NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Berkman ND, Lohr KN, Morgan LC, et al. Reliability Testing of the AHRQ EPC Approach to Grading the Strength of Evidence in Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 May.

Reliability Testing of the AHRQ EPC Approach to Grading the Strength of Evidence in Comparative Effectiveness Reviews [Internet].
Show detailsANTIDEPRESSANTS: FLUOXETINE VS. PAROXETINE
1Benefits: HAM-D Response:
Key Question: For adults with MDD, do commonly used medications for depression differ in efficacy or effectiveness in treating depressive symptoms?
| 5 RCTs/TOTAL N=690 | |||
|---|---|---|---|
| Study | N | Quality | HAM-D Responders: Fluoxetine vs. Paroxetine |
| De Wilde 19939 RCT | 100 | Fair | 63% vs. 68% (p=NS) |
| Gagiano 199312 RCT | 90 | Fair | 63% vs. 70% (p=NR) |
| Fava 199810 RCT | 109 | Fair | 57% vs. 58% (p=NS) |
| Chouinard 19998 RCT | 203 | Fair | 68% vs. 67% (p=0.93) |
| Fava 200211 RCT | 188 | Fair | 65% vs. 69% (p=NS) |
Abbreviations: NR = not reported; NS = not sufficient; RCT = randomized controlled trial = vs. = versus.
For this exercise, we also provide the pooled data analysis (forest plot below).
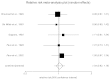
Figure
Relative risk meta-analysis of response rates comparing fluoxetine with paroxetine on the HAM-D. Heterogeneity (Non-combinability) of studies Cochran Q=0.668662 (df=4) p=0.9551
2Benefits: Response in elderly subpopulation
Key Question: How does the efficacy of treatment with antidepressants differ in elderly or very elderly patients with MDD?
| 1 RCT/TOTAL N=108 | |||
|---|---|---|---|
| Study | N | Quality | Results: Fluoxetine vs. Paroxetine |
| Schöne 199313 RCT | 108 | Fair | HAM-D: Significantly more paroxetine responders (Results reported in bar graph only; p=0.03) MADRS: Significantly more paroxetine responders (Results reported in bar graph only; p=0.04) |
Abbreviations: HAM-D = Hamilton Rating Scale for Depression; MADRS = Montgomery Asberg Depression Rating Scale; RCT = randomized controlled trial.
3Harms: Sexual dysfunction:
Key Question: For adults with MDD, do commonly used antidepressants differ in the occurrence of the adverse event sexual dysfunction?
| 4 RCTs/TOTAL N=904 | |||
|---|---|---|---|
| 2 observational studies/TOTAL N=3,154 | |||
| Study | N | Quality | Results: Fluoxetine vs. Paroxetine |
| Fava 199810 RCT | 109 | Fair | Rate of spontaneously reported sexual dysfunction events (not explicitly defined);7% vs. 25%; (p=0.01†) |
| Chouinard 19998 RCT | 203 | Fair | Abnormal ejaculation: 12.2% vs. 24.3%; (p=0.16†) Impotence: 7.3% vs. 10.8%; (p=0.59†) |
| Fava 200211 RCT | 188 | Fair | Libido decrease: 14% vs. 15.6%; (p=0.77†) Abnormal ejaculation (corrected for gender): 11.8% vs. 20%; (p=0.34†) |
| Kroenke 200114 Open-label RCT | 404 | Fair | Sexual function (based on 4 individual items constituting sexual functioning scale: sexual satisfaction, ED or inadequate lubrication, difficulty having orgasm, and ability to satisfy sexual partner)—difference between groups (p=NS) |
| Montejo 200116 Prospective cohort study | 487 | Fair | Incidence of sexual dysfunction (assessed by Psychotropic-Related Sexual Dysfunction Questionnaire): 57.7% vs. 70.7%; (p=0.003†) Observed frequency of sexual dysfunction: Decreased libido: 50.2 vs. 63.9; (p=0.003†) Delayed orgasm/ejaculation: 49.5 vs. 63.9; (p=0.002†) Anorgasmia/no ejaculation: 39.1 vs. 52.8; (p=0.002†) Erectile dysfunction/decreased vaginal lubrication: 21.8 vs. 41.4; (p<0.0001†) |
| Clayton 200215 Cross-sectional survey | 2,667 | Fair | Odds of sexual dysfunction by antidepressant taken (reference drug is bupropion SR): Fluoxetine: OR, 2.23; 95% CI, 1.75 to 2.87 Paroxetine: 2.89 (95% CI, 2.24 to 3.73) Prevalence of sexual dysfunction based on CSFQ total scores lower in FLUOX-treated patients; difference reached statistical significance |
- †
p-value calculated post-hoc by RTI.
