NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
Obesity is associated with a wide range of public health and economic problems throughout diverse demographic groups. As there is a high incidence of insulin resistance and type-2 diabetes among the obese, a mechanistic understanding of the relationship between insulin resistance and caloric consumption / obesity is needed. In cases of obesity and insulin resistance, triacylglycerides (TAG) accumulate within skeletal muscle cell; however, the mechanism of this relationship is not well understood. The research program described herein therefore sought small molecule probes that inhibit TAG accumulation. Toward this end, screening of 227,000 compounds resulted in the identification of MLS-0308942, which inhibited TAG accumulation with an IC50 of 1.7 μM. Subsequent medicinal chemistry follow up identified MLS-0472732 (ML377), which had similar potency (IC50 1.1 μM) with some improved ADME/T properties. Data were confirmed in the H9c2 high content assay (IC50 = 0.84 and 0.79 μM, respectively, with full response) and in a human primary cell assay. Furthermore, the compounds tested negatively for DGAT inhibition and cytotoxicity. Several additional analogs with comparable potency were observed, and the established SAR suggests the synthesis of new analogs in the future, which may address the poor microsomal stability exhibited thus far with the new series of compounds.
Assigned Assay Grant #: 1 R24 DK084969 (Fast Track - FT1012)
Screening Center Name & PI: Sanford-Burnham Medical Research Institute & Michael R. Jackson, Ph.D.
Chemistry Center Name & PI: Sanford-Burnham Medical Research Institute & Michael R. Jackson, Ph.D.
Assay Submitter & Institution: Daniel P. Kelly, Sanford-Burnham Center for Chemical Genomics at Sanford-Burnham Medical Research Institute, Orlando, Florida 32827, USA
PubChem Summary Bioassay Identifier (AID): 651587
Probe Structure & Characteristics
This Center Probe Report describes ML377, a selective inhibitor of triacylglyceride accumulation. The chemical structure and data summary are shown in (Table 1).
Table 1Potency and selectivity characteristics for probe ML377
| CID/ML# | Target Name | IC50 (nM) [SID, AID] | Anti-target Name(s) | LC50 (μM) [SID, AID] | Fold-Selective | Secondary Assay(s) Names IC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| CID 71677755/ML377 | TAG pathway | 1,100 ± 100 nM (n=4) (100% Emax) | H9c2 cytotox Nuclei cell count DAPI fluorescence | >50 μM (n=2) SID 163875672 AID 743160 | >45-fold | TAG pathway By orthogonal HCS |
| SID 163875672 AID 743161 | DGAT-1 Pathway By radiolabel | >50 μM (n=2) SID 163875672 AID 743168 | > 45-fold | 790 ± 10 nM (n=2) SID 163875672 AID 743170 |
1. Recommendations for Scientific Use of the Probe
Across nations, cultures, and demographic groups, we are witnessing a dramatic increase in the prevalence of obesity.1-4 This poses enormous public health problems and economic costs due to associated burdens of chronic disease and disability related largely to the high incidence of insulin resistance and, ultimately type 2 diabetes, among the obese. Rational therapeutic approaches to this pervasive medical problem require a comprehensive understanding of the molecular mechanisms that link insulin resistance to excess caloric consumption and obesity.
In the obese state, complex lipids, chiefly triacylglycerides (TAG), accumulate within cells of tissues that do not typically store fat, including skeletal muscle. An association between muscle lipid accumulation and insulin resistance is widely recognized.5,6 However, the development of effective therapeutics aimed at this linkage has been hampered because the mechanistic underpinnings of the association remain elusive, and experimental evidence to support a clear causal relationship is lacking. Indeed, there are circumstances where intramyocellular TAG accumulation is associated with improved muscle performance and metabolic flexibility. Taken together, these observations have led to dogma and confusion, defining a critical gap in scientific knowledge. We hypothesize that myocellular lipid accumulation can trigger both adaptive and maladaptive (lipotoxic) responses relevant to muscle insulin resistance, and that a dynamic balance between these responses determines the evolution of muscle insulin resistance and diabetes. We believe this problem demands an unbiased approach. To this end, we sought to identify molecular probes of cellular processes that control skeletal myocyte lipid homeostasis, and its cross-talk with insulin-stimulated glucose utilization in our R24 Seed Grant proposal (NIDDK R24-DK084969).
