NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Weichbrod RH, Thompson GAH, Norton JN, editors. Management of Animal Care and Use Programs in Research, Education, and Testing. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. doi: 10.1201/9781315152189-10

Management of Animal Care and Use Programs in Research, Education, and Testing. 2nd edition.
Show detailsIntroduction
Animal care and use program administrators and managers are responsible for both promoting animal well-being and facilitating the research. However, since animal welfare laws and regulations primarily articulate edicts intended to mitigate harm to animals, it can be difficult to see how an institution’s regulatory compliance activities can both protect animal subjects and serve the research enterprise. While it can be a challenge to balance these seemingly competing goals, one should not conclude that ensuring high program achievement in terms of compliance precludes concomitant highly productive research.
In this chapter, we explore how the expanding environment of compliance”“driven by both legal requirements and the associated institutional regulatory processes”“drives the development of programs for oversight of animal care and use and impacts the work of scientists using animals. We describe some opportunities and strategies that program administrators and managers can use to facilitate the research enterprise without compromising compliance demands. Two general approaches are presented: (1) alleviating unnecessary regulatory burden, which conserves institutional and researcher resources, and (2) selecting appropriate standards and methods for assessing outcomes, which promotes program flexibility. It is worth noting at the outset that whatever the approach, it is crucial for the program manager or administrator to engage with the institutional animal care and use committee (IACUC) and other institutional oversight entities, such as the responsible institutional official (IO) and attending veterinarian (AV), as well as other program stakeholders. (Note: The term institutional animal care and use committee, or IACUC, is used in a generic sense to denote the institutional body, regardless of name, charged with oversight and evaluation of the animal care and use program.)
Impact of Regulatory Compliance Burden
Regulatory Burden
National and regional laws and regulations pertaining to the use and care of animals in research vary considerably with respect to the compliance responsibilities required of the regulated institutions. For example, in the United States, the federal regulatory system relies heavily on self-enforcement at the institutional level, while in countries of the European Union, there is greater reliance on direct enforcement of compliance by the state. Regardless of the explicit local regulatory environment, however, each well-managed institutional program will not only design animal care and use processes that conform to regulatory standards, but also exercise some degree of internal oversight to ensure those standards are being met. Thus, regulatory burden can be considered the total effort expended and concomitant cost incurred to achieve and maintain compliance. With regard to animal research, regulatory burden encompasses, but is not limited to, the activities and expenses of the IACUC, committee administrative support, government interface (hosting inspections, preparing and filing required applications and reports, etc.), and providing animal care procedures and facilities that meet standards. Likewise, the researchers themselves devote considerable effort to fulfilling compliance requirements. These include securing and maintaining licensures, preparing animal use protocols for IACUC review, maintaining IACUC approvals, hosting laboratory audits, fulfilling program orientation and training requirements, and similar activities. Quite obviously, satisfying compliance requirements comes at considerable cost to the institution.
The scientific community clearly accepts, as one of many social obligations, the regulatory burden necessary for safeguarding proper treatment and care of research animals. Without question, institutions recognize that maintaining their privilege to conduct research with animals depends on their ability to ensure compliance with regulatory standards. But as necessary as compliance may be, when regulatory burden becomes excessive, it can impinge upon research productivity while failing to yield any further enhancements to animal welfare. The impact of expanding regulatory requirements on research in the United States has been described (Federal Demonstration Partnership 2014; National Science Board 2014; Federation of American Societies for Experimental Biology 2015; Committee on Federal Research Regulations and Reporting Requirements et al. 2016). For example, in a recent survey of principal investigators of federally sponsored research projects in the United States, it was reported that they spend more than 40% of their time on administrative tasks associated with compliance (Federal Demonstration Partnership 2014). Global data on regulatory burden are not available, but it is reasonable to assume it has significant impact on research output in many countries. So the total cost of regulatory burden is limited not only to resource outlay, but also to lost productivity. As such, program managers should implement processes that effectively ensure regulatory compliance while minimizing regulatory burden so that research output is protected and unnecessary costs are contained.
Self-Imposed Compliance Burden
Haywood and Greene (2008) considered three sources of regulatory burden: explicit legal and regulatory requirements, additional interpretive guidance given by regulatory agencies, and internal, self-imposed burden. For the purposes of this discussion, however, we consider just two: external requirements imposed by law, regulation, or regulatory agency, and internal, self-imposed requirements not explicitly required by law, regulation, or regulatory guidance. In other words, we differentiate between compulsory and elective standards and processes. This distinction is important because program administrators and managers have relatively little opportunity to alleviate the burden stemming from compulsory standards, while they can have a significant influence on diminishing burden originating from elective institutional requirements.
Examples of self-imposed burdens adopted by institutions and opinions regarding how these burdens arise have been described (Haywood and Greene 2008; Plante and James 2008; Thulin et al. 2014; Pritt et al. 2016). Those facets of the program commonly augmented by elective practices include, but are not limited to, animal use protocol review, institutional policies, investigator training, documentation, and monitoring of laboratories. Maintaining accreditation by AAALAC International is another common example of voluntary program enhancement. All these result in burden beyond that stemming from the minimum external requirements, and most probably originate from both a desire for excellence and aversion to the risk of noncompliance.
