Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
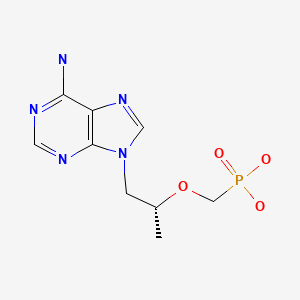
CASRN: 147127-20-6
Drug Levels and Effects
Summary of Use during Lactation
Tenofovir is available in the U.S. in two forms, tenofovir disoproxil fumarate and tenofovir alafenamide. Both release tenofovir, but tenofovir disoproxil fumarate releases tenofovir in the bloodstream whereas tenofovir alafenamide enters cells before releasing tenofovir. Most published experience is with tenofovir disoproxil fumarate in HIV therapy and prophylaxis. Exposure of the breastfed infant to tenofovir is trivial in HIV-positive mothers and HIV-negative mothers treated for HIV prophylaxis or hepatitis B infection.[1,2] Some data indicate that tenofovir milk levels decrease with time after delivery. Among HIV-positive mothers who have breastfed during tenofovir disoproxil fumarate therapy no infant adverse effects have occurred up to 2 years of age. Tenofovir alfenamide use results in even lower milk levels and infant dosages than tenofovir disoproxil fumarate. Achieving and maintaining viral suppression with antiretroviral therapy decreases breastfeeding transmission risk to less than 1%, but not zero. Individuals with HIV who are on antiretroviral therapy with a sustained undetectable viral load and who choose to breastfeed should be supported in this decision. If a viral load is not suppressed, banked pasteurized donor milk or formula is recommended.[3,4]
Pre-exposure prophylaxis (PrEP) regimens containing tenofovir are acceptable for use in HIV-negative nursing mothers.[5] Maternal use of prophylactic vaginal tenofovir (investigational in the U.S.) also does not appear to present a risk to the breastfed infant.[6] In hepatitis B, expert reviews of available data and most professional guidelines state that there is no justification for contraindicating the use of tenofovir during breastfeeding.[7-11] One guideline suggests discussing the lack of long-term safety data with the mother.[10] No differences exist in infection rates between breastfed and formula-fed infants born to hepatitis B-infected women, as long as the infant receives hepatitis B immune globulin and hepatitis B vaccine at birth.
Drug Levels
Tenofovir has poor bioavailability and is available commercially as the more bioavailable tenofovir disoproxil fumarate and tenofovir alfenamide. Tenofovir disoproxil fumarate releases tenofovir in the bloodstream whereas tenofovir alafenamide enters cells before releasing tenofovir. Both are metabolized intracellularly to the active metabolite tenofovir diphosphate. The bioavailability of tenofovir and tenofovir diphosphate from breastmilk are not known, but presumed to be extremely low. Tenofovir (not in a salt form) is also being investigated as a gel for vaginal use in prophylaxis.
Maternal Levels. Tenofovir disoproxil fumarate. Five exclusively breastfeeding mothers received oral tenofovir disoproxil fumarate 300 mg plus emtricitabine 200 mg and nevirapine 200 mg at the start of labor, then oral tenofovir disoproxil fumarate 300 mg daily and emtricitabine 200 mg for 7 days postpartum. A total of 16 concurrent maternal blood and milk samples were collected on days 1, 2, 3, and 7 postpartum between 10 minutes and 21 hours after the mothers’ doses. Median peak and trough tenofovir concentrations in breastmilk were 14.1 mcg/L and 6.8 mcg/L, respectively. The authors estimated that an exclusively breastfed infant would receive about 0.03% of the proposed infant dose for tenofovir and achieve trivial infant serum concentrations that would likely have no adverse consequences.[12]
In a multicenter study in Malawi and Brazil, mothers were given a single dose of either 600 mg or 900 mg of tenofovir disoproxil fumarate during labor. Breastmilk samples were collected from mothers at various times postpartum. Tenofovir was detected (>2.5 mcg/L) in three-fourths of samples collected from 25 mothers during the first 2 days postpartum. Levels ranged from 6.3 to 17.8 mcg/L. At 4 to 6 days postpartum, only one milk sample of 21 had a detectable tenofovir level of 15.7 mcg/L.[13]
