NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Identification of Carcinogenic Hazards to Humans. Gentian Violet, Leucogentian Violet, Malachite Green, Leucomalachite Green, and CI Direct Blue 218. Lyon (FR): International Agency for Research on Cancer; 2022. (IARC Monographs on the Identification of Carcinogenic Hazards to Humans, No. 129.)

Gentian Violet, Leucogentian Violet, Malachite Green, Leucomalachite Green, and CI Direct Blue 218.
Show details1. Exposure Characterization
1.1. Identification of the agent
Gentian violet is a cationic triphenylmethane dye. Leucogentian violet, the leuco base or reduced form of gentian violet, is formed by the chemical or enzymatic reduction of gentian violet. Gentian violet and its leuco base are susceptible to oxidation−reduction and demethylation reactions.
1.1.1. Gentian violet
(a) Nomenclature
- Chem. Abstr. Serv. Reg. No.: 548-62-9
- Chem. Abstr. Serv. name: N-[4-[bis[4-(dimethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-methylmethanaminium chloride (1 : 1)
- EC No.: 208-953-6
- IUPAC systematic name: [4-[bis[4-(dimethylamino)phenyl]methylidene]cyclohexa-2,5-dien-1-ylidene]-dimethylazanium chloride; (4-[4,4-bis(dimethylamino)benzhydrylidene]cyclohexa-2,5-dien-1-ylidene)dimethylammonium chloride; tris(4-(dimethylamino)phenyl)methylium chloride
- Synonyms: CI Basic Violet 3, CI 42555, basic violet, crystal violet, hexamethyl-para-rosaniline chloride, methyl violet 10B, methylrosanilium chloride, aniline violet (ECHA, 2020a; NCBI, 2020).
(b) Structural and molecular formulae, and relative molecular mass
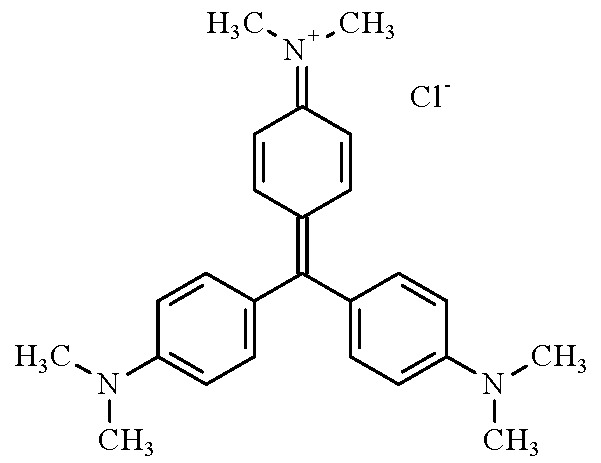
- Molecular formula: C25H30ClN3
- Relative molecular mass: 407.98
(c) Chemical and physical properties of the pure substance
- Description: green to very dark green powder; dark purple in solution
- Boiling point: 631.92 °C (ECHA, 2020a)
- Melting point: 205–215 °C (decomposes) (NCBI, 2013); 198 °C (ECHA, 2020a)
- Density: 1.19 g/cm3 at 20 °C (OEHHA, 2019)
- Solubility: 4000 mg/L at 25 °C, and 10–50 g/L at 27 °C and pH 3.07, in water (ECHA, 2020a); soluble in ethanol and chloroform (NCBI, 2013)
- Vapour pressure: 1.02 × 10−13 mm Hg [1.36 × 10−14 kPa] at 25 °C (estimated) (NCBI, 2013); 0 Pa at 25 °C (ECHA, 2020a)
- Auto-ignition temperature: > 190 °C (United States Pharmacopeia, 2014)
- Stability and reactivity: stable under normal conditions; light-sensitive; incompatible with strong oxidizing agents, reducing agents, and strong acids (United States Pharmacopeia, 2014; Mani & Bharagava, 2016)
- Octanol/water partition coefficient (P): log Kow = 0.51 (NCBI, 2013)
- Henry’s law constant: 3.06 × 10−16 atm m3 mol−1 [3.10 × 10−10 Pa m3 mol−1] (estimated) at 25 °C (NLM, 2020)
- Ultraviolet maximum: 590 nm (water) (NCBI, 2013).
(d) Impurities
Gentian violet is composed primarily of hexamethyl-para-rosaniline (crystal violet) with impurities of pentamethyl-para-rosaniline and tetramethyl-para-rosaniline (Cooksey, 2017). The purity of gentian violet may range from > 76% to < 90% (w/w) (ECHA, 2012). The composition of commercial gentian violet is typically > 96% hexamethyl-para-rosaniline, < 4% pentamethyl-para-rosaniline, < 4% tetramethyl-para-rosaniline, and a trace amount of trimethyl-para-rosaniline (OEHHA, 2019). Unreacted reagents such as Michler’s ketone or Michler’s base may also be present (Cooksey, 2017).
1.1.2. Leucogentian violet
(a) Nomenclature
- Chem. Abstr. Serv. Reg. No.: 603-48-5
- Chem. Abstr. Serv. name: leucocrystal violet
- EC No.: 210-043-9
- IUPAC systematic name: 4-[bis[4-(dimethylamino)phenyl]methyl]-N,N-dimethylaniline
- Synonyms: leucocrystal violet, leuco Basic Violet 3, crystal violet leucobase, 4,4′,4′′-tris(dimethylamino)triphenylmethane, 4,4′,4′′-methylidynetris-N,N-dimethyl-benzenamine, 4,4′,4′′-methylidynetris-N,N-dimethyl-aniline, tris[para-(dimethylamino)phenyl]methane, N,N,N′,N′,N′′,N′′-hexamethyl-4,4′,4′′-methylidynetrianiline (NCBI, 2020).
(b) Structural and molecular formulae, and relative molecular mass
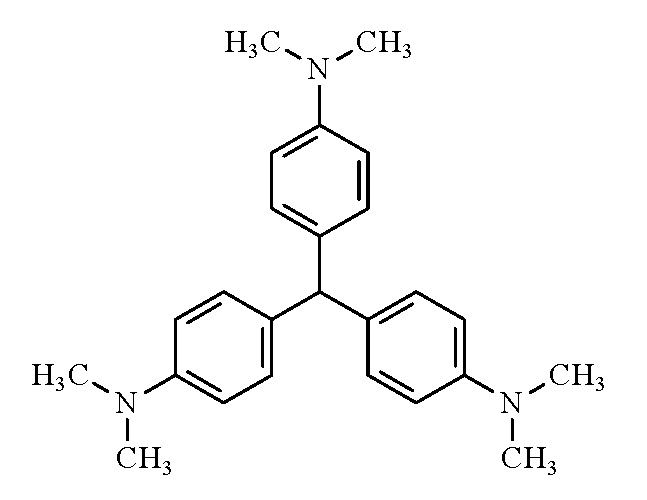
- Molecular formula: C25H31N3
- Relative molecular mass: 373.53
(c) Chemical and physical properties of the pure substance
- Description: white to very pale lavender powder
- Boiling point: decomposition at 227.8 °C, before reaching the boiling point (ECHA, 2020b)
- Melting point: 175–177 °C (NCBI, 2013); 176.8 °C (ECHA, 2020b)
- Density: 1.141 g/cm3 at 19.6 °C (ECHA, 2020b)
- Solubility: 1.3 mg/L at 20 °C and pH 7.4–8.7 in water (ECHA, 2020b); 0.6 mg/mL in ethanol (NCBI, 2013)
- Vapour pressure: 1.95 × 10−5 Pa at 20 °C (ECHA, 2020b)
- Stability and reactivity: stable under normal conditions; light- and air-sensitive; carbon and nitrogen oxides and hydrogen chloride may form from thermal decomposition (Chemical Book, 2017; ECHA, 2020b).
- Octanol/water partition coefficient (P): log Kow = 5.9 (ECHA, 2020b)
- Ultraviolet maximum: 260 nm (Merck, 2021).
(d) Impurities
Leucogentian violet is available with a purity ranging from 98% to > 99%.
1.2. Production and use
1.2.1. Gentian violet
(a) Production process
Several methods are reported to produce gentian violet, each resulting in different compositions of the N-methylated para-rosaniline dye components (Gessner & Mayer, 2000; Cooksey, 2017). High-purity hexamethyl-para-rosaniline is produced from the condensation of N,N-dimethylaniline with Michler’s ketone (4,4-bis(dimethylamino)benzophenone), which is an intermediate generated from the reaction of carbonyl dichloride (phosgene) with dimethylaniline (ECHA, 2012; Cooksey, 2017). Gentian violet can also be generated from the oxidation of leucogentian violet. In a “one-pot” reaction, leucogentian violet is produced from the condensation of N,N-dimethylaniline with formaldehyde, reaction with additional N,N-dimethylaniline, and oxidation in the presence of chloranil and a catalyst such as (dihydrodibenzotetraaza[14]annulene) iron, a vanadium or molybdenum compound, or a nitrous gas (Gessner & Mayer, 2000).
(b) Production volume
India and China are the largest producers of gentian violet (ECHA, 2012). [No information was found on production volumes in these countries.] In the USA, the production volumes of gentian violet were reported to be between > 500 000 and 1 million pounds [> 227–454 tonnes] per year in 1986 and 1990, and between 10 000 and 500 000 pounds [between 4.54 and 227 tonnes] per year in 1994, 1998, and 2002 (NCBI, 2013). Gentian violet is not produced in the European Union (EU), but the EU imports 210–230 tonnes of gentian violet per year (ECHA, 2012). In 2020, gentian violet was available from 36 suppliers in China, 15 suppliers in the USA, 9 suppliers in India, and 2 suppliers in Europe (Chemical Register, 2020a).
(c) Uses
Gentian violet has been in use for more than a century as a dye or pigment, biological stain, and topical antiseptic. It has numerous diverse applications because of its colouring and medicinal properties.
The deep blue-violet colour of gentian violet is used to dye numerous textiles including silk, cotton, wool, and nylon. Gentian violet is also used as a dye for paper and as a pigment for ballpoint pen and printer ink, paint, plastic, gasoline, varnish, oil, and wax (Gessner & Mayer, 2000; ECHA, 2012; Mani & Bharagava, 2016). Gentian violet can be used in food-packaging materials. Gentian violet is used to mark locations on the skin for body piercings (Skellie, 2020) and has also been used as a hair dye (Diamante et al., 2009). [The Working Group noted that more than 100 posts and videos can be found online describing the use of gentian violet as a cheap source of home-made hair dye.]
Gentian violet is used in clinical and bacteriological laboratories as a stain for biological specimens, because it permits visualization of cellular and histological morphology, and to distinguish Gram-positive from Gram-negative bacteria; gentian violet is the primary purple stain used in the Gram staining method (Boyanova, 2018). It is used in surgery as a skin-marking dye (Granick et al., 1987) and in chromoendoscopy to stain the gastrointestinal tract to distinguish lesions from normal tissue (Singh et al., 2020). It is used to detect the presence of bacteria in countless biological assays and is also a pH indicator, with a colour change from yellow at pH 0.0 to blue-violet at pH 2.0 (Cooksey, 2017).
The antibacterial, antifungal, and anthelmintic properties of gentian violet have resulted in numerous applications in medicine (Maley & Arbiser, 2013). As a topical treatment, gentian violet is effective against Gram-positive bacteria including Staphylococcus aureus and Streptococcus, and has been used for the treatment of eczema, impetigo, and to prevent infection and promote the healing of wounds, burns, inflammation resulting from radiotherapy, and the umbilical stumps of infants. Importantly, gentian violet has been effectively used to treat methicillin-resistant Staphylococcus aureus infections of the dermis, middle ear, chest cavity, nostrils, and vascular grafts. For decades, washing affected areas with a dilute solution of gentian violet has been used to treat fungal infections; notably, oral, oesophageal, vulvovaginal (Watson & Calabretto, 2007), nipple, and catheter infections caused by Candida. Coating invasive medical devices (e.g. catheters) with gentian violet reduces the adherence of pathogenic organisms to biofilms, which may lead to infection. Finally, gentian violet has been used against protozoa (e.g. Trypanosoma cruzi, which cause blood transfusion-associated Chagas disease, and Leishmania), nematodes (pinworms), and some viral infections (oral hairy leukoplakia), and may contribute to the inhibition of angiogenesis and tumour growth (Maley & Arbiser, 2013). The antimicrobial properties of gentian violet also have applications in veterinary medicine. Gentian violet has been used in poultry feed to inhibit the growth of moulds and fungi, as a topical treatment for bacterial and fungal infections of the skin and eyes in livestock, and as an immersion-bath treatment for fungal and parasitic infections in fish, including Ichthyophthirius multifiliis, the protozoan that causes white spot disease (WHO, 2014a). Although gentian violet is restricted for use in aquaculture, it is a common treatment for diseases in aquarium fish. Gentian violet is also used in aerosol sprays, in combination with antibiotics or insecticides, for the treatment of skin and hoof diseases in animals (Christodoulopoulos, 2009; Mutebi et al., 2016).
