks EV. 1978. Zinc deficiency: A cause of abnormal dark adaptation in cirrhotics. Am J Clin Nutr 31:276–281. [PubMed: 623051]
- Morriss-Kay GM, Sokolova N. 1996. Embryonic development and pattern formation. FASEB J 10:961–968. [PubMed: 8801178]
- Moss AJ, Levy AS, Kim I, Park YK. 1989. Use of Vitamin and Mineral Supplements in the United States: Current Users, Types of Products, and Nutrients . Advance Data, Vital and Health Statistics of the National Center for Health Statistics, Number 174. Hyattsville, MD: National Center for Health Statistics.
- Muhilal, Permeisih D, Idjradinata YR, Muherdiyantiningsih, Karyadi D. 1988. Vitamin A-fortified monosodium glutamate and health, growth, and survival of children: A controlled field trial. Am J Clin Nutr 48:1271–1276. [PubMed: 3189216]
- Munoz EC, Rosado JL, Lopez P, Furr HC, Allen LH. 2000. Iron and zinc supplementation improves indicators of vitamin A status of Mexican preschoolers. Am J Clin Nutr 71:789–794. [PubMed: 10702174]
- Napoli JL, Race KR. 1988. Biogenesis of retinoic acid from β-carotene: Differences between the metabolism of β-carotene and retinal. J Biol Chem 263:17372–17377. [PubMed: 3182850]
- Napoli JL, Boerman MH, Chai X, Zhai Y, Fiorella PD. 1995. Enzymes and binding proteins affecting retinoic acid concentrations. J Steroid Biochem Mol Biol 53:497–502. [PubMed: 7626500]
- Natadisastra G, Wittpenn JR, West KP Jr, Muhilal, Sommer A. 1987. Impression cytology for detection of vitamin A deficiency. Arch Ophthalmol 105:1224–1228. [PubMed: 3307720]
- Nauss KM, Newberne PM. 1985. Local and regional immune function of vitamin A-deficient rats with ocular herpes simplex virus (HSV) infections. J Nutr 115:1316–1324. [PubMed: 3876416]
- Naz JF, Edwards WM. 1952. Hypervitaminosis A: A case report. N Engl J Med 246:87–89. [PubMed: 14890815]
- Nierenberg DW, Dain BJ, Mott LA, Baron JA, Greenberg ER. 1997. Effects of 4 y of oral supplementation with beta-carotene on serum concentrations of retinol, tocopherol, and five carotenoids. Am J Clin Nutr 66:315–319. [PubMed: 9250109]
- Novotny JA, Dueker SR, Zech LA, Clifford AJ. 1995. Compartmental analysis of the dynamics of β-carotene metabolism in an adult volunteer. J Lipid Res 36:1825–1838. [PubMed: 7595103]
- NRC (National Research Council). 1980. Recommended Dietary Allowances , 9th ed. Washington, DC: National Academy Press.
- NRC. 1986. Nutrient Adequacy: Assessment Using Food Consumption Surveys . Washington, DC: National Academy Press. [PubMed: 25032431]
- NRC. 1989. Recommended Dietary Allowances , 10th ed. Washington, DC: National Academy Press.
- Nusser SM, Carriquiry AL, Dodd KW, Fuller WA. 1986. A semiparametric transformation approach to estimating usual daily intake distributions. J Am Stat Assoc 91:1440–1449.
- Olson JA. 1972. The prevention of childhood blindness by the administration of massive doses of vitamin A. Isr J Med Sci 8:1199–1206. [PubMed: 4647769]
- Olson JA. 1979. Liver vitamin A reserves of neonates, preschool children and adults dying of various causes in Salvador, Brazil. Arch Latinoam Nutr 29:521–545. [PubMed: 575876]
- Olson JA. 1982. New approaches to methods for the assessment of nutritional status of the individual. Am J Clin Nutr 35:1166–1168. [PubMed: 7044095]
- Olson JA. 1983. Adverse effects of large doses of vitamin A and retinoids. Semin Oncol 10:290–293. [PubMed: 6364354]
- Olson JA. 1987. Recommended dietary intakes (RDI) of vitamin A in humans. Am J Clin Nutr 45:704–716. [PubMed: 3565297]
- Olson JA. 1991. Vitamin A. In: Machlin LJ, editor. , ed. Handbook of Vitamins , 2nd ed. New York: Marcel Dekker. Pp.1–57.
