Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
CASRN: 102767-28-2
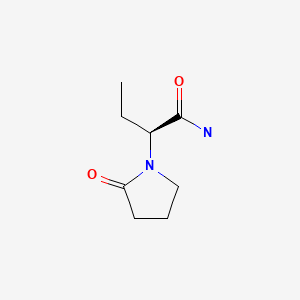
Drug Levels and Effects
Summary of Use during Lactation
Levels of levetiracetam in milk can be relatively high in some women and can occasionally cause sedation and other adverse effects in their breastfed infants. If levetiracetam is required by the mother, it is not necessarily a reason to discontinue breastfeeding. However, the infant should be monitored for drowsiness, adequate weight gain, and developmental milestones, especially in younger, exclusively breastfed infants and when using combinations of anticonvulsants. Maternal serum level monitoring and dosage adjustment is advisable in the early postpartum period if the drug was taken throughout pregnancy and breastfeeding.[1] Some evidence suggests that levetiracetam might reduce the maternal breastmilk supply in some women.
Drug Levels
In published reports of anticonvulsant use during breastfeeding, many women were taking a combination of anticonvulsants. Some other anticonvulsants (e.g., phenytoin, carbamazepine) stimulate the metabolism of other drugs including anticonvulsants, whereas others (e.g., valproic acid) inhibit the metabolism of other drugs. Therefore, the relationship of the maternal dosage to the concentration in breastmilk can be quite variable, making calculation of the weight-adjusted percentage of maternal dosage less meaningful than for other drugs in this database.
Maternal Levels. A woman who was taking phenytoin, valproic acid and an unspecified dosage of levetiracetam had a milk levetiracetam level of 16.9 mg/L 3 hours after a dose, which was 3.1 times her simultaneous serum level.[2]
Breastmilk levels in 12 mothers who were monitored at 4 days and 2 to 3 months postpartum were "significantly lower" than maternal blood levels. Further details were not published in the abstract.[3]
Seven women taking an average of 2430 mg daily (range 1500 to 3500 mg daily) of levetiracetam plus various other anticonvulsants for epilepsy at the time of delivery had foremilk levels measured 3 to 5 days postpartum. Average milk levels were 12.5 mg/L (range 4.8 to 26 mg/L). Milk levels were again measured in 5 of the women plus another woman at one or more of the following times: 2, 4, 6 to 8 weeks and 4 or 10 months postpartum. Specific milk levels were not reported at those times, but the milk to plasma ratio was very similar at those times to the values at 3 to 5 days postpartum.[4]
Eleven mothers who were 4 to 23 days postpartum and taking levetiracetam provided milk samples before nursing. Three women taking 100 mg daily had an average milk levetiracetam concentration of 8.68 mg/L (range 5.79 to 10.55 mg/L); 2 women taking 2000 mg daily had milk levels of 11.7 and 35.7 mg/L; 4 women taking 2500 mg daily had an average milk levetiracetam concentration of 13.95 mg/L (range 10 to 20.4 mg/L); and 2 women taking 3000 mg daily had milk concentrations of 17.4 and 29.1 mg/L. The authors estimated that a fully breastfed infant would receive 7.9% of the maternal weight-adjusted dosage.[5]
A pregnant woman was treated with levetiracetam 1000 mg and lacosamide 100 mg twice daily as well as enoxaparin and labetalol for the rest of her pregnancy and postpartum. Levetiracetam was undetectable (<3 mg/L) in a milk sample on day 5 postpartum (exact time not specified).[6]
Fifty-four women taking levetiracetam during pregnancy and postpartum had measurements of levetiracetam in colostrum taken on day 2 to 4 postpartum for routine monitoring. Colostrum levetiracetam levels ranged from 1.7 to 27.3 mg/L. Another 8 mothers had milk samples taken between day 7 and 31 (median 12 days) postpartum, mostly in the morning before their first dose. Their milk levels ranged from 2.8 to 21.6 mg/L.[7]
Twenty women taking immediate-release levetiracetam collected steady-state milk samples before and 1, 3, 6, 9 and 12 hours after a dose. Average peak milk levels of 55 mg/L (range 32 to 76 mg/L) occurred at a mean of 1 hour (range 1 to 3 hours) after the dose. The average milk concentration was 36 mg/L and the trough breastmilk levetiracetam concentrations averaged 24.4 mg/L (range 8.6 to 42.3 mg/L). The infant daily dosage of levetiracetam was estimated to be 5.39 mg/kg daily in the fully breastfed infants and 2.7 mg/kg daily for the partially breastfed infants, which represents 12.8% and 6.4% of the maximum therapeutic daily dose of levetiracetam for 1- to 6 month-old infants. The relative weight-adjusted dosages were 13.8% and 6.9% of maternal dosage in the fully and partially breastfed infants, respectively.[8]
