NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Testicular cancer: Does routine screening for men aged 16 years and older lead to better treatment outcomes? IQWiG Reports – Commission No. HT18-01 [Internet] Cologne (Germany): Institute for Quality and Efficiency in Health Care (IQWiG); 2021 Apr 14.

Testicular cancer: Does routine screening for men aged 16 years and older lead to better treatment outcomes? IQWiG Reports – Commission No. HT18-01 [Internet]
Show details5.1. Supplementary presentation of results from diagnostic studies
The aim of the supplementary presentation was to identify diagnostic studies which revealed information on the diagnostic quality of testicular cancer screening by TSE or clinical palpation in combination with scrotal ultrasound with regard to the detection of testicular cancer in an asymptomatic screening population or the frequency of harms due to false-positive and false-negative screening results.
The focused information retrieval for the supplementary presentation of diagnostic studies found no study which met all inclusion criteria and provided usable data on the quality of the investigated screening methods in an asymptomatic screening population.
However, the search found several studies which contain information on relevant aspects of the investigated methods and are usable for roughly estimating the potential harm associated with their use. These excluded studies were marked and extracted as additional literature of interest. In addition to 8 studies providing additional information of interest from the focused search for diagnostic studies [14,32–38], 4 other diagnostics-related studies were found in the systematic search for the benefit assessment and included [39–42]. These studies provided data on the positive predictive value (PPV) – i.e. the probability of the disease being present in men with abnormal test results – as well as on unnecessary testicular exploration or removal resulting from diagnostic evaluation after a medical examination. The medical examination consisted of either a combined clinical exam and scrotal ultrasound, or a palpation exam alone, or scrotal ultrasound due to a clinically suspected tumour. Two studies [32,39] were used to collect evidence on the magnitude of the potentially adverse impacts of TSE.
5.1.1. Adverse impacts of clinical testicular examination (testicular anomalies requiring diagnostic evaluation and unnecessary testicular exploration)
Potential harms from screening are predominantly the result of the limited capability of sonography to differentiate between malignant and benign lesions [43,44]. Hence, definitive diagnostics often require histopathological examination of the surgically exposed or removed testis. Since, in case of non-malignant findings, testicular exploration or removal can be deemed unnecessary harm, this investigation aimed to collect data on the frequency of unnecessary invasive procedures resulting from the use of screening. Noncancerous anomalies detected during a targeted testicular examination, such as varicoceles, hydroceles, and cysts, represent another problem: While they typically do not require treatment, they might worry patients and lead to the increased use of medical resources for further diagnostic evaluation. Any adverse impacts of clinical testicular exams were determined on the basis of studies providing data for calculating PPVs of screening exams or data on the prevalence of testicular anomalies found in the examinations.
Table 3
PPV and proportion of unnecessary testicular explorations or removals following clinical palpation and scrotal ultrasound
The data extracted from the 12 studies on clinical testicular exams involving a total of 7297 patients, (see Table 3) show that about 12–67% of the testicular explorations carried out as part of diagnostic evaluation of testicular cancer resulted in benign findings and hence might have been unnecessary. Three studies (3781 patients) had investigated an asymptomatic population. However, no testicular cancer occurred in 2 of these studies. Therefore, it was possible to calculate a PPV for asymptomatic participants solely on the basis of the Peterson study. It had the lowest PPV of 33%, i.e. 33% of men with abnormal findings actually had testicular cancer [41]. Even in a selected population of men with scrotal swelling, 12% of ultrasound findings suspicious for malignancy were false positive, and 33% of the findings deemed benign were false negative [37]. Further, it was found that a substantial number of noncancerous anomalies are discovered by clinical palpation and particularly by scrotal ultrasound; this causes worries in the affected men and might contribute to a greater consumption of medical resources due to further diagnostic evaluation. Isidori [33] illustrated that a very high percentage of nonpalpable lesions detectable only by ultrasound are benign and showed that, due to the various reasons for referral, the results of diagnostic studies with patients referred for ultrasound are difficult to transfer to a screening population. The considered studies fail to show whether and to what extent clinical screening contributes to the prevention of advanced stages of testicular cancer and mortality. Likewise, the studies do not allow comparing clinical screening versus no clinical screening in terms of unnecessary testicular exploration. False-negative findings were rare and found primarily in studies histologically examining even ultrasound findings which were not suspicious for malignancy [36,37].