Internal, elective standards are not always without merit”“many may be worth the additional effort they create. This is especially true when they address gaps in the regulatory standards or promote program quality in ways not specifically addressed by the regulations. For example, while participation in the AAALAC International accreditation program is voluntary and requires considerable effort, many institutions find that the value of the periodic peer reviews outweighs the costs. Nonetheless, it is likely the case that some institutions have implemented various elective standards and processes without ever thoroughly vetting them for benefit and cost. Consequently, the compliance programs at many of these institutions may unnecessarily stymie research activities without enhancing animal well-being or even reducing institutional risk.
Minimizing Regulatory Compliance Burden
Regulatory agencies view compliance oversight as a means to ensure rules are being followed, and institutions often view this as a process to help ensure they remain compliant and therefore avoid “trouble” with regulatory agencies or the public. Typically, the research community views compliance as a hurdle rather than a facilitative process. The challenge for leadership in the animal care and use program at an institution, therefore, is to promote a culture in which compliance truly does facilitate the research process while at the same time protecting the institution from noncompliance events that may threaten its privilege to conduct animal research and secure associated grant funding (Bayne and Garnett 2008). To promote such a culture, it is imperative that stakeholders understand the goals of compliance processes, and that the processes are clear and without unnecessary and burdensome steps. In addition, flexibility in achieving compliance goals should be maintained when feasible, and compliance outcomes assessed routinely to improve the process as needed over time. Imposing new processes without input from stakeholders, that result in additional work, are inflexible, and do not have obvious benefits will not be embraced. This can lead to erosion of the relationship between the research community and institutional compliance efforts.
Perhaps the most important way compliance programs can become more facilitative of the research is to minimize the regulatory burden placed on the researchers. A basic approach to reducing regulatory burden involves determining first whether a compliance requirement or process is mandated or elective, then eliminating elective practices based on benefit–cost analysis, and finally, determining if remaining mandated or elective practices can be done more efficiently (Haywood and Greene 2008). With thorough knowledge of the regulatory standards, establishing whether a process is mandatory should be relatively straightforward. But weighing the benefits and costs of elective procedures will be more complex. A rigorous assessment will require questions such as
- What are the intended benefits of the policy, procedure, or requirement?
- What entities, for example, animals, researchers, IACUC members, or institution, benefit from the policy, procedure, or requirement?
- Are the intended benefits being realized, and can the achievement be measured?
- What are the costs, for example, staffing, researcher time (correlates to time that cannot be devoted to research), space, or equipment, of the policy, procedure, or requirement?
- Can the costs be measured?
The answers to these questions will provide the data or measures from which objective decisions can be made about the value of policies and procedures and whether they should be retained or retired.
Finding more efficient ways to perform the remaining compliance procedures can be a significant challenge. Adopting a standardized approach to process improvement can be helpful in this regard. One such approach is “Lean thinking,” a method by which the value of processes (or steps within processes) is assessed from the perspective of defined customers, for example, the researchers or the animals, and waste within processes identified, thus facilitating process improvements that achieve desired outcomes (Kim et al. 2009). With respect to compliance, it is important to understand that more compliance effort does not necessarily lead to “better” compliance outcomes; rather, there is a point at which added effort likely results in diminishing returns and wasted resources. (Figure 10.1 illustrates the effect of compliance effort on risk noncompliance and regulatory burden.) As we will point out later, inspections are not the best way to achieve desired outcomes. With this in mind, program managers and oversight bodies should strive for that balance between too little and too much compliance effort.
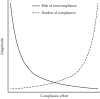
Figure 10.1
Relationship of overall compliance effort to risk of noncompliance and compliance burden. With little or no compliance effort, the risk of noncompliance is high, while the compliance burden is low. As the effort increases, risk decreases while burden (more...)
Assessing Outcomes
It is imperative to “measure” the effectiveness of the activities associated with animal care and research procedures to ensure animal well-being and promote scientific integrity. It is not sufficient to simply design a program that ought to work well. An institution should also have some means to verify that animal activities have appropriate outcomes. Yet, institutions should not fall prey to the notion that measuring outcomes alone achieves quality. Program quality is achieved by implementing well-designed and effective procedures, while outcome measures simply provide the information needed to assess effectiveness and drive improvement where needed. Developing appropriate outcome metrics requires a thorough understanding of the different types of standards that apply to the animal care and use program.
Engineering Standards
Engineering standards are often described in the regulations and specify both the characteristics and technical details required in order to meet the standard. An engineering standard not only specifies what the standard or outcome must be, but also how it is achieved. They dictate the methods to be used in order to achieve the standard or outcome. The benefit of such an approach is that there is uniformity of methods across the animal program and among animal programs. It is easier to assess compliance with engineering standards because they require fixed methods for achieving outcomes, but they do not allow flexibility to adopt methods that may be more effective for a particular program. An engineering standard leaves little or no room for interpretation about what must be done and, as a result, avoids the onus of careful and accurate interpretation that is required when implementing performance standards.