1.2.2. Leucogentian violet
(a) Production process
Leucogentian violet is produced by the condensation of formaldehyde with N,N-dimethylaniline to form 4,4′-methylenebis(N,N-dimethylaniline), which is reacted with additional N,N-dimethylaniline to yield the leuco base of gentian violet (Gessner & Mayer, 2000).
(b) Production volume
Leucogentian violet is manufactured in and/or imported to the European Economic Area in a volume of between 1 and 10 tonnes per annum (ECHA, 2020b). In 2020, leucogentian violet was available from 22 suppliers in China, 5 suppliers in the USA, 2 suppliers in India, and 1 supplier in Canada (Chemical Register, 2020b). [Data on quantities produced and used elsewhere in the world were not found by the Working Group.]
(c) Uses
Leucogentian violet is used as a precursor in the production of gentian violet dye (Gessner & Mayer, 2000). Leucogentian violet has been used as a chromogenic reagent for several analytical applications. Leucogentian violet is colourless and reacts quickly with oxidizers and free radicals to yield gentian violet, which is strongly coloured. The reaction can be readily observed by visualization or spectrophotometric analysis. Leucogentian violet is used in forensic analysis to enhance blood-impression evidence from fingerprints and footwear. Fixation with a 5-sulfosalicylic acid solution denatures proteins in the blood, allowing leucogentian violet to react with haem on the surface of the print. In the presence of hydrogen peroxide, haem catalyses the oxidation of leucogentian violet to gentian violet, producing the characteristic purple colour that results in enhanced print visualization (Spence & Asmussen, 2003; Bossers et al., 2011). Although other forensic dyes react with proteins and amino acids, the haem-sensitive reaction of leucogentian violet indicates the presence of blood. In analytical chemistry, the oxidation reaction of leucogentian violet to gentian violet has been used for sensitive spectrophotometric determination of hypochlorite, hydrogen peroxide, iodine/iodide, and metals (Borges & Reis, 2011). In a method for antimony determination, based on the reaction of antimony (III) with potassium iodate under acidic conditions to generate iodine, iodine oxidizes leucogentian violet to enable colorimetric detection (Tiwari et al., 2006). Leucogentian violet has also been used as a radiochromic indicator to enable the measurement of radiation exposure by dosimeters. Free radical production from gamma-radiation on a matrix can cause radiolytic oxidation of leucogentian violet, which generates a visible measure of radiation exposure (Dhevi et al., 2020).
Leucogentian violet is a metabolite resulting from the veterinary use of gentian violet for the treatment of fish and poultry. Residues of leucogentian violet may be found in fatty muscle and skin (WHO, 2014a).
1.3. Methods of detection and quantification
Representative methods for the detection and quantification of gentian violet and leucogentian violet are summarized in Table 1.1.
Table 1.1
Representative methods for the detection and quantification of gentian violet and leucogentian violet in various matrices .
1.3.1. Air
No methods for the detection and quantification of gentian violet or leucogentian violet particulates in air were found.
1.3.2. Water
Gentian violet is measured in water for environmental monitoring and to determine the efficiency of physical, chemical, and biological methods to remove, decolourize, or degrade gentian violet in wastewater (Mani & Bharagava, 2016). Ultraviolet-visible absorbance techniques are commonly used to measure the reduction of the purple colour from highly concentrated wastewater samples, while liquid chromatography with spectroscopic or mass spectrometry detection is a more sensitive technique (Tkaczyk et al., 2020). For residue analysis in environmental water samples, pre-treatment procedures are required to concentrate gentian violet residues before analysis. Magnetic, ionic liquid, nanoparticle material, and microextraction techniques such as magnetic solid-phase extraction, dispersive liquid−liquid microextraction, micro-cloud point extraction, and monolithic fibre-based solid-phase microextraction have been used to isolate gentian violet residues from aqueous samples before analysis, with detection limits ranging from 0.03 to 5 μg/L (Šafařík & Šafaříková, 2002; Zhang et al., 2012; Wang et al., 2015; Ghasemi & Kaykhaii, 2016; Moradi Shahrebabak et al., 2020).
1.3.3. Soil
Leucogentian violet has been identified in soil near waste discharged from a dye-manufacturing plant by means of Soxhlet extraction with 2-propanol and analysis by gas chromatography-tandem mass spectrometry (Nelson & Hites, 1980).
1.3.4. Food, beverages, and consumer products
Gentian violet is not permitted for use as a food additive, but numerous methods have been developed to determine residues of gentian violet and its metabolite, leucogentian violet, in animal products as a result of veterinary treatment with gentian violet (WHO, 2014a; Verdon & Andersen, 2017). In gentian violet-treated fish, the major metabolite (leucogentian violet) has a longer residence time (> 79 days) than gentian violet (~5 days) in fish (Thompson et al., 1999). Thus, leucogentian violet is the marker residue used to monitor gentian violet use in aquaculture, and seafood analysis methods must assess both compounds. Many early methods of residue analysis were based on the extraction of muscle with an acidic buffer and acetonitrile, liquid−liquid partitioning, and solid-phase clean-up with alumina, followed by high-performance liquid chromatography (HPLC) separation (Roybal et al., 1990). Several approaches have been used to enable the detection of both the chromatic dye and the colourless leuco base, including electrochemical detection, post-column oxidation of leucogentian violet with lead oxide (Ascari et al., 2012), and simultaneous visible (gentian violet absorbs at 588 nm) and fluorescence (leucogentian violet excitation at 265 nm with emission at 360 nm) detection (Verdon & Andersen, 2017). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods have largely replaced HPLC to meet low-concentration regulatory monitoring levels (e.g. 0.5 μg/kg) for direct quantification of the dye and leuco ions (Hurtaud-Pessel et al., 2011; Xu et al., 2012; Andersen et al., 2018; Eich et al., 2020). Some multiresidue LC-MS/MS methods for the detection of therapeutic dyes in seafood include the oxidation of leuco compounds with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone to ensure that dye metabolites are also detected (Tarbin et al., 2008; Andersen et al., 2009; Reyns et al., 2014; Dubreil et al., 2019). A method has been developed to extract gentian violet and leucogentian violet from zebrafish using a solid-phase microextraction probe, which detects residues via direct ionization mass spectrometry from the probe (Xiao et al., 2020). [The Working Group noted that the novel method employed in the study of zebrafish (which are not typically eaten) could have applicability in fish species that are consumed by humans.] Additional multidye LC-MS/MS methods that include sensitive quantification of gentian violet (0.09–2 μg/kg) have been applied to the analysis of foods such as dried tofu and sauces (Hu et al., 2020), and beef, chicken, pork, eggs, and milk (Park et al., 2020). High-resolution mass spectrometry has also been used for the detection and quantification of gentian violet and leucogentian violet (Amelin et al., 2017).
Surface-enhanced Raman scattering and direct mass spectrometry techniques have also been used to detect gentian violet. Silver nanoparticle films and pastes have been used to detect gentian violet on the surface of fish skin and in ballpoint pen ink (Alyami et al., 2019; Saviello et al., 2019). A surface-assisted laser desorption/ionization mass spectrometry method has been used to analyse gentian violet in printed supermarket receipts (Gao et al., 2019).
1.3.5. Biological specimens
Methods for the detection and quantification of gentian violet and leucogentian violet in human biological specimens are similar to those used for food (as described in Section 1.3.4). Gentian violet and leucogentian violet have been determined in human urine via extraction of neutralized urine with dichloromethane, extract clean-up with sodium sulfate, and analysis by HPLC with absorbance or electrochemical detection (Sagar et al., 1995). [The Working Group noted that the methods used for gentian violet and leucogentian violet detection in fish described in Section 1.3.4 could be useful for analysing material from humans or experimental animals. For biological specimen analysis, it might be more important to monitor N-demethylated and/or N-oxide metabolites of gentian violet and leucogentian violet.]
1.4. Occurrence and exposure
1.4.1. Environmental occurrence
Gentian violet is not known to occur naturally in the environment. Gentian violet and leucogentian violet production and their use (e.g. during the production of ink cartridges and coloured paper, and during the recycling of printed paper) may result in the release of these compounds into the environment via streams of both industrial and municipal wastewater (Health Canada, 2020; Tkaczyk et al., 2020).
When released into the environment, gentian violet exists in cationic form. Considering its physicochemical properties, gentian violet exists only in the particulate phase in the atmosphere. [The Working Group also noted that the water solubility of gentian violet is several orders of magnitude higher than that of leucogentian violet and that the octanol/water partition coefficient of gentian violet is one order of magnitude higher, which has implications for its fate in the environment.] Particulate-phase gentian violet is removed from the atmosphere by wet and dry deposition and may be susceptible to direct photolysis by sunlight. Gentian violet is expected to be immobile if released into soil. Soils containing organic carbon and clay will adsorb gentian violet’s cationic form more strongly than its neutral counterpart. Volatilization from moist soil is not expected. According to the transformation rates observed during a river die-away test, biodegradation may be an important environmental process in soil and water. If released into water, gentian violet is expected to adsorb on suspended solids and sediment, and the non-adsorbed fraction will exist almost entirely in the cationic form; therefore volatilization from water is not expected. Gentian violet is not expected to undergo hydrolysis in the environment (NCBI, 2013).
Leucogentian violet was detected in a soil sample taken near a bank of the Buffalo River, New York, close to a dyestuff-manufacturing plant (Nelson & Hites, 1980). Theoretical estimations of concentrations of non-sulfonated triarylmethane dyes in surface water (also representing drinking-water) were calculated for three industrial sources in Canada based on the maximum production capacities of these industries: 3.2 × 10−4 mg/L from the paper-dyeing industry, 9.5 × 10−4 mg/L from the de-inking industry, and 2.1 × 10−4 mg/L from the general formulation industry. These conservative estimates were made for gentian violet, malachite green, and two other triarylmethane dyes collectively, assuming that any one of the four dyes could be substituted for another (Health Canada, 2020). In the National Water Pollution Control and Treatment Project in Dong Lin, China, gentian violet concentrations of 0.87 and 0.049 µg/L were found in the water from turtle farming ponds and effluent environmental water, respectively (Zhang et al., 2012). Gentian violet absorbs light at an ultraviolet maximum of 590 nm with potential for direct photolysis. In water, the photoreaction is reported to give para-dimethylamino phenol and 4,4′-bis dimethylamino benzophenone, the leuco and demethylated derivatives of gentian violet. The bioconcentration in aquatic organisms is low, as suggested by the estimated bioconcentration factor of 3 L/kg in fish (NCBI, 2013), but such models may not be appropriate for triarylmethane dyes because of their cationic nature. For these triarylmethanes, partitioning to proteins in the cell membranes is more likely to occur than partitioning to lipids (Health Canada, 2020).
A study was performed to analyse the presence of 16 dyes, which included triarylmethanes and their metabolites such as gentian violet and leucogentian violet, in wild fish in Belgium. Muscle samples were analysed from individual yellow-phased European eels (Anguilla anguilla) from 91 locations in rivers, canals, and lakes sampled between 2000 and 2009. Gentian violet and leucogentian violet were detected in samples from 58.2% and 50.5% of the locations, respectively. The concentrations of gentian violet and leucogentian violet ranged between 0.12 and 2.60 µg/kg (Belpaire et al., 2015). In an earlier study conducted in Germany, gentian violet and leucogentian violet were found in tissue samples from wild eels caught in seven out of eight receiving waters of effluents from municipal sewage treatment plants. The concentrations of gentian violet and leucogentian violet ranged from 0.06 to 6.71 µg/kg (Schuetze et al., 2008).
