Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
CASRN: 541-15-1; 3040-38-8; 17298-37-2
Drug Levels and Effects
Summary of Use during Lactation
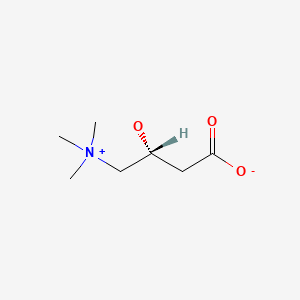
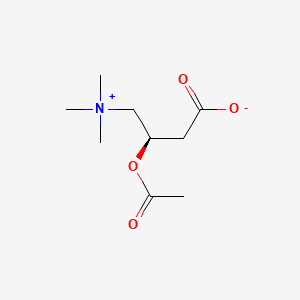
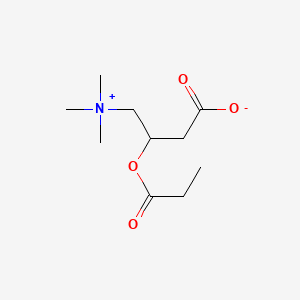
Levocarnitine and acetyl-l-carnitine (acetlycarnitine) are normal components of human milk that are required for fat metabolism. The body can use only levocarnitine; dextrocarnitine can be an antagonist of levocarnitine. Acetyl-l-carnitine, and propionyl-l-carnitine can be converted to levocarnitine by the body. The bioavailability of levocarnitine is less than 20%, but acetylcarnitine and propionlycarnitine may be higher. These substances have no specific lactation-related uses. Within the normal range of dietary intake, excretion of levocarnitine into breastmilk is relatively constant. Women with carnitine deficiency appear to secrete insufficient amounts of carnitine into their breastmilk for their breastfed infants, who may require levocarnitine supplementation.[1] Preterm infants are often deficient in levocarnitine and require supplementation.[2] No data exist on the safety and efficacy levocarnitine supplementation in nursing mothers or infants without carnitine deficiency. Levocarnitine and its derivatives are generally well tolerated in adults with occasional gastrointestinal upset and restlessness. A fishy odor to the breath, sweat and urine has been reported. Although data are very limited, poor bioavailability might limit absorption by the breastfed infant. It appears unlikely that maternal levocarnitine supplements during nursing would be harmful to the infant, but until more data are available, it is probably best to avoid levocarnitine supplementation unless it is prescribed by a healthcare professional.
Pasteurization (method not stated) had little effect on the concentration of endogenous carnitine in one study. Pasteurization followed by refrigeration at 5 degrees C for 48 hours reduced the carnitine concentration by about 13%.[3]
Drug Levels
Levocarnitine and acetylcarnitine are normal components of breastmilk, and are transported into milk via organic cation transporters.[4] As of the revision date, no published information was found regarding breastmilk levels following levocarnitine used as a dietary supplement by nursing mothers.
Maternal Levels. A woman diagnosed with carnitine uptake deficiency following the birth of her infant was given oral carnitine 500 mg 3 times daily. This dose failed to increase her breastmilk carnitine concentration, with a persistently low concentration of 3.4 mg/L (21 micromoles/L) before supplementation and after 18 days of supplementation.[1]
The carnitine concentration was measured in the breastmilk of 27 normal mothers who were not taking carnitine as a supplement. Average carnitine concentrations were 10.1 mg/L over the first 21 days postpartum, which was higher than the maternal serum concentration. The concentration decreased by about 50% by postpartum days 40 to 50. No difference in concentration was found between fore- and hindmilk.[3] Another study in normal mothers found a similar average carnitine concentration of 10.6 mg/L.[5]
In a study that followed 37 healthy, nonsmoking mothers who were not taking carnitine as a supplement, carnitine and acetylcarnitine in breastmilk were measured over time. Immediately postpartum, the average milk carnitine concentration was 12.6 mg/L and acetylcarnitine was 5.7 mg/L. From 2 months postpartum to 12 months postpartum, carnitine and acetylcarnitine milk concentrations were lower, in the range of 6.1 to 8.9 mg/L for carnitine and 1.2 to 2 mg/L for acetylcarnitine.[6]
Ten mothers of fullterm infants and mothers of preterm infants (36 weeks gestation or less) provided blood and milk samples for measurement of carnitine at 4 times during the first month postpartum. None were taking carnitine as a supplement. In the mothers of fullterm infants, total breastmilk carnitine averaged 11.7 mg/L at 2 to 3 days postpartum, 11.8 mg/L at 5 to 7 days postpartum, 11.3 mg/L at 2 weeks postpartum, and 10.5 mg/L at 4 weeks postpartum. About one-third to one-half of the carnitine was free carnitine and the remainder was acetylcarnitine. Breastmilk concentrations were about 50 to 100% greater than simultaneous plasma concentrations. The mothers of preterm infants had higher breastmilk carnitine levels, averaging about 12 mg/L throughout the first month postpartum.[7]
Carnitine and acetylcarnitine were measured in the breastmilk of 14 lactating women who were 1 to 10 months postpartum and not taking carnitine as a supplement. The average milk concentration of free carnitine was 5.9 mg/L and of total carnitine was 7.2 mg/L. Maternal dietary intake of carnitine had no effect on breastmilk carnitine concentrations. The authors estimated that an exclusively breastfed infant would receive about 6 mg daily of carnitine from breastmilk.[8]
Infant Levels. A retrospective study of 12,000 (6,000 male and 6,000 female) breastfed neonates in Greece found that carnitine and it various esters in the serum of 3-day-old infants was related to their birth weight. In general, levels of carnitine and its esters increased with increasing birthweight, although the differences between birthweight groups varied by ester.[9]
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
References
- 1.