Women in Malawi received the option B+ regimen for prevention of mother-to-child transmission of HIV consisting of tenofovir disoproxil fumarate, lamivudine and efavirenz between 6 and 8 pm daily. The tenofovir disoproxil fumarate dose was not stated, but was presumably 300 mg daily. Milk samples collected in the morning from 33 women at month 1 postpartum had a median tenofovir concentration of 5 mcg/L (IQR 0 to 6.1 mcg/L). Milk samples collected in the morning from 47 women at month 12 postpartum had a median tenofovir concentration of 2.5 mcg/L (IQR 0 to 5.5 mcg/L).[14]
Fifty HIV-negative women who were nursing their infants were given pre-exposure prophylaxis daily with the combination of tenofovir disoproxil fumarate 300 mg and emtricitabine 200 mg by directly observed therapy for 10 days. On days 7 and 10 of therapy, peak milk samples were obtained 1 to 2 hours after a dose and trough samples were obtained 23 to 24 hours after the previous dose. The median peak milk tenofovir concentration was 3.2 mcg/L and the trough concentration was 3.3 mcg/L. These values represent an estimated daily dosage of 0.47 to 0.49 mcg/kg, which is less than 0.01% of the proposed infant therapeutic dosage.[15]
Tenofovir was measured in 6 HIV-positive nursing mothers after a 300 mg dose of tenofovir disoproxil fumarate during ongoing therapy. Tenofovir reached a peak breastmilk concentration of 5.9 mcg/L (range 5.5 to 8.0 mcg/L) at an average of 3 hours (range 1 to 7) hours after the dose.[16]
Forty-eight Nigerian and Ugandan women took 300 mg of tenofovir disoproxil fumarate once daily as part of a combination therapy for HIV. Expressed milk samples were taken before the dose and at several times in the 12 hours after the morning dose (n = 30) or at 12, 16 and 20 hours after a dose given the previous evening (n = 18). The median peak breastmilk concentration from dried breastmilk spots was 5.98 mcg/L (IQR 0 to 8.05 mcg/L) at a median of 4 hours after the dose (IQR 1 to 6 hours).[17]
A meta-analysis of 4 previous studies[12,14,15,17] calculated that breastfed infants would receive only 0.03% of the recommend dose of tenofovir.[1]
Eleven mothers with chronic hepatitis B who had been taking tenofovir disoproxil fumarate 245 mg daily for at least one month donated breastmilk samples at a median of 12 hours (IQR 10 to 17 hours) after a dose. The median concentration in breastmilk was 6.69 mcg/L (IQR 4.88 to 7.03) mcg/L. The mean concentration was 6.41 mcg/L (range 4.44 to 10 mcg/L).[18]
Twenty-four mothers taking tenofovir disoproxil fumarate 300 mg once daily provided milk samples at a median of 16 hours after a dose. The median drug concentration in milk was 5 mcg/L, which resulted in an estimated infant dosage of 1 mcg/kg daily and a relative infant dose of 0.01% of the maternal weight-adjusted dosage.[19]
Thirty women with hepatitis B were treated with tenofovir disoproxil fumarate 300 mg daily from week 24 to 32 of pregnancy and until three months postpartum. Twenty-seven women collected milk samples at 24, 48 and 72 hours after a dose. Three women provide milk samples before a dose and at 0.5, 1, 2, 4, 8, 12, 24, 48 and 72 hours after a dose. The highest tenofovir concentration in 81 milk samples was 14.5 mcg/L on the first day after the dose. The median concentrations of tenofovir at 24, 48, and 72 hours after the last dose were 4 mcg/L, 2 mcg/L, and 1.1 mcg/L, respectively. The AUC 0-48 hours of tenofovir at three months postpartum was 3.1 times lower than at one month postpartum. Milk levels were 11.6% and 1% of maternal blood and amniotic fluid levels, respectively. The authors estimated that a fully breastfed infant would receive a dose of 0.21 to 1.76 mcg/kg daily, which represents up to 0.029% of the proposed pediatric therapeutic daily oral dose.[2]
Tenofovir alfenamide. Fifty-two pregnant women with hepatitis B were given either tenofovir alfenamide (n = 26) or tenofovir disoproxil fumarate (n = 26) from 28 weeks of pregnancy to delivery to prevent mother-to-child transmission. Dosages were not stated. Milk samples obtained 6 hours after delivery from the women taking tenofovir alfenamide contained no detectable (<0.5 mcg/L) tenofovir. Milk samples obtained 6 hours after delivery from the women taking tenofovir disoproxil fumarate contained a median of 12.83 (IQR 7.46–29.46) mcg/L of tenofovir.[20]