An example of a commonly used engineering standard is the space recommendations in the eighth edition of the Guide for the Care and Use of Laboratory Animals (Guide) specified in the tables 3.2 through 3.6 (NRC 2011). These tables provide specific floor space metrics required to meet Guide standards, and it is a straightforward process to determine whether these standards are achieved. A process to establish outcome assessment is unnecessary.
Performance Standards
In many ways, performance standards are considered the opposite of engineering standards in that they often have qualitative outcomes, whereas engineering standards usually have quantitative outcomes. “Performance standards” or the “performance-based approach” is a concept that has been part of animal research management for many years. The term performance approach became a prominent feature of the seventh edition of the Guide (NRC 1996). However, in previous editions of the Guide, as early as the fourth edition (NRC 1972), the term professional judgment was used in the context of how the recommendations are to be used and interpreted. Professional judgment is a key element of performance standards, so the fundamental concepts of the performance-based approach are not new. It has been said that professional judgment is the collective judgment of a profession, and not simply the judgment of one professional. This is an important distinction as one develops performance standards.
The key advantage of the performance-based approach is the programmatic latitude it affords. A performance standard identifies a specific goal or outcome that is to be achieved, but does not specify how the goal is achieved. The intent of such a standard is to allow latitude about the best, most effective manner to meet the standard given the unique aspects of each program. A variety of methods might all result in an outcome that meets the specified goal or standard. The performance standard approach is a key element of the eighth edition of the Guide. The Guide provides much detail about what constitutes appropriate standards (goals) of animal care and use. It is very clear on what must, or should, be done, but it is virtually silent about how the standards are to be achieved. This was done intentionally to allow flexibility in methods, which is considered to be a positive, less prescriptive approach. However, with this flexibility comes greater institutional responsibility to ensure that the standards, or goals, are correctly and accurately interpreted. When using performance standards in the animal program, it is critical to neither under- nor overinterpret what a particular standard or goal is.
When considering performance-based standards, three basic steps are required to ensure a standard is clearly identified and achievable:
- Identify a specific and precise definition of the standard.
- Establish the assessment criteria to determine that the standard is achieved.
- Develop and implement methods for ongoing evaluation.
An example of commonly used performance standards is the criteria described in the Guide regarding adequate cage space. In contrast to the Guide’s tables 3.2 through 3.6 that specify engineering metrics for cage space, there is much language in the Guide that describes the qualitative, performance-based aspects of the provision of adequate cage space (e.g., the need to accommodate postural adjustments, the importance of cage volume, the provision of environmental enrichment, and space considerations for social grouping and breeding). The Guide highlights the need for professional judgment when assessing the performance-based criteria for adequate cage space. Because there are a number of ways to achieve these performance standards, it is important that institutions develop specific criteria to assess whether the performance standards are met: (1) precisely defining adequate cage space characteristics (relying on Guide parameters), (2) developing assessment criteria to benchmark adequacy of space, and (3) implementing methods for ongoing assessments of cage space provided.
This kind of approach embodies the performance-based concept of operational latitude while still satisfying the requirements specified in the Guide. Of course, the definition of the standard and criteria for monitoring and methods of assessment for an entire animal program will be much more complex, but the above example illustrates the utility of the performance-based approach. When considering this approach, however, one should be mindful of the fact that procedures deemed acceptable for achieving a performance standard in one program may not be the most effective in another program. In addition, it is important to note that implementation of performance standards may require more effort than a corresponding engineering standard.
Practice Standards
In addition to describing performance and engineering standards, the Guide also refers to “practice standards,” a term that has recently been used to denote another aspect of benchmarking practices. Practice standard is a term that designates a time-proven, effective practice that is the result of collective professional judgment that usually evolves from practical experience and information in peer-reviewed scientific literature and textbooks. An example of a practice standard is the common practice of a cage-change interval once every 2 weeks for ventilated mouse cages. This is a widely accepted practice that has arisen from a combination of scientific literature, cage manufacturer information, and years of practical experience across a variety of institutions and research programs.
Because the field of laboratory animal science is continuously evolving, widespread practices emerge and become commonly accepted by the profession as sound, effective, and beneficial. Practice standards are usually improved as a natural result of scientific progress, improvements in technology, and advances in management rather than by regulatory requirement.
Institutions may implement practice standards that have evolved over time because the practices have proven to be effective in the context of their animal care and use program, and are based on the specific needs of the animals and experimental requirements. It is important to validate practice standards to ensure they are effective and promote animal well-being and good science. Furthermore, this validation will ensure that these practice standards are not simply based on tradition and convenience. Validation of institution-specific practice standards can be done in much the same way that performance standards are assessed, although practice standards, having been time proven, typically require less rigorous analysis of the outcomes.
Selection of Appropriate Standards
Most animal care and use programs employ a combination of engineering, performance, and practice standards. Each type of standard has its benefit, and the choice to use one versus another is best made after careful consideration of the regulations and guidelines required by external agencies, the scientific needs of the program, and the overall management and organization of the animal care and use program. Engineering standards typically result from regulatory requirements. Performance and practice standards are often employed to allow appropriate flexibility, often required in diverse, dynamic, and complex research programs.