- Olson JA, Hayaishi O. 1965. The enzymatic cleavage of β-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Nat Acad Sci USA 54:1364–1370. [PMC free article: PMC219905] [PubMed: 4956142]
- Olson JA, Gunning D, Tilton R. 1979. The distribution of vitamin A in human liver. Am J Clin Nutr 32:2500–2507. [PubMed: 506974]
- Oren R, Ilan Y. 1992. Reversible hepatic injury induced by long-term vitamin A ingestion. Am J Med 93:703–704. [PubMed: 1361304]
- Panfili G, Manzi P, Pizzoferrato L. 1998. Influence of thermal and other manufacturing stresses on retinol isomerization in milk and dairy products. J Dairy Res 65:253–260. [PubMed: 9627844]
- Papiz MZ, Sawyer L, Eliopoulos EE, North AC, Findlay JB, Sivaprasadarao R, Jones TA, Newcomer ME, Kraulis PJ. 1986. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. Nature 324:383–385. [PubMed: 3785406]
- Parker RS, Swanson JE, You CS, Edwards AJ, Huang T. 1999. Bioavailability of carotenoids in human subjects. Proc Nutr Soc 58:155–162. [PubMed: 10343353]
- Pasatiempo AM, Kinoshita M, Taylor CE, Ross AC. 1990. Antibody production in vitamin A-depleted rats is impaired after immunization with bacterial polysaccharide or protein antigens. FASEB J 4:2518–2527. [PubMed: 2110538]
- Patton S, Kelly JJ, Keenan TW. 1980. Carotene in bovine milk fat globules: Observations on origin and high content in tissue mitochondria. Lipids 15:33–38. [PubMed: 7360008]
- Persson B, Tunell R, Ekengren K. 1965. Chronic vitamin A intoxication during the first half year of life. Acta Paediatr Scand 54:49–60. [PubMed: 14248225]
- Persson B, Krook M, Jornvall H. 1995. Short-chain dehydrogenases/reductases. In: Weiner H, editor; , Holmes RS, editor; , Wermuth B, editor. , eds. Enzymology and Molecular Biology of Carbonyl Metabolism 5 . New York: Plenum Press. Pp.383–395.
- Pilch SM. 1987. Analysis of vitamin A data from the health and nutrition examination surveys. J Nutr 117:636–640. [PubMed: 3585513]
- Pinnock CB, Alderman CP. 1992. The potential for teratogenicity of vitamin A and its congeners. Med J Aust 157:804–809. [PubMed: 1454015]
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC, Ramaswamy K, Rahmathullah R, Babu G. 1990. Reduced mortality among children in southern India receiving a small weekly dose of vitamin A. N Engl J Med 323:929–935. [PubMed: 2205798]
- Raica N Jr, Scott J, Lowry L, Sauberlich HE. 1972. Vitamin A concentration in human tissues collected from five areas in the United States. Am J Clin Nutr 25:291–296. [PubMed: 5011912]
- Reddy V. 1985. Vitamin A Requirements of Preschool Children . Joint FAO/WHO Expert Group on Requirement for Vitamin A, Iron, Folate and Vitamin B12, Doc. No. 6. Geneva: World Health Organization.