- Studinski AL, Pino GB, Grycki E, et al. Correlation of plasma and breast milk carnitine levels and the effect of carnitine supplementation in a lactating woman with carnitine uptake deficiency (CUD). Mol Genet Metab 2011;102:315. Abstract. doi: 10.1016/j.ymgme.2010.11.160. [CrossRef]
- 2.
- Pekala J, Patkowska-Sokola B, Bodkowski R, et al. L-carnitine - Metabolic functions and meaning in humans life. Curr Drug Metab. 2011;12:667–78. [PubMed: 21561431]
- 3.
- Sandor A, Pecsuvac K, Kerner J, et al. On carnitine content of the human breast milk. Pediatr Res. 1982;16:89–91. [PubMed: 7058085]
- 4.
- Kwok B, Yamauchi A, Rajesan R, et al. Carnitine/xenobiotics transporters in the human mammary gland epithelia, MCF12A. Am J Physiol Regul Integr Comp Physiol. 2006;290:R793–802. [PubMed: 16195500]
- 5.
- Cederblad G, Svenningsen N. Plasma carnitine and breast milk carnitine intake in premature infants. J Pediatr Gastroenterol Nutr. 1986;5:616–21. [PubMed: 3735012]
- 6.
- Rovamo LM, Salmenpera L, Arjomaa P, et al. Carnitine during prolonged breast feeding. Pediatr Res. 1986;20:806–9. [PubMed: 3737295]
- 7.
- Penn D, Dolderer M, Schmidt-Sommerfeld E. Carnitine concentrations in the milk of different species and infant formulas. Biol Neonate. 1987;52:70–9. [PubMed: 3651516]
- 8.
- Mitchell ME, Snyder EA. Dietary carnitine effects on carnitine concentrations in urine and milk in lactating women. Am J Clin Nutr. 1991;54:814–20. [PubMed: 1951151]
- 9.
- Manta-Vogli PD, Schulpis KH, Loukas YL, et al. Perinatal free carnitine and short chain acylcarnitine blood concentrations in 12,000 full-term breastfed newborns in relation to their birth weight. Pediatr Neonatol. 2020 [PubMed: 32771364]
Substance Identification
Substance Name
Levocarnitine
CAS Registry Number
541-15-1; 3040-38-8; 17298-37-2
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Beta-Carotene.[Drugs and Lactation Database (...]Review Beta-Carotene.. Drugs and Lactation Database (LactMed®). 2006
- Review Vitamin B(12).[Drugs and Lactation Database (...]Review Vitamin B(12).. Drugs and Lactation Database (LactMed®). 2006
- Review Ceftazidime and Avibactam.[Drugs and Lactation Database (...]Review Ceftazidime and Avibactam.. Drugs and Lactation Database (LactMed®). 2006
- Review Ceftazidime.[Drugs and Lactation Database (...]Review Ceftazidime.. Drugs and Lactation Database (LactMed®). 2006
- Review Marine Oils.[Drugs and Lactation Database (...]Review Marine Oils.. Drugs and Lactation Database (LactMed®). 2006
- Levocarnitine - Drugs and Lactation Database (LactMed®)Levocarnitine - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