For engineering standards, the institution must simply ensure that the methods and standards imposed by the regulatory statutes are fulfilled as directed, a relatively straightforward process. Other guidelines, such as the Guide, expect programs to implement performance and practice standards that meet the specified outcomes. Finally, there may be institutional guidelines and standards added to those required by external agencies, and these can be engineering, performance, or practice standards. The latitude afforded by implementing performance-based standards comes with choosing the best means to achieve the goal. However, once the most appropriate methods are established, adherence to them is important to ensure that standards are achieved.
The implementation of performance-based standards requires a well-reasoned, thoughtful approach to achieve high-quality animal care and use, while at the same time ensuring that performance-based methods are not overly cumbersome and onerous. It is imperative that the methods and practices employed promote animal welfare and high-quality science, and are straightforward so that meeting the standards is an inherent part of daily animal care and use. Conversely, overreliance on a compliance monitoring program to “enforce” the standards is usually less effective, inefficient, and tedious.
Evaluation of Outcomes
Once appropriate outcome metrics have been established (i.e., what to measure), the next step is to determine how the outcomes will be measured. The process for measuring how well an institution meets animal program standards requires routine, ongoing commitment and attention to detail. It can also require much time and effort. However, the benefit to a well-implemented and efficient program of monitoring provides valuable information on animal well-being, animal use activities, and institutional commitment. Effective monitoring will provide information that can be used to validate strengths and address weaknesses while avoiding unnecessary, burdensome processes that have little or no impact on animal care and use. Although monitoring is a necessary part of attaining quality, emphasis should be on implementing procedures that effectively achieve the outcome.
Personnel in each area of the animal program will likely have a role in the evaluation of outcome metrics. However, the IACUC plays a central role and has the responsibility to evaluate all aspects of the program. The IACUC may rely primarily on its periodic reviews of the program and facilities for evaluation or, in larger, more diverse and complex programs, delegate monitoring to others. In recent years in the United States, employing dedicated personnel to monitor the program on behalf of the IACUC has become common (see below), although there is no requirement that such personnel or formal monitoring program be implemented.
Alternatively, the IACUC can rely on other program personnel to help with monitoring activities. Investigators play a key role in monitoring animal use activities. Ongoing monitoring of animal activities throughout the experiment is part of the scientific process, and this level of monitoring by investigators also aids the IACUC in ensuring compliance with regulations and guidelines. Many institutions have advisory committees comprised of scientists to help provide information and feedback to the IACUC and animal program personnel. The IACUC should also rely on veterinarians to monitor aspects of animal health and well-being, as well as some aspects of animal use, such as surgery or emerging and unexpected health issues. Facility managers and operational staff can supply objective information on metrics involving the animal environment and facilities, husbandry, sanitation, and day-to-day animal care. Maintenance staff are also important and can provide assessments of critical mechanical systems, such as heating, ventilation, and air-conditioning (HVAC), electrical power, and lighting. Institutional security personnel are instrumental in providing information on physical and personal security and regulating appropriate access to animal facilities. Occupational health and safety professionals are integral for providing information on the occupational health and safety program (OHSP) for personnel with animal contact, and they should provide ongoing feedback to the IACUC regarding the overall functionality of the OHSP. Finally, careful tracking of issues and error rate analysis can highlight common areas of concern that may emerge and help to focus institutional resources in the appropriate areas.
Application of Performance Standards to Oversight of the Animal Care and Use Program
For most animal care and use programs, the application of performance standards across all components of the program is a mainstay for day-to-day function. The IO, IACUC, and AV are responsible for ensuring that performance standards satisfy regulatory requirements, promote animal welfare, are tailored to the institution’s needs, and are not overly burdensome or costly. This responsibility creates a significant workload, but when done effectively, everyone benefits: the animals, animal caregivers, investigators, management, and IACUC itself.
Animal Care Program
The main components of the animal care and use program include institutional administration and management, the IACUC (protocol review and postapproval monitoring [PAM]), the program of veterinary care, animal environment and management, facilities and physical plant, and the OHSP. Although the IACUC does not perform or implement all the program components, the IACUC must ensure that performance standards in all these component areas are in place and functional. Regardless of the specific component of the program being evaluated, the collection of objective, relevant information, and error rate analysis are critical. Remember that part 3 of developing a performance standard involved developing methods for ongoing assessment of performance criteria. It is that ongoing evaluation of criteria that provides objective data as to whether a performance standard has been met, and provides the IACUC with key information needed for overall evaluation of the program.
It is beyond the scope of this chapter to detail how the IACUC provides oversight of all program components; however, some examples are provided that illustrate practical methods for the IACUC to ensure that performance-based standards are appropriate.
- Institutional administration and management: The institution must provide sufficient resources to the animal care and use program to ensure that the research enterprise is viable and high standards of animal welfare are in place. A straightforward method for the IACUC to evaluate the overall institutional support of the program involves a careful review of several semiannual reports. It is the responsibility of the IACUC to identify problematic trends and work with management to ensure corrections are made. When several annual or semiannual reports are reviewed over time, concerning trends may become evident that indicate that the institution’s administration may need to augment the resources allocated to the program. If problematic items are repeatedly identified by the IACUC without resolution, this may be a good indication that administrative support may be insufficient. On the other hand, if issues identified by the IACUC are consistently resolved, administrative support of the program is likely sound.