There are no current selective and specific inhibitors of the pathways associated with striated muscle lipotoxicity, other than inhibitors of the TAG synthesis pathway (e.g. A-922500, an inhibitor of diacylglycerol acyltransferase (DGAT-1). We specifically are not interested in TAG synthesis pathway inhibitors, so will eliminated them through countersceening against DGAT-1, and in our literature and patent search terms.
The probe, ML377, identified in this proposal will take us to the next step of mechanistic exploration. The following types of tertiary assays and follow-up studies will be conducted (and are described in greater detail in our full R24 proposal, R24 DK092781).
- Assessment of the effects of the molecular probe hits on cultured primary skeletal myocyte insulin-stimulated myotube glucose uptake (ISGU) and glycogen synthesis (ISGS) to define whether the probe is linked to adaptive versus maladaptive responses.
- Given the importance of mitochondrial function in linking skeletal myotube lipid accumulation to insulin sensitivity and glucose utilization, rigorous studies of the effects of the probes on mitochondrial respiratory function and mitochondrial biogenesis in primary skeletal myotubes in culture will be performed.
- Further pharmacologic analyses and optimization will be conducted in order to assess the suitability of the candidate probes for in vivo studies of insulin sensitivity and glucose tolerance in rodents.
- In parallel, the probes will be used as a perturbagen in primary skeletal myocytes in culture to identify downstream signaling responses and corresponding target pathways. This will involve exposing primary human skeletal myotubes in culture to a relevant concentration of the probe followed by transcriptional profiling, metabolomic profiling, and measurement of substrate fluxes. This approach has worked well for our independent studies focused on the effects of gene “knockout” or overexpression to identify pathways affected by the perturbation.
- Based on the results of the studies described above, candidate targets will then be assessed. Standard approaches will be used including evaluation of direct interaction of the molecular probe with putative candidate target proteins using biochemical approaches, including mass spectrometry.
In the long run, based on the results of these next-phase experiments, we hope to establish molecular probes of adaptive/maladaptive responses of the skeletal myocyte to lipid overload. These probes will allow us, as delineated above, to identify new pathways and corresponding mechanisms that link alterations in myotube lipid metabolism to glucose utilization and insulin sensitivity. Such probes will serve as highly valuable experimental tools for the field. In addition, the targets identified in these planned experiments could prove useful as new targets for the development of novel therapeutic approaches aimed at the early stages of insulin resistance in the obese population. Further development of “hit” to “lead” will involve continued collaboration with medicinal chemists and pharmacologists. In the long-term, this work could lead to the development of a new class of drugs aimed at preventing the development of insulin resistance, and reducing the end-organ complications of type 2 diabetes.
Examples of the types of scientists in our broad scientific community that would likely utilize this probe are as follows:
- Mitochondrial biologists interested in changing mitochondrial function and biogenesis in cells in culture.
- Scientists with interests in conducting small animal studies aimed at assessing the effects of changing muscle lipid accumulation relevant to the development of insulin resistance.
- Biomedical engineers and nanoscientists who are developing innovative tissue culture techniques to assess muscle cell function in vitro, including assessing fatigability and tension development as a surrogate for exercise performance.
- iPSC-based studies conducted in human cells to assess the effects of changing lipid metabolism and mitochondrial function on progenitor cell differentiation lineages and specific disease phenotypes (e.g. metabolic diseases, cardiovascular diseases, muscular diseases).
- Studies by chemists interested in the structure-function relationship of molecules that change cellular capacity to handle lipid load and alter insulin responsiveness.
- Molecular biologists interested in the gene regulatory control of fat metabolism, who would conduct combined functional genomics (siRNA or cDNA screens) with the effects of the probe to identify relevant pathways downstream of each. This would be a first step towards novel therapeutic target discovery relevant to the broad area of metabolic and cardiovascular disease.