Abbreviations: CI = confidence interval; CSFQ = Changes in Sexual Functioning Questionnaire; ED = erectile dysfunction; FLUOX = fluoxetine; NS = not sufficient; OR = odds ratio; RCT = randomized controlled trial = SR = slow release; vs. versus.
4Harms: Suicidality
Key Question: For adults with MDD, do commonly used antidepressants differ in the occurrence of the adverse event suicidality?
| 1 RCT/TOTAL N=90 | |||
|---|---|---|---|
| 2 observational studies/TOTAL N=11,350 | |||
| Study | N | Quality | Results: Fluoxetine vs. Paroxetine |
| Gagiano 199312 RCT | 90 | Fair | Suicidal ideation (HAM-D item 3) score increase: Fluoxetine: 6 (13.3%) Paroxetine: 0 (p=0.026†) Score decrease: Fluoxetine: 29 (64.4%) Paroxetine: 31 (70.5%) No patient attempted suicide |
| Jick 200417 Case-control study | 1,299 nonfatal suicidal behavior cases and controls from cohort that were prescribed fluoxetine or paroxetine | Fair | Relation between fluoxetine or paroxetine and nonfatal suicidal behaviors OR (95% CI), comparing each with dothiepin as the reference group: Fluoxetine: 1.16 (95% CI, 0.91 to 1.50) [cases: 31.7%, controls: 28.5%] Paroxetine: 1.29 (95% CI, 0.97 to 1.70) [cases: 24.3%, controls: 19.4%] |
| Martinez 200518 Nested case-control study | Cases and controls, nonfatal self harm: 10,051 Cases and controls, completed suicides312: | Good | Risk of non-fatal self harm in people prescribed fluoxetine (compared with paroxetine as reference): adjusted* OR, 0.94 (95% CI, 0.79 to 1.11) Risk of completed suicides in people prescribed fluoxetine (compared with paroxetine as reference): adjusted* OR, 0.42 (95% CI, 0.13 to 1.39) *Adjusted for severity of depression, time depression was diagnosed in relation to start of txt, referral to psychiatrist or psychologist before index day, history of self harm, diagnosis of or txt for anxiety or panic disorder, schizophrenia, antipsychotic drugs, drug misuse, and alcohol misuse |
- †
p-value calculated post-hoc by RTI
5Harms: Nausea
Key Question: For adults with MDD, do commonly used antidepressants differ in the occurrence of the adverse event nausea?
| 6 RCTs/TOTAL N=689 | |||
|---|---|---|---|
| Study | N* | Quality | Results: Fluoxetine vs. Paroxetine |
| De Wilde 19939 RCT | 100 | Fair | 20% vs. 20.4% (p=NS) |
| Gagiano 199312 RCT | 90 | Fair | 33.3% vs. 36.4% (p=0.764†) |
| Schöne 199313 RCT | 108 | Fair | 11.5% vs. 9.3% (p=0.701†) |
| Chouinard 19998 RCT | 203 | Fair | 31.7% vs. 37.3% (p=0.404†) |
| Fava 200211 RCT | 188 | Fair | 15.2% vs. 25.0% (p=0.095†) |
- *
N=total fluoxetine and paroxetine patients only
- †
p-value calculated post-hoc by RTI
ARTHRITIS DRUGS: BIOLOGICS VS. ORAL DMARDS
6Benefits: ACR20 Response
Key Question: For patients with RA, do drug therapies differ in their ability to reduce patient-reported symptoms, to slow or limit progression of radiographic joint damage, or to maintain remission?