5.1.2. Adverse impacts of TSE (testicular anomalies requiring diagnostic evaluation and unnecessary testicular exploration)
The search did not find any studies on the prevalence of TSE in Germany or on the number of clinical evaluations performed based on concerns from TSE.
The study which comes closest to answering the research question is a Hungarian study by Geczi et al. [32], which was listed as additional literature of interest. The study describes the findings from 5056 men who voluntarily underwent a testicular exam and ultrasound at a hospital specializing in testicular cancer. Their participation followed a 1995 media awareness campaign about the importance of testicular cancer screening and performing TSE. In case of findings which were suspicious for cancer, tumour markers were additionally measured and invasive diagnostics performed, if appropriate. A total of 2342 of the 5056 volunteers presented due to various complaints. We assume this to be precisely the group which would see a physician after TSE. Outside a study setting, the other, no-complaints group would presumably not see a physician and hence, no harm from diagnostic evaluation could arise. Therefore, we used the group with complaints as the basis for calculating potential harms. In 1810 men (77%) of this group, the medical examination revealed a testicular anomaly. Further urological evaluation was necessary in 3.9% of the complaints group. In 31 (1.3%) of the men presenting with complaints, clinical examination and ultrasound resulted in suspected testicular cancer, which was confirmed by subsequent invasive diagnostic evaluation in 26 cases. Nineteen (73%) of the 26 malignant tumours were in stage I. In the remaining 5 suspected cases (16%), the invasive examination did not reveal any malignancies. Assuming that the study’s group with complaints represents men who – even outside a study setting – would visit a physician if they found abnormalities in the TSE, a PPV of 1.1% (26/2342) can be derived for TSE (Table 4). The PPV of clinical examination and scrotal ultrasound in a population preselected through TSE would be 84% (26/31) (also see Table 3). The study provides only part of the information necessary to estimate negative impacts in a TSE setting since it does not reveal which percentage of the population reached by the media awareness campaign is represented by the complaints group included in the study. Since no comparison is available with men who were not exposed to the awareness campaign, it is impossible to determine whether the media-based encouragement to perform TSE contributed to the reduction of advanced stages of testicular cancer and mortality. Given the unproven and likely small benefit and the large number of incidental findings, the authors recommend limiting TSE to high-risk groups.
Table 4
PPV and proportion of unnecessary testicular exploration or removal following TSE
5.2. Supplementary presentation of results on the benefit of earlier treatment
Studies based on cancer registries were used because the screening of 16 systematic reviews of therapeutic studies on testicular cancer showed there are presumably no therapeutic studies allowing a comparison of treatment effects in patients with early treatment start (corresponding to a screening situation) versus late treatment start (corresponding to a no-screening situation).
The supplementary presentation of results from cancer registries is intended to explore the potential added benefit resulting from starting treatment in earlier disease stages. For this purpose, the distribution of testicular cancer stages and stage-specific mortality were determined from registry data.
Two studies with appropriate data from cancer registries were found [46,47].
Minicozzi (2017) [47] analysed the quality of stage information for 15 cancer types diagnosed in 2000–2007 as reported to EUROCARE by 62 European cancer registries and provided age-standardized relative 5-year survival rates for local, regional, and metastatic tumour stages. The overall assignment to the three stages of tumour spread was carried out either by the reporting tumour registries themselves or else determined on the basis of detailed or condensed TNM data. Results were reported by the type of reported data. Table 5 shows the average distribution of testicular cancer stages at the time of diagnosis on the basis of data from 12 881 testicular cancer cases from 8 selected cancer registries with qualitatively acceptable TNM information as well as the distribution of summarized stages reported from Austria, which was included in the table to replace missing data from Germany. On the basis of the data, the percentage of testicular cancer cases detected in the prognostically unfavourable metastatic stage can be estimated as 6–11%. Age-standardized relative 5-year survival for the local, regional, and metastatic testicular cancer stage was presented in the appendix of the publication in the form of box plots stratified by the type of reporting data.