Thirty-six lactating women with hepatitis B were given tenofovir alfenamide 25 mg once a day with food and another 36 were given 300 mg of tenofovir disoproxil fumarate once a day. Breast milk samples from both groups were collected at 48 hours postpartum, but the time with respect to doses was not reported. Tenofovir measured by mass spectrometry was undetectable (lower limit of assay not specified) in breastmilk in all of the women given tenofovir alfenamide, while only 4 of the women who received tenofovir disoproxil fumarate had undetectable tenofovir levels. The average milk tenofovir levels in the remaining 32 mothers was 19.2 mcg/L.[21]
Women with hepatitis B received either tenofovir alfenamide 25 mg (n = 12) or tenofovir disoproxil fumarate 300 mg (n = 4) daily from 24 to 28 weeks of gestation until the 4th week postpartum. Milk samples were collected before the dose and 1, 2, 4, 6, 8 hours after the dose on the 3rd day postpartum. Trough and 1-hour post-dose milk samples were also collected on days 15 and 30 postpartum in mothers who received tenofovir alfenamide and on the 7th day postpartum in mothers receiving tenofovir disoproxil fumarate. Mothers who received tenofovir alfenamide had an average peak milk concentration of 101 mcg/L at a median peak time of 4 hours after the dose. Mothers who received tenofovir disoproxil fumarate had a lower average peak milk concentration of 22 mcg/L at a median peak time of 4 hours after the dose. Trough and 1-hour milk tenofovir levels decreased by about 45% at 15 and 30 days postpartum in the tenofovir alfenamide group and by more than half in the tenofovir disoproxil fumarate group at 7 days postpartum.[22] Using the highest milk level on day 3 postpartum, infants would receive a maximum of 15 mcg/kg daily; the average level would be considerably lower.
Eight women with chronic hepatitis B took oral tenofovir alafenamide 25 mg daily. Milk samples were taken pre-dose, and 0.5 hours, 1 to 1.5 hours, 2,5 to 3.5 hours, 5 to 6 hours, 8 hours and 24 hours after a dose and analyzed for tenofovir alfenamide and tenofovir. For tenofovir alafenamide, the median steady-state peak breastmilk level was 1.7 mcg/L (IRQ 0.22 to 5.65 mcg/L) at a median of 0.5 hours after a dose. The median half-life in milk was 0.5 hours and by 6 to 8 hours milk levels were undetectable (<0.05 mcg/L) in 7 of the 8 women. The median breastmilk tenofovir alafenamide concentration was 0.125 mcg/L (range 0.06 to 11.6 mcg/L), which resulted in an estimated infant dose of 18.75 ng/kg daily and a relative infant dose of 0.005% of the maternal weight-adjusted dose. For tenofovir, the median steady-state peak breastmilk level was 53.1 mcg/L (IRQ 29.6 to 57.8 mcg/L) at a median of 5.5 hours after a dose. The median half-life of tenofovir in milk was 27.7 hours. The mean tenofovir concentration in milk was 33.3 mcg/L in breastmilk, which would result in an estimated infant dosage of about 5 mcg/kg daily.[23]
Five mothers taking tenofovir alfenamide 25 mg once daily provided milk samples at a median of 8.5 hours after a dose. The median drug concentration in milk was 45 mcg/L, which resulted in an estimated infant dosage of 7 mcg/kg daily.[19]
Tenofovir vaginal gel. Seventeen women received 40 mg vaginal doses of 1% tenofovir gel daily for 6 days, with the first and last doses applied in clinic under observation. Two women reported using four doses at home, eleven women reported five doses, and two reported six doses. Breastmilk samples were collected before and 2, 4, and 6 hours after the first and last doses. Only 25% of milk samples contained detectable (>1 mcg/L) tenofovir post-dose on day 0, 12.5% at pre-dose on day 6, and 37.5% post-dose on day 6. Breastmilk tenofovir concentrations ranged from 0 to 0.75 mcg/L on day 0 and from 0 to 1.6 mcg/L on day 6.[6]
Infant Levels. Tenofovir disoproxil fumarate. Five infants were exclusively breastfed by 4 mothers who took tenofovir 245 mg (presumably 300 mg of tenofovir disoproxil fumarate) daily. At an average of 1.8 months of age, infant serum tenofovir concentrations were measured. Tenofovir was undetectable (<0.005 mg/L) in the serum of 4 of the infants, and 0.0055 mg/L in the serum of one infant.[24]