- Program of veterinary care: The program of veterinary care must ensure that healthy animals are acquired and maintained. Programs that meet current veterinary practice standards must be in place for animal clinical care and preventive medicine, emergency care, health monitoring and biosecurity, anesthesia and analgesia, surgery, pathology, and euthanasia. Metrics for ensuring an adequate veterinary care program often include evaluating the timeliness of veterinary clinical care for sick or injured animals; the monitoring and control of endemic animal pathogens; the procurement of high-quality, healthy animals; adequate veterinary support for services such as surgery and pathology; and the programs and strategies for control of animal pain or distress. By simply tracking and collecting data regarding these, and other veterinary activities, the effectiveness of the program of veterinary care can be assessed.
- Animal environment and management: Standards for animal environment and management include species-appropriate housing that promotes psychological and physical well-being and minimizes experimental variables that confound research data. The needs for socialization, environmental enrichment, quality feed and water, husbandry, and sanitation are important to ensure animal welfare. Metrics to evaluate these aspects of the program are often part of the semiannual review process. Considerations when evaluating the program of day-to-day care of animals often include the assessment of the overall adequacy of animal facility operations management: careful review of husbandry records to ensure key elements of daily care are regularly performed; review of sanitation intervals for cages and pens and the facility, as well as records and data relative to verification of sanitation effectiveness; review of the implementation and effectiveness of programs for social housing and environmental enrichment; review of feed storage and feeding practices; review of methods to provide quality drinking water and reports of water quality assessments; evaluation of standard operating procedures (SOPs) that impact animal well-being and assess work practices to ensure that SOPs are followed; assessment of staffing needs and the levels of expertise required; and evaluating events impacting animal well-being or research integrity.
- Animal facilities: The animal facilities must support the research activities involving animals, be well designed to promote a healthy environment for animals, have properly designed and functioning mechanical systems, maintain biosecurity, be clean and well maintained, and provide a safe working environment for personnel. They should contain adequate space for animal housing, procedural areas, specialized areas (e.g., surgery, necropsy, and quarantine), bio- and hazard containment as necessary, and adequate support areas (e.g., storage and cage-washing facilities). Evaluation of the animal facilities often is done concurrently with assessment of the animal environment and management, as these two components of the program are linked and interdependent. Evaluation of the facilities is relatively straightforward and involves ongoing assessment for needs relative to overall space, design, and maintenance in support of the institution’s research enterprise; regular evaluation of critical mechanical systems, such as HVAC, lighting, electrical power, and disaster planning safeguards; careful monitoring of the animal environment to ensure stable environments for research integrity, as well as animal safety; review of environmental monitoring data to ensure stable temperature and humidity according to the needs of the animals and scientific goals; review of adequacy of caging and pen systems to support animal well-being and the scientific needs of the program; and operation of animal facilities to foster animal and human safety.
- Protocol review and approval processes: Discussions of performance standards typically focus on how the IACUC or other oversight body can leverage them in the evaluation of the animal care and use program and facilities. However, the performance-based approach can also be applied to the processes used to support what is arguably the most important responsibility of the IACUC”“protocol review. Researchers have indicated that animal care and use protocol approval and revision are among the most significant regulatory burdens associated with animal-based research (Federal Demonstration Partnership 2014). As such, careful design and ongoing evaluation of these processes are imperative not only for ensuring regulatory compliance, but also for facilitating the conduct of research.There are myriad regulatory standards governing animal use protocol review and approval. In some cases, the regulations are noncommittal or even silent on the processes that must be used. Under these circumstances, institutions have tremendous opportunity for the application of performance-based standards in planning, evaluating, and improving protocol review processes. Unfortunately, many institutions fail to take full advantage of the opportunity afforded here because protocol review processes tend to be developed with a singular focus on regulatory standards and relatively little consideration for efficiency for either the IACUC or investigator. As a result, the institution may achieve its regulatory compliance objectives, but with greater burden than is necessary. To address this, institutions should adopt outcome-based standards for protocol review that not only give credence to the regulatory requirements and ethical responsibilities to the animal subjects, but also do so as efficiently as possible. This means careful examination of processes and eliminating waste by modifying or purging activities or requirements that do not add value.Haywood and Greene (2008) provide a lengthy list of self-imposed burdens associated with protocol review. Considering these suggested items, most programs will readily identify areas where processes can be improved. For purposes of illustration here, we explore two common problem areas in the process: overly complex protocol forms and excessive need for revisions to protocol submissions. These two interrelated facets are both sources of undue waste in the process.Completing the protocol form, which is the primary tool used by most IACUCs for review of animal activities, can be a daunting task for researchers. While the regulatory standards usually specify the content or specific considerations (e.g., identification of species, description of animal use, alternatives to painful procedures, and number of animals used) that must be addressed in animal use protocols, the forms at many institutions require information far in excess of the regulatory standards, or that the information be provided in a more complex format than the regulatory standards require. For instance, a standard required by regulation might be for the protocol to include a complete description of animal use; however, protocol forms