1.4.2. Occurrence in food and feed
Gentian violet is used in veterinary medicine and in the aquaculture industry for the control of ectoparasites, and fungal and bacterial infections. Residues of both gentian violet and leucogentian violet may be present in muscle and skin after gentian violet treatment. Although gentian violet metabolizes within days of treatment, leucogentian violet persists in fish muscle and skin for months and is considered to be the marker residue (Thompson et al., 1999). [The Working Group noted that in the reports described below, the methods either detected gentian violet and leucogentian violet separately, or detected total residues as the sum of gentian violet and leucogentian violet after leucogentian violet had been oxidized to gentian violet.]
According to the European Food Safety Authority reports published between 2015 and 2020, few Member States (one to four) reported one or two samples that were non-compliant for the presence of gentian violet and leucogentian violet in their national veterinary drug residue control plan (EFSA, 2015, 2016, 2017, 2018, 2019, 2020). In the European Rapid Alert System for Food and Feed, very few notifications of non-compliant samples associated with imports or trade between Member States have been reported. Since 2005, 15 notifications of gentian violet or leucogentian violet residue violations have been made by EU Member States in eel, salmon, tilapia, rainbow trout, catfish, pangasius, and sturgeon (caviar). Residue concentrations have typically ranged from 0.8 to 6.6 µg/kg, although two high-concentration (41.1 and 654.6 µg/kg) samples were reported for eel from Indonesia in 2006 (European Commission, 2020).
In a study of processed fish and shrimp samples in Korean local markets, gentian violet was detected (168.4 µg/kg) in 1 of 67 eel samples tested. It was not detected in the other 186 processed fish and shrimp samples, which originated from the Republic of Korea, China, Thailand, Viet Nam, Norway, Peru, and the Russian Federation, or were of unknown origin (Lee et al., 2010). Among fish obtained from a local market in China, 7.15 µg/kg of gentian violet was detected in tilapia; none was detected in carp, sea cucumber, or seashell (Xu et al., 2012). Among 20 salmon and shrimp samples purchased from different markets in China, 1.2 µg/kg of gentian violet and 2.5 µg/kg of leucogentian violet were detected in one salmon muscle sample (Tao et al., 2011). Leucogentian violet (0.6–1.0 µg/kg) was detected in 5 out of 208 samples of rainbow trout obtained from local fish retailers and supermarkets in Turkey (Kaplan et al., 2014). In the Russian Federation, 5.3 µg/kg of gentian violet was detected in black caviar (Amelin et al., 2017). Gentian violet and leucogentian violet residues have also been reported for samples tested in the USA, Canada, and Jordan (Table 1.2; WHO, 2014a; Gammoh et al., 2019). In the EU and USA, respectively, 3% and 6% of reported veterinary drug violations detected in finfish in 2001–2008 and 2001–2006, respectively, were due to the detection of gentian violet. The concentrations detected in the EU and the USA did not differ (Love et al., 2011).
Table 1.2
Detection and quantification of gentian violet and leucogentian violet in aquaculture products available on the international marketa.
In a screening study of 19 commercially available processed animal products (salmon feed ingredients) from central Europe, leucogentian violet was detected in one poultry blood-meal sample (Nácher-Mestre et al., 2016).
1.4.3. Occupational exposure
Occupational exposure to gentian violet is expected to occur via dermal contact during paper dyeing, via inhalation of dust or aerosols produced during the formulation of dye or ink, or during the filling of containers such as ink cartridges and ballpoint pens (ECHA, 2012). [The Working Group noted that occupational exposure to gentian violet and leucogentian violet may occur through dermal contact and inhalation at workplaces where the compounds are produced or applied (see Sections 1.1.2 and 1.2.2).] In a survey conducted in the USA in 1981–83, 75 632 people were estimated to be potentially occupationally exposed to gentian violet: 69% of them working in health services, 12% in printing and publishing, and 8% in agricultural services (NIOSH, 2017). [The Working Group noted that it is unclear whether these percentages reflect modern exposure patterns, given the age of the study.]
1.4.4. Exposure in the general population
The predominant source of exposure to dye substances in the triarylmethanes group is from the use of products that contain them that are available to consumers (Health Canada, 2020). Exposure of the general population can potentially occur during the use of the consumer products described in Section 1.1.2, such as ballpoint and marker pens (orally by sucking or via dermal contact), topical treatments for animals (inhalation or dermal), coloured paper, hair dye, aquarium fish treatments, or through the consumption of contaminated drinking-water or residue-containing fish (Table 1.2). A screening assessment performed by Health Canada suggested exposure via drinking-water to be the main route of exposure to gentian violet. A potential dose of 0.0001 mg/kg body weight (bw) per day was estimated for the Canadian general population on the basis of predicted surface water concentrations as a result of environmental release by the paper de-inking industry. Other exposure scenarios considered, but not included in the estimation because of lower estimated exposures, were surface water due to industrial release from paper dyeing in paper mills and production facilities, and consumer “down-the-drain” releases, consumption via food, and the use of consumer products such as paper products, mixtures, or manufactured items in which gentian violet is used as a pigment (Health Canada, 2020).
[The Working Group noted that despite the multitude of sources, no quantitative exposure data were available.]
1.5. Regulations and guidelines
1.5.1. Exposure limits and guidelines
Gentian violet is listed by the European Chemicals Agency as a carcinogen (Category 2) and as a carcinogen (Category 1B) when the Michler’s ketone or Michler’s base impurity is present at 0.1% or more (ECHA, 2012). It is classified as a substance of very high concern (ECHA, 2012). Gentian violet is very toxic to aquatic life (acute H400 and chronic H410), is harmful if swallowed (H302), causes serious eye damage (H318), and is suspected of causing cancer (H350) (ECHA, 2020a).
The Joint Food and Agriculture Organization of the United Nations/WHO Expert Committee on Food Additives (JEFCA) concluded that there is no acceptable daily intake or maximum residue limit for gentian violet and its marker leucogentian violet (WHO, 2014a). Gentian violet is not authorized for use as a veterinary drug in the Australia, Brazil, Canada, Chile, the EU, New Zealand, or the UK, and there is zero tolerance for residues of gentian violet in food for human consumption (Verdon & Andersen, 2017; Health Canada, 2019). In the USA, gentian violet is not permitted for use in animal feeds or as a veterinary drug for food-producing animals (US FDA, 2007). Gentian violet and leucogentian violet are not permitted for use as food additives or in food packaging in the USA (US FDA 2020, 2021). In Canada, gentian violet is not permitted for use in animal feeds or in aquaculture production (Health Canada, 2018).
In food products derived from animals where gentian violet is prohibited for use, there is zero tolerance for residues of gentian violet and/or its metabolite leucogentian violet, which is the marker residue that indicates the use of gentian violet (WHO, 2014a). Reference points for action range from 0.5 to 2.0 μg/kg, as determined by the detection capabilities of the analytical methods used in national and international residue monitoring programmes for each compound, or for the sum of gentian violet and leucogentian violet residues (Verdon & Andersen, 2017).
Gentian violet is not permitted for use as a hair dye in the European Economic Area (European Commission, 2009), and it is not approved for any cosmetic use in Canada, New Zealand, or Singapore (Health Canada, 2018; NZ EPA, 2019; HSA, 2020). United States Food and Drug Administration regulations require that hair dyes containing gentian violet are accompanied by a cautionary statement for skin and eye irritation, with instructions to perform a skin patch test before use (Diamante et al., 2009).
No stand-alone regulations were found for leucogentian violet.
1.5.2. Reference values for biological monitoring of exposure
No reference values for biological monitoring of gentian violet or leucogentian violet exposure were found.
2. Cancer in Humans
No data were available to the Working Group.
3. Cancer in Experimental Animals
3.1. Gentian violet
See Table 3.1.
Table 3.1
Studies of carcinogenicity with gentian violet in experimental animals.
3.1.1. Mouse
Oral administration (feed)
In a study of chronic toxicity and carcinogenicity that complied with Good Laboratory Practice (GLP) (NCTR, 1984; Littlefield et al., 1985), a total of 720 male and 720 female B6C3F1 mice (age, approximately 4–5 weeks) were given feed containing gentian violet (purity, 99%; methyl violet, 1%) at a concentration of 0, 100, 300, or 600 ppm [approximately equivalent to 0, 12.5, 33.9, and 66.1 mg/kg bw per day for males, and 0, 14.3, 37.5, and 71.4 mg/kg bw per day for females] for the control group and the groups at the lowest, intermediate, and highest dose, respectively, for up to 24 months. The feed containing gentian violet was certified to be within 10% of the target dose. For the mice treated for 24 months, there were 192 males and 192 females in the control group and 96 males and 96 females in each group treated with gentian violet. For the mice treated for 12 or 18 months, there were 48 males and 48 females in the control group and 24 males and 24 females in each group treated with gentian violet. Mortality was very low until approximately 450 days (15 months), after which there was a significant positive dose-related trend in males (P = 0.01288, Cochran–Armitage test) and females (P = 0.00005, Cochran–Armitage test), with mortality being significantly higher in all treated groups of females compared with controls. At study termination, survival was 167/192, 83/96, 77/96, and 74/96 in males, and 167/192, 69/96, 70/96, and 35/96 in females, for the control group and the groups at the lowest, intermediate, and highest dose, respectively. Treatment with gentian violet did not influence the terminal body weights of males or females. Complete necropsies and histopathological examinations were performed.
In male mice at 24 months, there was a significant positive trend in the incidence of hepatocellular adenoma [P < 0.001, Cochran–Armitage trend test] and of hepatocellular carcinoma (P < 0.001, trend test), with a significant increase in the incidence of hepatocellular adenoma at the intermediate and highest dose [P < 0.01 and P < 0.001, respectively, one-tailed Fisher exact test], and of hepatocellular carcinoma at the highest dose [P < 0.01, one-tailed Fisher exact test]. The incidence of Harderian gland adenoma was also significantly increased at the intermediate and highest dose (P < 0.05 and [P = 0.0362], respectively, one-tailed Fisher exact test). At 12 or 18 months, no treatment-associated neoplasms were reported in males.
In female mice at 24 months, there was a significant positive trend in the incidence of hepatocellular adenoma and of hepatocellular carcinoma (both P < 0.001, Cochran–Armitage trend test), with a significant increase in the incidence of hepatocellular adenoma [P < 0.01 and P < 0.001, one-tailed Fisher exact test] and of hepatocellular carcinoma (both P < 0.001, one-tailed Fisher exact test) at the intermediate and highest dose, respectively, when compared with controls. Treatment with gentian violet caused a significant positive trend in the incidence of Harderian gland adenoma (P = 0.001, Cochran–Armitage trend test), with the incidence being significantly higher at the lowest, intermediate, and highest dose [P < 0.05, P < 0.001, and P < 0.005, respectively, one-tailed Fisher exact test] than in controls. Significant positive trends in the incidence of type A reticulum cell sarcoma [histiocytic sarcoma] were reported for the urinary bladder, ovaries, uterus, and vagina [P < 0.0005, P = 0.009, P < 0.001, P = 0.001, respectively, Cochran–Armitage trend test], with a significant increase in incidence (urinary bladder, P < 0.05 and P < 0.01; ovaries, P = 0.036 and P = 0.04; uterus, P < 0.01 and P < 0.001; and vagina, P = 0.04 and P < 0.001, Fisher exact test) at the intermediate and highest dose, respectively. At 18 months, a significant positive trend in the incidence of hepatocellular adenoma (P = 0.002, Cochran–Armitage trend test) was observed, with the increase being significant (P = 0.005, one-tailed Fisher exact test) at the highest dose. At 12 months, treatment with gentian violet did not cause a significant increase in the incidence of tumours in female mice.
Regarding non-neoplastic lesions observed at 24 months, exposure to gentian violet caused a significant positive trend and an increase in the incidence of erythropoiesis in the spleen and atrophy of the ovaries in females treated with gentian violet compared with controls. [The Working Group noted that this was a well-conducted study that complied with GLP, males and females were used, the duration of exposure and observation was adequate, and a high number of mice per group was used.]
3.1.2. Rat
(a) Oral administration
In a study in rats [age and strain not reported], oral administration [regimen not reported] of 4:4′:4′′-hexamethyltriaminotriphenylmethane [gentian violet, purity not reported] for more than 300 days caused gastric papilloma and adenomatous proliferation in the hepatic tissue (Kinosita, 1940). [The Working Group noted that the study lacked details on study design and primary data and was considered inadequate for the evaluation of the carcinogenicity of gentian violet in experimental animals.]