In a study of women and their infants receiving antiretroviral therapy for HIV infection, mothers who received a tenofovir disoproxil fumarate-containing regimen were compared to those who did not. The risk of infant death was reduced by 57% among infants who were breastfed and exposed to tenofovir compared to those who were not breasted. No alterations in growth and development were seen among breastfed infants in 2 years of follow-up.[25]
Blood samples were taken from 25 breastfed infants of mothers who were receiving option B+ regimen for prevention of mother-to-child transmission of HIV consisting of tenofovir disoproxil fumarate, lamivudine and efavirenz between 6 and 8 pm daily. The tenofovir dose was not stated, but was presumably 300 mg daily. The median morning infant plasma concentration of tenofovir at 6 months of age was 24 mcg/L (IQR 0 to 51.6 mcg/L). The median morning infant plasma concentration of tenofovir at 12 months of age was 0 mcg/L.[14]
Fifty HIV-negative women who were nursing their infants were given pre-exposure prophylaxis daily with the combination of tenofovir disoproxil fumarate 300 mg and emtricitabine 200 mg by directly observed therapy for 10 days. A single infant blood sample was obtained after the mother’s 7th dose. Of 49 infant blood samples collected, 46 had an undetectable (<0.31 mcg/L) concentration of tenofovir. The 3 with detectable levels contained 0.9, 0.9 and 17.4 mcg/L of tenofovir.[15]
Tenofovir disoproxil fumarate 300 mg daily was given to 6 HIV-positive nursing mothers. None of the breastfed infants had detectable tenofovir serum levels.[16]
Forty-eight Nigerian and Ugandan women took 300 mg of tenofovir disoproxil fumarate once daily either in the morning or evening as part of a combination therapy for HIV. Their exclusively breastfed infants were fed on demand and had blood samples taken at 2 and 8 hours after the dose. Dried blood spots were analyzed and no infants had a measurable (>4.2 mcg/L) tenofovir blood concentration.[17]
Eleven mothers with chronic hepatitis B who had been taking tenofovir disoproxil fumarate 245 mg daily for at least one month were breastfeeding their infants, 7 exclusively. Infant blood samples were obtained at a median of 12 hours (IQR 10 to 17 hours) after a dose. Infants had a median age of 3 months (IQR 2 to 6 months). All infants had undetectable (<4 mcg/L) tenofovir in their plasma.[18]
Ten infants were breastfed by mothers taking tenofovir disoproxil fumarate 300 mg once daily, although the extent of breastfeeding was not sated. Infant serum concentrations taken between 2 and 19 hours after maternal drug intake at 1 month of age were undetectable.[19]
Tenofovir alfenamide. Eight mothers with chronic hepatitis B who were predominantly breastfeeding took oral tenofovir alafenamide 25 mg daily. Infant urine was collected from the time of the dose to 8 hours after the dose. Tenofovir was detectable in 3 of 7 infant urine samples with the remaining 4 samples having undetectable (<10 mcg/L) levels. The three detectable infant urine concentrations of tenofovir were 12, 24 and 25 mcg/L.[23]
An infant was breastfed by a mother taking tenofovir alafenamide 25 mg daily, although the extent of breastfeeding was not sated. The infant’s serum concentrations taken19 hours after maternal drug intake at 1 month of age was undetectable.[19]
Tenofovir vaginal gel. Seventeen women received 40 mg vaginal doses of 1% tenofovir gel daily for 6 days, with the first and last doses applied in clinic under observation. Two women reported four doses at home, eleven women reported inserting five doses, and two reported six doses. Infant blood was collected 6 hours after the maternal dose, which ranged from 1 to 4 hours after breastfeeding. Six infants (37.5%) had detectable tenofovir levels after the maternal dose on day 0, and 12 (75%) of infants post-dose on day 6. In infants with detectable tenofovir serum concentrations, day 6 levels were higher (median 2.4 mcg/L) than on day 0 concentrations (median 0). No difference was seen in the anti-HIV activity of breastmilk between day 0 and day 6.[6]
Effects in Breastfed Infants
