By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsIn order to be activated, a naive T cell must recognize a foreign peptide bound to a self MHC molecule. But this is not, on its own, sufficient for activation. That requires the simultaneous delivery of a co-stimulatory signal by a specialized antigen-presenting cell. Only dendritic cells, macrophages, and B cells are able to express both classes of MHC molecule as well as the co-stimulatory cell-surface molecules that drive the clonal expansion of naive T cells and their differentiation into armed effector T cells.
The most potent activators of naive T cells are mature dendritic cells and these are thought to initiate most, perhaps all, T-cell responses in vivo. As we will describe in this part of the chapter, immature dendritic cells in the tissues take up antigen at sites of infection and are activated to travel to local lymphoid tissue. Here they mature into cells that express high levels of co-stimulatory molecules and the adhesion molecules that mediate interactions with the naive T cells continually recirculating through these tissues. The activation and clonal expansion of a naive T cell on initial encounter with antigen on the surface of an antigen-presenting cell is often called priming, to distinguish it from the responses of armed effector T cells to antigen on their target cells, and the responses of primed memory T cells.
8-1. T-cell responses are initiated in peripheral lymphoid organs by activated antigen-presenting cells
Adaptive immune responses are not initiated at the site where a pathogen first establishes a focus of infection. They occur in the organized peripheral lymphoid tissues through which naive T cells are continually migrating. Pathogens or their products are transported to lymphoid tissue in the lymph that drains the infected tissue, or, more rarely, by the blood. Pathogens infecting mucosal surfaces accumulate in lymphoid tissues such as the Peyer's patches of the gut or the tonsils; those that enter the blood are trapped in the spleen; while those infecting peripheral sites are trapped in the lymph nodes directly downstream of the site of infection (see Section 1-3). All these lymphoid organs contain cells specialized for capturing antigen and presenting it to T cells. The most important of these are the dendritic cells, which capture antigen at the site of infection and then migrate to the downstream lymph node.
The delivery of antigen from a site of infection to downstream lymphoid tissue and its subsequent presentation to naive T cells is actively aided by the innate immune response to infection. As discussed in Chapter 2, this is rapidly triggered at the site of infection by nonclonotypic receptors that recognize molecular patterns that are associated with pathogens but not host cells. One of the induced responses of innate immunity is an inflammatory reaction that increases the entry of plasma into the infected tissues and the consequent drainage of tissue fluids into the lymph. Another is the induced maturation of tissue dendritic cells that have been taking up particulate and soluble antigens at the site of infection (Fig. 8.2). These cells are activated through receptors that signal the presence of pathogen components bound by dendritic cell receptors, or by cytokines produced during the inflammatory response. The dendritic cells respond by migrating to the lymph node and expressing the co-stimulatory molecules that are required, in addition to antigen, for the activation of naive T cells. Macrophages, which are phagocytic cells found in the tissues and scattered throughout lymphoid tissue, and B cells, which bind pathogen components, may be similarly induced through nonspecific receptors to express co-stimulatory molecules and act as antigen-presenting cells. Thus the innate immune response to infection hastens the transport of antigens to the local lymphoid tissue, and enables those cells that have taken up antigen to present it effectively to the naive T cells that migrate through this tissue.
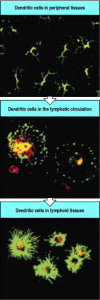
Figure 8.2
Immature dendritic cells take up antigen in the tissues. Immature dendritic cells in the tissues have a very dendritic morphology, with many long processes, as shown in the top panel, where the tissue is stained for MHC class II molecules in green and for (more...)
The distribution of dendritic cells, macrophages, and B cells in a lymph node is shown in Fig. 8.3. Dendritic cells are present mainly in the T-cell areas. These cells are named after their fingerlike processes, which form a network of branches among the T cells. By the time they arrive in the lymph nodes, dendritic cells have lost their ability to capture new antigen. They are, however, able to present the antigens they ingested at the site of infection and in their mature, activated form they are the most potent antigenpresenting cells for naive T cells.

Figure 8.3
Antigen-presenting cells are distributed differentially in the lymph node. Dendritic cells are found throughout the cortex of the lymph node in the T-cell areas. Macrophages are distributed throughout but are mainly found in the marginal sinus, where (more...)
Macrophages are found in many areas of the lymph node, especially in the marginal sinus, where the afferent lymph enters the lymphoid tissue, and in the medullary cords, where the efferent lymph collects before flowing into the blood. Here they can actively ingest microbes and particulate antigens and so prevent them from entering the blood. As most pathogens are particulate, macrophages in the T-cell areas may stimulate immune responses to many sources of infection.
Finally, the B cells, which recirculate through the lymphoid tissues and concentrate in the lymphoid follicles, are particularly efficient at taking up soluble antigens such as bacterial toxins by the specific binding of antigen to the B-cell surface immunoglobulin. The antigen:receptor complex is internalized by receptor-mediated phagocytosis, and degraded fragments of the antigen can return to the B-cell surface complexed with MHC class II molecules. Antigen-specific B cells can thus activate naive CD4 T cells if the B cells are also induced to express co-stimulatory molecules. B cells are, however, very inefficient at initiating adaptive immune responses. This is because only those with the appropriate receptor specificity can internalize and present a particular antigen at high frequency, and these will be very scarce. Thus, the probability of their encountering a naive T cell specific for the same antigen is very low.
The antigen-presenting function of dendritic cells, macrophages, and B cells will be discussed in more detail in Sections 8-5 to 8-7. Only these three cell types express the specialized co-stimulatory molecules required to activate naive T cells; furthermore, all of these cell types express these molecules only when suitably activated in the context of a response to infection. Dendritic cells can take up, process, and present a wide variety of pathogens and antigens and appear to be the most important activators of naive T cells, whereas macrophages and B cells specialize in processing and presenting antigens from ingested pathogens and soluble antigens, respectively, and are also the targets of subsequent actions of armed effector CD4 T cells.
8-2. Naive T cells sample the MHC:peptide complexes on the surface of antigen-presenting cells as they migrate through peripheral lymphoid tissue
Naive T cells enter lymphoid tissue by crossing the walls of specialized venules known as high endothelial venules (HEV). They circulate continuously from the bloodstream to the lymphoid organs and back to the blood, making contact with many thousands of antigen-presenting cells in the lymphoid tissues every day. These contacts allow the sampling of MHC:peptide complexes on the surface of these antigen-presenting cells, which is important for two reasons. One is that it appears to reinforce the process of positive selection for self MHC recognition that occurred during T-cell development. As we discussed in Chapter 7, T-cell receptors are selected for their ability to interact with self MHC:self peptide complexes during T-cell development. In this way, a repertoire of mature T cells is selected that can be activated by nonself peptides bound to the same MHC molecules. Recent experiments show that T-cell survival in the periphery also depends on contact with self MHC:self peptide ligands (see Section 7-32), and that the signals required for survival are delivered effectively through interactions with MHC:peptide complexes on dendritic cells. Thus, as naive T cells migrate through peripheral lymphoid tissue, they receive survival signals through their interactions with dendritic cells. At the same time, the sampling of MHC:peptide ligands ensures that each T cell has a high probability of encountering antigens derived from pathogens at any site of infection. This is crucial for the initiation of an adaptive immune response, as only one naive T cell in 104-106 is likely to be specific for a particular antigen, and adaptive immunity depends on the activation and expansion of such rare antigen-specific T cells (Fig. 8.4). The T cells that do not encounter their antigen eventually reach the medulla of the lymph node, from where they are carried by the efferent lymphatics back to the blood to continue recirculating through other lymphoid organs. Naive T cells that recognize their antigen on the surface of a dendritic cell cease to migrate, and embark on the steps that generate armed effector cells. The generation of effector cells from a naive T cell takes several days. At the end of this period, the armed effector T cells leave the lymphoid organ and reenter the bloodstream to migrate to sites of infection.