Infant Levels. An infant (aged approximately 1 to 2 weeks) of a mother taking phenytoin, valproic acid and levetiracetam (dosage unspecified) had a serum levetiracetam level of 1 mg/L 96 hours after the mother discontinued breastfeeding.[2]
Seven breastfed infants whose mothers were taking an average dosage of 2430 mg daily (range 1500 to 3500 mg daily) of levetiracetam plus various other anticonvulsants during pregnancy and lactation had serum levetiracetam measured before the mother's morning dose at 3 to 5 days of age. In 6 infants, levetiracetam was undetectable (<1.7 mg/L), although one of them had a serum level of 13 mg/L at the age of 1 day. A seventh infant who was fully breastfed had a serum level of 2.5 mg/L at 3 to 5 days of age. This infant and had serum levels ranging from 2.5 to 2.9 mg/L during the first 8 weeks of life and an undetectable serum level at 4 months of age.[4]
Ten infants who were 4 to 23 days old were breastfed by mothers taking levetiracetam in dosages of 1000 to 3000 mg daily. Infant plasma levels were obtained 30 to 120 minutes after nursing and before their mother's morning dose (10 to 15 hours after the last evening dose), except for one infant whose plasma was sampled before nursing. The average levetiracetam plasma concentration was 1.9 mg/L (range 0.7 to 3.4 mg/L). The infants' plasma levels averaged 13.5% of the maternal plasma concentration taken before their morning dose. The authors found that 13 newborns' levetiracetam elimination half-life averaged 18 hours, which is 2 to 3 times that of adults.[5]
The 10-day-old breastfed (extent not stated) infant of a mother who was taking levetiracetam 3000 mg daily had a serum concentration of 2.1 mg/L, which was less than half of the low end of the therapeutic range. Timing of the sample was not stated.[9]
In a multicenter study of nursing mother-infant pairs, 58 infants had blood samples taken at about the same time as maternal blood samples. Only 18 of the infants had blood levels of levetiracetam above the lower limit of quantification (1.8 mg/L). The authors estimated the average infant levetiracetam serum concentration to be 0.9 mg/L (range 0.9 to 4.5 mg/L), assuming unquantifiable serum concentrations to be 50% of the lower limit of quantification. Median infant blood levels were 5.3% (range 2.1 to 20.4%) of their mothers’ blood levels.[10]
Fifty-four infants whose mothers were taking levetiracetam during pregnancy and postpartum had measurements of levetiracetam in serum taken on day 2 to 4 postpartum for routine monitoring. The infant serum concentration ranged from 0.5 to 2.3 mg/L, which averaged 19% of the maternal serum concentration. Another 10 infants had serum levetiracetam levels taken between day 7 and 31 (median 12 days) postpartum. Their serum levels ranged from 0.5 to 51 mg/L. Infant serum levels averaged 14% of simultaneous maternal serum levels.[7]
Effects in Breastfed Infants
A woman with epilepsy took phenytoin and valproic acid during pregnancy. She began breastfeeding on day 3 postpartum and had a seizure on day 7 postpartum. Levetiracetam (dosage not reported) was started and the infant became increasingly hypotonic and nursed poorly. Breastfeeding was discontinued and the infant was discharged from the hospital in a healthy condition.[2]
Seven exclusively breastfed infants whose mothers were taking an average dosage of 2430 mg daily (range 1500 to 3500 mg daily) of levetiracetam plus various other anticonvulsants during pregnancy and lactation appeared healthy to the investigators throughout the 6 to 8 week study period. An eighth partially breastfed infant whose mother was taking valproate and oxcarbazepine started taking levetiracetam 9 months postpartum appeared healthy at 10 months of age.[4]
No adverse effects were reported in 10 newborns who were 4 to 23 days old who were breastfed during maternal intake of levetiracetam 1000 to 3000 mg daily. Four mothers were also taking lamotrigine; 1 was taking carbamazepine; and one was taking tiagabine, clobazam and oxcarbazepine.[5]
A woman with long-standing seizure disorder was taking primidone and levetiracetam became pregnant. The dosage of her medications were reduced during pregnancy to provide a levetiracetam serum concentration of 40.5 mg/L and a primidone (phenobarbital) serum concentration of 3.4 mg/L. The mother was instructed to discontinue breastfeeding after 3 days. The following day her infant developed withdrawal seizures. After reinstituting breastfeeding, the infant's seizures stopped and did not recur. The infant had no abnormal findings and was thriving and seizure free at 6 months of age.[11]