2. Materials and Methods
Primary assay: “HTS identification of small molecule Triacylglycerol inhibitors in a fluorescence assay (from PubChem website (http://pubchem.ncbi.nlm.nih.gov/). Comprises a small molecule screening campaign to identify molecular probes relevant to muscle lipid accumulation and insulin resistance (lipotoxicity) and of cellular processes that control skeletal myocyte lipid homeostasis, and its crosstalk with insulin-stimulated glucose utilization (see AID 651582 In Table 2)
Table 2
Summary of Assays and AIDs.
2.1. Assays
Table 2 summarizes details for the assays that enabled this probe discovery project. A detailed description of the Primary assay can be found in the Appendix 6.1 and in the PubChem AIDs listed.
2.2. Probe Chemical Characterization
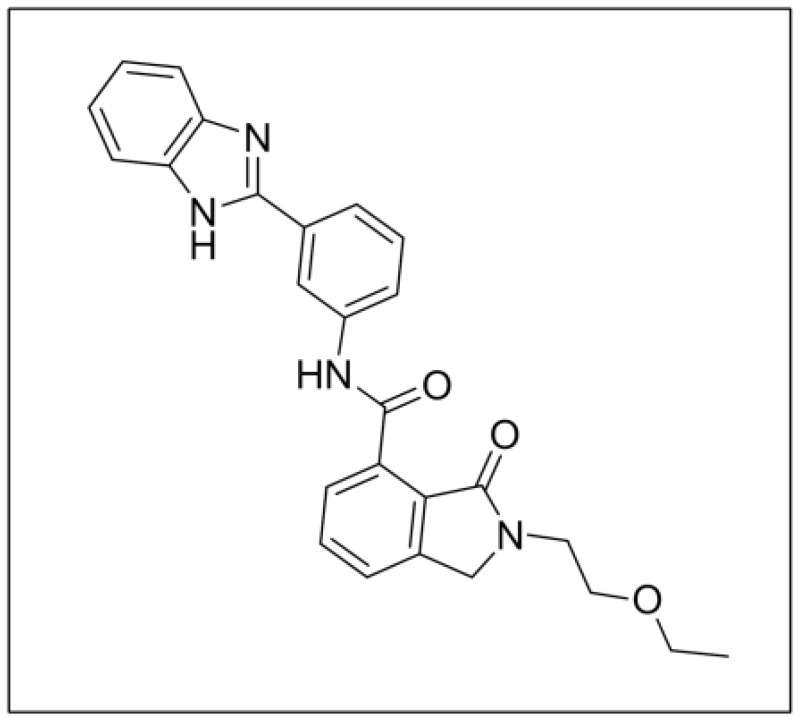
Chemical name of probe compound. The IUPAC name of the probe is N-(3-(1H-benzo[d]imidazol-2-yl)phenyl)-2-(2-ethoxyethyl)-3-oxoiso-indoline-4-carboxamide. The actual batch prepared, tested and submitted to the MLSMR is archived as SID 163875672 corresponding to CID 71677755 (Figure 1). ML377 does not have any stereogenic centers. This probe is not commercially available. A 25 mg sample of ML377 synthesized at SBCCG has been deposited in the MLSMR (Evotec) (see Probe Submission Table 3, that summarizes the deposition of the Probe and 5 analogs).

Figure 1
Structure of ML377.
Table 3
Probe and Analog Submissions to MLSMR (Evotec) for TAG inhibitors.
Solubility and Stability of ML377 in PBS at room temperature. The stability of ML377 was investigated (Figure 2) in aqueous buffers at room temperature by monitoring the amount of starting ML377 apparently remaining after incubation at room temperature in either PBS (pH 7.4) or 1:1 PBS:acetonitrile (v/v). ML377 was stable in PBS: acetonitrile with 91.9% of the parent compound remaining after 48 hrs. The apparently lower stability (45.7%) in neat PBS is a reflection of relatively low solubility. Thus, the stability value is artificially low due to compound precipitation vs. degradation. As noted in the Summary of in vitro ADME/T properties, ML377 has ∼20 μM solubility in pH 5.0, 6.2 and 7.4 pION buffer. In pH 7.4 PBS, the solubility of ML377 was 8.4 μM. The scaffold structure represented by ML377 has no substantial chemical liabilities.7

Figure 2
Stability of ML377 in 1× PBS at RT.