| 5 RCTs/TOTAL N=1,639 | ||||
|---|---|---|---|---|
| 2 observational studies/TOTAL N=2,461 | ||||
| Study/Design | N | Comparison | Quality | Results: Biologics vs. Oral DMARDs |
| ERA study, 200019,24 RCT | 424 | ETN 25 mg twice/wk vs. MTX (mean 19mg/wk) | Fair | At 12 months: 72% vs. 65%, (p=0.16) |
| Edwards 200422 RCT | 80 | RTX vs. MTX | Fair | 24 weeks: 65% vs. 38%; (p=0.025) 48 weeks: 33% vs. 20%; (p=0.204†) |
| TEMPO, 200525–27 RCT | 451 | ETN 25 mg twice/wk vs. MTX | Fair | At 52 weeks: 76% vs. 75%; (p=NS) |
| Combe 200621 RCT | 153 | ETN 25 mg twice/wk vs. SSZ | Fair | At 24 weeks: 73.8% vs. 28.0%, (p<0.01) |
| PREMIER, 200620 RCT | 531 | ADA 40 mg biweekly vs. MTX 20 mg/wk | Fair | Year 1: 54% vs. 63%, (p=0.043) |
| Geborek 200228 Nonrandomized open-label trial (Effectiveness trial) | 269 | ETN 25 mg twice/wk vs. LEF | Fair | Greater ACR20/50 responses for ETN at 3 months (p<0.001) and 6 months (p<0.05) [data reported in bar graph only] |
| Weaver 200629 Prospective cohort study | 2192 | ETN vs. MTX | Fair | ETN patients significantly more likely to achieve ‡mACR20 response at 12 months: adjusted* OR, 1.23 (95% CI, 1.02 to 1.47); (p<0.05) *adjusted for baseline covariates: age, baseline HAQ score (>1.5 vs. ≤1.5), comorbid disease (presence vs. absence), physician’s judgment of disease severity, duration of RA, employment/disability status, Medicare coverage, race, sex, previous txt with DMARDs, previous txt with biologics, insurance status, and highest educational level attained |
- †
p-value calculated post-hoc by RTI
- ‡
Excludes ESR/CRP criterion from ACR
Abbreviations: CI = confidence interval; DMARD = disease modifying antirheumatic drug; ETN = etanercept = HAQ = Health Assessment Questionnaire; mACR20 = Modified American College of Rheumatology; MTX = methotrexate; NS = not sufficient; OR = odds ratio; RA = rheumatoid arthritis; txt = treatment; vs. = versus.
7Benefits: ACR70 Response
Key Question: For patients with RA = do drug therapies differ in their ability to reduce patient-reported symptoms = to slow or limit progression of radiographic joint damage = or to maintain remission?
| 5 RCTs/TOTAL N=1 =639 | ||||
|---|---|---|---|---|
| 1 observational study/TOTAL N=269 | ||||
| Study/Design | N | Comparison | Quality | Results: Biologics vs. Oral DMARDs |
| ERA Study = 200019,24 RCT | 424 | ETN 25 mg twice/wk vs. MTX (mean 19mg/wk) | Fair | At 12 months: no significant differences between txt groups |
| Edwards 200422 RCT | 80 | RTX vs. MTX | Fair | 24 weeks: 15% vs. 5%; (p=NS) 48 weeks: 10% vs. 0%; (p=NS) |
| TEMPO = 200523,25–27 RCT | 451 | ETN 25 mg twice/wk vs. MTX | Fair | 52 weeks: 24% vs. 19%; (p=0.166†) |
| Combe 200621 RCT | 153 | ETN 25 mg twice/wk vs. SSZ | Fair | 24 weeks: 21.4% vs. 2% = (p<0.01) |
| PREMIER = 200620 RCT | 531 | ADA 40 mg biweekly vs. MTX 20 mg/wk | Fair | Year 1: 26% vs. 28% = (p=0.585†) |
| Geborek 200228 Nonrandomized open-label trial (Effectiveness trial) | 269 | ETN 25 mg twice/wk vs. LEF | Fair | 12 months: differences between txt groups: (p=NS) |
- †
p-value calculated post-hoc by RTI
Abbreviations: ETN = etanercept = LEF = lefluonomide; mg = milligram; MTX = methotrexate; NR: not reported; NS = not sufficient; RCT = randomized controlled trials; SSZ = sulfasalazine; txt = treatment; vs. = versus; wk = week.