Table 6 shows the approximate medians read off of the figures for the distribution of stage-specific relative survival based on data reported to various tumour registries. The box plots did not show which registries were included in each analysis. When compared to the high relative survival rates of 99% in the local stage and 86% in the regional stage, survival rates in the metastatic stage were far lower, at values between 67% and 81%.
Table 5
Distribution of testicular cancer stages at diagnosis based on the reporting data from various European tumour registries in 2000–2007 by type of underlying stage information (in percent)[47]
Available U.S. data paint a similar picture. Gandalia (2014) [46] analysed data of 31 330 patients from the Surveillance Epidemiology and End Results (SEER) database who were diagnosed with testicular cancer between 1993 and 2009. Cancer-specific 15-year mortality rates for seminoma patients were 0.4% when diagnosed in the local stage, 2.4% when diagnosed in the regional stage, and 10.8% when diagnosed in the metastatic stage. Cancer-specific 15-year mortality rates for non-seminoma patients were 1.6% for patients diagnosed in the local stage, 3.1% in the regional stage, and 19.6% in the metastatic stage. SEER data from 2009–2015 are available online [48] and show that testicular cancer was detected in the local stage in 68% of cases, in the regional stage in 19% of cases, and in the metastatic stage in 12% of cases. The relative 5-year mortality rate for testicular cancer in the local stage is 99%, in the regional stage, 96%, and in metastatic stage, 73%.
Since European and U.S. registry data show the survival rates of patients in the local stage and regional stage to be reduced very little, any medical benefit of testicular cancer screening would have to largely result from the prevention of metastatic stages. However, alongside differences in survival rates, the assessment of potential benefit must take into account the low prevalence of metastatic stages, of approximately 6–11%, even in the absence of screening. Furthermore, it must be noted that even metastatic testicular cancer is classified by IGCCCG into tumours with good, intermediate, and poor prognoses [9], thereby further reducing the target group of men whose lives might be saved by earlier diagnosis. It is important to be aware that the stage distributions and survival rates derived from the registry data reflect the current situation without established screening. The registry data do not show whether and to what extent screening programmes might contribute to the prevention of advanced stages of testicular cancer and the reduction of mortality. However, the information obtained as part of this supplementary presentation does allow roughly estimating the maximum theoretically possible benefit of screening based on the assumption that all cases currently detected in the metastatic stage are preventable by way of screening and that the survival rate correspondingly increases as a result of earlier treatment.
5.3. Supplementary presentation of results from modelling studies
The information retrieval found no modelling studies on testicular cancer screening. The most recent search was conducted on 7 November 2018.
5.4. Discussion
The systematic search for comparative interventional studies covering the entire screening chain was unsuccessful. Therefore, no robust evidence is available to draw any conclusions on any added benefit of screening via TSE or via clinical palpation and scrotal ultrasound when compared to no screening in asymptomatic men from 16 years of age. There is no hint of (greater) benefit or (greater) harm. The present report did not explicitly investigate screening programmes focusing exclusively on men at higher risk of testicular cancer. This was because the majority of men with known risk factors (prior unilateral testicular cancer, father or brother with testicular cancer, infertility examinations) are believed to already be specifically examined for testicular cancer. Our extensive search shows, however, that no interventional studies on the benefit of screening high-risk populations are available either, and hence, no conclusions can be drawn even for this population. However, in general, the benefit–harm relationship of screening measures increases with the risk of disease.
The absence of corresponding benefit studies is confirmed by the systematic reviews included as part of the search [18,49,50]. In addition, the more current, unchanged evidence situation in 2014 and March 2019 is reported on the websites of USPSTF and the PDQ Screening and Prevention Editorial Board of the U.S. National Cancer Institute [51]. All reviews conclude that the potential added benefit of screening is low due to the low incidence and high cure rates of the disease.
No references meeting the inclusion criteria were found in the searches conducted as part of the supplementary presentation to the benefit assessment; these searches looked for studies on the accuracy of the investigated screening procedures in an asymptomatic screening population or on the benefit of earlier treatment or for modelling studies. The supplementary searches did, however, find several studies containing some information relevant for assessing the screening; these studies were recorded and analysed as additional literature of interest in the individual parts of the supplementary presentations.