- Reddy V, Srikantia SG. 1966. Serum vitamin A in kwashiorkor. Am J Clin Nutr 18:105–109. [PubMed: 4951495]
- Rock CL, Lovalvo JL, Emenhiser C, Ruffin MT, Flatt SW, Schwartz SJ. 1998. Bioavailability of β-carotene is lower in raw than in processed carrots and spinach in women. J Nutr 128:913–916. [PubMed: 9567003]
- Roels OA, Trout M, Dujacquier R. 1958. Carotene balances on boys in Ruanda where vitamin A deficiency is prevalent. J Nutr 65:115–127. [PubMed: 13550006]
- Roels OA, Djaeni S, Trout ME, Lauw TG, Heath A, Poey SH, Tarwotjo MS, Suhadi B. 1963. The effect of protein and fat supplements on vitamin A deficient children. Am J Clin Nutr 12:380–387. [PubMed: 13982350]
- Rohde CM, Manatt M, Clagett-Dame M, DeLuca HF. 1999. Vitamin A antagonizes the action of vitamin D in rats. J Nutr 129:2246–2250. [PubMed: 10573558]
- Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. 1996. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res 37:962–971. [PubMed: 8725149]
- Rosales FJ, Jang JT, Pinero DJ, Erikson KM, Beard JL, Ross AC. 1999. Iron deficiency in young rats alters the distribution of vitamin A between plasma and liver and between hepatic retinol and retinyl esters. J Nutr 129:1223–1228. [PubMed: 10356091]
- Ross AC. 1996. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin Immunol Immunopathol 80:S63–S72. [PubMed: 8811065]
- Ross AC. 1999. Vitamin A and retinoids. In: Shils ME, editor; , Olson JA, editor; , Shike M, editor; , Ross AC, editor. , eds. Modern Nutrition in Health and Disease , 9th ed. Baltimore, MD: Williams & Wilkins. Pp.305–328.
- Rothman KJ, Moore LL, Singer MR, Nguygen UDT, Mannino S, Milunsky B. 1995. Teratogenicity of high vitamin A intake. N Engl J Med 333:1369–1373. [PubMed: 7477116]
- Saari JC. 1994. Retinoids in photosensitive systems. In: Sporn MB, editor; , Roberts AB, editor; , Goodman DS, editor. , eds. The Retinoids: Biology, Chemistry, and Medicine , 2nd ed. New York: Raven Press. Pp.351–385.
- Salazar-Lindo E, Salazar M, Alvarez JO. 1993. Association of diarrhea and low serum retinol in Peruvian children. Am J Clin Nutr 58:110–113. [PubMed: 8317381]
- Sanchez AM, Congdon NG, Sommer A, Rahmathullah L, Venkataswamy PG, Chandravathi PS, Clement L. 1997. Pupillary threshold as an index of population vitamin A status among children in India. Am J Clin Nutr 65:61–66. [PubMed: 8988914]
- Sato M, Lieber CS. 1981. Hepatic vitamin A depletion after chronic ethanol consumption in baboons and rats. J Nutr 111:2015–2023. [PubMed: 7197710]
- Sauberlich HE, Hodges HE, Wallace DL, Kolder H, Canham JE, Hood J, Raica N, Lowry LK. 1974. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm 32:251–275. [PubMed: 4617402]
- Schindler R, Friedrich DH, Kramer M, Wacker HH, Feldheim W. 1988. Size and composition of liver vitamin A reserves of human beings who died of various causes. Int J Vitam Nutr Res 58:146–154. [PubMed: 3170086]
- Semba RD, Muhilal, Scott AL, Natadisastra G, Wirasasmita S, Mele L, Ridwan E, West KP Jr, Sommer A. 1992. Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr 122:101–107. [PubMed: 1729457]
- Semba RD, Bulterys M, Munyeshuli V, Gatsinzi T, Saah A, Chao A, Dushimimana A. 1996. Vitamin A deficiency and T-cell subpopulations in children with meningococcal disease. J Trop Pediatr 42:287–290. [PubMed: 8936960]
- Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, Alpers MP, West KP Jr. 1999. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua, New Guinea: A randomised trial. Lancet 354:203–209. [PubMed: 10421302]
- Shaw GM, Wasserman CR, Block G, Lammer EJ. 1996. High maternal vitamin A intake and risk of anomalies of structures with a cranial neural crest cell contribution. Lancet 347:899–900. [PubMed: 8622412]
- Shingwekar AG, Mohanram M, Reddy V. 1979. Effect of zinc supplementation on plasma levels of vitamin A and retinol-binding protein in malnourished children. Clin Chim Acta 93:97–100. [PubMed: 108038]