(b) Transplacental and perinatal exposure, followed by oral administration (feed)
In a study of chronic toxicity and carcinogenicity that complied with GLP (NCTR, 1988; Littlefield et al., 1989), groups of male and female Fischer 344 rats (F0 generation) (180 controls and 90 treated rats per group) were given feed containing gentian violet (purity, 99%; methyl violet, 1%) at a concentration of 0, 100, 300, or 600 ppm, for the control group, and the groups at the lowest, intermediate, and highest dose, respectively, for at least 80 days. While still receiving treated feed, female rats were mated with males that were receiving the same doses of gentian violet. Two offspring (F1 generation) of each sex were randomly selected from each litter and three rats allocated per cage as weanlings [age, not reported] to the study of chronic toxicity and carcinogenicity. The F1 rats were exposed to the same doses as their respective F0 parents for up to 24 months. [These dose levels were approximately equivalent to 0, 4.3, 11.4, and 22.9 mg/kg bw per day for male F1 rats, and 0, 5.7, 14.3, and 28.6 mg/kg bw per day for female F1 rats.] The feed containing gentian violet was certified to be within 10% of the target dose. For the interim evaluation at 24 months, there were 180 F1 males and 180 F1 females in the control group and 90 F1 males and 90 F1 females in each dose group. For the interim evaluation at 12 or 18 months, there were 15 F1 males and 15 F1 females in each group. Mortality was significantly increased in male rats at the intermediate dose, and there was a significant dose-related increase in mortality in female rats, with the increase in mortality being significant for females at the intermediate and highest dose. Survival was 121/180, 60/90, 47/90, and 55/90 in males, and 121/180, 56/90, 36/90 and 31/90 in females, for the control group and the groups at the lowest, intermediate, and highest dose, respectively. At 24 months, the terminal body weights of male and female rats receiving gentian violet at the highest dose were significantly lower than those of the controls [with the final mean body weights being 92% and 86% of those of the male and female control rats, respectively]. Complete necropsies and histopathological examinations were performed.
In male rats at 24 months, there was a significant positive trend in the incidence of hepatocellular adenoma (P < 0.01, Peto trend test), with incidence being significantly increased at the intermediate and highest dose (both P < 0.01, Peto test and Bonferroni correction). Such a significant positive trend was also observed for the incidence of follicular cell adenocarcinoma of the thyroid gland (P < 0.01, Peto trend test), with the incidence being significantly increased in rats at the lowest and the highest dose (P < 0.05 and P < 0.01, respectively, Peto test and Bonferroni correction). There was a significant positive trend in the incidence of follicular cell adenoma or adenocarcinoma (combined) of the thyroid gland [P < 0.05, Cochran–Armitage trend test], with incidence being significantly increased at the highest dose [P < 0.01, one-tailed Fisher exact test]. Mesothelioma of the testis or epididymis was observed with an incidence of 3%, 2%, 6%, and 9% in the control group and in the groups receiving the lowest, intermediate, and highest dose, respectively [statistical analysis of the incidence of mesothelioma could not be performed, because the incidence was not reported as the number of rats with lesions per number of rats examined microscopically]. At 12 or 18 months, treatment did not cause a significant increase in the incidence of tumours in male rats. However, mesothelioma of the testis or epididymis was observed at 18 months with an incidence of 0%, 0%, 13%, and 13% in the control group and in groups at the lowest, intermediate, and highest dose, respectively [statistical analysis of the incidence of mesothelioma could not be performed because the incidence was not reported as the number of rats with lesions per number of rats examined microscopically].
In female rats, at 24 months, there was a significant positive trend in the incidence of follicular cell adenocarcinoma of the thyroid gland (P < 0.01, Peto trend test), and a significant increase in incidence at the two higher doses (P < 0.05 and P < 0.01, respectively, Peto test and Bonferroni correction). There was a significant positive trend in the incidence of follicular cell adenoma or adenocarcinoma (combined) of the thyroid gland [P < 0.01, Cochran–Armitage trend test], with a significant increase in incidence at the two higher doses [P < 0.01 and P < 0.001, respectively, one-tailed Fisher exact test]. Adenomas and adenocarcinomas of the clitoral gland were also observed with an incidence of 12%, 6%, 18%, and 33% in the control group and in groups at the lowest, intermediate, and highest dose, respectively [statistical analysis of the incidence of adenoma or adenocarcinoma (combined) of the clitoral gland could not be performed because the incidence was not reported as the number of rats with lesions per number of rats examined microscopically]. At 18 months, there was a significant positive trend in the incidence of mononuclear cell leukaemia (P < 0.05, Cochran–Armitage trend test), with a significant increase in incidence in females at the highest dose (P < 0.01, one-tailed Fisher exact test). At 12 months, no treatment-associated neoplasms were reported in females.
Regarding non-neoplastic lesions observed at 24 months, most were reported in the liver. Gentian violet caused a significant positive trend in the incidence and an increase in the incidence of hepatocyte regeneration and of mixed cell foci in all treated groups of male and female rats. Other lesions listed below also showed at least a significant positive trend in incidence, with incidence being significantly increased in one or two dose groups. In males, these other non-neoplastic lesions included clear cell foci, eosinophilic foci, basophilic foci, cytoplasmic vacuolization, and centrilobular necrosis of the liver, follicular cysts of the thyroid gland, red pulp hyperplasia of the spleen, and hyperplasia of the mesenteric lymph nodes. In females, these other non-neoplastic lesions included eosinophilic foci, haematopoietic cell proliferation, centrilobular fatty change and necrosis, and bile duct hyperplasia of the liver, and hyperplasia of the bone marrow. [The Working Group noted that this was a well-conducted study that complied with GLP, males and females were used, the duration of exposure and observation was adequate, and a high number of rats per group was used.]
3.2. Leucogentian violet
No studies were available to the Working Group.
3.3. Evidence synthesis for cancer in experimental animals
3.3.1. Gentian violet
The carcinogenicity of gentian violet has been assessed in male and female mice exposed by oral administration (in the feed) in one study, in male and female rats exposed in utero, followed by lactational exposure and oral administration (in the feed) in another study, and in rats exposed by oral administration in a third study.
In one study that complied with GLP (NCTR, 1984; Littlefield et al., 1985), male and female B6C3F1 mice were treated with gentian violet in the feed for up to 24 months. Gentian violet caused a significant increase, with a significant positive trend, in the incidence of hepatocellular adenoma and hepatocellular carcinoma in males and females at 24 months, and of hepatocellular adenoma in females at 18 months. In female mice, gentian violet caused significant increases, and significant positive trends, in the incidence of histiocytic sarcoma for the urinary bladder, ovaries, uterus, and vagina at 24 months. In males and females, there was a significant increase in the incidence of Harderian gland adenoma at 24 months.
In one study that complied with GLP (NCTR, 1988; Littlefield et al., 1989), male and female Fischer 344 rats were exposed to gentian violet in utero, followed by lactational exposure and oral administration (in the feed), for up to 24 months. In male and female rats, gentian violet caused a significant increase, and significant positive trend, in the incidence of follicular cell adenocarcinoma of the thyroid gland and follicular cell adenoma or adenocarcinoma (combined) of the thyroid gland at 24 months. In females, gentian violet caused a significant increase, and significant positive trend, in the incidence of mononuclear cell leukaemia at 18 months. In males, gentian violet caused a significant increase, and a significant positive trend, in the incidence of hepatocellular adenoma at 24 months.
A study in rats, where gentian violet was given by oral administration, was considered inadequate for the evaluation of the carcinogenicity of gentian violet in experimental animals (Kinosita, 1940).
3.3.2. Leucogentian violet
No studies were available to the Working Group.
4. Mechanistic Evidence
4.1. Absorption, distribution, metabolism, and excretion
4.1.1. Humans
No data were available to the Working Group.
4.1.2. Experimental systems
The absorption, distribution, metabolism, and excretion of gentian violet has been reviewed in Docampo & Moreno (1990), WHO (2014b), and OEHHA (2019).
(a) In vivo
Radiolabelled gentian violet was administered orally to rats and mice. Male and female Fischer 344 rats treated by gavage were given a single dose of [14C]-labelled gentian violet (4.8 mg/kg bw for males, 5.2 mg/kg bw for females). The distribution of the [14C]-labelled dye was measured in the liver, kidney, fatty tissue, gonads, muscle, urine, and faeces at 2, 4, 14, 24, and 36 hours after administration. Maximal residue levels were found at 4 hours in the liver, kidney, muscle, and gonads; a plateau was reached in fatty tissue after 24 hours. The depletion half-lives in male and female livers were 14.5 and 17.0 hours, respectively. The recovery values for males and females (males/females) were 2.2/2.2% and 72.9/63.8% of the single gentian violet dose in the urine and the faeces, respectively. In bile collected from cannulated rats, 5.7–6.4% of the single oral dose was recovered (McDonald et al., 1984a; NCTR, 1989).
Radiolabelled ([14C]) gentian violet was also administered in multiple doses (twice per day for 7 days) to both male and female Fischer 344 rats and B6C3F1 mice by gavage. Maximal residue levels were found in fatty tissues of females of both species, and a statistically significant sex difference (P < 0.01) was noted. Residue levels in kidney and muscle tissues from both species, and in mouse livers, also showed sex differences. The recovery values for males and females (males/females) were 2.2%/1.6% and 65.5%/72.8% in the urine and the faeces of rats, respectively, and 5.9%/8.1% and 65.9%/67.4% in the urine and faeces of mice (McDonald et al., 1984a; NCTR, 1989).
Regarding the metabolism of gentian violet, McDonald & Cerniglia (1984) showed that leucogentian violet was excreted in the faeces collected from a female Fischer 344 rat that was given [14C]-labelled gentian violet by gavage for 4 days. The metabolites of gentian violet were also analysed in mice and rats by NCTR (1989) and identified as three demethylated metabolites (pentamethyl para-rosaniline and N,N,N′,N′- and N,N,N′,N′′-tetramethyl para-rosanilines) and two reduced metabolites (leucogentian violet and leuco-pentamethyl para-rosaniline). A summary of the proposed metabolism of gentian violet and leucogentian violet is provided in Fig. 4.1.
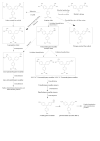
Fig. 4.1
Metabolic pathways for gentian violet and leucogentian violet
(b) In vitro
In bacteria, McDonald & Cerniglia (1984) demonstrated that gentian violet was transformed to leucogentian violet after incubation under anaerobic conditions with microflora isolated from human faeces, and from the intestinal contents of rats and chickens.
When metabolized by rat liver microsomes, gentian violet appears to undergo one-electron reduction by cytochrome P450 to produce a carbon-centred free radical (Harrelson & Mason, 1982). This carbon-centred radical can be formed by photoreduction of gentian violet after exposure to visible light (Docampo et al., 1988).
McDonald et al. (1984b) studied the metabolism of gentian violet in the presence of liver microsomes obtained from both sexes of four mouse strains, three rat strains, hamster, guinea-pig, and chicken: the main metabolites identified were pentamethyl para-rosaniline and the isomeric N,N,N′,N′- and N,N,N′,N′′-tetramethyl para-rosanilines. Comparable patterns of demethylated metabolites were observed between species. [The Working Group noted that information about the relative amounts of the different metabolites, including leucogentian violet, was sparse.]
4.2. Evidence relevant to key characteristics of carcinogens
This section summarizes the evidence for the key characteristics of carcinogens (Smith et al., 2016), including whether gentian violet (and leucogentian violet) is electrophilic or can be metabolically activated to an electrophile; is genotoxic; or induces oxidative stress. Insufficient data were available for the evaluation of other key characteristics of carcinogens.
4.2.1. Is electrophilic or can be metabolically activated to an electrophile
Through measurement of sedimentation and viscosity, it was shown that gentian violet binds externally to the surface of the DNA helix, with a high degree of preference for two adjacent A−T base pairs, and that it induces severe bending accompanied by unwinding of the DNA helix (Müller & Gautier, 1975; Wakelin et al., 1981).
The ability of gentian violet to bind to bovine haemoglobin was demonstrated in vitro by Liu et al. (2013) using several spectroscopic and molecular modelling methods. A change in the spatial conformation of bovine haemoglobin was observed after binding of gentian violet (Liu et al., 2013).
4.2.2. Is genotoxic
Table 4.1, Table 4.2, Table 4.3, and Table 4.4 summarize the available studies of the genetic and related effects of gentian violet.
Table 4.1
Genetic and related effects of gentian violet in human primary cells and human cell lines in vitro.
Table 4.2
Genetic and related effects of gentian violet in non-human mammals in vivo.