The supplementary presentations on the benefit assessment reveal that some important information which would be required for a model-based evaluation of the benefit of testicular cancer screening is missing. For instance, no evidence is available on whether and to what extent screening actually contributes to the prevention of advanced testicular cancer stages and the associated mortality and how it impacts the number of unnecessary testicular exposures. With the aid of assumptions, the information obtained as part of the supplementary presentations can be used to assess the theoretically possible benefit and harm. However, due to missing data on individual subareas, this is possible only to a limited extent. The data and data gaps found in the weighing of benefit and harm can be presented in the form of flow diagrams, which use the available data to compare the number of expected benefit and harm events in case of TSE, clinical examination, and no screening.
The flow diagram in Figure 1 presents the available data for estimating the effects of screening on the expected distribution of tumour stages at diagnosis and cancer-related mortality and hence focuses on the benefit aspects of screening. The flow diagram illustrates the lack of data on screening exams which would be necessary for the benefit assessment. However, relying on the incidence and mortality rates in Germany in addition to assumptions, the researchers were able to use the data on the no-screening situation to estimate the maximum possible benefit of screening. The figure shows that in the current no-screening situation, 11 cases of testicular cancer per 100 000 men are detected annually [1]. On the basis of the stage distribution extracted from Minicozzi [47] (Table 5), this population would include 0.7–1.2 patients with metastatic tumours and substantially reduced survival rates when compared to the normal population. Every year, there are 0.4 deaths from testicular cancer per 100 000 men [1]. Under the extreme assumption that all tumours detected in the local and regional stages are curable and all cases detected in the metastatic stage could be prevented by screening, for every 100 000 men participating in screening every year, no more than 1.2 advanced tumours and 0.4 deaths could be prevented. This would mean that the estimated maximum benefit of testicular cancer screening is one hundred times lower than the benefit of the established colon cancer screening with colonoscopy, which has been reported as 30–60 prevented deaths per 100 000 annually in 55-year-old men [52].

Figure 1
Flow diagram for estimating the benefit of testicular cancer screening
The flow diagram in Figure 2 focuses on the harm aspects of screening. It shows the available data for estimating the effects of screening on the number of discovered testicular abnormalities, more extensive urological evaluations, and testicular exploration/removal with malignant and benign definitive findings. The flow diagram illustrates that there is a lack of the data required to evaluate the harm caused by the current no-screening situation. Due to the missing data, the added harm to be expected in case of screening cannot be quantified. The data found as part of the supplementary presentation can be used only for estimating harm events in case of screening. However, this again requires making assumptions and using incidence rates from Germany. The figure shows that, in case of TSE screening, 991 of 100 000 men notice a worrisome change during one of their TSEs, resulting in an office visit. No empirical data on the prevalence of worrisome findings in TSEs were available. Therefore, the number of worrisome findings per 100 000 men was calculated backwards on the basis of the prevalence of confirmed cases of testicular cancer (n = 26) found in the Gezci study [32] within the population of men with office visits due to complaints after being instructed on how to perform TSE (n = 2342) and the testicular cancer incidence in Germany (11/100 000) as follows (2342/26)*11=991. It is important to note that the use of German incidence rates for testicular cancer in this step affects all subsequent calculations as well. However, using these figures seems justifiable since screening programmes are unlikely to substantially affect the observed incidence of testicular cancer, particularly since they do not prevent the development of testicular cancer and since its rapid growth [53] and typically extracorporeal location suggest a comparatively small pool of latent cases which might be additionally discovered through screening. Calculations based on data from Geczi et al. likewise showed that 763 of 991 men with worrisome TSEs (77%) have testicular abnormalities, and more extensive urological evaluation is required in 39 of them (5%). In 13 of these 39 cases (33%), the examination leads to a suspicion of cancer, resulting in testicular exploration or removal; in 2 cases, the testicular exploration or removal ultimately reveals benign findings and might therefore be deemed unnecessary. The quantity of unnecessary testicular explorations is calculated by multiplying the 13 cases of suspected cancer requiring invasive diagnostic evaluation by Geczi’s reported proportion of suspected tumour cases not confirmed by