rarely ask simply for a complete description of animal use. Instead, forms often attempt to illicit a complete description of animal use through the use of many questions and form fields, such as overview of the animal procedures, description of nonsurgical procedures, description of survival surgical procedures, description of postsurgical care, description of nonsurvival surgical procedures, table for frequency of volumes of blood collected, table of drugs administered, and listing of facility locations where animal procedures will be performed. If done well, some parsing of the base content requirements may actually make it easier for the investigator to provide the necessary information. But if done poorly, the parsing, along with the accompanying form field instructions, can result in needlessly lengthy and complex forms that investigators find difficult to complete. This in turn directly contributes to the need for revisions of submissions, which creates extra work for submitters and reviewers alike. For these reasons, it is advisable for the IACUC to evaluate the benefit and cost of the protocol form fields in a manner similar to the method described earlier.In the authors’ collective experience, it is relatively rare for protocols to wend their way through the review process without revisions and reworks being required. When reworks are commonly required in any process, the process itself becomes suspect. For example, if in the manufacturing of widgets 90% of them had to go back for rework before they were acceptable for market, one would consider the manufacturing process to be seriously flawed. Of course, preparation of an animal use protocol is not the same as the fabrication of a product in a factory, but when a high percentage of protocol submissions require revision, it still is indicative of underlying flaws in the process.For years, quality management specialists have been exhorting the recognition that inspection does not improve the quality of a product or service because it is retrospective in nature”“the product or service, whether of the desired quality or not, has already been produced or performed (Deming and Hollnagel 2000). Not to diminish the importance of the protocol review process, but much of it is indeed mere inspection. Is the form complete? Is the correct information provided? Are the descriptions sufficiently detailed? Are the proposed activities consistent with applicable regulations and policies? Is the literature search current? Imagine the time and effort that could be saved for both protocol authors and reviewers if protocols were completed to the IACUC’s satisfaction at the time of their original submission. More or better protocol reviews are not likely to lead to a significant reduction in the number of protocol reworks required; rather, only changes to the way protocols are produced or authored will accomplish this.Investigation into the root causes of inadequate protocol submissions may help uncover elements of the protocol authoring and submission process that can be revised in order to reduce the likelihood of revision requests. The causes may include unclear or overly complicated form instructions, language barriers, or poorly trained authors. In any case, identification of root causes will unveil those process elements that can be targeted for improvements and, subsequently, whether the improvements effect a decreased rate of protocol revisions.Ensuring the protocol form is easy to complete and does not ask for superfluous information, and changing the protocol authoring and submission process to reduce the need for revisions are just two ways whereby improved overall efficiency can be garnered. Of course, there are many other aspects of the protocol review process that can and should be evaluated using a performance-based standard of optimized efficiency. One payoff for this approach is reduced compliance burden for the researchers, which frees more time that can be devoted to the conduct of research. In addition, these types of process improvements can free the IACUC to concentrate more on the matters, such as harm–benefit analysis, that are of greatest import to animal welfare and research success.
- PAM: The investigator has responsibility to ensure activities are being conducted in congruence with the IACUC-approved protocol. In the United States, the IACUC is further tasked with “continued oversight” of approved activities. Historically, this monitoring has been achieved primarily via the semiannual program review and facilities inspections, communications with the animal care staff, and external agency inspections. More recently, many U.S. institutions have implemented a PAM program in which a dedicated staff is tasked with assessing congruency between the IACUC-approved activities and actual activities in the animal care and use program. In some cases, this dedicated monitoring program includes oversight of the animal care operations, in addition to research activities. Regardless of how the PAM program is conducted, it is critical that the program identifies problem areas and addresses them in a timely fashion to ensure a quality program. Instituting a PAM system without using the information gained to refine the program, and thereby prevent noncompliance from happening, is wasteful and directs resources away from more beneficial components of the animal care program (Plante and James 2008).When performance standards (outcomes focus) are applied to PAM, the leadership of the animal care and use program can more effectively determine how noncompliance items are typically identified at their institution, the types of noncompliance identified by the various monitoring programs, and the approximate associated costs. This approach allows the program to minimize those non-value-added components and minimize the regulatory burden when possible.Semiannual facility evaluations, which are a regulatory requirement in the United States, typically identify physical facility issues associated with the animal care and use program. The quality of this inspection process can vary greatly, and represents an opportunity for the IACUC to self-assess its effectiveness. One possible outcome measure or performance standard for semiannual inspections would be to identify with a high level of accuracy direct animal care deficiencies present in the animal housing areas. For example, at Institution A, the AAALAC site visitors identified facility problems that were not noted in the IACUC semiannual inspections. Upon introspection, Institution A noted that the semiannual inspections appeared to be cursory in nature, as the time required to complete the inspection was minimal. To address this concern, Institution A refined its inspection process to ensure that the inspection team included one of the veterinarians, and provided additional education to the IACUC members regarding their role in the semiannual inspection and items to look for as they conduct inspections. Over the course of the next year, the