2.3. Probe Preparation
ML377 probe SID 163875672 corresponding to CID 71677755 was synthesized according to Scheme 1.

Scheme 1
Synthesis of ML377.
Experimental
A: A solution of 4-methylisobenzofuran-1,3-dione (3.4 g, 21 mmol) in 100 mL methanol was treated with 1 mL concentrated sulfuric acid and was stirred at reflux overnight. The solvent was removed in vacuo and the resulting oil was partitioned with ethyl acetate and aqueous sodium bicarbonate. Evaporation of the organic phase provided dimethyl 3-methylphthalate (A) as a clear oil (2.0 g. 46%), which was, used without further purification. 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 7.6 Hz, 1H), 7.34 (d, J = 7.4 Hz, 1H), 7.29 (t, J = 7.7 Hz, 1H), 3.88 (s, 3H), 3.82 (s, 3H), 2.28 (s, 3H). MS (ESI+ve): Calculated for C11H13O4, [M+H] = 209.08, observed [M+H] = 209.07.
B: A flask was charged with A (2.0 g, 9.6 mmol) followed by carbon tetrachloride (80 mL), N-bromosuccinimide (1.8 g, 10 mmol), and AIBN (0.1 g, 0.6 mmol). The mixture was placed in a preheated bath and was stirred at 80 °C overnight. The solvent was removed in vacuo prior to partitioning the residue with ethyl acetate and aqueous sodium bicarbonate. The organic phase was condensed in vacuo to provide a residual oil which was purified by chromatography on silica gel eluted with 3-25% ethyl acetate in hexanes to afford 1.9 g (69%) of dimethyl 3-(bromomethyl)phthalate (B) as a clear oil. 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 7.8 Hz, 1H), 7.66 (d, J = 7.6 Hz, 1H), 7.50 (t, J = 7.8 Hz, 1H), 4.57 (s, 2H), 4.00 (s, 3H), 3.93 (s, 3H). MS (ESI+ve): Calculated for C11H12BrO4, [M+H] = 286.99, observed [M+H] = 286.99.
C: Intermediate B (82 mg, 0.28 mmol) was dissolved in 3.5 mL of acetonitrile and charged with 0.06 mL (0.43 mmol) of triethylamine. 0.50 mL of acetonitrile containing 28 mg (0.31 mmol) of 2-ethoxyethylamine was added and the mixture was heated at 80 C. LC-MS analysis indicated clean conversion after 1 hour. The mixture was diluted with 15 mL of water and extracted with three 3 mL portions of ethyl acetate. The organics were concentrated to give 62 mg of a clear film which was purified by flash chromatography (25 mL silica gel, eluting with 25% and then 50% ethyl acetate / hexanes followed by neat ethyl acetate) to return 49.2 mg (67%) of methyl 2-(2-ethoxyethyl)-3-oxoisoindoline-4-carboxylate (C) as a clear film 1H NMR (500 MHz, Chloroform-d) δ 7.55 – 7.45 (m, 3H), 4.49 (s, 2H), 3.93 (s, 3H), 3.71 (t, J = 5.1 Hz, 2H), 3.60 (t, J = 5.1 Hz, 2H), 3.42 (q, J = 7.0 Hz, 2H), 1.12 (t, J = 7.0 Hz, 3H). MS (ESI+ve): Calculated for C14H18NO4, [M+H] = 264.12, observed [M+H] = 264.11.
D: The ester intermediate C (49 mg, 0.19 mmol) was dissolved in 3 mL of tetrahyrofuran and treated with 1 mL of 0.5 M aqueous lithium hydroxide. After 40 hours, the mixture was diluted with 5 mL water, acidified with 1 mL 1N aqueous hydrochloric acid, and extracted thrice (4 mL, 2 mL, 2 mL) with chloroform. The extracts were filtered through magnesium sulfate and concentrated to give 129 mg of crude white solid product, which was triturated with chloroform. Concentration of the solution phase returned 50.4 mg (ca. 100%) of 2-(2-ethoxyethyl)-3-oxoisoindoline-4-carboxylic acid (D) as a white solid. 1H NMR (500 MHz, Chloroform-d) δ 15.83 (s, 1H), 8.44 – 8.26 (m, 1H), 7.77 – 7.54 (m, 2H), 4.65 (s, 2H), 3.81 (t, J = 5.1 Hz, 2H), 3.65 (t, J = 5.1 Hz, 2H), 3.45 (q, J = 7.0 Hz, 2H), 1.13 (t, J = 7.0 Hz, 3H). MS (ESI+ve): Calculated for C13H16NO4, [M+H] = 250.10, observed [M+H] = 250.09.