testicular exploration (5/31). For the clinical screening for testicular cancer, data from Roemer et al. [42] allow estimating that testicular abnormalities will be found in 1700 of 100 000 screening participants (1.7%), and more extensive urological evaluations will be required in 200 cases (12%). The actual case numbers in this area are likely even higher since, in light of missing data, it was not possible to include clinical examinations performed due to changes found by the patient in the estimate. The number of necessary and unnecessary testicular explorations/removals as well as the resulting sum of surgical procedures can be estimated based on the range of PPVs of 33–88% (Table 3) [37,41], which were extracted as part of the supplementary presentation, and the incidence of testicular cancer (11/100 000) observed in Germany. The estimate suggests that for every 100 000 men participating in clinical screening, approximately 12–33 testicular explorations/removals can be expected, of which 1–22 will reveal benign findings and might therefore be deemed unnecessary. The estimate of 22 unnecessary procedures is based on the predictive value reported by Peterson [41]. Since this was the only study conducted in asymptomatic men, an estimate based on its data possesses higher credibility. Despite the lack of comparison with the current no-screening situation, the estimate shown in the flow chart reveals that, in case of TSE-based screening, no more than negligible added harm in the form of unnecessary testicular exploration would be expected; this harm could be offset or outweighed even by slight gains in benefits. Recommendations in favour of regular TSE, such as from the German Society of Urology, the German Cancer Society, and the Federal Centre for Health Education [54–56], appear to adopt this perspective, despite the lack of scientific evidence of benefit. However, it should also be noted that even TSE-based screening can lead to the consumption of additional medical resources for the diagnostic evaluation of suspected cases or as a result of testicular abnormalities, which are often discovered upon targeted examination. As illustrated by the estimate in the flow diagram, clinical screening would be expected to be many times more resource-intensive than TSE. Furthermore, there would be a risk of a substantial increase in unnecessary testicular exploration, representing harm which would need to be balanced out by higher gains on the benefit side.
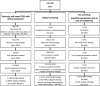
Figure 2
Flow diagram for estimating the harm from testicular cancer screening RKI: Robert Koch Institute
The presented calculations exhibit a series of fundamental limitations:
- Even on the basis of the data from the supplementary presentations, it is not possible to draw any comparative conclusions on the potential benefit and harm of the three options – namely no screening (or clinical palpation of the testis upon request from the 45th year of life), screening by TSE, and screening by clinical palpation and scrotal ultrasound – because no data on benefits (prevented deaths, prevented cases of metastatic testicular cancer) were available or derivable for the screening situation and no data on harm (cases with unnecessary testicular exploration or removal) were available or derivable for the no-screening status quo. In principle, differences in quality of life resulting from the prevention of advanced cancer stages, burdensome treatment forms, and their late toxicities should be included in the comparison as well. Due to the lack of available evidence, this was impossible to do.
- The assumptions made regarding the PPVs for clinical examination are largely from diagnostic studies with preselected patients rather than from a population of asymptomatic men, as would be the case in a clinical screening exam. This typically leads to overestimates of the PPV when compared with an unselected population and hence to underestimates of the potential harm. No systematic assessment of the studies’ risk of bias was carried out, and therefore, any further potential weaknesses of the studies were not included in the assessment.
- The assumptions made on stage distribution at the time of diagnosis and on stage-specific survival rates are based on registry data of limited quality: In up to 30% of data, staging information may be missing [47]. Only a small proportion of registry data are from Germany. However, 82% of the 12 881 included cases are from the Netherlands, Austria, Norway, Germany, and Switzerland and hence from countries with similar living conditions and health systems.
- The calculation of benefit and harm required assumptions reflecting the actual situation to an unknown or only limited extent. For instance, it was assumed that screening could discover all metastatic tumours in the local or regional stage. Most assumptions were made in such a way that the potential bias would be in favour of the effectiveness of screening.
- Results: Supplementary presentations to the benefit assessment - Testicular canc...Results: Supplementary presentations to the benefit assessment - Testicular cancer
- Results: Benefit assessment - Testicular cancerResults: Benefit assessment - Testicular cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...