Figure 8.4
Naive T cells encounter antigen during their recirculation through peripheral lymphoid organs. Naive T cells recirculate through peripheral lymphoid organs, such as the lymph node shown here, entering through specialized regions of vascular endothelium (more...)
8-3. Lymphocyte migration, activation, and effector function depend on cell-cell interactions mediated by cell-adhesion molecules
The migration of naive T cells through the lymph nodes, and their initial interactions with antigen-presenting cells, depend on cells binding to each other through interactions that are not antigen-specific. Similar interactions eventually guide the effector T cells into the peripheral tissues and play an important part in their interaction with target cells. Binding of T cells to other cells is controlled by an array of adhesion molecules on the surface of the T lymphocyte that recognize a complementary array of adhesion molecules on the surface of the interacting cell. The main classes of adhesion molecule involved in lymphocyte interactions are the selectins, the integrins, members of the immunoglobulin superfamily, and some mucinlike molecules. We have already encountered members of the first three classes in the recruitment of neutrophils and monocytes to sites of infection during an innate immune response (see Section 2-22). Most adhesion molecules play fairly broad roles in the generation of immune responses. Many that are involved in lymphocyte migration and the interactions of armed effector T cells with their targets are also involved in interactions between other leukocytes. Adhesion molecules are important in getting lymphocytes together in adaptive immune responses that involve T-cell-B-cell interactions, and we will describe these in Chapter 10, where we present an integrated view of the immune response.
The selectins (Fig. 8.5) are particularly important for leukocyte homing to particular tissues, and can be expressed either on leukocytes (L-selectin, CD62L) or on vascular endothelium (P-selectin, CD62P, and E-selectin, CD62E). L-Selectin is expressed on naive T cells and guides their exit from the blood into peripheral lymphoid tissues. P-Selectin and E-selectin are expressed on the vascular endothelium at sites of infection and serve to recruit effector cells into the tissues at these sites (see Sections 2-21 and 2-22). Selectins are cell-surface molecules with a common core structure, distinguished from each other by the presence of different lectinlike domains in their extracellular portion (see Fig. 2.34). The lectin domains bind to particular sugar groups, and each selectin binds to a cell-surface carbohydrate. L-Selectin binds to the carbohydrate moiety, sulfated sialyl-Lewisx, of mucinlike molecules called vascular addressins, which are expressed on the surface of vascular endothelial cells. Two of these addressins, CD34 and GlyCAM-1, are expressed as sulfated sialyl-Lewisx molecules on high endothelial venules in lymph nodes. A third, MAdCAM-1, is expressed on endothelium in mucosa, and guides lymphocyte entry into mucosal lymphoid tissue such as that of the gut.
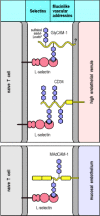
Figure 8.5
L-Selectin and the mucinlike vascular addressins direct naive lymphocyte homing to lymphoid tissues. L-Selectin is expressed on naive T cells, which bind to sulfated sialyl-Lewisx moieties on the vascular addressins CD34 and GlyCAM-1 on high endothelial (more...)
The interaction between L-selectin and the vascular addressins is responsible for the specific homing of naive T cells to lymphoid organs but does not, on its own, enable the cell to cross the endothelial barrier into the lymphoid tissue. For this, proteins from two other families—the integrins and the immunoglobulin superfamily—are required. These proteins also play a critical part in the subsequent interactions of lymphocytes with antigen-presenting cells and later with their target cells.
The integrins comprise a large family of cell-surface proteins that mediate adhesion between cells, and between cells and the extracellular matrix, in normal development as well as in immune and inflammatory responses. Integrins bind tightly to their ligands after receiving signals that induce a change in conformation. For example, as we saw in Chapter 2, signaling by chemokines activates integrins on leukocytes to bind tightly to the vascular surface during migration of leukocytes into sites of inflammation. Chemokines similarly activate T-cell integrins during the migration of T lymphocytes into lymphoid organs and in the migration of activated T lymphocytes to sites of infection.
The migration of naive T cells into lymphoid tissues is mediated by the chemokine SLC (secondary lymphoid tissue chemokine). This is expressed by the high vascular endothelium, stromal cells, and dendritic cells in lymphoid tissue, and binds to the CCR7 chemokine receptor on naive T cells. This interaction, by a mechanism as yet unknown, is able to increase the affinity of integrin binding, arresting the T cell's progress through the blood and enabling it to enter the lymphoid tissue. Similar interactions with chemokines produced at sites of inflammation direct effector T-cell migration into the tissues; this will be discussed in more detail when we describe the functions of effector T cells in Chapter 10. Chemokines are not the only molecules able to signal the upregulation of integrin affinity; later in this chapter we will see how signaling through the T-cell receptor also triggers T-cell integrins to adhere tightly to their ligands on the antigen-presenting cell.
The integrins were introduced in Chapter
2, so we will just review their most important characteristics here.
An integrin molecule consists of a large α chain that pairs noncovalently with a
smaller β chain. There are several subfamilies of integrins, broadly defined by
their common β chains. We will be concerned chiefly with the leukocyte integrins, which have a
common β2 chain with distinct α chains (Fig. 8.6). All T cells express a β2 integrin
known as lymphocyte function-associated antigen-1
(LFA-1). This leukocyte integrin is also found on macrophages
and neutrophils, and is involved in their recruitment to sites of infection (see
Sections 2-21 and 2-22). LFA-1 plays a similar role in the
migration of both naive and effector T cells out of the blood. In addition, it
is thought to be the most important adhesion molecule for T-lymphocyte
activation, because antibodies to LFA-1 effectively inhibit the activation of
both naive and armed effector T cells. ( Leukocyte Adhesion Deficiency, in
Case Studies in Immunology, see Preface for details)
Leukocyte Adhesion Deficiency, in
Case Studies in Immunology, see Preface for details)

Figure 8.6
Integrins are important in T-lymphocyte adhesion. Integrins are heterodimeric proteins containing a β chain, which defines the class of integrin, and an α chain, which defines the different integrins within a class. The α chain (more...)
Surprisingly, T-cell responses can be normal in patients lacking the β2 integrin chain and hence all integrins that contain β2, such as LFA-1. This is probably because T cells also express other adhesion molecules, including CD2 and β1 integrins, which may be able to compensate for the absence of LFA-1. Expression of the β1 integrins increases significantly at a late stage in T-cell activation, and they are thus often called VLAs, for very late activation antigens; we will see in Chapter 10 that they play an important part in directing armed effector T cells to their inflamed target tissues.
Many cell-surface adhesion molecules are members of the immunoglobulin superfamily, which also includes the antigen receptors of T and B cells, the co-receptors CD4, CD8, and CD19, and the invariant domains of MHC molecules. At least five adhesion molecules of the immunoglobulin superfamily are especially important in T-cell activation (Fig. 8.7). Three very similar intercellular adhesion molecules (ICAMs)—ICAM-1, ICAM-2, and ICAM-3—all bind to the T-cell integrin LFA-1. ICAM-1 and ICAM-2 are expressed on endothelium as well as on antigen-presenting cells; binding to these molecules enables lymphocytes to migrate through blood vessel walls. ICAM-3 is expressed only on leukocytes and is thought to play an important part in adhesion between T cells and antigen-presenting cells, particularly dendritic cells. In addition to binding LFA-1, ICAM-3 binds with high affinity to a recently discovered lectin called DC-SIGN, which is found only on dendritic cells. Another interaction involving immunoglobulin superfamily molecules is mediated by LFA-3 on the antigen-presenting cell binding to CD2 on the T cell; this interaction synergizes with that between LFA-1 and ICAM-1 and ICAM-2.