8Benefits: DAS Remission
Key Question: For patients with RA = do drug therapies differ in their ability to reduce patient-reported symptoms = to slow or limit progression of radiographic joint damage = or to maintain remission?
| 2 RCTs/N=982 | ||||
|---|---|---|---|---|
| 1 observational study/N=1 =083 | ||||
| Study/Design | N | Comparison | Quality | Results: Biologics vs. Oral DMARDs |
| TEMPO = 200523,25–27 RCT | 451 | ETN 25 mg twice/wk vs. MTX | Fair | Remission at week 24: DAS<1.6: 13.0% vs. 13.6% (p=NS) DAS28<2.6: 13.9% vs. 13.6% (p=NS) Remission at week 52: DAS <1.6: 17.5% vs. 14% = (p=NS) DAS28<2.6: 17.5% vs. 17.1% = (p=NS) |
| PREMIER = 200620 RCT | 531 | ADA 40 mg biweekly vs. MTX 20 mg/wk | Fair | Clinical remission (DAS28<2.6) at 1 year: 23% vs. 21% = (p=0.582†) |
| Listing 200630 Prospective cohort study | 1083 | Biologics vs. conventional DMARDs | Fair | Odds of achieving remission (DAS28<2.6) at 12 months: Adjusted* OR = 1.95 (95% CI = 1.20 to 3.19); (p=0.006) *Adjusted for age = sex = # of previous DMARDs = DAS28 = ESR = FFbH = osteoporosis = previous txt with cyclosporine A. Matched pairs analysis‡ DAS28 remission at 12 months: 24.9% vs. 12.4% = (p=0.004) |
- †
p-value calculated post-hoc by RTI
- ‡
Pairs of biologic and oral DMARD patients differing by less than 0.05 on propensity score
Abbreviations: ADA = adalimumab; CI = confidence interval; DAS = Disease Activity Score; DMARD = disease modifying antirheumatic drug; ESR = erythrocyte sedimentation rate; FFbH = Funktionsfragebogen Hannover Functional Status Questionnaire; ETN = etanercept; mg = milligram; MTX = methotrexate; OR = odds ratio; NS = not sufficient; RCT = randomized controlled trials; txt = treatment; vs. = versus; wk = week.
9Harms: Serious Infections
Key Question: For patients with RA = do drug therapies differ in harms = tolerability = adherence = or adverse effects?
| 4 RCTs/TOTAL N=1 =215 | ||||
|---|---|---|---|---|
| 2 observational studies/TOTAL N=7 =695 | ||||
| Study/Design | N | Comparison | Quality | Results: Biologics vs. Oral DMARDs |
| Edwards 200422 RCT | 80 | RTX vs. MTX | Fair | 2 patients (5%) vs. 1 patient (2.5%) (p=NR) |
| TEMPO = 200523,25–27 RCT | 451 | ETN 25 mg twice/wk vs. MTX | Fair | 4% vs. 4% (p=NS) |
| Combe 200621 RCT | 153 | ETN 25 mg twice/wk vs. SSZ | Fair | ETN: 3 serious infections in 2 patients SSZ: 0 serious infections (p=NS) |
| PREMIER = 200620 RCT | 531 | ADA 40 mg biweekly vs. MTX 20 mg/wk | Fair | TEAEs -# of events per 100 patient-years Any serious infection: 0.7 vs. 1.6; (p=NS) TB: 0 vs. 0 |
| Curtis 200731 Retrospective cohort study | 5 =326 | Biologics vs. MTX | Fair | Serious infections during entire study period: 2.7% vs. 2.0%; number needed to harm=143 Crude HR (95% CI) biologic treatment association with hospitalization with a definite bacterial infection: HR = 1.39 (95% CI = 0.97 to 1.98) Adjusted* HR (95% CI) biologic treatment association with hospitalization with a definite bacterial infection: HR = 1.94 (95% CI = 1.32 to 2.83) *Adjusted for age = sex = U.S. region of residence = insurance status = comorbid diseases = corticosteroid use = MTX use |