- Siegel NJ, Spackman TJ. 1972. Chronic hypervitaminosis A with intracranial hypertension and low cerebrospinal fluid concentration of protein. Two illustrative cases. Clin Pediatr 11:580–584. [PubMed: 4263318]
- Sivakumar B, Reddy V. 1972. Absorption of labelled vitamin A in children during infection. Br J Nutr 27:299–304. [PubMed: 5015249]
- Sivakumar B, Reddy V. 1975. Absorption of vitamin A in children with ascariasis. J Trop Med Hyg 78:114–115. [PubMed: 1152102]
- Smith FR, Goodman DS. 1976. Vitamin A transport in human vitamin A toxicity. N Engl J Med 294:805–808. [PubMed: 943041]
- Smith JE, Brown ED, Smith JC Jr. 1974. The effect of zinc deficiency on the metabolism of retinol-binding protein in the rat. J Lab Clin Med 84:692–697. [PubMed: 4283791]
- Smith SM, Levy NL, Hayes CE. 1987. Impaired immunity in vitamin A-deficient mice. J Nutr 117:857–865. [PubMed: 3495650]
- Solomons NW, Morrow FD, Vasquez A, Bulux J, Guerrero AM, Russell RM. 1990. Test-retest reproducibility of the relative dose response for vitamin A status in Guatemalan adults: Issues of diagnostic sensitivity. J Nutr 120:738–744. [PubMed: 2366107]
- Sommer A. 1982. Nutritional Blindness. Xerophthalmia and Keratomalacia . New York: Oxford University Press.
- Sommer A, West KP Jr. 1996. Vitamin A Deficiency: Health, Survival, and Vision . New York: Oxford University Press.
- Sommer A, Tarwotjo I, Hussaini G, Susanto D. 1983. Increased mortality in children with mild vitamin A deficiency. Lancet 2:585–588. [PubMed: 6136744]
- Sommer A, Katz J, Tarwotjo I. 1984. Increased risk of respiratory disease and diarrhea in children with pre-existing mild vitamin A deficiency. Am J Clin Nutr 40:1090–1095. [PubMed: 6496388]
- Sommer A, Tarwotjo I, Djunaedi E, West KP Jr, Loeden AA, Tilden R, Mele L. 1986. Impact of vitamin A supplementation on childhood mortality: A randomized controlled community trial. Lancet 1:1169–1173. [PubMed: 2871418]
- Sporn MB, Roberts AB, Goodman DS. 1984. The Retinoids . Orlando: Academic Press.
- Staab DB, Hodges RE, Metcalf WK, Smith JL. 1984. Relationship between vitamin A and iron in the liver. J Nutr 114:840–844. [PubMed: 6726453]
- Stauber PM, Sherry B, VanderJagt DJ, Bhagavan HN, Garry PJ. 1991. A longitudinal study of the relationship between vitamin A supplementation and plasma retinol, retinyl esters, and liver enzyme activities in a healthy elderly population. Am J Clin Nutr 54:878–883. [PubMed: 1951160]
- Stephensen CB, Blount SR, Schoeb TR, Park JY. 1993. Vitamin A deficiency impairs some aspects of the host response to influenza A virus infection in BALB/ c mice. J Nutr 123:823–833. [PubMed: 8487093]
- Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI, Gammon RB. 1994. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr 60:388–392. [PubMed: 8074070]
- Stewart BE, Young RS. 1989. Pupillary response: An index of visual threshold. Appl Optics 28:1122–1127. [PubMed: 20548629]
- Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. 1993. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet 342:1325–1328. [PubMed: 7901636]
- Suthutvoravoot S, Olson JA. 1974. Plasma and liver concentration of vitamin A in a normal population of urban Thai. Am J Clin Nutr 27:883–891. [PubMed: 4846412]
- Takyi EE. 1999. Children's consumption of dark green, leafy vegetables with added fat enhances serum retinol. J Nutr 129:1549–1554. [PubMed: 10419989]
- Tang G, Qin J, Dolnikowski GG, Russell RM. 2000. Vitamin A equivalence of β-carotene in a woman as determined by a stable isotope reference method. Eur J Nutr 39:7–11. [PubMed: 10900552]
- Tanumihardjo SA. 1993. The modified relative dose-response assay. In: A Brief Guide to Current Methods of Assessing Vitamin A Status . A report of the International Vitamin A Consultative Group (IVACG). Washington, DC: The Nutrition Foundation. Pp.14–15.