IACUC then analyzed the effectiveness of this change by scrutinizing the number of findings on the semiannual inspection, as well as the type of finding. Overall, findings increased by 200%, and furthermore, the findings themselves more frequently involved a direct animal care concern or animal welfare issue. The cost of this effort was strictly personnel in nature, and included additional veterinary time, preparation time, and IACUC member time. Institution A’s IACUC determined that this additional time cost was worth the effort since it identified findings that directly impacted the animals at a higher rate than before the change was implemented. Most importantly, however, Institution A was able to use the information gained on the inspections to leverage facility repairs and enhance preventive maintenance procedures in the vivarium to minimize problems from occurring in the first place.Another example is Institution B, whose IACUC recognized a concern with their oversight of investigator lab areas as identified by an external accreditation agency. In response to the agency findings, Institution B implemented a change to include all animal use investigator areas that utilize non–U.S. Department of Agriculture (USDA)–covered species in the 6-month facility inspections. Note that regulations require those areas to be inspected “regularly” rather than “every 6 months” to ensure adequate IACUC oversight. Since many of the lab findings related to rodent surgery procedures, additional training was implemented to target aseptic technique and postoperative care. During the first several years of this change, the IACUC noted that findings in these investigator areas were common; however, after about the third year, it was uncommon to identify a problem in these locations. Based on this outcome, the IACUC at Institution B redesigned the inspection process to lower the frequency of inspections for areas that had no findings on multiple inspections, and maintained the 6-month frequency for laboratory areas that had findings. The use of this continuous process improvement approach as applied to the semiannual inspection process has allowed both institutions to achieve an effective and efficient program of oversight for animal care and use.The animal care and veterinary staff are an integral component of the PAM program, and frequently are the individuals who identify serious noncompliance issues that impact animal welfare. The IACUC should ensure that animal care and veterinary staffs are familiar with the research protocols and activities and know how to report problems to the IACUC as applicable. On an international level, it is the animal care and veterinary staff to whom monitoring and compliance activities often are delegated (Whittaker 2014).Institutions that are considering use of a dedicated PAM specialist should determine what areas of the animal care and use program may require this additional oversight, and develop a feedback mechanism to facilitate correction of the root causes of the noncompliance. The qualifications and reporting structure of the specialist may vary depending on the institution’s oversight needs. Investing in dedicated PAM personnel can direct resources away from direct animal care and research support. Therefore, institutions should make an informed decision on the cost–benefit of this nonrequired activity for their institution (Thulin et al. 2014).As Haywood and Green (2008) advocated, the IACUC should consider the following three questions: Why do we do this? Does it help the animals? Can the end be achieved in a more efficient, cost-effective manner? As the PAM program is structured, it is useful to consider these three items to ensure efforts are directed toward the most important areas for their institution.
Organizational Considerations
Institutions provide support of the animal care and use program utilizing a management structure in which all support activities are centralized or a structure in which support is distributed among various units at the institution. The approach to management varies between academics and private industry based on a survey done in 2013, with private industry having a higher predominance of centralized support, with all functional areas of the animal care program being provided from the same administrative unit. In academia, the predominant administrative structure in support of the animal care and use program is a distributed model with functional areas (animal care administration and IACUC support) utilizing separate but parallel reporting structures (Bradfield and Thulin 2013). Pinson (2012) suggested that this separation may weaken the programmatic authority and fiscal stability in the animal care program. When studying the impact of organizational culture on the animal care and use program, it becomes easy to understand how communication in a distributed administrative structure can be especially challenging. Specifically, each administrative group is responsible for separate yet complementary functional components of the animal care program (Ellenberger and Corning 1999). On one hand, the animal care program provides administrative support for direct animal care, while on the other, the IACUC office provides administrative support for regulatory documentation associated with animal use. The latter function is typically removed from direct animal care, and the focus becomes more regulatory, or bureaucratic in nature. The consequence of this organizational structure can be a disconnect of the IACUC support staff from both the direct animal care program and the researcher, and lead to disharmony within the functional components of the animal care program (Thulin et al. 2014). Regardless of which organizational model is utilized, the institutional culture and personnel involved (Bronstad and Tronsdal Berg 2011) will ultimately determine how well the organizational structure functions at an individual institution.
Summary
Ensuring the animal care and use program conforms to the regulatory standards does indeed come at considerable cost and effort to the institution and the investigators conducting the science. The challenge for program managers and oversight bodies is to conduct the compliance activities in a way that not only ensures proper animal welfare, but also is no more restrictive to research than is necessary. Facilitation then is about minimizing compliance burden and better accommodating science through flexibility”“not just about making sure the institution retains its research privileges. Reduction in compliance burden can be achieved through careful examination of the attendant processes to eliminate those that are unnecessary or do not add value, and to find more efficient ways to perform all others. Embracing performance standards paired with careful evaluation of outcomes is important for achieving program flexibility. Programs that successfully realize these approaches will certainly be viewed as facilitating both animal welfare and the research enterprise.