Figure 8.7
Adhesion molecules involved in leukocyte interactions. Several structural families of adhesion molecules play a part in lymphocyte migration, homing, and cell-cell interactions; most have already been introduced in Fig. 2.34. One new member, described (more...)
8-4. The initial interaction of T cells with antigen-presenting cells is mediated by cell-adhesion molecules
As they migrate through the cortical region of the lymph node, naive T cells bind transiently to each antigen-presenting cell they encounter. Antigenpresenting cells, and dendritic cells in particular, bind naive T cells very efficiently through interactions between LFA-1, CD2, and ICAM-3 on the T cell, and ICAM-1, ICAM-2, LFA-3, and DC-SIGN on the antigen-presenting cell (Fig. 8.8). The binding of ICAM-3 to DC-SIGN is unique to the interaction between dendritic cells and T cells, while the other molecules synergize in the binding of lymphocytes to all three types of antigen-presenting cell. Perhaps because of this synergy, the precise role of each adhesion molecule has been difficult to distinguish. People lacking LFA-1 can have normal T-cell responses, and this also seems to be the case for genetically engineered mice lacking CD2. It would not be surprising if there were enough redundancy in the molecules mediating T-cell adhesive interactions to enable immune responses to occur in the absence of any one of them; such molecular redundancy has been observed in other complex biological processes.

Figure 8.8
Cell-surface molecules of the immunoglobulin superfamily are important in the interactions of lymphocytes with antigen-presenting cells. In the initial encounter of T cells with antigen-presenting cells, CD2 binding to LFA-3 on the antigen-presenting (more...)
The transient binding of naive T cells to antigen-presenting cells is crucial in providing time for T cells to sample large numbers of MHC molecules on each antigen-presenting cell for the presence of specific peptide. In those rare cases in which a naive T cell recognizes its peptide:MHC ligand, signaling through the T-cell receptor induces a conformational change in LFA-1, which greatly increases its affinity for ICAM-1 and ICAM-2. This conformational change is the same as that induced by signaling through chemokine receptors during the migration of leukocytes to sites of infection (see Section 2-20), although its mechanism is not known. The change in LFA-1 stabilizes the association between the antigen-specific T cell and the antigen-presenting cell (Fig. 8.9). The association can persist for several days, during which time the naive T cell proliferates and its progeny, which also adhere to the antigen-presenting cell, differentiate into armed effector T cells.
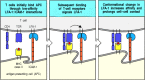
Figure 8.9
Transient adhesive interactions between T cells and antigen-presenting cells are stabilized by specific antigen recognition. When a T cell binds to its specific ligand on an antigen-presenting cell, intracellular signaling through the T-cell receptor (more...)
Most T-cell encounters with antigen-presenting cells do not, however, result in recognition of an antigen. In these encounters, the T cells must be able to separate efficiently from the antigen-presenting cells so that they can continue to migrate through the lymph node, eventually leaving via the efferent lymphatic vessels to reenter the blood and continue circulating. Dissociation, like stable binding, may also involve signaling between the T cell and the antigen-presenting cells, but little is known of its mechanism.
8-5. Both specific ligand and co-stimulatory signals provided by an antigen-presenting cell are required for the clonal expansion of naive T cells
We saw in Chapter 3 that armed effector T cells are triggered when their antigen-specific receptors and either the CD4 or CD8 co-receptors bind to peptide:MHC complexes. By contrast, ligation of the T-cell receptor and co-receptor does not, on its own, stimulate naive T cells to proliferate and differentiate into armed effector T cells. The antigen-specific clonal expansion of naive T cells requires a second, or co-stimulatory, signal (Fig. 8.10), which must be delivered by the same antigen-presenting cell on which the T cell recognizes its antigen. CD8 T cells appear to require a stronger co-stimulatory signal than CD4 cells and, as we will see later, their clonal expansion is aided by CD4 cells interacting with the same antigen-presenting cell.

Figure 8.10
Activation of naive T cells requires two independent signals. Binding of the peptide:MHC complex by the T-cell receptor and, in this example, the CD4 co-receptor, transmits a signal (arrow 1) to the T cell that antigen has been encountered. Activation (more...)
The best-characterized co-stimulatory molecules are the structurally related glycoproteins B7.1 (CD80) and B7.2 (CD86). We will call them the B7 molecules from here on, as functional differences between the two have yet to be defined. The B7 molecules are homodimeric members of the immunoglobulin superfamily that are found exclusively on the surfaces of cells that can stimulate T-cell proliferation. Their role in co-stimulation has been demonstrated by transfecting fibroblasts that express a T-cell ligand with genes encoding B7 molecules and showing that the fibroblasts could then stimulate the clonal expansion of naive T cells. The receptor for B7 molecules on the T cell is CD28, yet another member of the immunoglobulin superfamily (Fig. 8.11). Ligation of CD28 by B7 molecules or by anti-CD28 antibodies co-stimulates the clonal expansion of naive T cells, whereas anti-B7 antibodies, which inhibit the binding of B7 molecules to CD28, inhibit T-cell responses. Although other molecules have been reported to co-stimulate naive T cells, so far only the B7 molecules have been shown definitively to provide costimulatory signals for naive T cells in normal immune responses.

