NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. Lyon (FR): International Agency for Research on Cancer; 1999. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 71.)
Data were last reviewed in IARC (1986) and the compound was classified in IARC Monographs Supplement 7 (1987).
1. Exposure Data
1.1. Chemical and physical data
1.1.1. Nomenclature
- Chem. Abstr. Serv. Reg. No.: 78-87-5
- Chem. Abstr. Name: 1,2-Dichloropropane
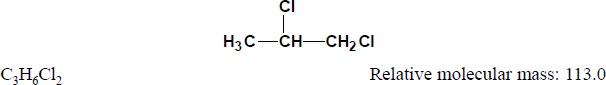
1.1.2. Structural and molecular formulae and relative molecular mass
1.1.3. Physical properties (for details, see IARC, 1986)
- (a) Boiling-point: 96.4°C (Lide, 1995)
- (b) Melting-point: −100.4°C (Lide, 1995)
- (c) Conversion factor: mg/m3 = 4.6 × ppm
1.2. Production, use and human exposure
1,2-Dichloropropane has been used as an industrial solvent, as a chemical intermediate and in soil fumigants. Human exposure may occur during its production and industrial and domestic use, and due to the presence of low levels in ambient air and in water (IARC, 1986).
2. Studies of Cancer in Humans
No data were available to the Working Group.
3. Studies of Cancer in Experimental Animals
1,2-Dichloropropane was tested for carcinogenicity by gavage in one experiment in mice and one experiment in rats. A dose-related increase in the incidence of hepatocellular tumours was observed in male and female mice. Inconclusive results were obtained in female rats, and no effect was seen in male rats (IARC, 1986).
4. Other Data Relevant to an Evaluation of Carcinogenicity and its Mechanisms
4.1. Absorption, distribution, metabolism and excretion
4.1.1. Humans
No data were available to the Working Group.
4.1.2. Experimental systems
Rats receiving single oral doses of 1,2-dichloro[1-14C]propane excreted about 50% in the urine and 5% in faeces in 24 h. There was little further excretion over the next 72 h. A total of 19% of the dose was excreted as 14CO2 and 23% as other volatile substances. At 96 h, 5% remained in the carcass. The major urinary metabolite found after oral dosing was N-acetyl-S-(2-hydroxypropyl)-l-cysteine. Identified minor metabolites were β-chlorolactate and N-acetyl-S-(2,3-dihydroxypropyl)-l-cysteine (IARC, 1986).
In later studies, male and female Fischer 344 rats were either exposed to atmospheres of 5, 50 or 100 ppm [23, 230 or 460 mg/m3] of singly labelled 1,2-dichloro[14C]propane for a 6-h period or dosed orally once with 1 or 100 mg/kg bw or on seven consecutive days with 1 mg/kg bw. During inhalation exposure, maximum blood concentrations were reached after 2 h, the values being approximately 0.06, 0.9 and 4.0 µg/g blood, respectively. Once exposure stopped, 1,2-dichloropropane was rapidly eliminated from blood. Analysis of expired air provided evidence for saturation of metabolism, the proportion of expired 1,2-dichloro[14C]propane increasing with dose. For both gavage and inhalation administration, the principal routes of elimination were urine (37–65%) and expired air (18–40%), most of the radioactivity being eliminated within 24 h, irrespective of the route or sex. Tissues, faeces and the cage wash accounted for < 11%, about 10% and about 4% of the dose, respectively. The major urinary metabolites were N-acetyl-S-(2-hydroxypropyl)-l-cysteine (I), N-acetyl-S-(2-oxopropyl)-l-cysteine (II) and N-acetyl-S-(1-carboxyethyl)-l-cysteine (III) (Timchalk et al., 1991). It has been proposed that these metabolites could arise as follows (Figure 1): oxidation of C-1 and subsequent conjugation on C-2 gives III; conjugation on C-1 and oxidation of C-2 gives II; reduction of II gives I. This mechanism is supported by studies with D6-labelled compounds (Bartels & Timchalk, 1990) as well as studies that strongly suggest that 1,2-dichloropropane is activated by human CYP2E1, by oxidation, to a product trapped as a glutathione conjugate (Guengerich et al., 1991).

Figure 1
Proposed metabolic scheme for the formation of mercapturic acid metabolites of 1,2-dichloropropane in the rat.