References
- Bayne, K.A., and Garnett, N.L.2008. Mitigating risk, facilitating research. ILAR J49:369–371. [PubMed: 18849589]
- Bradfield, J.F., and Thulin, J.D.2013. Institutional oversight: The role of the attending veterinarian. Presented at the American College of Laboratory Animal Medicine Forum, Williamsburg, VA, April 14–17.
- Bronstad, A., and Tronsdal Berg, A.2011. The role of organizational culture in compliance with the principles of the 3Rs. Lab Anim40:22–26. [PubMed: 21173772]
- Committee on Federal Research Regulations and Reporting Requirements: A New Framework for Research Universities in the 21st Century; Committee on Science, Technology, and Law; Board on Higher Education and Workforce; Policy and Global Affairs; and National Academies of Sciences, Engineering, and Medicine. 2016. Optimizing the Nation’s Investment in Academic Research: A New Regulatory Framework for the 21st Century. Washington, DC: National Academies Press. https://www
.nap.edu/download/21824 (accessed September 14, 2016). [PubMed: 27386613] - Deming, W.D., and E.Hollnagel. 2000. Out of the Crisis. Cambridge, MA: MIT Press.
- Ellenberger, M.A., and Corning, B.R.1999. The Animal Care and IACUC offices: United or divided?Lab Anim28:44–47.
- Federation of American Societies for Experimental Biology. 2015. Sustaining discovery in biological and medical sciences: A framework for discussion. http://www
.faseb.org /Portals/2/PDFs/opa/2015/10 .23.15%20Sustaining %20Discovery%20for %20print%2031Aug15.pdf (accessed September 14, 2016). - Federal Demonstration Partnership. 2014. 2012 faculty workload survey: Research report. http://sites
.nationalacademies .org/cs/groups /pgasite/documents /webpage/pga_087667.pdf (accessed September 14, 2016). - Haywood, J.R., and Greene, M.2008. Avoiding an overzealous approach: A perspective on regulatory burden. ILAR J49:426–434. [PubMed: 18849596]
- Kim, C.S., Spahlinger, D.A., and Billi, J.E.2009. Creating value in health care: The case for Lean thinking. J Clin Outcomes Manag16:557–562.
- National Research Council. 1972. Guide for the Care and Use of Laboratory Animals. 4th edition. Washington: US Government Printing Office.
- National Research Council. 1996. Guide for the Care and Use of Laboratory Animals. 7th edition. Washington: National Academy Press. [PubMed: 25121211]
- National Science Board. 2014. Reducing investigators’ administrative workload for federally funded research. March10. http://www
.nsf.gov/pubs /2014/nsb1418/nsb1418.pdf (accessed September 18, 2015). - NRC (National Research Council). 2011. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press. [PubMed: 21595115]
- Pinson, D.2012. A training primer for institutional officials. Lab Animal41:198–203. [PubMed: 22718241]
- Plante, A., and James, M.L.2008. Program oversight enhancements (POE): The big PAM. ILAR J49:419–425. [PubMed: 18849595]
- Pritt, S., McNulty, J.A.Greene, M., Light, S., and Brown, M.2016. Decreasing institutionally imposed regulatory burden for animal research. Lab Anim45(8):297–300. [PubMed: 27439099]
- Thulin, J.D., Bradfield, J.F., Bergdall, V.K.et al.2014. The cost of self-imposed regulatory burden in animal research. FASEB J28:3297–3300. [PubMed: 24784580]
- Whittaker, A.2014. Animal research regulation in Australia”“Does it pass the test of robustness?Glob J Anim Law1:1–14.
- PubMedLinks to PubMed
- Review Culture of Care: Organizational Responsibilities.[Management of Animal Care and ...]Review Culture of Care: Organizational Responsibilities.Brown MJ, Symonowicz C, Medina LV, Bratcher NA, Buckmaster CA, Klein H, Anderson LC. Management of Animal Care and Use Programs in Research, Education, and Testing. 2018
- Review Fostering Collaborative Roles and Responsibilities for Members of an IACUC or Oversight Body.[Management of Animal Care and ...]Review Fostering Collaborative Roles and Responsibilities for Members of an IACUC or Oversight Body.Prentice ED, Blackburn ME, Dixon RS. Management of Animal Care and Use Programs in Research, Education, and Testing. 2018
- Avoiding an overzealous approach: a perspective on regulatory burden.[ILAR J. 2008]Avoiding an overzealous approach: a perspective on regulatory burden.Haywood JR, Greene M. ILAR J. 2008; 49(4):426-34.
- Review Compliance and Regulatory Programs.[Management of Animal Care and ...]Review Compliance and Regulatory Programs.King WW, Paramastri YA, GuillÁ©n J. Management of Animal Care and Use Programs in Research, Education, and Testing. 2018
- Training strategies for IACUC members and the institutional official.[ILAR J. 2007]Training strategies for IACUC members and the institutional official.Greene ME, Pitts ME, James ML. ILAR J. 2007; 48(2):131-42.
- Facilitating the Research Process: Limiting Regulatory Burden and Leveraging Per...Facilitating the Research Process: Limiting Regulatory Burden and Leveraging Performance Standards - Management of Animal Care and Use Programs in Research, Education, and Testing
Your browsing activity is empty.
Activity recording is turned off.
See more...