ML377: The acid D (49 mg, 0.20 mmol) and the solids ethyl dimethylaminopropylcarbodiimide hydrochloride (94 mg, 0.49 mmol) and 1-hydroxybenzotriazole (33 mg, 0.22 mmol) were charged with 1 mL of N,N-dimethylformamide. The mixture was treated with triethylamine (0.16 mL, 1.18 mmol) and stirred at room temperature for 15 minutes before adding solid 3-(1H-benzo[d]imidazol-2-yl)aniline (CAS #7596-74-9, 41 mg, 0.20 mmol). After stirring for 18 hours, the mixture was diluted with 10 mL water. After standing for 4 hours during which a white solid settled, the supernatant was drawn off. The residue was transferred with 33% methanol / chloroform. Concentration gave 85.4 mg of crude solid product, which was purified by flash chromatography (25 mL silica gel, eluting with 50% ethyl acetate / hexanes followed by neat ethyl acetate). The purified material was taken up in 4 mL of 75% acetonitrile / water and lyophilized to yield 64.5 mg of N-(3-(1H-benzo[d]imidazol-2-yl)phenyl)-2-(2-ethoxyethyl)-3-oxoisoindoline-4-carboxamide (ML377) as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 13.70 (s, 1H), 12.99 (br. s, 1H), 8.65 (br. s, 1H), 8.37 (d, J = 7.5 Hz, 1H), 8.05 – 7.80 (m, 4H), 7.60 (m, 3H), 7.28 – 7.20 (m, 2H), 4.75 (s, 2H), 3.87 (t, J = 5.4 Hz, 2H), 3.72 (t, J = 5.4 Hz, 2H), 3.52 (q, J = 7.0 Hz, 2H), 1.14 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 168.97, 162.74, 151.54, 144.34, 140.31, 132.35, 132.19, 131.40, 131.05, 130.04, 128.79, 127.25, 122.16, 121.73, 118.36, 68.11, 65.96, 51.40, 43.07, 15.57. HRMS (ESI+ve): Calculated for C26H25N4O3, [M+H] = 441.1882, observed [M+H] = 441.1928.
3. Results
3.1. Dose Response Curves for Probe
ML377 did not inhibit cell viability of H9c2 cells as determined by ATP levels and DAPI nuclei staining (Figure 3) while it was was efficacious for inhibition of TAG accumulation in both rat H9c2 skeletal in human primary myotube cells.

Figure 3
Potency of ML377 inhibition for triacylglycerol accumulation in both H9c2 cells and differentiated human skeletal myotubes. Error bars are standard deviation of triplicate runs tested on at least two separate days.
3.2. Cellular Activity
The differential dose responsiveness for inhibition of TAG accumulation in H9c2 and human primary cells while not affecting H9c2 viability are consistent with the ability of ML377 to penetrate cell membranes. Additional profiling indicated ML377 was not toxic to human hepatocytes at >50 μM (Table 4).
Table 4
Summary of in vitro ADME Properties of TAG inhibitor probe ML377.
3.3. Profiling Assays
ML377 was evaluated in a detailed in vitro pharmacology screen as shown in Table 4.:
ML377 achieved modest concentrations of 10 × IC50 in aqueous buffer over a pH range of 5.0-7.4. Its solubility was better than all other analogs tested, including the purchased singleton hit (Entry 1) which exhibited solubility approximately equal to its IC50. In pH 7.4 PBS, the solubility of ML377 was 8.4 μM.