4.2. Toxic effects
4.2.1. Humans
Human exposures resulting in toxicity indicate that the main target organs are liver and kidney (IARC, 1986). Sublethal exposure also causes central nervous system depression (Imberti et al., 1987; Lucantoni et al., 1992). In a case series of 10 painters or engineers with contact allergic dermatitis, all patients demonstrated a positive response to 1,2-dichloropropane (Baruffini et al., 1989).
4.2.2. Experimental systems
Liver damage follows short-term exposure of rats to 1,2-dichloropropane by inhalation (IARC, 1986).
Adult male Sprague-Dawley rats were dosed by gavage with 0, 100, 250, 500 or 1000 (750 in the 13-week study) mg/kg bw 1,2-dichloropropane per day for one day, for up to 10 days or for 13 weeks. In the single-dose study, the main effects were a reduction in body weight gain and central nervous system depression; morphological changes were restricted to centrilobular hepatocytes in rats of the 500 and 1000 mg/kg bw dose groups. Non-protein sulfhydryl (thiols) were decreased in the liver and increased in the kidney. Over the 10-day period, resistance to hepatotoxicity developed, but there was clear evidence of haemolytic anaemia and haemosiderosis. In the 13-week study, many deaths occurred in the groups given 500 and 750 mg/kg bw, but none occurred in the lower-dose groups. There was limited hepatotoxicity and no apparent nephrotoxicity, while splenic haemosiderosis was evident in most rats of all dose groups (Bruckner et al., 1989).
Exposure of rats by inhalation to 1,2-dichloropropane concentrations of 100 mg/m3 for 4 h produced blood concentrations of 0.2 µg/mL and resulted in a reduction of hepatic non-protein thiols immediately following the treatment, while there was no evidence of hepatic lipid peroxidation or change in total protein content (Di Nucci et al., 1990). A dose-dependent decrease in hepatic reduced glutathione content was also found after single intraperitoneal injections of rats with 1,2-dichloropropane. Daily dosing for four weeks resulted in a dose-dependent increase in reduced glutathione and glutathione-S-transferase activity and a decrease in cytochrome P450 content. Areas of focal necrosis observed after five days dosing tended to disappear after the longer dosing period. Steatosis was evident after five days' dosing at 100 mg/kg bw, while hyperplasia of the liver was seen in 5/5 rats examined at 10 mg/kg bw (Trevisan et al., 1989). Treatment of rats with buthionine sulfoximine, a glutathione-depleting agent, increased the lethal toxicity of 1,2-dichloropropane (2 mL/kg bw), while administration of N-acetylcysteine, a glutathione precursor, decreased the toxicity (Imberti et al., 1990).
In contrast to the increased concentration of non-protein thiols in the kidney, mentioned above, there has been report of a dose-dependent decrease in angiotensin-converting enzyme activity of the proximal tubule brush border, fraying of the microvilli and epithelial coagulative necrosis of the brush border after intraperitoneal treatment of rats with 250 and 500 mg/kg bw 1,2-dichloropropane. The earliest renal changes are alterations of the glomeruli, but the most sensitive parameter is angiotensin-converting enzyme activity. The biochemical changes are reversible (Trevisan et al., 1988, 1991). In-vitro studies in which rat renal cortical slices were exposed to 1,2-dichloropropane show that a depletion in glutathione content occurs which can be prevented by carbon monoxide and the loss of organic anion accumulation (lactate and 4-aminohippurate) can be partially inhibited by acivicin and aminooxyacetic acid, which are inhibitors of γ-glutamyltranspeptidase and β-lyase activities, respectively (Trevisan et al., 1993).
4.3. Reproductive and developmental effects
4.3.1. Humans
No data were available to the Working Group.
4.3.2. Experimental systems
Pregnant Sprague-Dawley rats and New Zealand White rabbits were dosed orally (gavage) with 1,2-dichloropropane on gestation days 6–15 and 7–19, respectively. Maternal toxicity in both rats and rabbits was observed at doses of 125 mg/kg bw and 150 mg/kg bw, respectively. At these maternally toxic doses only, there were increases in the incidence of delayed ossification of the skull of the fetuses. No teratogenic effects were observed in either rats or rabbits (Kirk et al., 1995).