Two newborn infants whose mothers were treated with tenofovir 245 mg (presumably 300 mg of tenofovir disoproxil fumarate) daily were exclusively breastfed for 3 months. At 4 months of age, neither showed any adverse outcomes on standard developmental parameters.[24]
Five women with hepatitis B infection were treated with tenofovir disoproxil fumarate 300 mg daily beginning in the third trimester of pregnancy and continuing postpartum. Although instructed not to breastfeed, 5 mothers breastfed (extent not stated) their newborn infants. No short-term adverse reactions were seen and the infants’ HBsAg was negative between 28 and 36 weeks of age.[26]
Fourteen mothers were treated with tenofovir disoproxil fumarate (dosage unspecified) during pregnancy (12 beginning in the first trimester) for hepatitis B. Three of the mothers breastfed while taking tenofovir. No adverse outcomes were noted in their breastfed infants up to 1 year of age.[27]
In a study of 50 infants breastfed by HIV-negative women who were given pre-exposure prophylaxis daily with the combination of tenofovir disoproxil fumarate 300 mg and emtricitabine 200 mg by directly observed therapy for 10 days, 2 infants reportedly had diarrhea lasting 2 to 3 days. No other side effects were reported.[15]
A study of 136 breastfed infants of mothers who took tenofovir disoproxil fumarate, efavirenz and lamivudine during pregnancy and postpartum (Option B+) in Malawi measured bone markers at 1, 6 and 12 months of age. Markers included bone-specific alkaline phosphatase and C-terminal telopeptide of type I collagen. Although tenofovir is known to affect bone density and bone mineral density in adults, no effects were seen on infants’ bone markers in the study.[28]
In a long-term study of tenofovir disoproxil fumarate for chronic hepatitis B, 3 women reportedly breastfed their infants (extent not stated). None of the infants had any adverse effects up to 1 year of age.[29]
A study of pregnant women with hepatitis B infection in China enrolled 143 women. Tenofovir disoproxil fumarate 300 mg daily was given starting at 22 to 33 weeks of pregnancy and continued postpartum. Thirty-one mothers breastfed (extent not stated) their infants who received standard hepatitis B prophylaxis. At 28 weeks postpartum, infant physical and neurologic development was within national standards and none had developed hepatitis B infection. Mild side effects of cough and fever were reported in >5% of infants. Less frequent reactions included skin rash, diarrhea, vomiting, jaundice and pneumonia. All adverse effects were judged not to be related to the drug by the authors.[30]
In a study of 17 nursing mothers who receive 40 mg of 1% vaginal tenofovir gel daily for 6 days, 4 of 17 infants had one or more adverse effects. There were a total of 8 adverse reactions. Seven were mild, and one had diarrhea that was thought to be related to tenofovir exposure.[6]
A prospective cohort study in Malawi compared the infants of HIV+ mothers taking tenofovir disoproxil fumarate and efavirenz (n = 260) to infants of mothers who were HIV negative (n = 125). Infants were followed for growth and development for up to 18 months at which time there were 169 mother-infant pairs in the treatment group and 54 in the HIV-negative group. No difference was found in the growth and development of the breastfed infants of treated women compared to the infants of untreated mothers.[31]
Thirty women with hepatitis B were treated with tenofovir disoproxil fumarate 300 mg daily from week 24 to 32 of pregnancy and until three months postpartum. Their breastfed infants (extent not stated) had no abnormal signs or symptoms reported during maternal therapy. The physical growth parameters (height, weight and head circumference) stratified by sex of infants at birth, 3, 6 and 12 months postpartum were normal.[2]
An open-label, controlled, multicenter phase 3 trial women who were confirmed HIV-positive were randomized to receive one of 3 regimens: dolutegravir, emtricitabine, and tenofovir alafenamide (n = 208); dolutegravir, emtricitabine, and tenofovir disoproxil fumarate (n = 202); or efavirenz, emtricitabine, and tenofovir disoproxil fumarate (n = 207). The regimens were started at 14 to 28 weeks of pregnancy and continued postpartum. Of the 617 liveborn infants, 99% were breastfeeding at time of last infant HIV test, which was as late as 50 weeks of age. The mean infant duration on the study was 47.6 weeks of age. Infants who had any clinical or laboratory adverse event of grade 3 or higher ranged from 25 to 31%, but was not statistically significant between groups. Dolutegravir-containing regimens resulted in lower rates of virological failure, HIV drug resistance, and infant mortality up to 50 weeks postpartum compared with efavirenz, emtricitabine, and tenofovir disoproxil fumarate.[32]