Figure 8.11
The principal co-stimulatory molecules expressed on antigen-presenting cells are B7 molecules, which bind the T-cell protein CD28. Binding of the T-cell receptor (TCR) and its co-receptor CD4 to the peptide:MHC class II complex on the antigen-presenting (more...)
Once a naive T cell is activated, however, it expresses a number of proteins that contribute to sustaining or modifying the co-stimulatory signal that drives clonal expansion and differentiation. One such protein is CD40 ligand, so-called because it binds to CD40 on antigen-presenting cells. Binding of CD40 ligand by CD40 transmits activating signals to the T cell and also activates the antigen-presenting cell to express B7 molecules, thus stimulating further T-cell proliferation. CD40 and CD40 ligand belong to the TNF family of receptors and ligands and, as we will describe later in this chapter, have a central role in the effector function of fully differentiated T cells. Their earlier role in sustaining the development of a T-cell response is demonstrated by mice lacking CD40 ligand; when these mice are immunized, the clonal expansion of responding T cells is curtailed at an early stage. Another pair of TNF family molecules that appear to contribute to co-stimulation of T cells are the T-cell molecule 4-1BB (CD137) and its ligand 4-1BBL, which is expressed on activated dendritic cells, macrophages, and B cells. As with CD40L and CD40, the effects of this receptor-ligand interaction are bidirectional, with both the T cell and the antigen-presenting cell receiving activating signals; this process is sometimes referred to as the T-cell/antigen-presenting cell dialogue.
CD28-related proteins are also induced on activated T cells and serve to modify the co-stimulatory signal as the T-cell response develops. One is CTLA-4 (CD152), an additional receptor for B7 molecules. CTLA-4 closely resembles CD28 in sequence, and the two proteins are encoded by closely linked genes. However, CTLA-4 binds B7 molecules about 20 times more avidly than does CD28 and delivers an inhibitory signal to the activated T cell (Fig. 8.12). This makes the activated progeny of a naive T cell less sensitive to stimulation by the antigen-presenting cell and limits the amount of an autocrine T-cell growth factor, interleukin-2 (IL-2), that is produced. Thus, binding of CTLA-4 to B7 molecules is essential for limiting the proliferative response of activated T cells to antigen and B7. This was confirmed by producing mice with a disrupted CTLA-4 gene; such mice develop a fatal disorder characterized by massive lymphocyte proliferation.
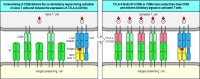
Figure 8.12
T-cell activation through the T-cell receptor and CD28 leads to the increased expression of CTLA-4, an inhibitory receptor for B7 molecules. Naive T cells express CD28, which delivers a co-stimulatory signal on binding B7 molecules (left panel), thereby (more...)
A third CD28-related protein is induced on activated T cells and can enhance T-cell responses; this inducible co-stimulator, or ICOS, binds a ligand known as LICOS, the ligand of ICOS, which is distinct from B7.1 and B7.2. LICOS is produced on activated dendritic cells, monocytes, and B cells, but its contribution to immune responses has not yet been clearly defined. Although it resembles CD28 in driving T-cell growth, it differs from CD28 in not inducing IL-2; instead it induces IL-10 (see Fig. 8.32).

Figure 8.32
The nomenclature and functions of well-defined T-cell cytokines. The major actions are noted in boxes. Each cytokine has multiple activities on different cell types. The mixture of cytokines secreted by a given cell type produces many effects through (more...)
Thus antigen-presenting cells engage in a co-stimulatory dialogue with T cells that recognize the antigens they display. This dialogue involves the delivery and receipt of signals through a number of different molecules, but appears to be initiated through the binding of B7 molecules to CD28 on a naive T cell. Antigen-presenting cells are activated to express B7 molecules on detecting the presence of infection through receptors of the innate immune system. The requirement for the simultaneous delivery of antigen-specific and co-stimulatory signals by one cell in the activation of naive T cells means that only such activated antigen-presenting cells, principally the dendritic cells that migrate into lymphoid tissue after being activated by binding and ingesting pathogens, can initiate T-cell responses. This is important, because not all potentially self-reactive T cells are deleted in the thymus; peptides derived from proteins made only in specialized cells in peripheral tissues might not be encountered during negative selection of thymocytes. Self-tolerance could be broken if naive autoreactive T cells could recognize self antigens on tissue cells and then be co-stimulated by an antigen-presenting cell, either locally or at a distant site. Thus, the requirement that the same cell presents both the specific antigen and the co-stimulatory signal is important in preventing destructive immune responses to self tissues. Indeed, antigen binding to the T-cell receptor in the absence of co-stimulation not only fails to activate the cell, it instead leads to a state called anergy, in which the T cell becomes refractory to activation by specific antigen even when the antigen is subsequently presented to it by a professional antigen-presenting cell (Fig. 8.13).

Figure 8.13
The requirement for one cell to deliver both the antigen-specific signal and the co-stimulatory signal is crucial in preventing immune responses to self antigens. In the upper panels, a T cell recognizes a viral peptide on the surface of an antigen-presenting (more...)
Now that we have discussed the molecular interactions that allow naive T cells to adhere transiently to antigen-presenting cells and scan their MHC:peptide complexes, and also the adhesion and co-stimulatory molecules that contribute to T-cell activation once a specific antigen is encountered, we will look more closely at the properties of the three types of antigen-presenting cell. Dendritic cells, macrophages, and B cells differ in their selectivity of antigen uptake, their antigen-processing properties, and their co-stimulatory and migratory behavior, and thus have distinctive functions in initiating T-cell responses.
8-6. Dendritic cells specialize in taking up antigen and activating naive T cells
The only known function of dendritic cells is to present antigen to T cells, and the mature dendritic cells found in lymphoid tissues are by far the most potent stimulators of naive T cells. This ability is not shared, however, by the immature dendritic cells found under most surface epithelia and in most solid organs such as the heart and kidneys. Dendritic cells arise from myeloid progenitors within the bone marrow, and emerge from the bone marrow to migrate in the blood to peripheral tissues. In these tissues, they have an immature phenotype that is associated with low levels of MHC proteins, and they lack co-stimulatory B7 molecules (Fig 8.14, top panel). They are not yet equipped to stimulate naive T cells. However, they share with their close relatives the macrophages, the ability to recognize and ingest pathogens through receptors that recognize features common to microbial surfaces, and they are very active in taking up antigens by phagocytosis using receptors such as DEC 205. Other extracellular antigens are taken up nonspecifically by macropinocytosis, in which large volumes of surrounding fluid are engulfed.

Figure 8.14
Dendritic cells mature through at least two definable stages to become potent antigen-presenting cells in lymphoid tissue. Dendritic cells arise from bone marrow progenitors and migrate via the blood to peripheral tissues and organs, where they are highly phagocytic (more...)
Typical of immature dendritic cells are the Langerhans' cells of the skin. These are actively phagocytic and contain large granules, known as Birbeck granules, which may be a type of phagosome. An infection triggers the migration of Langerhans' cells to the regional lymph nodes (Figs 8.15 and 8.2). Here, they rapidly lose the ability to take up and process antigen, but synthesize new MHC molecules that present peptides of pathogens at a high level. On arriving in the regional lymph node, they also express B7 molecules, which can co-stimulate naive T cells, and also large numbers of adhesion molecules, which enable them to interact with antigen-specific T cells. In this way the Langerhans' cells capture antigens from invading pathogens and differentiate into mature dendritic cells that are uniquely fitted for presenting these antigens and activating naive T cells.