The infants (including 3 preterm) of 18 nursing mothers who were taking levetiracetam and called the Pharmacovigilance Center in Lyon, France before breastfeeding were paired with 18 control infants. The median dosage was 1000 mg daily (range 500 to 3000 mg daily) and 8 were receiving at least one additional anticonvulsant. The median duration of breastfeeding was 40 days (range 10 to 224 days), and 13 newborns were exclusively breastfed. Breastfed infants were followed for a median of 9.1 months (range 0.75 to 73 months). One 25-day-old infant whose mother was taking levetiracetam 3000 mg daily plus clobazam was hospitalized for sedation, vomiting, and weight loss, and improved rapidly after breastfeeding discontinuation. Another infant exposed to levetiracetam and clobazam had poor weight gain, but it appeared to be caused by poor milk production. Other than these infants, all levetiracetam and control infants grew and developed normally.[9]
A pregnant woman suffered blood clots in the sinuses and 2 small intracranial hemorrhages followed by status epilepticus at 8 weeks of gestation. She was treated with levetiracetam 1000 mg and lacosamide 100 mg twice daily as well as enoxaparin and labetalol for the rest of her pregnancy and postpartum. Her infant was delivered at 36 weeks gestation and about 50% breastfed for the first days of life. The infant was sleepy and fed poorly, but pauses in breastfeeding did not improve the infant's condition. Breastfeeding was discontinued at 15 days postpartum and the infant gradually improved. The infant showed normal development at 7 months of age.[6]
A mother with epilepsy took levetiracetam 2000 mg daily plus lacosamide 200 mg twice daily while breastfeeding their infants. She breastfed (extent not stated) her infant for 7 months with no infant adverse effects at 24 months of age.[12]
A pregnant woman suffered blood clots in the sinuses and 2 small intracranial hemorrhages followed by status epilepticus at 8 weeks of gestation. She was treated with levetiracetam 1000 mg and lacosamide 100 mg twice daily as well as enoxaparin and labetalol for the rest of her pregnancy and postpartum. Her infant was delivered at 36 weeks gestation and about 50% breastfed for the first days of life. The infant was sleepy and fed poorly, but pauses in breastfeeding did not improve the infant's condition. Breastfeeding was discontinued at 15 days postpartum and the infant gradually improved. The infant showed normal development at 7 months of age.[6] Lacosamide and levetiracetam were probably the cause of the infant's sedation and poor feeding.
In a study of 20 mothers taking levetiracetam and their 21 infants, 3 women taking levetiracetam monotherapy in daily doses of 3750, 3000 and 1500 mg reported drowsiness in their fully breastfed infants. Drowsiness resolved shortly after switching to partial breastfeeding. No adverse effects were reported by 14 other mothers who were on levetiracetam monotherapy, and 3 mothers who were on polytherapy with sodium valproate, lacosamide, or topiramate. All the infants (n = 21) of the mothers participating in the study had age-appropriate weight gain.[8]
In a multicenter study of women in the United Kingdom exposed to anticonvulsants during pregnancy and breastfeeding, 84 women who were exposed to anticonvulsants breastfed their infants were compared to 81 women not exposed to antiseizure medications. Of the women taking anticonvulsants 22% were taking levetiracetam alone. No negative effect of breastfeeding was observed in cognitive, language or motor development in the breastfed infants at 1 and 2 years, although only 29% of women breastfed longer than 3 months.[13]
Effects on Lactation and Breastmilk
In a study of mothers taking levetiracetam during breastfeeding, 7 of 18 mothers discontinued or reduced breastfeeding because of poor milk output. The infant of one mother taking 3000 mg of levetiracetam daily plus clobazam had poor weight gain at day 15 of life.[9]
A retrospective study of 102 women with epilepsy found that women taking levetiracetam were more likely to initiate and continue breastfeeding at 3 months postpartum than women taking lamotrigine.[14]
Alternate Drugs to Consider
(Seizure Disorder) Carbamazepine, Divalproex, Gabapentin, Lamotrigine, Oxcarbazepine, Phenytoin, Valproic Acid
References
- 1.