Table 4.3
Genetic and related effects of gentian violet in non-human mammalian cells in vitro.
Table 4.4
Genetic and related effects of gentian violet in non-mammalian experimental systems.
(a) Humans
(i) Exposed humans
No data were available to the Working Group.
(ii) Human primary cells and human cell lines in vitro
See Table 4.1.
In human primary cells in vitro, a single concentration of gentian violet induced an increase in chromosomal aberration in cultured primary human peripheral blood lymphocytes from healthy donors (Au et al., 1978; Hsu et al., 1982), and from healthy individuals and patients with β-thalassaemia (Krishnaja & Sharma, 1995).
Au et al. (1978) also showed that gentian violet induced an increase in chromosomal aberrations in HeLa cells.
(b) Experimental systems
(i) Non-human mammals in vivo
See Table 4.2.
After injection of gentian violet in the tail veins of B6C3F1 mice up to a dose of 8 mg/kg bw, no DNA damage was observed in splenic lymphocytes (Aidoo et al., 1990). Gentian violet also failed to induce chromosomal aberrations in bone marrow erythrocytes of Swiss albino mice that received the substance via drinking-water for 4 weeks up to a dose of 8 mg/kg (Au et al., 1979).
(ii) Non-human mammalian cells in vitro
See Table 4.3.
Aidoo et al. (1990) showed that gentian violet induced DNA damage (nucleoid sedimentation) in cultured lymphocytes from the spleens of B6C3F1 mice and caused weak gene amplification in SV40-transformed Chinese hamster embryo (CO60) cells. Gentian violet induced DNA strand breaks in whole-blood samples collected from Sprague-Dawley rats (Díaz Gómez & Castro, 2013). When the rats were treated with antioxidants (α-tocopherol, lipoic acid, or N-acetylcysteine) before the blood samples were collected, the genotoxic effects induced by gentian violet were significantly decreased (Díaz Gómez & Castro, 2013). Gentian violet did not induce gene mutations at the hypoxanthine-guanine phosphoribosyltransferase (Hprt) locus of Chinese hamster ovary (CHO) CHO-K1-BH4 cells, but caused a slight increase at the glutamic-pyruvate transaminase (Gpt) locus of CHO AS52 cells (the increase was observed only at very toxic concentrations, and was not reproduced with different gentian violet batches) (Aidoo et al., 1990).
Au et al. (1978) demonstrated that gentian violet induced mitotic anomalies. Gentian violet consistently induced chromosomal aberrations in various cell lines: Mus musculus mouse fibroblast L cells, a fibroblast cell line derived from Peromyscus eremicus, and a fibroblast cell line derived from the Indian muntjac (Muntiacus muntjak) (Au et al., 1978). It also induced chromosomal aberrations in CHO cells (Au et al., 1978, 1979; Au & Hsu, 1979). The cytogenic effect observed in CHO cells decreased in the presence of the S9 metabolic activation system (Au et al., 1979).
(iii) Non-mammalian experimental systems
See Table 4.4.
At low concentrations, gentian violet binds to double-stranded DNA at AT-rich regions, while it binds at all available sites at high concentrations (Fox et al., 1992).
Cornell K-strain chicken embryos treated with gentian violet did not show sister-chromatid exchange (Au et al., 1979; Bloom, 1984).
In one study performed on Drosophila melanogaster, gentian violet did not induce mutations in a sex-linked recessive lethal assay (Mason et al., 1992).
Gentian violet induced DNA damage in Bacillus subtilis (Fujita et al., 1976; Choudhary et al., 2004). Grigg et al. (1984) observed that gentian violet induced DNA strand breaks in Escherichia coli B strain.
An overwhelming majority of the data show that gentian violet did not induce mutagenicity with or without metabolic activation in Salmonella typhimurium strains TA1535, TA100, TA1538, TA97, TA98, TA1537, YG1041, and YG1042 (Shahin & Von Borstel, 1978; Au et al., 1979; Bonin et al., 1981; Levin et al., 1982; Thomas & MacPhee, 1984; Hass et al., 1986; Aidoo et al., 1990; Malachová et al., 2006; Ackerman et al., 2009). However, a few authors reported a mutagenic effect without metabolic activation in TA98, TA100, and TA1535 (Fujita et al., 1976; Bonin et al., 1981), with metabolic activation in TA98 and TA100 (Fujita et al., 1976; Ayed et al., 2017), as well as in TA97 and TA104 (Aidoo et al., 1990). Malachová et al. (2006) described a mutagenic effect of crystal [gentian] violet with metabolic activation in TA98, which was associated with a cytotoxic effect. In E. coli strains, gentian violet caused mutagenicity with and without metabolic activation (Thomas & MacPhee, 1984; Hass et al., 1986), and induced mutagenicity without metabolic activation in a study by Fujita et al. (1976) (not tested with metabolic activation). In the Rosenkranz repairable DNA assay, gentian violet gave positive results in E. coli strains W3110 polA+ and P3478 polA– (Au et al., 1979; Levin et al., 1982), but negative results in the Saccharomyces cerevisiae XV185-14C strain (Shahin & Von Borstel, 1978).
4.2.3. Induces oxidative stress
As already mentioned above (Section 4.1.2.b), gentian violet can lead to the formation of a carbon-centred free radical, either by photoreduction (Reszka et al., 1986; Docampo et al., 1988) or by enzymatic reaction (Harrelson & Mason, 1982).
4.3. Other relevant evidence
4.3.1. Humans
Several studies using patch tests showed that gentian violet was among the least active sensitizers of several tested drugs, because contact hypersensitivity was rarely observed with gentian violet (Bajaj et al., 1982; Pasricha & Gupta, 1982; Bajaj & Gupta, 1986; Mahaur et al., 1987).
Bielicky & Novák (1969) observed that, in patients with eczema, gentian violet induced sensitization. Moreover, cross-sensitization between crystal violet and malachite green was possible, as the probable determinant groups for sensitization are -N(CH3)2 and -N(C2H5)2.
4.3.2. Experimental systems
No data were available to the Working Group.
4.4. Data relevant to comparisons across agents and end-points
The mechanistic characteristics common to carcinogens (the 10 key characteristics of carcinogens) can be investigated through biochemical and cell-based assays run by the United States Environmental Protection Agency (US EPA) and the United States National Institutes of Health Toxicity Forecaster/Toxicology in the 21st Century (ToxCast/Tox21) high-throughput screening programmes (Chiu et al., 2018; Guyton et al., 2018). Since 2017, the IARC Monographs have described the results of high-throughput screening assays to compare activity across agents and other in vitro and in vivo evidence relevant to the key characteristics.
Of the five compounds included in IARC Monographs Volume 129, three have been evaluated in at least some of the US EPA and United States National Institutes of Health high-throughput screening assays: gentian violet (CAS No. 548-62-9), malachite green (malachite green chloride, CAS No. 569-64-2, and malachite green oxalate, CAS No. 2437-29-8), and leucomalachite green (CAS No. 129-73-7) (US EPA, 2020a, b, c, d). Table 4.5 summarizes findings for assay end-points mapped to key characteristics for the compounds evaluated. Details of the specific assays (and end-points) run for each chemical in this volume and the mapping to the key characteristics can be found in the Supplementary Material (Annex 1, Supplementary material for Section 4, web only; available from: https://publications.iarc.fr/603). It is important to note that some assays either lacked, or had uncharacterized and generally low, xenobiotic metabolism, limiting observations primarily to effects elicited by parent compounds. The strengths of the high-throughput screening battery of assays are the standardization of the protocols applied across compounds, allowing comparisons across compounds and the evaluation of specificity of assay end-points to the key characteristics, and ultimately to the apical outcome of carcinogenesis (Becker et al., 2017; Chiu et al., 2018; Watford et al., 2019). The 299 ToxCast/Tox21 assay end-points mapped to key characteristics interrogated in this and other monographs are initially described in Chiu et al. (2018), with the most up-to-date mapping described in detail in IARC Monographs Volume 123 (IARC, 2019). All ToxCast/Tox21 data were downloaded from the US EPA CompTox Chemicals Dashboard 10th Release (US EPA, 2021) between 2 and 19 October 2020 or on 24 February 2021 (malachite green oxalate).
Table 4.5
Summary of results of ToxCast/Tox21 high-throughput screening assays linked to key characteristics of carcinogens for agents reviewed in IARC Monographs Volume 129a .
The individual assessments for each compound are included in the corresponding monographs in the present volume.
4.4.1. Gentian violet
Results were available for 280 assay end-points (out of the 299 that were mapped to key characteristics) for gentian violet (US EPA, 2020a). Gentian violet was considered active in 126 assay end-points, including the one assay end-point mapped to “is electrophilic or can be metabolically activated to an electrophile”, 10 of the 12 assay end-points mapped to “is genotoxic”, 2 of the 5 mapped to “induces epigenetic alterations”, 8 of 16 end-points mapped to “induces oxidative stress”, 27 of the 90 assay end-points mapped to “modulates receptor-mediated effects”, and 78 of 109 end-points mapped to “alters cell proliferation, cell death, or nutrient supply”.
Assays within the “is genotoxic” key characteristic provide measurements of DNA damage or repair in human liver (HepG2), kidney (HEK293T), and intestinal (HCT-116) cell lines, as well as a CHO cell line (CHO-K1) and a chicken lymphoblast cell line (DT40). Gentian violet (purity, > 90%) elicited TP53 activation measured through reporter assays in HCT-116 and HepG2 cells. Gentian violet was considered active, as measured by phosphorylated histone H2AX (γH2AX) assay detecting H2AX protein phosphorylation, consistent with DNA double-strand breaks in a CHO cell line (CHO-K1). Gentian violet was also considered active as measured by assays using DT40 chicken lymphoblastoid cell lines deficient for the DNA-repair genes REV3 and KU70/RAD54. Gentian violet was not considered active as determined by the ATAD5-luc assay in HEK293T cells, which measures levels of ATAD5 protein that localize to the site of stalled replication forks resulting from DNA damage in replicating cells. It is important to note that both positive (e.g. etoposide, 5-fluorouridine, tetra-N-octylammonium bromide, and mitomycin C) and negative (dimethyl sulfoxide) controls are run concurrently, and subsequent analyses and activity calls are normalized against data for positive and negative controls run on the same plates (Hsieh et al., 2019).
4.4.2. Leucogentian violet
Leucogentian violet has not been evaluated in high-throughput screening assays.
4.4.3. Summary
Gentian violet has been evaluated in ToxCast or Tox21 assays with end-points mapped to key characteristics of carcinogens. It was active in a significant fraction of mapped end-points in which it had been tested (45%). Specifically, gentian violet was considered active in most of the “is genotoxic” assay end-points. It was also considered active for a variety of the assay end-points mapped to the following key characteristics: induces epigenetic alterations, induces oxidative stress, modulates receptor-mediated effects, and alters cell proliferation, cell death, or nutrient supply. Relevant to findings in other sections, gentian violet was considered active in an assay measuring thyroid receptor antagonism in GH3, a rat pituitary gland cell line, and was considered to give negative results in an assay measuring thyroid hormone receptor-agonist activity in the same cell line. Gentian violet was considered to give negative results in an assay measuring thyroid hormone receptor-mediated transcription in HepG2 cells. Leucogentian violet has not been evaluated in high-throughput screening assays.
5. Summary of Data Reported
5.1. Exposure characterization
Gentian violet is a cationic triphenylmethane dye. The reduced form of gentian violet is leucogentian violet, which can be formed by chemical or enzymatic reduction of gentian violet. Gentian violet is widely used as a textile dye, a pigment for consumer and industrial products (inks, papers, and coatings), as a biological stain (Gram stain), and for cosmetic purposes (hair dyes and body piercing). The antibacterial, antifungal, and anthelmintic properties of gentian violet make it an important agent in human medicine as an antiseptic to prevent infection and promote wound healing, and as a topical treatment for fungal and bacterial infections. Gentian violet also has several veterinary applications for the treatment of fungal and parasite disease in fish, disinfection of aquariums, topical treatment for bacterial and fungal infections in livestock, and the prevention of growth of mould and fungi in poultry feeds. Leucogentian violet is a precursor in the production of gentian violet dye, and is used as an analytical reagent to enhance blood impression evidence in forensic analysis, for laboratory determination of anions and metal ions, and as a radiochromic indicator in dosimeters to detect radiation exposure. As gentian violet may be used to control fish diseases, residues of its major metabolite, leucogentian violet, might be found in treated fish or shellfish, and have a longer residence time than the parent compound.