Figure 8.15
Langerhans' cells can take up antigen in the skin and migrate to lymphoid organs where they present it to T cells. Langerhans' cells can ingest antigen by several means, but have no co-stimulatory activity. In the presence of infection, they take up antigen (more...)
Immature dendritic cells persist in the peripheral tissues for variable lengths of time. When an infection occurs, they are stimulated to migrate via the lymphatics to the local lymphoid tissues, where they have a completely different phenotype. The dendritic cells in lymphoid tissue are no longer able to engulf antigens by phagocytosis or by macropinocytosis. However, they now express very high levels of long-lived MHC class I and MHC class II molecules; this enables them to stably present peptides from proteins acquired from the infecting pathogens. They also express very high levels of adhesion molecules, including DC-SIGN, as well as high levels of B7 molecules (Fig. 8.14, center panel). They also secrete a chemokine that specifically attracts naive T cells; this chemokine, called DC-CK, is expressed only in dendritic cells in lymphoid tissues. These properties help to explain dendritic cells' ability to stimulate strong naive T-cell responses.
Although activated mature dendritic cells will also present some self peptides, the T-cell receptor repertoire has been purged in the thymus of receptors that recognize self peptides presented by dendritic cells (see Chapter 7), and thus T-cell responses against ubiquitous self antigens are avoided. In addition, tissue dendritic cells reaching the end of their life-span without having been activated by infection also travel via the lymphatics to local lymphoid tissue. Because they do not express the appropriate costimulatory molecules, these cells induce tolerance to any self antigens derived from peripheral tissues that they display.
The signals that activate tissue dendritic cells to migrate and mature after taking up antigen are clearly of key importance in determining whether an adaptive immune response will be initiated. These signals can be generated through direct interactions with pathogens or by cytokine stimulation, but in both cases they are thought to be a consequence of the recognition of invading pathogens by nonclonotypic receptors of the innate immune system. The best-understood example is the response to gram-negative bacteria, whose cell walls contain lipopolysaccharide (LPS). Receptors that recognize LPS are found on dendritic cells and macrophages, and these associate with the Toll-like signaling receptor TLR-4, which then activates the transcription factor NFκB (see Sections 2-17 and 6-15). Signaling through this pathway induces the expression of B7 molecules, and of cytokines such as TNF-α, which stimulate the migration of tissue dendritic cells. Thus an immature tissue dendritic cell that binds and internalizes a gram-negative bacterium is induced to migrate to local lymphoid tissue and present bacterium-derived peptide antigens to naive T cells. Other members of the TLR family are expressed on tissue dendritic cells, and are thought to be involved in detecting and signaling the presence of other classes of pathogen. Other types of receptor that can bind pathogens, such as receptors for complement, or phagocytic receptors such as the mannose receptor, are also expressed on dendritic cells and may contribute to their activation.
Pathogens that have evolved to escape recognition by phagocytic receptors are taken up by tissue dendritic cells through the process of macropinocytosis, and can then be presented to T cells. This is thought to occur after intracellular degradation of the pathogen to reveal components that trigger activation of the dendritic cell. Bacterial DNA containing unmethylated CpG dinucleotide motifs induces the rapid activation of dendritic cells. This probably occurs after recognition of the DNA by an intracellular receptor called TLR-9. Exposure to bacterial DNA activates NFκB and mitogen-activated protein kinase (MAP kinase) signaling pathways, leading to the production of cytokines such as IL-6, IL-12, IL-18, and interferon (IFN)-α and IFN-γ. In turn, these induce and augment the expression of co-stimulatory molecules. Bacterial heat-shock proteins are another internal bacterial constituent that can activate the antigen-presenting function of dendritic cells. Some viruses are thought to be recognized inside the dendritic cell, as a consequence of the production of double-stranded RNA in the course of their replication. As discussed in Section 2-25, viral infection also induces the production of IFN-α by infected cells. IFN-α is one of the cytokines that can activate dendritic cells to express co-stimulatory molecules.
Dendritic cells are likely to be particularly important in stimulating T-cell responses to viruses, which fail to induce co-stimulatory activity in other types of antigen-presenting cell. Viruses may infect dendritic cells by binding to any of several molecules on the cell surface, or after being engulfed but not destroyed by immature dendritic cells. Such viruses synthesize their proteins using the dendritic cell's own protein synthesis machinery, leading to surface expression of viral peptides by MHC class I molecules just as in other types of infected cell. Viral peptides will also be presented on both MHC class I and MHC class II molecules as a result of uptake of viral particles by phagocytic receptors such as the mannose receptor, which can recognize many viruses, or through macropinocytosis. The mechanism by which peptides generated by degradation of viral proteins in the endosomal pathway can be presented by MHC class I molecules is not known, nor, in fact, whether there is only one such mechanism. Nevertheless, it is clear that extracellular proteins taken up by dendritic cells can give rise to peptides presented by MHC class I molecules. In this way, viruses that are not able to infect dendritic cells are still able to stimulate effective immune responses. Thus, any virus-infected cell is able to activate naive CD8 T cells, generating cytotoxic CD8 effector T cells that can kill infected cells, and also to activate CD4 T cells that can stimulate the production of antibodies.
Dendritic cells are believed to present antigens from fungal as well as viral and bacterial pathogens. Indeed, they are thought to initiate immune responses to a wide range of pathogens, and to be able to distinguish between different classes of pathogen. This is reflected in the synthesis of different effector molecules by the activated dendritic cells, which in turn influence the differentiation of the responding T cells into different subclasses, which is discussed further in Section 10-5. In addition to pathogen-associated antigens, dendritic cells are thought to present protein antigens from environmental sources that trigger allergic reactions upon inhalation (see Chapter 12), and alloantigens deriving from a transplanted organ, which form the basis for graft rejection (see Chapter 13). In principle, any nonself antigen will be immunogenic if it is taken up and presented by a dendritic cell that is activated to migrate to nearby lymphoid tissues and mature. The normal physiology of dendritic cells is to migrate, and this is increased by stimuli that activate the linings of the lymphatics, like transplantation, which is why dendritic cells are so potent at stimulating allograft reactions.
8-7. Macrophages are scavenger cells that can be induced by pathogens to present foreign antigens to naive T cells
As we learned in Chapter 2, many of the microorganisms that enter the body are engulfed and destroyed by phagocytes, which provide an innate, antigen-nonspecific first line of defense against infection. Microorganisms that are destroyed by phagocytes without additional help from T cells do not cause disease and do not require an adaptive immune response. Pathogens, by definition, have developed mechanisms to avoid elimination by innate immunity, and the targeting and removal of such pathogens is the function of the adaptive immune response. Mononuclear phagocytes or macrophages that have bound and ingested microorganisms but have failed to destroy them, contribute to the adaptive immune response by acting as antigenpresenting cells. As we will see later in this chapter and in Chapter 10, the adaptive immune response is in turn able to stimulate the microbicidal and phagocytic capacities of these cells.
Resting macrophages have few or no MHC class II molecules on their surface, and do not express B7 molecules. The expression of both MHC class II and B7 molecules is induced by the ingestion of microorganisms and recognition of their foreign molecular patterns. Macrophages, like tissue dendritic cells, have a variety of receptors that recognize microbial surface components, including the mannose receptor, the scavenger receptor, complement receptors, and several Toll-like receptors (see Chapter 2). These receptors function in the innate immune defense mediated by macrophages; they are involved in the uptake of microorganisms by phagocytosis and in signaling for the secretion of pro-inflammatory cytokines that recruit and activate more phagocytes. In addition, they can play the same role as tissue dendritic cells, and allow the macrophage to function as an antigen-presenting cell. Once bound, microorganisms are engulfed and degraded in the endosomes and lysosomes, generating peptides that can be presented by MHC class II molecules. At the same time, the receptors recognizing these microorganisms transmit a signal that leads to expression of MHC class II molecules and B7 molecules.
Thus the induction of co-stimulatory activity by common microbial constituents occurs in both dendritic cells and macrophages. This is believed to allow the immune system to distinguish antigens borne by infectious agents from antigens associated with innocuous proteins, including self proteins. Indeed, many foreign proteins do not induce an immune response when injected on their own, presumably because they fail to induce costimulatory activity in antigen-presenting cells. When such protein antigens are mixed with bacteria, however, they become immunogenic, because the bacteria induce the essential co-stimulatory activity in cells that ingest the protein (Fig. 8.16). Bacteria used in this way are known as adjuvants (see Appendix I, Section A-4). We will see in Chapter 13 how self tissue proteins mixed with bacterial adjuvants can induce autoimmune diseases, illustrating the crucial importance of the regulation of co-stimulatory activity in discrimination of self from nonself.

