Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
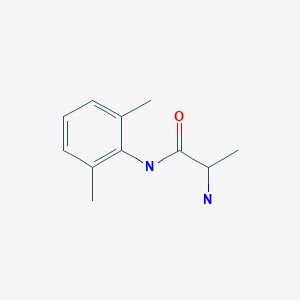
CASRN: 41708-72-9
Drug Levels and Effects
Summary of Use during Lactation
Tocainide was removed from the market in the United States in 2003 because it can cause serious and potentially fatal hematological adverse effects. Limited data indicate that rather large amounts of tocainide are excreted into breastmilk. Because of the relative lack of data concerning breastfeeding during maternal tocainide therapy and is potential toxicity, tocainide should be avoided during breastfeeding.
Drug Levels
Maternal Levels. One woman taking tocainide 400 mg every 8 hours orally during the early postpartum period had levels of 12 mg/L at 0.5 hour before a dose and 28 mg/L at 2 hours after a dose.[1] These data indicate that an exclusively breastfed infant would receive between 9 and 21% of the maternal weight-adjusted dosage.
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
References
- 1.
- Wilson JH. Breast milk tocainide levels. J Cardiovasc Pharmacol. 1988;12:497. Letter. [PubMed: 2465453]
Substance Identification
Substance Name
Tocainide
CAS Registry Number
41708-72-9
Drug Class
- Breast Feeding
- Antiarrhythmics
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Prilocaine.[Drugs and Lactation Database (...]Review Prilocaine.. Drugs and Lactation Database (LactMed®). 2006
- Review Mepivacaine.[Drugs and Lactation Database (...]Review Mepivacaine.. Drugs and Lactation Database (LactMed®). 2006
- Review Naltrexone.[Drugs and Lactation Database (...]Review Naltrexone.. Drugs and Lactation Database (LactMed®). 2006
- Review Mefenamic Acid.[Drugs and Lactation Database (...]Review Mefenamic Acid.. Drugs and Lactation Database (LactMed®). 2006
- Review Butorphanol.[Drugs and Lactation Database (...]Review Butorphanol.. Drugs and Lactation Database (LactMed®). 2006
- Tocainide - Drugs and Lactation Database (LactMed®)Tocainide - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