D-D, a commercial mixture of chlorinated hydrocarbons that contained 25.6% 1,2-dichloropropane (other major components being cis(Z)-1,3-dichloropropene, 28.1%, and trans(E)-1,3-dichloropropene, 25.6%) was tested for effects on reproduction in male and female rats exposed by inhalation to D-D concentrations up to 90 ppm (v/v) for 6 h per day on five days per week for 10 weeks before they were mated. There were decreases in body weight gain and slight increases in the weights of liver and kidney in 90-ppm rats of both sexes, but there were no effects upon reproductive performance (Linnett et al., 1988).
4.4. Genetic and related effects
4.4.1. Humans
No data were available to the Working Group.
4.4.2. Experimental systems (see Table 1 for references)
Table 1
Genetic and related effects of 1,2-dichloropropane.
1,2-Dichloropropane was mutagenic to Salmonella typhimurium but not to Streptomyces coelicolor. It induced mutations weakly but not chromosomal effects in Aspergillus nidulans. It did not induce sex-linked recessive lethal mutations in Drosophila melanogaster.
In Chinese hamster ovary CHO cells in culture, it induced sister chromatid exchanges and chromosomal aberrations.
5. Evaluation
No epidemiological data relevant to the carcinogenicity of 1,2-dichloropropane were available.
There is limited evidence in experimental animals for the carcinogenicity of 1,2-dichloropropane.
Overall evaluation
1,2-Dichloropropane is not classifiable as to its carcinogenicity to humans (Group 3).
6. References
- Bartels M.J., Timchalk C. 1,2-Dichloropropane: investigation of the mechanism of mercapturic acid formation in the rat. Xenobiotica. 1990;20:1035–1042. [PubMed: 2082593]
- Baruffini A., Cirla A.M., Pisati G., Ratti R., Zedda S. Allergic contact dermatitis from 1,2-dichloropropane. Contact Derm. 1989;20:379–380. [PubMed: 2527719]
- Bruckner J.V., Mackenzie W.F., Ramanathan R., Muralidhara S., Kim H.J., Dallas C.E. Oral toxicity of 1,2-dichloropropane: acute, short-term, and long-term studies in rats. Fundam. appl. Toxicol. 1989;12:713–730. [PubMed: 2744274]
- Crebelli R., Conti G., Conti L., Carere A. Induction of somatic segregation by halogenated aliphatic hydrocarbons in Aspergillus nidulans. Mutat. Res. 1984;138:33–38. [PubMed: 6387478]
- De Lorenzo F., Degl'Innocenti S., Ruocco A., Silengo L., Cortese R. Mutagenicity of pesticides containing 1,3-dichloropropene. Cancer Res. 1977;37:1915–1917. [PubMed: 322862]
- Di Nucci A., Gregotti C., Manzo L., Imbriani M., Ghittori S., Bianco L., Maestri L., Capodaglio E. 1,2-Dichloropropane hepatotoxicity in rats after inhalation exposure. J. appl. Toxicol. 1990;10:391–394. [PubMed: 2084176]
- Galloway S.M., Armstrong M.J., Reuben C., Colman S., Brown B., Cannon C., Bloom A.D., Nakamura F., Ahmed M., Duk S. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ. mol. Mutagen. 1987;10:1–175. [PubMed: 3319609]
- Guengerich F.P., Kim D.H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem. Res. Toxicol. 1991;4:168–179. [PubMed: 1664256]
- Haworth S., Lawlor T., Mortelmans K., Speck W., Zeiger E. Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. 1983;5:1–142. [PubMed: 6365529]
- IARC (1986) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 41, Some Halogenated Hydrocarbons and Pesticides Exposures, Lyon, pp. 131–147. [PubMed: 3473022]
- IARC (1987) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Supplement 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, p. 62. [PubMed: 3482203]
- Imberti R., Calabrese S.R., Emilio G., Marchi L., Giuffrida L. Acute poisoning with solvents: chlorinated aliphatic hydrocarbons. Minerva Anestesiol. 1987;53:399–403. (in Italian) [PubMed: 3444546]
