Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
CASRN: 22839-47-0
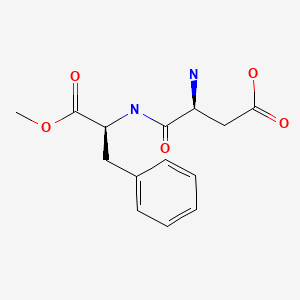
Drug Levels and Effects
Summary of Use during Lactation
Aspartame is not detectable in breastmilk after maternal ingestion because it is rapidly broken down in the mother's body. An extremely large intake of aspartame (equivalent to 17 cans of soda or 100 packets of Equal Sweetener) can slightly increase the amount of phenylalanine in breastmilk. Phenylalanine concentrations in milk return to baseline by 12 hours after a large single dose of aspartame. Although it is prudent to avoid the use of aspartame in women who are nursing an infant with phenylketonuria, amounts that are typically ingested in aspartame-sweetened foods and beverages do not result in any additional risk to breastfed infants with phenylketonuria. Ingestion of diet drinks containing low-calorie sweeteners might increase the risk of vomiting in breastfed infants. An association between low-calorie sweeteners, and especially aspartame, and the risk of autism in boys has been found, but more data are needed to establish a cause-and-effect relationship.
Drug Levels
Aspartame is rapidly metabolized in the body to 2 amino acids that occur naturally in breastmilk, aspartic acid and phenylalanine and methanol.
Maternal Levels. Six lactating women an average of 98 days postpartum (range 42 to 159 days) were given aspartame or lactose orally in a dose of 50 mg/kg (equivalent to 17 aspartame-sweetened sodas or 100 packets of Equal in a 68 kg [150 lb.] adult[1]) on 2 separate occasions 2 weeks apart in a randomized crossover trial. Breastmilk samples were collected over the next 24 hours. Breastmilk phenylalanine levels increased to a maximum of about 6 mg/L between 1 and 8 hours after the dose of aspartame and returned to baseline by about 12 hours after the dose. Lactose had no effect on breastmilk phenylalanine levels. Aspartame was not detected (<1.4 mg/L) in breastmilk at any time. The authors calculated that if the milk phenylalanine concentration were elevated for 24 hours to the maximum found in this study, a fully breastfed infant would receive an additional 0.5 mg/kg daily above the normal intake of 79 mg/kg of phenylalanine that occurs naturally in breastmilk.[2]
Twenty lactating women completed background questionnaires about breastfeeding and the intake of nonnutritive sweeteners in the prior 24 hours. Each then donated a milk sample that was analyzed for the presence of nonnutritive sweeteners. Sweetener intake was primarily from diet sodas and sweetener packets. Of the 14 women who reported intake of a nonnutritive sweetener, none had quantifiable levels of aspartame in their breastmilk.[3]
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
A cross-sectional survey assessed the dietary history of US mothers nursing infants between 11 and 15 weeks of age. The survey was used to estimate the amount of diet soda and fruit drinks consumed by the women. There were no statistically significant differences in infants’ weight or z-scores based on low calorie sweetener exposure. However, infants exposed to low calorie sweetener in milk once or less per week had a statistically significantly higher risk of vomiting than those who were not exposed. Greater exposure was not associated with vomiting. It was not possible to assess the effects of specific sweeteners.[4]
A retrospective dietary recall study compared the use of diet soda and aspartame during pregnancy and/or lactation to the risk of autism in the children. Among boys, autism was associated with three times the likelihood of exposure to aspartame. No statistically significant associations were found in girls.[5] The contribution of exposure during breastfeeding was not separated from the risk of exposure during pregnancy, and intact aspartame is usually not found in milk, so breastmilk exposure cannot be claimed to cause autism based on these data. The authors propose that the methanol metabolite might have an impact on infants.
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
References
- 1.
- Franz M. Is it safe to consume aspartame during pregnancy? A review. Nutrition update. Diabetes Educ 1986;12:145-7. [PubMed: 3634700]
- 2.
- Stegink LD, Filer LJ, Jr, Baker GL. Plasma, erythrocyte and human milk levels of free amino acids in lactating women administered aspartame or lactose. J Nutr 1979;109:2173-81. [PubMed: 512705]
- 3.
- Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health A 2015;78:1029-32. [PMC free article: PMC5583633] [PubMed: 26267522]
- 4.
- Huang Q, Murphy J, Smith ER, Sylvetsky AC. Diet beverage intake during lactation and associations with infant outcomes in the infant feeding practices study II. Nutrients 2021;13:3154. [PMC free article: PMC8472746] [PubMed: 34579031]
- 5.
- Fowler SP, Gimeno Ruiz de Porras D, Swartz MD, et al. Daily early-life exposures to diet soda and aspartame are associated with autism in males: A case-control study. Nutrients 2023;15:3772. [PMC free article: PMC10490529] [PubMed: 37686804]
Substance Identification
Substance Name
Aspartame
CAS Registry Number
22839-47-0
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Effect of repeated ingestion of aspartame-sweetened beverage on plasma amino acid, blood methanol, and blood formate concentrations in normal adults.[Metabolism. 1989]Effect of repeated ingestion of aspartame-sweetened beverage on plasma amino acid, blood methanol, and blood formate concentrations in normal adults.Stegink LD, Filer LJ Jr, Bell EF, Ziegler EE, Tephly TR. Metabolism. 1989 Apr; 38(4):357-63.
- Metabolism of aspartame and its L-phenylalanine methyl ester decomposition product by the porcine gut.[Metabolism. 1991]Metabolism of aspartame and its L-phenylalanine methyl ester decomposition product by the porcine gut.Burgert SL, Andersen DW, Stegink LD, Takeuchi H, Schedl HP. Metabolism. 1991 Jun; 40(6):612-8.
- Repeated ingestion of aspartame-sweetened beverage: effect on plasma amino acid concentrations in individuals heterozygous for phenylketonuria.[Metabolism. 1989]Repeated ingestion of aspartame-sweetened beverage: effect on plasma amino acid concentrations in individuals heterozygous for phenylketonuria.Stegink LD, Filer LJ Jr, Baker GL, Bell EF, Ziegler EE, Brummel MC, Krause WL. Metabolism. 1989 Jan; 38(1):78-84.
- Review A review of the metabolism of the aspartyl moiety of aspartame in experimental animals and man.[J Environ Pathol Toxicol. 1979]Review A review of the metabolism of the aspartyl moiety of aspartame in experimental animals and man.Ranney RE, Oppermann JA. J Environ Pathol Toxicol. 1979 Mar-Apr; 2(4):979-85.
- Review Acesulfame.[Drugs and Lactation Database (...]Review Acesulfame.. Drugs and Lactation Database (LactMed®). 2006
- Aspartame - Drugs and Lactation Database (LactMed®)Aspartame - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