Gentian violet may be released into the environment from waste discharged by textile mills and by other industrial processing, and persists in soil and aquatic species primarily as leucogentian violet.
Overall, data on exposure to gentian violet and leucogentian violet are sparse. The potential for occupational exposure to gentian violet and leucogentian violet exists through dermal contact and inhalation at workplaces where the compound is produced or applied; however, no current data on exposed occupational populations or occupational exposure levels were identified.
In the general population, exposure can occur through contact with textiles, paper, and inks containing gentian violet, medicinal or ornamental fish treatment, cosmetic application for hair dyeing and body piercing, and through the consumption of drinking-water, fish, or shellfish containing residues of gentian violet and leucogentian violet. One study indicated that drinking-water may be an important route of exposure to gentian violet.
Gentian violet is listed by the European Chemicals Agency as a carcinogen (Category 2) and is a substance of very high concern. Gentian violet is not authorized for use as a veterinary drug or for cosmetic applications in many countries, and there is zero tolerance for residues of gentian violet, or its marker leucogentian violet, in food for human consumption.
5.2. Cancer in humans
No data were available to the Working Group.
5.3. Cancer in experimental animals
5.3.1. Gentian violet
Exposure to gentian violet caused an increase in the incidence of malignant neoplasms in both sexes of two species (mouse and rat).
In B6C3F1 mice exposed to gentian violet in the feed, there was a significant positive trend and significant increase in the incidence of hepatocellular carcinoma in males and females, and of histiocytic sarcoma of the urinary bladder, ovaries, uterus, and vagina in females in a study that complied with Good Laboratory Practice (GLP).
In Fischer 344 rats exposed to gentian violet in utero, followed by lactational exposure and oral administration (in the feed), there was a significant positive trend and significant increase in the incidence of follicular cell adenocarcinoma of the thyroid gland in males and females, and of mononuclear cell leukaemia in females in a study that complied with GLP.
5.3.2. Leucogentian violet
No studies were available to the Working Group.
5.4. Mechanistic evidence
No data on absorption, distribution, metabolism, or excretion in humans were available. In mice and rats, orally administered gentian violet is distributed to the liver, kidney, and fatty tissue, and is excreted primarily in faeces. Various demethylated and reduced metabolites have been detected in rats and mice, and in vitro experiments using microsomal preparations from different species. Bacteria have been shown to transform gentian violet to the metabolite leucogentian violet, but data from mammalian species are sparse.
For gentian violet, the mechanistic evidence is suggestive but incoherent across studies in experimental systems, and no data in humans were available. Regarding the key characteristics of carcinogens, gentian violet binds to isolated DNA and to haemoglobin, but no data on DNA adducts were available. Gentian violet induced chromosomal aberrations in human primary cells and in various cultured mammalian cell lines in a few studies. It was considered active in various high-throughput in vitro assays indicative of DNA damage including TP53 activation and γH2AX. However, it did not induce DNA damage or chromosomal aberrations in orally exposed mice in the few studies available. In rodent cells in vitro, it induced DNA damage but not gene mutations. It gave negative results in tests in chicken embryos and in Drosophila melanogaster, and largely negative results across various Salmonella typhimurium strains, including TA1535, TA100, TA1538, TA97, TA98, TA1537, TA104, YG1041, and YG1042. In Escherichia coli strains, gentian violet caused mutagenicity with and without metabolic activation. For other key characteristics of carcinogens, there is a paucity of available data.
For leucogentian violet, data were scarce.
6. Evaluation and Rationale
6.1. Cancer in humans
There is inadequate evidence in humans regarding the carcinogenicity of gentian violet.
There is inadequate evidence in humans regarding the carcinogenicity of leucogentian violet.
6.2. Cancer in experimental animals
There is sufficient evidence in experimental animals for the carcinogenicity of gentian violet.
There is inadequate evidence in experimental animals regarding the carcinogenicity of leucogentian violet.
6.3. Mechanistic evidence
For gentian violet, there is limited mechanistic evidence.
For leucogentian violet, there is inadequate mechanistic evidence.
6.4. Overall evaluation
Gentian violet is possibly carcinogenic to humans (Group 2B).
Leucogentian violet is not classifiable as to its carcinogenicity to humans (Group 3).
6.5. Rationale
The Group 2B evaluation for gentian violet is based on sufficient evidence for cancer in experimental animals. The evidence regarding cancer in humans is inadequate as no studies were available. The mechanistic evidence is limited for gentian violet, based on suggestive but incoherent evidence in experimental systems pertinent to key characteristics of carcinogens. The sufficient evidence for cancer in experimental animals is based on an increase in the incidence of malignant neoplasms in males and females of two species in two studies that comply with GLP.
Leucogentian violet was evaluated as Group 3 because the evidence regarding cancer in humans and in experimental animals, as well as mechanistic evidence, is inadequate, since no studies were available.
References
- Ackerman J, Sharma R, Hitchcock J, Hayashi T, Nagai Y, Li S, et al. (2009). Inter-laboratory evaluation of the bioluminescent Salmonella reverse mutation assay using 10 model chemicals. Mutagenesis. 24(5):433–8. 10.1093/mutage/gep026 [PubMed: 19581339] [CrossRef]
- Aidoo A, Gao N, Neft RE, Schol HM, Hass BS, Minor TY, et al. (1990). Evaluation of the genotoxicity of gentian violet in bacterial and mammalian cell systems. Teratog Carcinog Mutagen. 10(6):449–62. 10.1002/tcm.1770100604 [PubMed: 1982909] [CrossRef]
- Alyami A, Quinn AJ, Iacopino D (2019). Flexible and transparent surface enhanced raman scattering (SERS)-active Ag NPs/PDMS composites for in-situ detection of food contaminants. Talanta. 201:58–64. 10.1016/j.talanta.2019.03.115 [PubMed: 31122461] [CrossRef]
- Amelin VG, Korotkov AI, Andoralov AM (2017). Simultaneous determination of dyes of different classes in aquaculture products and spices using HPLC–high-resolution quadrupole time-of-flight mass spectrometry. J Anal Chem. 72(2):183–90. 10.1134/S1061934817020034 [CrossRef]
- Andersen WC, Turnipseed SB, Karbiwnyk CM, Lee RH, Clarck SB, Rowe DW, et al. (2009). Multiresidue method for the triphenylmethane dyes in fish: Malachite green, crystal (gentian) violet, and brilliant green. Analytica Chemica Acta. 637:279–89. 10.1134/S1061934817020034 [PubMed: 19286041] [CrossRef]
- Andersen WC, Casey CR, Nickel TJ, Young SL, Turnipseed SB (2018). Dye residue analysis in raw and processed aquaculture products: matrix extension of AOAC IOM International Official Method 2012.25. J AOAC Int. 101(6):1927–39. 10.5740/jaoacint.18-0015 [PubMed: 29776453] [CrossRef]
- Ascari J, Dracz S, Santos FA, Lima JA, Diniz MHG, Vargas EA (2012). Validation of an LC-MS/MS method for malachite green (MG), leucomalachite green (LMG), crystal violet (CV) and leucocrystal violet (LCV) residues in fish and shrimp. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 29(4):602–8. 10.1080/19440049.2011.653695 [PubMed: 22325002] [CrossRef]
- Au W, Butler MA, Bloom SE, Matney TS (1979). Further study of the genetic toxicity of gentian violet. Mutat Res. 66(2):103–12. 10.1016/0165-1218(79)90054-5 [PubMed: 372796] [CrossRef]
- Au W, Hsu TC (1979). Studies on the clastogenic effects of biologic stains and dyes. Environ Mutagen. 1(1):27–35. 10.1002/em.2860010109 [PubMed: 95447] [CrossRef]
- Au W, Pathak S, Collie CJ, Hsu TC (1978). Cytogenetic toxicity of gentian violet and crystal violet on mammalian cells in vitro. Mutat Res. 58(2–3):269–76. 10.1016/0165-1218(78)90019-8 [PubMed: 745616] [CrossRef]
- Ayed L, Bakir K, Ben Mansour H, Hammami S, Cheref A, Bakhrouf A (2017). In vitro mutagenicity, NMR metabolite characterization of azo and triphenylmethanes dyes by adherents bacteria and the role of the “cna” adhesion gene in activated sludge. Microb Pathog. 103:29–39. 10.1016/j.micpath.2016.12.016 [PubMed: 27998733] [CrossRef]
- Bajaj AK, Govil DC, Bajaj S, Govil M, Tewari AN (1982). Contact hypersensitivity to topical antimicrobial and antifungal agents. Indian J Dermatol Venereol Leprol. 48(6):330–2. [PubMed: 28193915]
- Bajaj AK, Gupta SC (1986). Contact hypersensitivity to topical antibacterial agents. Int J Dermatol. 25(2):103–5. 10.1111/j.1365-4362.1986.tb04548.x [PubMed: 3699951] [CrossRef]
- Becker RA, Dreier DA, Manibusan MK, Cox LAT, Simon TW, Bus JS (2017). How well can carcinogenicity be predicted by high throughput “characteristics of carcinogens” mechanistic data? Regul Toxicol Pharmacol. 90:185–96. 10.1016/j.yrtph.2017.08.021 [PubMed: 28866267] [CrossRef]
- Belpaire C, Reyns T, Geeraerts C, Van Loco J (2015). Toxic textile dyes accumulate in wild European eel Anguilla. Chemosphere. 138:784–91. 10.1016/j.chemosphere.2015.08.007 [PubMed: 26291760] [CrossRef]
- Bielicky T, Novák M (1969). Contact-group sensitization to triphenylmethane dyes. Gentian violet, brilliant green, and malachite green. Arch Dermatol. 100(5):540–3. 10.1001/archderm.1969.01610290024005 [PubMed: 5350405] [CrossRef]