| Schneeweiss 200732 Retrospective cohort study | 2 =369 | Biologics vs. MTX | Good | Compared with MTX no higher rates of serious bacterial infections in elderly patients; adjusted* model: RR = 1.0 (95% CI = 0.6 to 1.71) *Adjusted for age = sex = race = nursing home residence = hospitalization = # of physician visits = # of distinct prescription drugs = Charlson comorbidity score = RA severity = independent predictors of serious infections = previous antibiotic use = influenza vaccination and pneumococcal vaccination. |
Abbreviations: CI = confidence interval; ETN = etanercept; mg = milligram; HR = hazard ratio; MTX = methotrexate; NR = not reported; NS = not sufficient; RCT = randomized controlled trials; RA = rheumatoid arthritis; RTX = TB = tuberculosis; TEAE = treatment emergent adverse event; vs. = versus; wk. = week.
10Harms: Infusion or Injection Reaction
Key Question: For patients with RA = do drug therapies differ in harms = tolerability = adherence = or adverse effects?
| 4 RCTs/TOTAL N=1 =108 | ||||
|---|---|---|---|---|
| Study/Design | N | Comparison | Quality | Results: Biologics vs. Oral DMARDs |
| ERA = 200019,24 RCT | 424 | ETN 25 mg twice/wk vs. MTX (mean 19mg/wk) | Fair | 37% vs. 7% (p<0.001) |
| Edwards 200422 RCT | 80 | RTX vs. MTX | Fair | Any event associated with first infusion: 45% vs. 30% (p=0.166†) |
| TEMPO = 200523,25–27 RCT | 451 | ETN 25 mg twice/wk vs. MTX | Fair | 21% vs. 2% (p<0.0001) |
| Combe 200621 RCT | 153 | ETN vs. SSZ | Fair | 32.0% vs. 2.0% (p<0.05) |
- †
p-value calculated post-hoc by RTI
Abbreviations: ETN = etanercept; MTX = methotrexate; RTX = Rituximab; SSZ = sulfasalazine; vs. = versus; wk = week
- Strength of Evidence Summary Tables Used To Complete the Exercises - Reliability...Strength of Evidence Summary Tables Used To Complete the Exercises - Reliability Testing of the AHRQ EPC Approach to Grading the Strength of Evidence in Comparative Effectiveness Reviews
- Homo sapiens v-myc myelocytomatosis viral oncogene homolog 1, lung carcinoma der...Homo sapiens v-myc myelocytomatosis viral oncogene homolog 1, lung carcinoma derived (avian), mRNA (cDNA clone MGC:20172 IMAGE:4541675), complete cdsgi|33877814|gb|BC011864.2|Nucleotide
- PREDICTED: Homo sapiens filamin A interacting protein 1 like (FILIP1L), transcri...PREDICTED: Homo sapiens filamin A interacting protein 1 like (FILIP1L), transcript variant X1, mRNAgi|2217341624|ref|XM_047447376.1|Nucleotide
- Mimulus washingtonensis large ribosomal protein 16 (rpl16) gene, intron; chlorop...Mimulus washingtonensis large ribosomal protein 16 (rpl16) gene, intron; chloroplastgi|68349041|gb|DQ090901.1|Nucleotide
- Comolia edmundoi voucher M.J.R.Rocha 975 (BHCB) acetyl-CoA carboxylase beta subu...Comolia edmundoi voucher M.J.R.Rocha 975 (BHCB) acetyl-CoA carboxylase beta subunit (accD) gene, partial cds; accD-psaI intergenic spacer, complete sequence; and photosystem I subunit VIII (psaI) gene, partial cds; chloroplastgi|1018565655|gb|KU501176.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...