- Tanumihardjo SA, Olson JA. 1991. The reproducibility of the modified relative dose response (MRDR) assay in healthy individuals over time and its comparison with conjunctival impression cytology (CIC). Eur J Clin Nutr 45:407–411. [PubMed: 1761001]
- Terhune MW, Sandstead HH. 1972. Decreased RNA polymerase activity in mammalian zinc deficiency. Science 177:68–69. [PubMed: 5041777]
- Thatcher AJ, Lee CM, Erdman JW Jr. 1998. Tissue stores of β-carotene are not conserved for later use as a source of vitamin A during compromised vitamin A status in Mongolian gerbils (Meriones unguiculatus). J Nutr 128:1179–1185. [PubMed: 9649603]
- Tomlinson JE, Hemken RW, Mitchell GE, Tucker RE. 1976. Mammary transfer of vitamin A alcohol and ester in lactating dairy cows. J Dairy Sci 59:607–613. [PubMed: 1262574]
- Torronen R, Lehmusaho M, Hakkinen S, Hanninen O, Mykkanen H. 1996. Serum β-carotene response to supplementation with raw carrots, carrot juice or purified β-carotene in healthy non-smoking women. Nutr Res 16:565–575.
- Trechsel U, Evequoz V, Fleisch H. 1985. Stimulation of interleukin 1 and 3 production by retinoic acid in vitro. Biochem J 230:339–344. [PMC free article: PMC1152623] [PubMed: 3931632]
- Underwood BA. 1984. Vitamin A in animal and human nutrition. In: Sporn MB, editor; , Roberts AB, editor; , Goodman DS, editor. , eds. The Retinoids , Vol. 1. New York: Academic Press. Pp.281–392.
- Underwood BA. 1994. Hypovitaminosis A: International programmatic issues. J Nutr 124:1467S–1472S. [PubMed: 8064405]
- Underwood BA, Siegel H, Weisell RC, Dolinski M. 1970. Liver stores of vitamin A in a normal population dying suddenly or rapidly from unnatural causes in New York City. Am J Clin Nutr 23:1037–1042. [PubMed: 5504960]
- Van den Berg H, van Vliet T. 1998. Effect of simultaneous, single oral doses of β-carotene with lutein or lycopene on the β-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr 68:82–89. [PubMed: 9665100]
- Van het Hof KH, Gartner C, West CE, Tijburg LB. 1998. Potential of vegetable processing to increase the delivery of carotenoids to man. Int J Vitam Nutr Res 68:366–370. [PubMed: 9857263]
- Van het Hof KH, Brouwer IA, West CE, Haddeman E, Steegers-Theunissen RP, van Dusseldorp M, Weststrate JA, Ekes TK, Hautvast JG. 1999. Bioavailability of lutein from vegetables is five times higher than that of β-carotene. Am J Clin Nutr 70:261–268. [PubMed: 10426704]
- Von Lennep E, El Khazen N, De Pierreux G, Amy JJ, Rodesch F, Van Regemorter N. 1985. A case of partial sirenomelia and possible vitamin A teratogenesis. Prenat Diagn 5:35–40. [PubMed: 3156316]
- Wagner KH. 1940. Die experimentelle avitaminose a bein menschen. Ztschf Physiol Chem 264:153–188.