- Bloom SE (1984). Sister chromatid exchange studies in the chick embryo and neonate: actions of mutagens in a developing system. In: Tice RR, Hollaender A, Lambert B, Morimoto K, Wilson CM, editors. Sister chromatid exchanges. Boston (MA), USA: Springer; pp. 509–33. 10.1007/978-1-4684-4892-4_2 [PubMed: 6397191] [CrossRef]
- Bonin AM, Farquharson JB, Baker RS (1981). Mutagenicity of arylmethane dyes in Salmonella. Mutat Res. 89(1):21–34. 10.1016/0165-1218(81)90127-0 [PubMed: 6165887] [CrossRef]
- Borges SS, Reis BF (2011). An environmental friendly procedure for photometric determination of hypochlorite in tap water employing a miniaturized multicommuted flow analysis setup. J Autom Methods Manag Chem. 2011:1–6. 10.1155/2011/463286 [PMC free article: PMC3124835] [PubMed: 21747732] [CrossRef]
- Bossers LCAM, Roux C, Bell M, McDonagh AM (2011). Methods for the enhancement of fingermarks in blood. Forensic Sci Int. 210(1–3):1–11. 10.1016/j.forsciint.2011.04.006 [PubMed: 21658871] [CrossRef]
- Boyanova L (2018). Direct Gram staining and its various benefits in the diagnosis of bacterial infections. Postgrad Med. 130(1):105–10. 10.1080/00325481.2018.1398049 [PubMed: 29091518] [CrossRef]
- Chemical Book (2017). Leucocrystal violet. Available from: https://www
.chemicalbook .com/ChemicalProductProperty _EN_CB7145919.htm, accessed 12 May 2021. - Chemical Register (2020a). Gentian violet. Chemical Register. The online chemical buyer’s guide [online database]. Cary (NC), USA. Available from: https://www
.chemicalregister .com/find/Find .asp?SearchTy=Product&cid =-1&SearchSu =gentian %20violet&SearchKe =AllKey&SearchLo =ALL&SearchPa=1, accessed 11 May 2021. - Chemical Register (2020b). CAS No. 603-485 [leucogentian violet]. Chemical Register. The online chemical buyer’s guide [online database]. Cary (NC), USA. Available from: https://www
.chemicalregister .com/find/Find .asp?SearchTy=Product&SearchSu =603-48-5&SearchKe =AllKey&SearchLo =ALL&x=0&y=0, accessed 12 May 2021. - Chiu WA, Guyton KZ, Martin MT, Reif DM, Rusyn I (2018). Use of high-throughput in vitro toxicity screening data in cancer hazard evaluations by IARC Monograph Working Groups. ALTEX. 35(1):51–64. 10.14573/altex.1703231 [PMC free article: PMC5783793] [PubMed: 28738424] [CrossRef]
- Choudhary E, Capalash N, Sharma P (2004). Genotoxicity of degradation products of textile dyes evaluated with rec-assay after PhotoFenton and ligninase treatment. J Environ Pathol Toxicol Oncol. 23(4):279–85. 10.1615/JEnvPathToxOncol.v23.i4.40 [PubMed: 15511215] [CrossRef]
- Christodoulopoulos G (2009). Foot lameness in dairy goats. Res Vet Sci. 86(2):281–4. 10.1016/j.rvsc.2008.07.013 [PubMed: 18774149] [CrossRef]
- Cooksey CJ (2017). Quirks of dye nomenclature. 7. Gentian violet and other violets. Biotech Histochem. 92(2):134–40. 10.1080/10520295.2017.1286038 [PubMed: 28296546] [CrossRef]
- Dhevi GGK, Sanyal B, Ghosh SK (2020). Radiation response studies of acetonitrile solutions of crystal violet and leuco crystal violet. Radiat Phys Chem. 177:109068. 10.1016/j.radphyschem.2020.109068 [CrossRef]
- Diamante C, Bergfeld WF, Belsito DV, Klaassen CD, Marks JG Jr, Shank RC, et al. (2009). Final report on the safety assessment of Basic Violet 1, Basic Violet 3, and Basic Violet 4. Int J Toxicol. 28(Suppl 3):193S–204S.10.1177/1091581809354649 [PubMed: 20086192] [CrossRef]
- Díaz Gómez MI, Castro JA (2013). [Genotoxicity in leukocytes by blood chemoprophylaxis with gentian violet and its prevention with antioxidants.] Acta Bioquim Clin Latinoam. 47(4):719–26. [Spanish]
- Docampo R, Moreno SNJ (1990). The metabolism and mode of action of gentian violet. Drug Metab Rev. 22(2–3):161–78. 10.3109/03602539009041083 [PubMed: 2272286] [CrossRef]
- Docampo R, Moreno SNJ, Gadelha FR, de Souza W, Cruz FS (1988). Prevention of Chagas’ disease resulting from blood transfusion by treatment of blood: toxicity and mode of action of gentian violet. Biomed Environ Sci. 1:406–13. [PubMed: 3151757]
- Dubreil E, Mompelat S, Kromer V, Guitton Y, Danion M, Morin T, et al. (2019). Dye residues in aquaculture products: targeted and metabolomics mass spectrometric approaches to track their abuse. Food Chem. 294:355–67. [Erratum in Food Chem. 2020; 306:125539]10.1016/j.foodchem.2019.05.056 [PubMed: 31126475] [CrossRef]
- ECHA (2012). Proposal for identification of a substance as a CMR 1A or 1B, PBT, vPvB or a substance of an equivalent level of concern. Annex XV dossier. Substance name: [4-[4,4ʹ-bis(dimethyl-amino)benzhydrylidene]cyclohexa-2,5-dien-1-ylidene]dimethylammonium chloride (CI Basic Violet 3). Helsinki, Finland: European Chemicals Agency. Available from: https://echa
.europa.eu /documents/10162/2842450 /svhc_axvrep_c _i_basic_violet_3_pub_14287_en .pdf/1ffe8b3f-bb77-e050-ca70-89b5c17fdfe5, accessed 11 May 2021. - ECHA (2020a). Substance infocard [4-[4,4ʹ-bis(dimethylamino)benzhydrylidene]cyclohexa-2,5-dien-1-ylidene]dimethylammonium chloride. Helsinki, Finland: European Chemicals Agency. Available from: https://echa
.europa.eu /fr/substance-information /-/substanceinfo/100 .008.140#CAS_NAMEScontainer, accessed 13 May 2021. - ECHA (2020b). Substance infocard. N,N,Nʹ,Nʹ,Nʺ,Nʺ-Hexamethyl-4,4ʹ,4ʺ-methylidynetrianiline. Helsinki, Finland: European Chemicals Agency. Available from: https://echa
.europa.eu /fr/substance-information /-/substanceinfo/100.009.131, accessed 11 May 2021. - EFSA (2015). Report for 2013 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. Parma, Italy: European Food Standards Agency. Available from: https://www
.efsa.europa .eu/fr/supporting/pub/en-723. - EFSA (2016). Report for 2014 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. Parma, Italy: European Food Standards Agency. Available from: https://www
.efsa.europa .eu/en/supporting/pub/en-923. - EFSA (2017). Report for 2015 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. Parma, Italy: European Food Standards Agency. Available from: https://www
.efsa.europa .eu/en/supporting/pub/en-1150. - EFSA (2018). Report for 2016 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. Parma, Italy: European Food Standards Agency. Available from: https://www
.efsa.europa .eu/en/supporting/pub/en-1358. - EFSA (2019). Report for 2017 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. Parma, Italy: European Food Standards Agency. Available from: https://www
.efsa.europa .eu/en/supporting/pub/en-1578. - EFSA (2020). Report for 2018 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. Parma, Italy: European Food Standards Agency. Available from: https://www
.efsa.europa .eu/en/supporting/pub/en-1775. - Eich J, Bohm DA, Holzkamp D, Mankertz J (2020). Validation of a method for the determination of triphenylmethane dyes in trout and shrimp with superior extraction efficiency. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 37(1):84–93. 10.1080/19440049.2019.1671611 [PubMed: 31697217] [CrossRef]
- European Commission (2009). Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. OJ L 342, 22 December 2009. Available from: https://eur-lex
.europa .eu/legal-content/EN /TXT/PDF/?uri=CELEX :02009R1223-20200501&from=EN, accessed 13 May 2021. - European Commission (2020). RASFF - food and feed safety alerts. Available from: https://ec
.europa.eu /food/safety/rasff_en, accessed on 13 May 2021. - Fox KR, Higson SL, Scott JE (1992). Methyl green and its analogues bind selectively to AT-rich regions of native DNA. Eur J Histochem. 36:263–70. [PubMed: 1281008]
- Fujita H, Mizuo A, Hiraga K (1976). [Mutagenicity of dyes in the microbial system.] Ann Rep Tokyo Metr Res Lab PH. 27(2):153–158. [Japanese]
- Gammoh S, Alu’datt MH, Alhamad MN, Rababah T, Ammari ZA, Tranchant CC, et al. (2019). Analysis of triphenylmethane dye residues and their leuco-forms in frozen fish by LC-MS/MS, fish microbial quality, and effect of immersion in whole milk on dye removal. J Food Sci. 84(2):370–80. 10.1111/1750-3841.14434 [PubMed: 30640981] [CrossRef]
- Gao C, Zhen D, He N, An Z, Zhou Q, Li C, et al. (2019). Two-dimensional TiO2 nanoflakes enable rapid SALDI-TOF-MS detection of toxic small molecules (dyes and their metabolites) in complex environments. Talanta. 196:1–8. 10.1016/j.talanta.2018.11.104 [PubMed: 30683337] [CrossRef]
- Gessner T, Mayer U (2000). Triarylmethane and diarylmethane dyes. In: Ullmann’s encyclopedia of industrial chemistry. 2nd ed. New York (NY), USA: Wiley-VCH Verlag GmbH & Co. 10.1002/14356007.a27_179 [CrossRef]
- Ghasemi E, Kaykhaii M (2016). Application of micro-cloud point extraction for spectrophotometric determination of malachite green, crystal violet and rhodamine B in aqueous samples. Spectrochim Acta A Mol Biomol Spectrosc. 164:93–7. 10.1016/j.saa.2016.04.001 [PubMed: 27085294] [CrossRef]
- Granick MS, Heckler FR, Jones EW (1987). Surgical skin-marking techniques. Plast Reconstr Surg. 79(4):573–80. 10.1097/00006534-198704000-00011 [PubMed: 2434965] [CrossRef]
- Grigg GW, Gero AM, Sasse WH, Sleigh MJ (1984). Inhibition and enhancement of phleomycin-induced DNA breakdown by aromatic tricyclic compounds. Nucleic Acids Res. 12(23):9083–93. 10.1093/nar/12.23.9083 [PMC free article: PMC320439] [PubMed: 6083550] [CrossRef]
- Guyton KZ, Rusyn I, Chiu WA, Corpet DE, van den Berg M, Ross MK, et al. (2018). Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis. 39(4):614–22. 10.1093/carcin/bgy031 [PMC free article: PMC5888955] [PubMed: 29562322] [CrossRef]
- Harrelson WG Jr, Mason RP (1982). Microsomal reduction of gentian violet. Evidence for cytochrome P-450-catalyzed free radical formation. Mol Pharmacol. 22(2):239–42. [PubMed: 6292686]
- Hass BS, Heflich RH, McDonald JJ (1986). Evaluation of the mutagenicity of crystal violet and its metabolites in Salmonella typhimurium and Escherichia coli. Environ Mutagen. 8(Suppl 6):36.