The PAMPA (Parallel Artificial Membrane Permeability Assay) assay is used as an in vitro model of passive, transcellular permeability. An artificial membrane immobilized on a filter is placed between a donor and acceptor compartment. At the start of the test, drug is introduced in the donor compartment. Following the permeation period, the concentration of drug in the donor and acceptor compartments is measured using UV spectroscopy. Consistent with its solubility data, ML377 exhibited good permeability across the pH range of the donor compartment. The screening hit 1 also had good permeability. ML377 was more permeable than the other analogs.
Plasma protein binding is a measure of a drug's efficiency to bind to the proteins within blood plasma. The less bound a drug is, the more efficiently it can traverse cell membranes or diffuse. Highly plasma protein bound drugs are confined to the vascular space, thereby having a relatively low volume of distribution. In contrast, drugs that remain largely unbound in plasma are generally available for distribution to other organs and tissues. All analogs were highly plasma protein bound.
Plasma stability is a measure of the stability of small molecules and peptides in plasma and is an important parameter, which can strongly influence the in vivo efficacy of a test compound. Drug candidates are exposed to enzymatic processes (proteinases, esterases) in plasma, and they can undergo intramolecular rearrangement or bind irreversibly (covalently) to proteins. All analogs showed modest stability in both human and mouse plasma.
The microsomal stability assay is commonly used to rank compounds according to their metabolic stability. This assay addresses the pharmacologic question of how long the parent compound will remain circulating in plasma within the body. All analogs showed poor stability in both human and mouse human liver microsomes after 1 hour.
ML377 showed no toxicity (>50 μM) towards immortalized Fa2-N4 human hepatocytes.
4. Discussion
4.1. Comparison to Existing Art and How the New Probe is an Improvement
ML377 is the first reported selective small molecule inhibitor of TAG accumulation. It has improved ADME/T properties relative to the HTS hit compound (1, 26), and offers a useful starting point for optimization efforts and eventual in vivo studies.
Intramyocellular lipid (IMCL) accumulation in obese and diabetic patients is associated with insulin resistance. Although the precise mechanisms remain to be fully understood, IMCL is thought to contribute to skeletal muscle insulin resistance through the generation of lipotoxic intermediates that alter insulin signaling, possibly at the level of the insulin receptor substrate (IRS). However, other theories exist and the mechanistic basis for the link between IMCL and insulin resistance remains largely enigmatic. Therefore, strategies designed to lower levels of IMCL in obese and insulin resistant patients would be predicted to improve whole body insulin sensitivity and counteract the lipotoxic effects in the muscle. This probe compound will allow us to test this hypothesis.. As a first step towards this goal, ML377 will be tested in separate cellular assays including fatty acid oxidation (FAO) and glucose uptake assays. Measurements of FAO will provide additional information as to the fate of the fatty acids and to determine if they are diverted towards oxidation rather than storage. The glucose uptake assay will allow us to determine if inhibition of lipid accumulation is associated with enhanced glucose uptake, either basal or insulin-stimulated uptake. ML377 will also prove useful in the identification of novel targets and pathways involved in cellular lipid storage and homeostasis.
5. References
- 1.
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. [PubMed: 11742409]
- 2.
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145. [PubMed: 15784668]
- 3.
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847–2850. [PubMed: 15199035]
- 4.
- Palamara KL, Mogul HR, Peterson SJ, Frishman WH. Obesity: new perspectives and pharmacotherapies. Cardiol Rev. 2006;14(5):238–258. [PubMed: 16924165]
- 5.
- Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annual Review of Nutrition. 2002;22(1):325–346. [PubMed: 12055349]
- 6.
- Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 Suppl 2:S9–S15. [PMC free article: PMC2995546] [PubMed: 17130651]
- 7.
- Baell JB, Holloway GA. J. Med. Chem. 2010;53:2719–2740. [PubMed: 20131845]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Inhibitors of Myocyte Triacylglyceride Accumulation - Probe Reports from the NIH...Inhibitors of Myocyte Triacylglyceride Accumulation - Probe Reports from the NIH Molecular Libraries Program
- Gene Links for GEO Profiles (Select 117750580) (1)Gene
- ATP5ME ATP synthase membrane subunit e [Homo sapiens]ATP5ME ATP synthase membrane subunit e [Homo sapiens]Gene ID:521Gene
- Gene Links for GEO Profiles (Select 116308958) (1)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...