- Wallingford JC, Underwood BA. 1987. Vitamin A status needed to maintain vitamin A concentrations in nonhepatic tissues of the pregnant rat. J Nutr 117:1410–1415. [PubMed: 3625312]
- Wang XD. 1999. Chronic alcohol intake interfers with retinoid metabolism and signaling. Nutr Rev 57:51–59. [PubMed: 10079703]
- Watkins M, Moore C, Mulinare J. 1996. Teratogenicity of high vitamin A intake. N Engl J Med 334:1196–1197. [PubMed: 8602193]
- Weber FL Jr, Mitchell GE Jr, Powell DE, Reiser BJ, Banwell JG. 1982. Reversible hepatotoxicity associated with hepatic vitamin A accumulation in a protein-deficient patient. Gastroenterology 82:118–123. [PubMed: 7198069]
- Wendling O, Chambon P, Mark M. 1999. Retinoid X receptors are essential for early mouse development and placentogenesis. Proc Natl Acad Sci USA 96:547–551. [PMC free article: PMC15173] [PubMed: 9892670]
- Werler MM, Lammer EJ, Mitchell AA. 1996. Teratogenicity of high vitamin A intake. N Engl J Med 334:1195–1196. [PubMed: 8602191]
- West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, Pradhan EK, Tielsch JM, Pandey MR, Sommer A. 1991. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 338:67–71. [PubMed: 1676467]
- West KP Jr, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, Conner PB, Dali SM, Christian P, Pokhrel RP, Sommer A. 1999. Double blind, cluster randomized trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. Br Med J 318:570–575. [PMC free article: PMC27760] [PubMed: 10037634]
- WHO (World Health Organization). 1950. Expert Committee on Biological Standardisation . Technical Report Series, No. 3. Geneva: WHO.
- WHO. 1966. WHO Expert Committee on Biological Standardization Eighteenth Report . Technical Report Series, No. 329. Geneva: WHO.
- WHO. 1982. Control of Vitamin A Deficiency and Xerophthalmia . Technical Report Series No. 672. Geneva: WHO. [PubMed: 6803444]
- WHO. 1995. Global Prevalence of Vitamin A Deficiency . Micronutrient Deficiency Information System Working Paper, No. 2. Geneva: WHO.
- WHO. 1997. Vitamin A Supplements: A Guide to Their Use in the Treatment of Vitamin A Deficiency and Xerophthalmia . Geneva: WHO.
- Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. 1993. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology 80:581–586. [PMC free article: PMC1422259] [PubMed: 8307607]
- Wilson JG, Roth CB, Warkany J. 1953. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat 92:189–217. [PubMed: 13030424]
- Wittpenn JR, Tseng SC, Sommer A. 1986. Detection of early xerophthalmia by impression cytology. Arch Ophthalmol 104:237–239. [PubMed: 3511895]
- Wolde-Gebriel Z, West CE, Gebru H, Tadesse AS, Fisseha T, Gabre P, Aboye C, Ayana G, Hautvast JG. 1993. Interrelationship between vitamin A, iodine and iron status in schoolchildren in Shoa Region, central Ethiopia. Br J Nutr 70:593–607. [PubMed: 8260484]
- Woodard WK, Miller LJ, Legant O. 1961. Acute and chronic hypervitaminosis in a 4-month-old infant. J Pediatr 59:260–264. [PubMed: 13786524]
- Zafrani ES, Bernuau D, Feldmann G. 1984. Peliosis-like ultrastructural changes of the hepatic sinusoids in human chronic hypervitaminosis A: Report of three cases. Hum Pathol 15:1166–1170. [PubMed: 6500549]
- Zahar M, Smith DE, Martin F. 1995. Vitamin A distribution among fat globule core, fat globule membrane, and serum fraction in milk. J Dairy Sci 78:498–505. [PubMed: 7782507]
- Zhao Z, Ross AC. 1995. Retinoic acid repletion restores the number of leukocytes and their subsets and stimulates natural cytotoxicity in vitamin A-deficient rats. J Nutr 125:2064–2073. [PubMed: 7643240]
- Zhao Z, Murasko DM, Ross AC. 1994. The role of vitamin A in natural killer cell cytotoxicity, number and activation in the rat. Nat Immun 13:29–41. [PubMed: 8111191]
- Vitamin A - Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, ...Vitamin A - Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc
Your browsing activity is empty.
Activity recording is turned off.
See more...