A study compared the bone mineral densities of the infants of mothers with HIV infections who were exposed to maternal tenofovir disoproxil fumarate, emtricitabine and lopinavir-ritonavir or infant treatment with nevirapine for HIV prophylaxis throughout breastfeeding. Infants in the tenofovir disoproxil fumarate group had a slightly lower lumbar spine bone mineral content at week 26 postpartum than those receiving nevirapine, but the difference was judged to be not clinically relevant.[33]
Effects on Lactation and Breastmilk
A preliminary study of Ugandan women compared the milk composition of women receiving a tenofovir-based regimen for HIV to that of women who were not infected with HIV. Women with HIV on tenofovir-based antiretroviral therapy had higher milk calcium in the first months of lactation (193 and 188 mg/L compared to 177 and 172 mg/L at 2 and 14 weeks postpartum, respectively) and a greater overall reduction in the first year of lactation than women without HIV (10 and 23% decrease compared to 8% and 16% decrease at 6 and 12 months). However, the only statistically significant differences were at 14 weeks postpartum for serum calcium and at 6 to 12 months for the percentage decrease in serum calcium in the HIV-infected women.[34]
Alternate Drugs to Consider
(Hepatitis B) Interferon Alfa, Lamivudine
References
- 1.
- Bierhoff M, Smolders EJ, Tarning J, et al. Pharmacokinetics of oral tenofovir disoproxil fumarate in pregnancy and lactation: A systematic review. Antivir Ther 2019;24:529-40. [PubMed: 31868655]
- 2.
- Li S, Jin J, Jiang Y, et al. The low level of tenofovir in breast milk supports breastfeeding in HBV-infected mothers. Int J Antimicrob Agents 2023:106726. [PubMed: 36646229]
- 3.
- World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. https://www
.who.int/publications /i/item/9789240031593 [PubMed: 34370423] - 4.
- Department of Health and Human Services. Recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the United States. Infant feeding for individuals with HIV in the United States. 2023. https:
//clinicalinfohivgov /en/guidelines /perinatal/infant-feeding-individuals-hiv-united-states?view=full - 5.
- Hodges-Mameletzis I, Fonner VA, Dalal S, et al. Pre-exposure prophylaxis for HIV prevention in women: Current status and future directions. Drugs 2019;79:1263-76. [PubMed: 31309457]
- 6.
- Noguchi LM, Montgomery ET, Biggio JR, et al. Detectable tenofovir levels in breastfeeding infants of mothers exposed to topical tenofovir. Antimicrob Agents Chemother 2016;60:5616-9. [PMC free article: PMC4997886] [PubMed: 27401570]
- 7.
- Ehrhardt S, Xie C, Guo N, et al. Breastfeeding while taking lamivudine or tenofovir disoproxil fumarate: A review of the evidence. Clin Infect Dis 2015;60:275-8. [PubMed: 25313254]
- 8.
- Mofenson LM, Baggaley RC, Mameletzis I. Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding. Aids 2017;31:213-32. [PubMed: 27831952]
- 9.
- Hu X, Wang L, Xu F. Guides concerning tenofovir exposure via breastfeeding: A comparison of drug dosages by developmental stage. Int J Infect Dis 2019;87:8-12. [PubMed: 31357055]
- 10.
- Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261-83. [PMC free article: PMC5987259] [PubMed: 26566064]
- 11.
- EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98. [PubMed: 28427875]
- 12.
- Benaboud S, Pruvost A, Coffie PA, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d'Ivoire, in the ANRS 12109 TEMAA Study, step 2. Antimicrob Agents Chemother 2011;55:1315-7. [PMC free article: PMC3067089] [PubMed: 21173182]
- 13.
- Mirochnick M, Taha T, Kreitchmann R, et al. Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J Acquir Immune Defic Syndr 2014;65:33-41. [PMC free article: PMC3912736] [PubMed: 23979002]
- 14.