- López-Fraile IP, Cid AO, Juste AO, Modrego PJ. Levetiracetam plasma level monitoring during pregnancy, delivery, and postpartum: clinical and outcome implications. Epilepsy Behav 2009;15:372-5. [PubMed: 19362602]
- 2.
- Kramer G, Hosli I, Glanzmann R, Holzgreve W. Levetiracetam accumulation in human breast milk. Epilepsia 2002;43 (Suppl 7):105. doi:10.1046/j.1528-1157.43.s7.1.x [CrossRef]
- 3.
- Greenhill L, Betts T, Yarrow H, Patsalos P. Breast milk levels of levetiracetam after delivery. Epilepsia 2004;45 (Suppl. 7):230. doi:10.1111/j.0013-9580.2004.t01-21-00001.x [PubMed: 15009224] [CrossRef]
- 4.
- Johannessen SI, Helde G, Brodtkorb E. Levetiracetam concentrations in serum and in breast milk at birth and during lactation. Epilepsia 2005;46:775-7. [PubMed: 15857447]
- 5.
- Tomson T, Palm R, Kallen K, et al. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia 2007;48:1111-6. [PubMed: 17381438]
- 6.
- Ylikotila P, Ketola RA, Timonen S, et al. Early pregnancy cerebral venous thrombosis and status epilepticus treated with levetiracetam and lacosamide throughout pregnancy. Reprod Toxicol 2015;57:204-6. [PubMed: 26187779]
- 7.
- Kacirova I, Grundmann M, Brozmanova H. Umbilical cord, maternal milk, and breastfed infant levetiracetam concentrations monitoring at delivery and during early postpartum period. Pharmaceutics 2021;13:398. [PMC free article: PMC8002441] [PubMed: 33802733]
- 8.
- Dinavitser N, Kohn E, Berlin M, et al. Levetiracetam in lactation: How much is excreted into the human breast milk? Br J Clin Pharmacol 2022;88:199-205. [PubMed: 34131926]
- 9.
- Paret N, Gouraud A, Bernard N, et al. Long-term follow-up of infants exposed to levetiracetam during breastfeeding: Comparison to a control group. Birth Defects Res A Clin Mol Teratol 2014;100:537-8. doi:10.1002/bdra.23258 [CrossRef]
- 10.
- Birnbaum AK, Meador KJ, Karanam A, et al. Antiepileptic drug exposure in infants of breastfeeding mothers with epilepsy. JAMA Neurol 2020;77:441-50. [PMC free article: PMC6990802] [PubMed: 31886825]
- 11.
- Rauchenzauner M, Kiechl-Kohlendorfer U, Rostasy K, Luef G. Old and new antiepileptic drugs during pregnancy and lactation - report of a case. Epilepsy Behav 2011;20:719-20. [PubMed: 21444249]
- 12.
- Lattanzi S, Cagnetti C, Foschi N, et al. Lacosamide during pregnancy and breastfeeding. Neurol Neurochir Pol 2017;51:266-9. [PubMed: 28385340]
- 13.
- Bromley RL, Bullen P, Campbell E, et al. Neurodevelopment of babies born to mothers with epilepsy: A prospective observational cohort study. Epilepsia 2023;64:2454-71. [PubMed: 37403560]
- 14.
- Al-Faraj AO, Pandey S, Herlihy MM, Pang TD. Factors affecting breastfeeding in women with epilepsy. Epilepsia 2021;62:2171-9. [PubMed: 34289107]
Substance Identification
Substance Name
Levetiracetam
CAS Registry Number
102767-28-2
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Brivaracetam.[Drugs and Lactation Database (...]Review Brivaracetam.. Drugs and Lactation Database (LactMed®). 2006
- Review Oxcarbazepine.[Drugs and Lactation Database (...]Review Oxcarbazepine.. Drugs and Lactation Database (LactMed®). 2006
- Review Topiramate.[Drugs and Lactation Database (...]Review Topiramate.. Drugs and Lactation Database (LactMed®). 2006
- Review Carbamazepine.[Drugs and Lactation Database (...]Review Carbamazepine.. Drugs and Lactation Database (LactMed®). 2006
- Profile of ucb L059, a novel anticonvulsant drug, in models of partial and generalized epilepsy in mice and rats.[Eur J Pharmacol. 1993]Profile of ucb L059, a novel anticonvulsant drug, in models of partial and generalized epilepsy in mice and rats.Löscher W, Hönack D. Eur J Pharmacol. 1993 Mar 2; 232(2-3):147-58.
- Levetiracetam - Drugs and Lactation Database (LactMed®)Levetiracetam - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