Figure 8.16
Microbial substances can induce co-stimulatory activity in macrophages. If protein antigens are taken up and presented by macro-phages in the absence of bacterial components that induce co-stimulatory activity in the macrophage, T cells specific for the (more...)
As macrophages continuously scavenge dead or dying cells, which are rich sources of self antigens, it is particularly important that they should not activate T cells in the absence of microbial infection. The Kupffer cells of the liver sinusoids and the macrophages of the splenic red pulp, in particular, remove large numbers of dying cells from the blood daily. Kupffer cells express little MHC class II and no TLR-4, the Toll-like receptor on human cells that signals the presence of LPS. Thus, although they generate large amounts of self peptides in their endosomes and lysosomes, these macrophages are not likely to elicit an autoimmune response.
8-8. B cells are highly efficient at presenting antigens that bind to their surface immunoglobulin
Macrophages cannot take up soluble antigens efficiently, whereas immature dendritic cells can take up large amounts of antigen from extracellular fluid by macropinocytosis. B cells, by contrast, are uniquely adapted to bind specific soluble molecules through their cell-surface immunoglobulin. B cells internalize the antigens bound by their surface immunoglobulin receptors and then display peptide fragments of antigen as peptide:MHC class II complexes. Because this mechanism of antigen uptake is highly efficient, and B cells constitutively express high levels of MHC class II molecules, high levels of specific peptide:self MHC class II complexes are generated at the B-cell surface (Fig. 8.17). This pathway of antigen presentation allows B cells to be targeted by antigen-specific CD4 T cells, which drive their differentiation, as we will see in Chapter 9. In circumstances in which the presenting B cells are induced to express co-stimulatory activity, it also allows B cells to activate naive T cells.

Figure 8.17
B cells can use their immunoglobulin receptor to present specific antigen very efficiently to T cells. Surface immunoglobulin allows B cells to bind and internalize specific antigen very efficiently. The internalized antigen is processed in intracellular (more...)
B cells do not constitutively express co-stimulatory activity but, as with dendritic cells and macrophages, they can be induced by various microbial constituents to express B7.1 and especially B7.2. Indeed, B7.1 was first identified as a molecule expressed on B cells activated by microbial lipopolysaccharide. These observations help explain why it is essential to co-inject bacterial adjuvants in order to produce an immune response to soluble proteins such as ovalbumin, hen egg-white lysozyme, and cytochrome c, which may require B cells as antigen-presenting cells.
The requirement for induced co-stimulatory activity also helps explain why, although B cells present soluble proteins efficiently, they are unlikely to initiate responses to soluble self proteins in the absence of infection. In the absence of co-stimulation, antigen not only fails to activate naive T cells but causes them to become anergic, or nonresponsive (see Fig. 8.13). This provides an additional safeguard to the mechanisms discussed in Chapter 7 whereby potentially self-reactive T and B cells are eliminated or inactivated as they develop in the thymus and bone marrow.
Although much of what we know about the immune system in general, and about T-cell responses in particular, has been learned from the study of immune responses to soluble protein immunogens presented by B cells, it is not clear how important B cells are in priming naive T cells in natural immune responses. Soluble protein antigens are not abundant during natural infections; most natural antigens, such as bacteria and viruses, are particulate, whereas soluble bacterial toxins act by binding to cell surfaces and so are present only at low concentrations in solution. Some natural immunogens enter the body as soluble molecules; examples are insect toxins, anticoagulants injected by blood-sucking insects, snake venoms, and many allergens. However, tissue dendritic cells could also be responsible for activating naive T cells that recognize these antigens. Although tissue dendritic cells could not concentrate these antigens in the same way as antigen-specific B cells, they may be more likely to encounter a naive T cell with the appropriate antigen specificity than the limited number of B cells able to bind and concentrate a particular antigen. The chances of a B cell encountering a T cell that can recognize the peptide antigens it displays is greatly increased once a naive T cell has been detained in lymphoid tissue by finding its antigen on the surface of a dendritic cell.
T-cell responses can thus be primed by three distinct types of antigen- presenting cell. Dendritic cells are optimally equipped to present a wide variety of antigens to naive T cells, while macrophages stimulate T-cell responses to the pathogens they take up but are unable to eliminate, and B cells specialize in presenting fragments of the antigen to which their surface immunoglobulin binds (Fig. 8.18). In each of these cell types, as we saw in Chapter 2, the expression of co-stimulatory activity is controlled so as to provoke responses against pathogens while avoiding immunization against self.

Figure 8.18
The properties of the various antigen-presenting cells. Dendritic cells, macrophages, and B cells are the main cell types involved in the initial presentation of foreign antigens to naive T cells. These cells vary in their means of antigen uptake, MHC (more...)
8-9. Activated T cells synthesize the T-cell growth factor interleukin-2 and its receptor
Naive T cells can live for many years without dividing. These small resting cells have condensed chromatin and a scanty cytoplasm and synthesize little RNA or protein. On activation, they must reenter the cell cycle and divide rapidly to produce the large numbers of progeny that will differentiate into armed effector T cells. Their proliferation and differentiation are driven by a cytokine called interleukin-2 (IL-2), which is produced by the activated T cell itself.
The initial encounter with specific antigen in the presence of the required co-stimulatory signal triggers entry of the T cell into the G1 phase of the cell cycle; at the same time, it also induces the synthesis of IL-2 along with the α chain of the IL-2 receptor. The IL-2 receptor has three chains: α, β, and γ (Fig. 8.19). Resting T cells express a form of this receptor composed of β and γ chains which binds IL-2 with moderate affinity, allowing resting T cells to respond to very high concentrations of IL-2. Association of the α chain with the β and γ chains creates a receptor with a much higher affinity for IL-2, allowing the cell to respond to very low concentrations of IL-2. Binding of IL-2 to the high-affinity receptor then triggers progression through the rest of the cell cycle. T cells activated in this way can divide two to three times a day for several days, allowing one cell to give rise to a clone composed of thousands of progeny that all bear the same receptor for antigen (Fig. 8.20). IL-2 also promotes the differentiation of these cells into armed effector T cells.

Figure 8.19
High-affinity IL-2 receptors are three-chain structures that are produced only on activated T cells. On resting T cells, the β and γ chains are expressed constitutively. They bind IL-2 with moderate affinity. Activation of T cells induces the (more...)