- Palombi L, Pirillo MF, Marchei E, et al. Concentrations of tenofovir, lamivudine and efavirenz in mothers and children enrolled under the Option B-Plus approach in Malawi. J Antimicrob Chemother 2016;71:1027-30. [PubMed: 26679247]
- 15.
- Mugwanya KK, Hendrix CW, Mugo NR, et al. Pre-exposure prophylaxis use by breastfeeding HIV-uninfected women: A prospective short-term study of antiretroviral excretion in breast milk and infant absorption. PLoS Med 2016;13:e1002132. [PMC free article: PMC5038971] [PubMed: 27676257]
- 16.
- Waitt C, Diliiy Penchala S, Olagunju A, et al. Development, validation and clinical application of a method for the simultaneous quantification of lamivudine, emtricitabine and tenofovir in dried blood and dried breast milk spots using LC–MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2017;1060:300-7. [PMC free article: PMC5588985] [PubMed: 28651173]
- 17.
- Waitt C, Olagunju A, Nakalema S, et al. Plasma and breast milk pharmacokinetics of emtricitabine, tenofovir and lamivudine using dried blood and breast milk spots in nursing African mother-infant pairs. J Antimicrob Chemother 2018;73:1013-9. [PMC free article: PMC5890695] [PubMed: 29309634]
- 18.
- Erturk US, Mete B, Ozaras R, et al. Plasma and breast milk pharmacokinetics of tenofovir disoproxil fumarate in nursing mother with chronic hepatitis B-infant pairs. Antimicrob Agents Chemother 2021;65:e0111021. [PMC free article: PMC8448110] [PubMed: 34310204]
- 19.
- Aebi-Popp K, Kahlert CR, Crisinel PA, et al. Transfer of antiretroviral drugs into breastmilk: A prospective study from the Swiss Mother and Child HIV Cohort Study. J Antimicrob Chemother 2022;77:3436-42. [PMC free article: PMC9704434] [PubMed: 36177836]
- 20.
- Bojun L, Ye G, Yan W, et al. Tenofovir (TFV) or tenofovir alafenamide (TAF) concentration in breast milk and infants' cord blood, with tenofovir disoproxil fumarate (TDF) or TAF treatment in pregnancy. Hepatol Int 2020;14 (Suppl 1):S78. doi:10.1007/s12072-020-10030-4 [CrossRef]
- 21.
- Li B, Liu Z, Liu X, et al. Efficacy and safety of tenofovir disoproxil fumarate and tenofovir alafenamide fumarate in preventing HBV vertical transmission of high maternal viral load. Hepatol Int 2021;15:1103-8. [PubMed: 34312798]
- 22.
- Yang N, Zhou G, Cheng X, et al. Distribution evaluation of tenofovir in the breast milk of mothers with HBeAg-positive chronic HBV infection after treatment with tenofovir alafenamide and tenofovir disoproxil fumarate by a sensitive UPLC-MS/MS method. Front Pharmacol 2021;12:734760. [PMC free article: PMC8414412] [PubMed: 34483946]
- 23.
- Kayes T, Crane H, Symonds A, et al. Plasma and breast milk pharmacokinetics of tenofovir alafenamide in mothers with chronic hepatitis B infection. Aliment Pharmacol Ther 2022;56:510-8. [PubMed: 35599363]
- 24.
- Gouraud A, Millaret A, Bernard N, et al. Tenofovir exposure through breast feeding: Serum concentrations in neonates and clinical follow-up. Fundam Clin Pharmacol 2012;26 (Suppl 1):9. doi:10.1111/j.1472-8206.2012.01032.x [CrossRef]
- 25.
- Gibb DM, Kizito H, Russell EC, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med 2012;9:e1001217. [PMC free article: PMC3352861] [PubMed: 22615543]
- 26.
- Pan CQ, Mi LJ, Bunchorntavakul C, et al. Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: A case series. Dig Dis Sci 2012;57:2423-9. [PMC free article: PMC7469074] [PubMed: 22543886]
- 27.
- Ganne-Carrie N, Causse X, Zarski JP, et al. Efficacy and safety results of tenofovir DF (TDF) treatment from the first trimester in HBV pregnant women in real-life clinical practice. Hepatology 2013;58 (Suppl 1):664A-665A. doi:10.1002/hep.26727 [CrossRef]
- 28.