- Imberti R., Mapelli A., Colombo P., Richelmi P., Berte F., Bellomo G. 1,2-Dichloropropane (DCP) toxicity is correlated with DCP-induced glutathione (GSH) depletion and is modulated by factors affecting intracellular GSH. Arch. Toxicol. 1990;64:459–465. [PubMed: 1980407]
- Kirk H.D., Berdasco N.M., Breslin W.J., Hanley T.R. Jr. Developmental toxicity of 1,2-dichloropropane (PDG) in rats and rabbits following oral gavage. Fundam. appl. Toxicol. 1995;28:18–26. [PubMed: 8566479]
- Linnett S.L., Clark D.G., Blair D., Cassidy S.L. Effects of subchronic inhalation of d-D (1,3-dichloropropene/1,2-dichloropropane) on reproduction in male and female rats. Fundam. appl. Toxicol. 1988;10:214–223. [PubMed: 3356308]
- Lucantoni C., Grottoli S., Gaetti R. 1,2-Dichloropropane is a renal and liver toxicant [Letter to the Editor]. Toxicol. appl. Pharmacol. 1992;117:133. [PubMed: 1440608]
- Principe P., Dogliotti E., Bignami M., Crebelli R., Falcone E., Fabrizi M., Conti G., Comba P. Mutagenicity of chemicals of industry and agricultural relevance in Salmonella, Streptomyces and Aspergillus. J. Sci. Food Agric. 1981;32:826–832. [PubMed: 7026896]
- Stolzenberg S.J., Hine C.H. Mutagenicity of 2- and 3-carbon halogenated compounds in the Salmonella/mammalian-microsome test. Environ. Mutagen. 1980;2:59–66. [PubMed: 7035158]
- Timchalk C., Dryzga M.D., Smith F.A., Bartels M.J. Disposition and metabolism of [14C]1,2-dichloropropane following oral and inhalation exposure in Fischer 344 rats. Toxicology. 1991;68:291–306. [PubMed: 1897000]
- Trevisan A., Rizzi E., Bungaro A., Pozzoben L., Gioffre F., Scapinello A., Valeri A., Chiesura P. Proximal tubule brush boarder angiotensin converting enzyme behaviour and nephrotoxicity due to 1,2-dichloropropane. Arch. Toxicol. 1988;Suppl. 12:190–192.
- Trevisan A., Rizzi E., Scapinello A., Gioffre F., Chiesura P. Liver toxicity due to 1,2-dichloropropane in the rat. Arch. Toxicol. 1989;63:445–449. [PubMed: 2619558]
- Trevisan A., Troso O., Maso S. Recovery of biochemical changes induced by 1,2-dichloropropane in rat liver and kidney. Hum. exp. Toxicol. 1991;10:241–244. [PubMed: 1679646]
- Trevisan A., Meneghetti P., Maso S., Troso O. In vitro mechanisms of 1,2-dichloropropane nephrotoxicity using the renal cortical slice model. Hum. exp. Toxicol. 1993;12:117–121. [PubMed: 8096708]
- von der Hude W., Scheutwinkel M., Gramlich U., Fissler B., Basler A. Genotoxicity of three-carbon compounds evaluated in the SCE test in vitro. Environ. Mutagen. 1987;9:401–410. [PubMed: 3582297]
- Woodruff R.C., Mason J.M., Valencia R., Zimmering S. Chemical mutagenesis testing in Drosophila. V. Results of 53 coded compounds tested for the National Toxicology Program. Environ. Mutagen. 1985;7:677–702. [PubMed: 3930237]
- PubMedLinks to PubMed
- Review 1,2-Dibromo-3-chloropropane.[IARC Monogr Eval Carcinog Risk...]Review 1,2-Dibromo-3-chloropropane.. IARC Monogr Eval Carcinog Risks Hum. 1999; 71 Pt 2(PT 2):479-500.
- Review 2-Nitropropane.[IARC Monogr Eval Carcinog Risk...]Review 2-Nitropropane.. IARC Monogr Eval Carcinog Risks Hum. 1999; 71 Pt 3(PT 3):1079-94.
- Review gamma-Butyrolactone.[IARC Monogr Eval Carcinog Risk...]Review gamma-Butyrolactone.. IARC Monogr Eval Carcinog Risks Hum. 1999; 71 Pt 2(PT 2):367-82.
- Review Vinylidene chloride.[IARC Monogr Eval Carcinog Risk...]Review Vinylidene chloride.. IARC Monogr Eval Carcinog Risks Hum. 1999; 71 Pt 3(PT 3):1163-80.
- Review 1,3-Propane sultone.[IARC Monogr Eval Carcinog Risk...]Review 1,3-Propane sultone.. IARC Monogr Eval Carcinog Risks Hum. 1999; 71 Pt 3(PT 3):1095-102.
- 1,2-Dichloropropane - Re-evaluation of Some Organic Chemicals, Hydrazine and Hyd...1,2-Dichloropropane - Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide
Your browsing activity is empty.
Activity recording is turned off.
See more...