Figure 8.20
Activated T cells secrete and respond to IL-2. Activation of naive T cells by the recognition of a peptide: MHC complex accompanied by co-stimulation induces expression and secretion of IL-2 and the expression of high-affinity IL-2 receptors. IL-2 binds (more...)
8-10. The co-stimulatory signal is necessary for the synthesis and secretion of IL-2
The production of IL-2 determines whether a T cell will proliferate and become an armed effector cell, and the most important function of the co-stimulatory signal is to promote the synthesis of IL-2. Antigen recognition by the T-cell receptor ultimately induces the synthesis of several transcription factors (see Chapter 6). One of these factors, NFAT (nuclear factor of activated T cells), binds to the promoter region of the IL-2 gene and is needed to activate its transcription. IL-2 gene transcription on its own, however, does not lead to the production of IL-2, which additionally requires CD28 ligation by B7. One effect of signaling through CD28 is thought to be the stabilization of IL-2 mRNA. Cytokine mRNAs are very short-lived because of an ‘instability’ sequence in their 3′ untranslated region. This instability prevents sustained cytokine production and release, and enables cytokine activity to be tightly regulated. The stabilization of IL-2 mRNA increases IL-2 synthesis by 20- to 30-fold. A second effect of CD28 ligation is to activate transcription factors (AP-1 and NFκB) that increase transcription of IL-2 mRNA by about threefold. These two effects together increase IL-2 protein production by as much as 100-fold. When a T cell recognizes specific antigen in the absence of co-stimulation through its CD28 molecule, little IL-2 is produced and the T cell does not proliferate.
The central importance of IL-2 in initiating adaptive immune responses is well illustrated by the drugs that are most commonly used to suppress undesirable immune responses such as transplant rejection. The immunosuppressive drugs cyclosporin A and FK506 (tacrolimus) inhibit IL-2 production by disrupting signaling through the T-cell receptor, whereas rapamycin (sirolimus) inhibits signaling through the IL-2 receptor. Cyclosporin A and rapamycin act synergistically to inhibit immune responses by preventing the IL-2-driven clonal expansion of T cells. The mode of action of these drugs will be considered in detail in Chapter 14.
8-11. Antigen recognition in the absence of co-stimulation leads to T-cell tolerance
Antigen recognition in the absence of co-stimulation inactivates naive T cells, inducing a state known as anergy. The most important change in anergic T cells is their inability to produce IL-2. This prevents them from proliferating and differentiating into effector cells when they encounter antigen, even if the antigen is subsequently presented by antigen-presenting cells. This helps to ensure the tolerance of T cells to self tissue antigens. Although anergy has only been demonstrated formally in vitro, there is sufficiently compelling evidence from studies in vivo showing peripheral tolerance to various antigens to assume that it happens in this setting as well.
As we saw in Section 7-24, any protein synthesized by all cells will be presented by antigen-presenting cells in the thymus and will cause clonal deletion of the T cells reactive to these ubiquitous self proteins. However, many proteins have specialized functions and are made only by the cells of certain tissues. Because MHC class I molecules present only peptides derived from proteins synthesized within the cell, such tissue-specific peptides will not be displayed on the MHC molecules of thymic cells, and T cells recognizing them are unlikely to be deleted in the thymus. An important factor in avoiding autoimmune responses to such tissue-specific proteins is the absence of co-stimulatory activity on tissue cells. Naive T cells recognizing self peptides on tissue cells are not activated; instead they may be induced to enter a state of anergy (Fig. 8.21).

Figure 8.21
T-cell tolerance to antigens expressed on tissue cells results from antigen recognition in the absence of co-stimulation. An antigen-presenting cell (APC) will neither activate nor inactivate a T cell if the appropriate antigen is not present on the APC surface, (more...)
Although the deletion of potentially autoreactive T cells is readily understood as a simple way to maintain self tolerance, the retention of anergic T cells specific for tissue antigens is less easy to understand. It would seem more economical and efficient to eliminate such cells; indeed, binding of the T-cell receptor on peripheral T cells in the absence of co-stimulators can lead to programmed cell death as well as to anergy. Nevertheless, some T cells persist in an anergic state in vivo. One possible explanation for this is that such anergic T cells have a role in preventing responses by naive, nonanergic T cells to foreign antigens that mimic self peptide:self MHC complexes. The persisting anergic T cells could recognize and bind to such peptide:MHC complexes on antigen-presenting cells without responding, and thus could compete with naive, potentially autoreactive cells of the same specificity. In this way, anergic T cells could serve to prevent the accidental activation of autoreactive T cells by infectious agents, thus actively contributing to tolerance.
8-12. Proliferating T cells differentiate into armed effector T cells that do not require co-stimulation to act
Late in the proliferative phase of the T-cell response induced by IL-2, after 4–5 days of rapid growth, activated T cells differentiate into armed effector T cells that can synthesize all the effector molecules required for their specialized functions as helper or cytotoxic T cells. In addition, all classes of armed effector T cells have undergone changes that distinguish them from naive T cells. One of the most critical is in their activation requirements: once a T cell has differentiated into an armed effector cell, encounter with its specific antigen results in immune attack without the need for co-stimulation (Fig. 8.22).
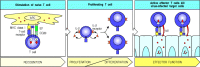
Figure 8.22
Armed effector T cells can respond to their target cells without co-stimulation. A naive T cell that recognizes antigen on the surface of an antigen-presenting cell and receives the required two signals (arrows 1 and 2, left panel) becomes activated, (more...)
This applies to all classes of armed effector T cells. Its importance is particularly easy to understand in the case of cytotoxic CD8 T cells, which must be able to act on any cell infected with a virus, whether or not the infected cell can express co-stimulatory molecules. However, it is also important for the effector function of CD4 cells, as armed effector CD4 T cells must be able to activate B cells and macrophages that have taken up antigen, even if, as is often the case, they have too little co-stimulatory activity to activate a naive CD4 T cell.
Changes are also seen in the cell-adhesion molecules expressed by armed effector T cells. They express higher levels of LFA-1 and CD2, but lose cell-surface L-selectin and thus cease to recirculate through lymph nodes. Instead, they express the integrin VLA-4, which allows them to bind to vascular endothelium at sites of inflammation. This allows the armed effector T cells to enter sites of infection and put their armory of effector proteins to good use. These changes in the T-cell surface are summarized in Fig. 8.23.