- Floridia M, Liotta G, Andreotti M, et al. Levels of bone markers in a population of infants exposed in utero and during breastfeeding to tenofovir within an Option B+ programme in Malawi. J Antimicrob Chemother 2016;71:3206-3211. [PubMed: 27494909]
- 29.
- Marcellin P, Zoulim F, Hezode C, et al. Effectiveness and safety of tenofovir disoproxil fumarate in chronic hepatitis B: A 3-year, prospective, real-world study in France. Dig Dis Sci 2016;61:3072-83. [PMC free article: PMC5020114] [PubMed: 26821154]
- 30.
- Wang M, Bian Q, Zhu Y, et al. Real-world study of tenofovir disoproxil fumarate to prevent hepatitis B transmission in mothers with high viral load. Aliment Pharmacol Ther 2019;49:211-7. [PubMed: 30506691]
- 31.
- Kapito-Tembo AP, Bauleni A, Wesevich A, et al. Growth and neurodevelopment outcomes in HIV, tenofovir and efavirenz exposed breastfed infants in PMTCT Option B+ program in Malawi. J Acquir Immune Defic Syndr 2021;86:81-90. [PubMed: 33027153]
- 32.
- Chinula L, Ziemba L, Brummel S, et al. Efficacy and safety of three antiretroviral therapy regimens started in pregnancy up to 50 weeks post partum: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet HIV 2023;10:e363-e374. [PMC free article: PMC10280394] [PubMed: 37167996]
- 33.
- Vhembo T, Baltrusaitis K, Tierney C, et al. Bone and renal health in infants with or without breastmilk exposure to tenofovir-based maternal antiretroviral treatment in the PROMISE randomized trial. J Acquir Immune Defic Syndr 2023;93:431-7. [PMC free article: PMC10337310] [PubMed: 37199427]
- 34.
- Nabwire F, Hamill MM, Fowler MG, et al. Milk calcium and phosphorus in Ugandan women with HIV on tenofovir-based antiretroviral therapy. J Hum Lact 2023;39:288-99. [PMC free article: PMC10115928] [PubMed: 36715180]
Substance Identification
Substance Name
Tenofovir
CAS Registry Number
147127-20-6
Drug Class
Breast Feeding
Milk, Human
Anti-Infective Agents
Antiviral Agents
Anti-HIV Agents
Anti-Retroviral Agents
Reverse Transcriptase Inhibitors
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Lamivudine.[Drugs and Lactation Database (...]Review Lamivudine.. Drugs and Lactation Database (LactMed®). 2006
- Review Emtricitabine.[Drugs and Lactation Database (...]Review Emtricitabine.. Drugs and Lactation Database (LactMed®). 2006
- Review Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF).[Biochem Pharmacol. 2016]Review Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF).De Clercq E. Biochem Pharmacol. 2016 Nov 1; 119:1-7. Epub 2016 Apr 29.
- Efficacy and safety of tenofovir disoproxil fumarate and tenofovir alafenamide fumarate in preventing HBV vertical transmission of high maternal viral load.[Hepatol Int. 2021]Efficacy and safety of tenofovir disoproxil fumarate and tenofovir alafenamide fumarate in preventing HBV vertical transmission of high maternal viral load.Li B, Liu Z, Liu X, Liu D, Duan M, Gu Y, Liu Q, Ma Q, Wei Y, Wang Y. Hepatol Int. 2021 Oct; 15(5):1103-1108. Epub 2021 Jul 26.
- Review Antivirals for prevention of hepatitis B virus mother-to-child transmission in human immunodeficiency virus positive pregnant women co-infected with hepatitis B virus.[Cochrane Database Syst Rev. 2023]Review Antivirals for prevention of hepatitis B virus mother-to-child transmission in human immunodeficiency virus positive pregnant women co-infected with hepatitis B virus.Ugwu EO, Eleje GU, Ugwu AO, Nwagha UI, Ikechebelu JI, Umeh UA, Okafor HU. Cochrane Database Syst Rev. 2023 Jun 12; 6(6):CD013653. Epub 2023 Jun 12.
- Tenofovir - Drugs and Lactation Database (LactMed®)Tenofovir - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
