Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
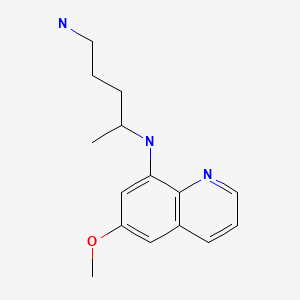
CASRN: 90-34-6
Drug Levels and Effects
Summary of Use during Lactation
Primaquine is poorly excreted into breastmilk of nursing mothers and undetectable in the serum of their breastfed infants. Breastfed infants beyond the neonatal period have shown no evidence of hemolysis. Neonates and infants with glucose-6-phosphate dehydrogenase (G6PD) deficiency have not been studied, but G6PD-deficient infants over 28 days of age appear to have a low risk of hemolysis from exposure in breastmilk.[1,2] If primaquine is required, testing the mother and infant for G6PD deficiency is advisable before the drug is given to a nursing mother.
United Kingdom malaria treatment guidelines recommend that primaquine be avoided in nursing mothers with malaria and that weekly chloroquine 500 mg be given until breastfeeding is completed.[3] However, these guidelines were developed before information on the excretion of primaquine into breastmilk and safety in breastfed infants was published. More recent information indicates that all mothers nursing infant over 28 days of age could safely receive primaquine.[1] The Centers for Disease Control and Prevention guidelines state that primaquine may be used in breastfeeding mothers and infants with normal G6PD levels.[4] Because the small amounts of primaquine transferred in breast milk are insufficient to provide adequate protection or treatment of malaria, infants who require chemoprophylaxis or therapy must receive the recommended dosages of primaquine.
Drug Levels
Primaquine's major metabolite is carboxyprimaquine, which has unknown activity against malaria. Primaquine's half-life is about 6 hours and carboxyprimaquine's half-life is 22 to 30 hours.
Maternal Levels. Twenty-one mothers with vivax malaria were give a dosage of primaquine 0.5 mg/kg daily for 14 days. Milk samples were taken on days 0 (first day of therapy), 3, 7, and 13 of therapy at various times after the dose. Peak breastmilk primaquine concentrations occurred about 1 hour after peak plasma concentrations (usually 3.4 to 3.9 hours after the dose) and averaged 44 mcg/L. Average peak breastmilk carboxyprimaquine concentrations 0f 7.2 mcg/L occurred 19 hours after the dose on day 0, and 12.1 mcg/L at hours 4 hours after the dose on day 13.[5]
A physiologically based pharmacokinetic model was constructed to simulate breastmilk and infant serum levels and compared to published milk level data. Results indicate that the relative infant dosage in infants would be less than 0.13%. the absolute infant dosage would be less than the dosage that might cause hemolysis in infants over 28 days of age with G-6-PD deficiency.[2]
Infant Levels. Twenty-one mothers with vivax malaria were give a dosage of primaquine 0.5 mg/kg daily for 14 days. Capillary blood samples were taken from the infants on days 0 (first day of therapy), 3, 7, and 13 of therapy at various times after the dose and after breastfeeding. All infant blood samples had unmeasurable amounts of primaquine (<1.14 mcg/L) and only 1 infant blood sample contained a measurable amount of carboxyprimaquine of 2.59 mcg/L on day 7 hour 0. Based on milk concentrations, the authors calculated median total cumulative primaquine dose expected to be ingested by the infant over the 14-day course based on measured breast milk concentrations to be approximately 0.042 mg/kg, corresponding to 2.98 mcg/kg daily (0.6% of a hypothetical infant daily dose of 0.5 mg/kg). The highest cumulative infant dose was estimated at 0.127 mg/kg, or 9.07 mcg/kg daily, which is 1.8% of a hypothetical infant daily dose of 0.5 mg/kg.[5]
Effects in Breastfed Infants
Twenty-one mothers with vivax malaria were give a dosage of primaquine 0.5 mg/kg daily for 14 days while breastfeeding their infants who were at least 28 days old. No alterations in hematocrit, Heinz body counts, serum bilirubin, oxygen saturation, or methemoglobinemia were seen in any of the infants.[5]
A woman with vivax malaria who was 5 months postpartum was given a dose of primaquine of 0.52 mg/kg daily for 7 days, then 0.46 mg/kg daily for 7 days after rechecking the patient’s weight. Shwas found to be heterozygous for glucose-6-phosphate dehydrogenase (G6PD) deficiency and experienced some hemolysis and anemia. Her female infant was being breastfed (extent not stated) during treatment and was found to be heterozygous for the G6PD Mahidol variant, but had no apparent hemolysis. The child’s vaccination schedule was completed, and the 6-month motor milestones were normal.[6]
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Alternate Drugs to Consider
Atovaquone and Proguanil, Chloroquine, Doxycycline, Mefloquine
References
- 1.
- Watson J, Taylor WRJ, Bancone G, et al. Implications of current therapeutic restrictions for primaquine and tafenoquine in the radical cure of vivax malaria. PLoS Negl Trop Dis 2018;12:e0006440. [PMC free article: PMC5931686] [PubMed: 29677199]
- 2.
- Pan X, Abduljalil K, Almond LM, et al. Supplementing clinical lactation studies with PBPK modeling to inform drug therapy in lactating mothers: Prediction of primaquine exposure as a case example. CPT Pharmacometrics Syst Pharmacol 2024;13:386-95. [PMC free article: PMC10941563] [PubMed: 38084656]
- 3.
- Lalloo DG, Shingadia D, Bell DJ, et al. UK Malaria Treatment Guidelines 2016. J Infect 2016;72:635-49. [PMC free article: PMC7132403] [PubMed: 26880088]
- 4.
- Centers for Disease Control and Prevention. CDC Yellow Book 2020: Health Information for International Travel. New York: Oxford University Press 2019. https://wwwnc
.cdc.gov /travel/yellowbook/2020 /travel-related-infectious-diseases /malaria - 5.
- Gilder ME, Hanpithakphong W, Hoglund RM, et al. Primaquine pharmacokinetics in lactating women and breastfed infant exposures. Clin Infect Dis 2018;67:1000–7. [PMC free article: PMC6137118] [PubMed: 29590311]
- 6.
- Brummaier T, Gilder ME, Gornsawun G, et al. Vivax malaria in pregnancy and lactation: A long way to health equity. Malar J 2020;19:40. [PMC free article: PMC6977346] [PubMed: 31969155]
Substance Identification
Substance Name
Primaquine
CAS Registry Number
90-34-6
Drug Class
Breast Feeding
Milk, Human
Anti-infective Agents
Antiparasitic Agents
Antimalarials
Antiprotozoal Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Chloroquine.[Drugs and Lactation Database (...]Review Chloroquine.. Drugs and Lactation Database (LactMed®). 2006
- Review Hydroxychloroquine.[Drugs and Lactation Database (...]Review Hydroxychloroquine.. Drugs and Lactation Database (LactMed®). 2006
- Review Melatonin.[Drugs and Lactation Database (...]Review Melatonin.. Drugs and Lactation Database (LactMed®). 2006
- Review Sulfasalazine.[Drugs and Lactation Database (...]Review Sulfasalazine.. Drugs and Lactation Database (LactMed®). 2006
- Review Metoclopramide.[Drugs and Lactation Database (...]Review Metoclopramide.. Drugs and Lactation Database (LactMed®). 2006
- Primaquine - Drugs and Lactation Database (LactMed®)Primaquine - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