Figure 8.23
Activation of T cells changes the expression of several cell-surface molecules. The example here is a CD4 T cell. Resting naive T cells express L-selectin, through which they home to lymph nodes, with relatively low levels of other adhesion molecules (more...)
8-13. The differentiation of CD4 T cells into TH1 or TH2 cells determines whether humoral or cell-mediated immunity will predominate.
Naive CD8 T cells emerging from the thymus are already predestined to become cytotoxic cells, even though they are not yet expressing any of the differentiated functions of armed effector cells. The case of CD4 T cells, however, is more complex. Naive CD4 T cells can differentiate upon activation into either TH1 or TH2 cells, which differ in the cytokines they produce and thus in their function. The decision on which fate the progeny of a naive CD4 T cell will follow is made during the clonal expansion that takes place after the first encounter with antigen (Fig. 8.24).
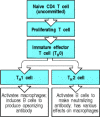
Figure 8.24
The stages of activation of CD4 T cells. Naive CD4 T cells first respond to their specific peptide:MHC class II complexes by making IL-2 and proliferating. These cells then differentiate into a cell type known as TH0, which has some of the effector functions (more...)
The factors that determine whether a proliferating CD4 T cell will differentiate into a TH1 or a TH2 cell are not fully understood. The cytokines elicited by infectious agents (principally IFN-γ, IL-12, and IL-4), the co-stimulators used to drive the response, and the nature of the peptide:MHC ligand all have an effect. In particular, because the decision to differentiate into TH1 versus TH2 cells occurs early in the immune response, the cytokines produced in response to pathogens by cells of the innate immune system play an important part in shaping the subsequent adaptive response; we will learn more about this in Chapter 10.
The consequences of inducing TH1 versus TH2 cells are
profound: the selective production of TH1 cells leads to
cell-mediated immunity, whereas the production of predominantly TH2
cells provides humoral immunity. A striking example of the difference this can
make to the outcome of infection is seen in leprosy, a disease caused by
infection with Mycobacterium leprae. M.
leprae, like M. tuberculosis, grows
in macrophage vesicles, and effective host defense requires macrophage
activation by TH1 cells. In patients with tuberculoid leprosy, in
which TH1 cells are preferentially induced, few live bacteria are
found, little antibody is produced, and, although skin and peripheral nerves are
damaged by the inflammatory responses associated with macrophage activation, the
disease progresses slowly and the patient usually survives. However, when
TH2 cells are preferentially induced, the main response is
humoral, the antibodies produced cannot reach the intracellular bacteria, and
the patients develop lepromatous leprosy ( Lepromatous Leprosy, in
Case Studies in Immunology, see Preface for details), in which M. leprae
grows abundantly in macrophages, causing gross tissue destruction that is
eventually fatal.
Lepromatous Leprosy, in
Case Studies in Immunology, see Preface for details), in which M. leprae
grows abundantly in macrophages, causing gross tissue destruction that is
eventually fatal.
8-14. Naive CD8 T cells can be activated in different ways to become armed cytotoxic effector cells
Naive CD8 T cells differentiate into cytotoxic cells, and perhaps because the effector actions of these cells are so destructive, naive CD8 T cells require more co-stimulatory activity to drive them to become armed effector cells than do naive CD4 T cells. This requirement can be met in two ways. The simplest is activation by dendritic cells, which have high intrinsic costimulatory activity. These cells can directly stimulate CD8 T cells to synthesize the IL-2 that drives their own proliferation and differentiation (Fig. 8.25). This has been exploited to generate cytotoxic T-cell responses against tumors, as we will see in Chapter 14.

Figure 8.25
Naive CD8 T cells can be activated directly by potent antigen-presenting cells. Naive CD8 T cells that encounter peptide:MHC class I complexes on the surface of dendritic cells, which express high levels of co-stimulatory molecules (left panel), are activated (more...)
Cytotoxic T-cell responses to some viruses and tissue grafts, however, seem to require the presence of CD4 T cells during the priming of the naive CD8 T cell. In these responses, both the naive CD8 T cell and the CD4 T cell must recognize related antigens on the surface of the same antigen-presenting cell. In this case, it is thought that the actions of the CD4 T cell may be needed to compensate for inadequate co-stimulation of naive CD8 T cells by the antigen-presenting cell. This compensatory effect is currently thought to occur by the recruitment of an armed effector CD4 T cell that activates the antigenpresenting cell to express higher levels of co-stimulatory activity. We have seen that this is one of the actions of the CD40 ligand, which is expressed once T cells have been activated. Binding of CD40 ligand on the CD4 T cell to CD40 on the antigen-presenting cell induces B7 expression and enables the antigen-presenting cell to co-stimulate the CD8 T cell directly (Fig. 8.26).
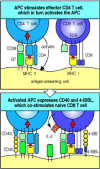
Figure 8.26
Some CD8 T-cell responses require CD4 T cells. CD8 T cells recognizing antigen on weakly co-stimulating cells may become activated only in the presence of CD4 T cells bound to the same antigen-presenting cell. This happens mainly by an effector CD4 T (more...)
Summary
The crucial first step in adaptive immunity is the activation of naive antigen-specific T cells by antigen-presenting cells. This occurs in the lymphoid tissues and organs through which naive T cells are constantly passing. The most distinctive feature of antigen-presenting cells is the expression of co-stimulatory molecules, of which the B7.1 and B7.2 molecules are the best characterized. Naive T cells will respond to antigen only when one cell presents both specific antigen to the T-cell receptor and a B7 molecule to CD28, the receptor for B7 on the T cell. The three cell types that can serve as antigen-presenting cells are dendritic cells, macrophages, and B cells. Each of these cells has a distinct function in eliciting immune responses. Tissue dendritic cells take up antigens by phagocytosis and macropinocytosis and are stimulated by infection to migrate to the local lymphoid tissue, where they differentiate into mature dendritic cells expressing co-stimulatory activity. They serve as the most potent activators of naive T-cell responses. Macrophages efficiently ingest particulate antigens such as bacteria and are induced by infectious agents to express MHC class II molecules and costimulatory activity. The unique ability of B cells to bind and internalize soluble protein antigens via their receptors may be important in activating T cells to this class of antigen, provided that co-stimulatory molecules are also induced on the B cell. In all three types of antigen-presenting cell, the expression of co-stimulatory molecules is activated in response to signals from receptors that also function in innate immunity to signal the presence of infectious agents (see Chapter 2).
The activation of T cells by antigen-presenting cells leads to their proliferation and the differentiation of their progeny into armed effector T cells. This depends on the production of cytokines, in particular the T-cell growth factor IL-2, which binds to a high-affinity receptor on the activated T cell. T cells whose antigen receptors are ligated in the absence of co-stimulatory signals fail to make IL-2 and instead become anergic or die. This dual requirement for both receptor ligation and co-stimulation helps to prevent naive T cells from responding to self antigens on tissue cells, which lack co-stimulatory activity. Proliferating T cells develop into armed effector T cells, the critical event in most adaptive immune responses. Once an expanded clone of T cells achieves effector function, its armed effector T-cell progeny can act on any target cell that displays antigen on its surface. Effector T cells can mediate a variety of functions. Their most important functions are the killing of infected cells by CD8 cytotoxic T cells and the activation of macrophages by TH1 cells, which together make up cell-mediated immunity, and the activation of B cells by both TH2 and TH1 cells to produce different classes of antibody, thus driving the humoral immune response.
- T-cell responses are initiated in peripheral lymphoid organs by activated antigen-presenting cells
- Naive T cells sample the MHC:peptide complexes on the surface of antigen-presenting cells as they migrate through peripheral lymphoid tissue
- Lymphocyte migration, activation, and effector function depend on cell-cell interactions mediated by cell-adhesion molecules
- The initial interaction of T cells with antigen-presenting cells is mediated by cell-adhesion molecules
- Both specific ligand and co-stimulatory signals provided by an antigen-presenting cell are required for the clonal expansion of naive T cells
- Dendritic cells specialize in taking up antigen and activating naive T cells
- Macrophages are scavenger cells that can be induced by pathogens to present foreign antigens to naive T cells
- B cells are highly efficient at presenting antigens that bind to their surface immunoglobulin
- Activated T cells synthesize the T-cell growth factor interleukin-2 and its receptor
- The co-stimulatory signal is necessary for the synthesis and secretion of IL-2
- Antigen recognition in the absence of co-stimulation leads to T-cell tolerance
- Proliferating T cells differentiate into armed effector T cells that do not require co-stimulation to act
- The differentiation of CD4 T cells into TH1 or TH2 cells determines whether humoral or cell-mediated immunity will predominate.
- Naive CD8 T cells can be activated in different ways to become armed cytotoxic effector cells
- Summary
- The production of armed effector T cells - ImmunobiologyThe production of armed effector T cells - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...