- Health Canada (2018). Risk management scope for certain triarylmethanes, specifically: Basic Violet 3 (CAS 548-62-9), Malachite Green (CAS 569-64-2), Basic Violet 4 (CAS 2390-59-2), Basic Blue 7 (CAS 2390-60-5). Ottawa (ON), Canada: Health Canada. Available from: https://www
.canada.ca /content/dam/eccc/documents /pdf/pded/triarylmethanes /Risk-management-scope-certain-triarylmethanes .pdf, accessed 13 May 2021. - Health Canada (2019). Health Canada warns Canadians of potential cancer risk associated with gentian violet. Ottawa (ON), Canada: Health Canada. Available from: https:
//recalls-rappels .canada.ca/en/alert-recall /health-canada-warns-canadians-potential-cancer-risk-associated-gentian-violet. - Health Canada (2020). Screening assessment triarylmethanes group. Chemical Abstracts Service Registry numbers 548-62-9, 569-64-2, 1324-76-1, 2390-59-2, 2390-60-5, 3844-45-9. Ottawa (ON), Canada: Health Canada. Available from: https://www
.canada.ca /content/dam/eccc/documents /pdf/pded/triarylmethanes /Screening-assessment-triarylmethanes-group.pdf, accessed 13 May 2021. - HSA (2020). Annexes of the ASEAN cosmetic directive. Singapore: Singapore Health Sciences Authority. Available from: https://www
.hsa.gov.sg /docs/default-source /hprg-cosmetics/annexes-of-the-asean-cosmetic-directive-(updated-nov20)-(1) .pdf, accessed 13 May 2021. - Hsieh JH, Smith-Roe SL, Huang R, Sedykh A, Shockley KR, Auerbach SS, et al. (2019). Identifying compounds with genotoxicity potential using Tox21 high-throughput screening assays. Chem Res Toxicol. 32(7):1384–401. 10.1021/acs.chemrestox.9b00053 [PMC free article: PMC6740247] [PubMed: 31243984] [CrossRef]
- Hsu TC, Cherry LM, Pathak S (1982). Induction of chromatid breakage by clastogens in cells in G2 phase. Mutat Res. 93(1):185–93. 10.1016/0027-5107(82)90134-8 [PubMed: 7062930] [CrossRef]
- Hu Z, Qi P, Wang N, Zhou QQ, Lin ZH, Chen YZ, et al. (2020). Simultaneous determination of multiclass illegal dyes with different acidic-basic properties in foodstuffs by LC-MS/MS via polarity switching mode. Food Chem. 309:125745. 10.1016/j.foodchem.2019.125745 [PubMed: 31678670] [CrossRef]
- Hurtaud-Pessel D, Couëdor P, Verdon E (2011). Liquid chromatography tandem mass spectrometry method for the determination of dye residues in aquaculture products: development and validation. J Chromatogr A. 1218(12):1632–45. 10.1016/j.chroma.2011.01.061 [PubMed: 21310421] [CrossRef]
- IARC (2019). Some nitrobenzenes and other industrial chemicals. IARC Monogr Eval Carcinog Risks Hum. 123:1–213. Available from https:
//publications.iarc.fr/584. - Kaplan M, Olgun EO, Karaoglu O (2014). A rapid and simple method for simultaneous determination of triphenylmethane dye residues in rainbow trouts by liquid chromatography tandem mass spectrometry. J Chromatogr A. 1349:37–43. 10.1016/j.chroma.2014.04.091 [PubMed: 24866565] [CrossRef]
- Kinosita R (1940). Studies on the cancerogenic azo and related compounds. Yale J Biol Med. 12(3):287–300. [PMC free article: PMC2602186] [PubMed: 21433884]
- Krishnaja AP, Sharma NK (1995). Heterogeneity in chemical mutagen-induced chromosome damage after G2 phase exposure to bleomycin, ara-C and gentian violet in cultured lymphocytes of β-thalassaemia traits. Mutat Res. 331(1):143–8. 10.1016/0027-5107(95)00060-V [PubMed: 7545265] [CrossRef]
- Lee JB, Kim HY, Jang YM, Song JY, Woo SM, Park MS, et al. (2010). Determination of malachite green and crystal violet in processed fish products. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 27(7):953–61. 10.1080/19440041003705839 [PubMed: 20544455] [CrossRef]
- Levin DE, Lovely TJ, Klekowski E (1982). Light-enhanced genetic toxicity of crystal violet. Mutat Res. 103(3–6):283–8. 10.1016/0165-7992(82)90055-0 [PubMed: 7045647] [CrossRef]
- Littlefield NA, Blackwell BN, Hewitt CC, Gaylor DW (1985). Chronic toxicity and carcinogenicity studies of gentian violet in mice. Fundam Appl Toxicol. 5(5):902–12. 10.1016/0272-0590(85)90172-1 [PubMed: 4065463] [CrossRef]
- Littlefield NA, Gaylor DW, Blackwell BN, Allen RR (1989). Chronic toxicity/carcinogenicity studies of gentian violet in Fischer 344 rats: two-generation exposure. Food Chem Toxicol. 27(4):239–47. 10.1016/0278-6915(89)90162-2 [PubMed: 2731819] [CrossRef]
- Liu Y, Lin J, Chen M, Song L (2013). Investigation on the interaction of the toxicant, gentian violet, with bovine hemoglobin. Food Chem Toxicol. 58:264–72. 10.1016/j.fct.2013.04.048 [PubMed: 23643798] [CrossRef]
- Love DC, Rodman S, Neff RA, Nachman KE (2011). Veterinary drug residues in seafood inspected by the European Union, United States, Canada, and Japan from 2000 to 2009. Environ Sci Technol. 45(17):7232–40. 10.1021/es201608q [PubMed: 21797221] [CrossRef]
- Mahaur BS, Sharma VK, Kumar B, Kaur S (1987). Prevalence of contact hyper sensitivity to common antiseptics, antibacterials and antifungals in normal persons. Indian J Dermatol Venereol Leprol. 53(5):269–72. [PubMed: 28145368]
- Malachová K, Pavlícková Z, Novotný C, Svobodová K, Lednická D, Musílková E (2006). Reduction in the mutagenicity of synthetic dyes by successive treatment with activated sludge and the ligninolytic fungus, Irpex lacteus. Environ Mol Mutagen. 47(7):533–40. 10.1002/em.20224 [PubMed: 16758470] [CrossRef]
- Maley AM, Arbiser JL (2013). Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol. 22(12):775–80. 10.1111/exd.12257 [PMC free article: PMC4396813] [PubMed: 24118276] [CrossRef]
- Mani S, Bharagava RN (2016). Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. Rev Environ Contam Toxicol. 237:71–104. 10.1007/978-3-319-23573-8_4 [PubMed: 26613989] [CrossRef]
- Mason JM, Valencia R, Zimmering S (1992). Chemical mutagenesis testing in Drosophila: VIII. Reexamination of equivocal results. Environ Mol Mutagen. 19(3):227–34. 10.1002/em.2850190307 [PubMed: 1572346] [CrossRef]
- McDonald JJ, Breeden CR, North BM, Roth RW (1984b). Species and strain comparison of the metabolism of gentian violet by liver microsomes. J Agric Food Chem. 32(3):596–600. 10.1021/jf00123a044 [CrossRef]
- McDonald JJ, Cerniglia CE (1984). Biotransformation of gentian violet to leucogentian violet by human, rat, and chicken intestinal microflora. Drug Metab Dispos. 12(3):330–6. [PubMed: 6145560]
- McDonald JJ, North BM, Breeden CR, Lai CC, Roth RW (1984a). Synthesis and disposition of 14C-labelled gentian violet in F344 rats and B6C3F1 mice. Food Chem Toxicol. 22(5):331–6. 10.1016/0278-6915(84)90360-0 [PubMed: 6539283] [CrossRef]
- Merck (2021). Leucocrystal violet. Darmstadt, Germany: Merck. Available from: https://www
.sigmaaldrich .com/catalog/product /aldrich/219215?lang =fr®ion=FR, accessed 12 May 2021. - Moradi Shahrebabak S, Saber-Tehrani M, Faraji M, Shabanian M, Aberoomand-Azar P (2020). Magnetic solid phase extraction based on poly(β-cyclodextrin-ester) functionalized silica-coated magnetic nanoparticles (NPs) for simultaneous extraction of the malachite green and crystal violet from aqueous samples. Environ Monit Assess. 192:262. 10.1007/s10661-020-8185-6 [PubMed: 32246207] [CrossRef]
- Müller W, Gautier F (1975). Interactions of heteroaromatic compounds with nucleic acids. A-T-specific non-intercalating DNA ligands. Eur J Biochem. 54(2):385–94. 10.1111/j.1432-1033.1975.tb04149.x [PubMed: 1175591] [CrossRef]
- Mutebi F, von Samson-Himmelstjerna G, Feldmeier H, Waiswa C, Bukeka Muhindo J, Krücken J (2016). Successful treatment of severe tungiasis in pigs using a topical aerosol containing chlorfenvinphos, dichlorphos and gentian violet. PLoS Negl Trop Dis. 10(10):e0005056. 10.1371/journal.pntd.0005056 [PMC free article: PMC5058476] [PubMed: 27727268] [CrossRef]
- Nácher-Mestre J, Ibáñez M, Serrano R, Boix C, Bijlsma L, Lunestad BT, et al. (2016). Investigation of pharmaceuticals in processed animal by-products by liquid chromatography coupled to high-resolution mass spectrometry. Chemosphere. 154(2016):231–9. 10.1016/j.chemosphere.2016.03.091 [PubMed: 27058915] [CrossRef]
- NCBI (2020). Leucocrystal violet. PubChem compound summary for CID 69048. Bethesda (MD), USA: United States National Library of Medicine, National Center for Biotechnology Information. Available from: https://pubchem
.ncbi .nlm.nih.gov/compound /Leucocrystal-Violet, accessed 9 February 2022. - NCBI (2013). Gentian violet. Hazardous Substances Data Bank. PubChem. Bethesda (MD), USA: United States National Library of Medicine, National Center for Biotechnology Information. Available from: https://pubchem
.ncbi .nlm.nih.gov/source/hsdb/4366, accessed 11 May 2021. - NCTR (1984) Chronic toxicity and carcinogenicity studies of gentian violet in mice. NCTR technical report for experiment No. 304. Jefferson (AR), USA: National Center for Toxicological Research; pp. 1–52.
- NCTR (1988) Chronic toxicity and carcinogenicity studies of gentian violet in Fischer 344 rats. NCTR technical report for experiment No. 338. Jefferson (AR), USA: National Center for Toxicological Research; pp. 1–57.
- NCTR (1989). Metabolism of gentian violet in Fischer 344 rats and B6C3F1 mice. NCTR technical report for experiments 302, 303. Jefferson (AR), USA: National Center for Toxicological Research; pp. 1–114.
- Nelson CR, Hites RA (1980). Aromatic amines in and near the Buffalo River. Environ Sci Technol. 14(9):1147–9. 10.1021/es60169a020 [CrossRef]
- NIOSH (2017). CI 42555 - Basic Violet 3. Estimated numbers of employees potentially exposed to specific agents by 2-digit standard Industrial Classification (SIC). National Exposure Survey 1981–1983. Cincinnati (OH), USA: Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. Available from: https://web
.archive.org /web/20111026175055/http:/www .cdc.gov /noes/noes1/m1517sic.html, accessed 13 May 2021. - NLM (2020). Methylrosanilinium chloride. Chem ID Plus [online database]. Bethesda (MD), USA: United States National Library of Medicine. Available from: https://chem
.nlm.nih .gov/chemidplus/rn/startswith/548-62-9, accessed 13 May 2021. - NZ EPA (2019). Cosmetic products group standard. Additional schedules. Wellington, New Zealand: New Zealand Environmental Protection Authority. Available from: https://www
.epa.govt .nz/assets/Uploads/Documents /Hazardous-Substances /2017-Group-Standards /46a81f194f /Cosmetic-Products-Group-Standard-Schedules-4-8.pdf, accessed 13 May 2021. - OEHHA (2019). Proposition 65. Evidence on the carcinogenicity of gentian violet. Sacramento (CA), USA: Office of Environmental Health Hazard Assessment. Available from: https://oehha
.ca.gov /media/downloads/crnr /gentianviolethid011719.pdf, accessed 13 May 2021. - Park H, Kim J, Kang HS, Cho BH, Oh JH (2020). Multi-residue analysis of 18 dye residues in animal products by liquid chromatography tandem mass spectrometry. J Food Hyg Saf. 35(2):109–17. 10.13103/JFHS.2020.35.2.109 [CrossRef]
- Pasricha JS, Gupta R (1982). Contact hypersensitivity to brilliant green and gentian violet. Indian J Dermatol Venereol Leprol. 48(3):151–3. [PubMed: 28193943]
- Reszka K, Cruz FS, Docampo R (1986). Photosensitization by the trypanocidal agent crystal violet. Type I versus type II reactions. Chem Biol Interact. 58(2):161–72. 10.1016/S0009-2797(86)80095-3 [PubMed: 3013436] [CrossRef]
- Reyns T, Belpaire C, Geeraerts C, Van Loco J (2014). Multi-dye residue analysis of triarylmethane, xanthene, phenothiazine and phenoxazine dyes in fish tissues by ultra-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 953–954:92–101. 10.1016/j.jchromb.2014.02.002 [PubMed: 24583201] [CrossRef]
- Roybal JE, Munns RK, Hurlbut JA, Shimoda W (1990). Determination of gentian violet, its demethylated metabolites, and leucogentian violet in chicken tissue by liquid chromatography with electrochemical detection. J Assoc Off Anal Chem. 73(6):940–6. 10.1093/jaoac/73.6.940 [PubMed: 2289926] [CrossRef]
- Šafařík I, Šafaříková M (2002). Detection of low concentrations of malachite green and crystal violet in water. Water Res. 36(1):196–200. 10.1016/S0043-1354(01)00243-3 [PubMed: 11766795] [CrossRef]
- Sagar KA, Smyth MR, Rodriguez M, Blanco PT (1995). Determination of gentian violet in human urine and poultry feed by high performance liquid chromatography with electrochemical detection using a carbon fibre microelectrode flow cell. Talanta. 42(2):235–42. 10.1016/0039-9140(94)00233-I [PubMed: 18966222] [CrossRef]
- Saviello D, Trabace M, Alyami A, Mirabile A, Baglioni P, Giorgi R, et al. (2019). raman spectroscopy and surface enhanced raman scattering (SERS) for the analysis of blue and black writing inks: identification of dye content and degradation processes. Front Chem. 7:727. 10.3389/fchem.2019.00727 [PMC free article: PMC6823613] [PubMed: 31709241] [CrossRef]
- Schuetze A, Heberer T, Juergensen S (2008). Occurrence of residues of the veterinary drug crystal (gentian) violet in wild eels caught downstream from municipal sewage treatment plants. Environ Chem. 5(3):194–9. 10.1071/EN08008 [PubMed: 18602134] [CrossRef]
- Shahin MM, Von Borstel RC (1978). Comparisons of mutation induction in reversion systems of Saccharomyces cerevisiae and Salmonella typhimurium. Mutat Res. 53(1):1–10. 10.1016/0165-1161(78)90374-6 [CrossRef]
- Singh R, Chiam KH, Leiria F, Pu LZCT, Choi KC, Militz M (2020). Chromoendoscopy: role in modern endoscopic imaging. Transl Gastroenterol Hepatol. 5:39. 10.21037/tgh.2019.12.06 [PMC free article: PMC7063532] [PubMed: 32632390] [CrossRef]
- Skellie B (2020). Gentian violet concerns & alternatives. The Point: Journal of Body Piercing. 89:44–6. Available from: https:
//safepiercing .org/gentian-violet-concerns-alternatives, accessed 11 May 2021. - Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, et al. (2016). Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect. 124(6):713–21. 10.1289/ehp.1509912 [PMC free article: PMC4892922] [PubMed: 26600562] [CrossRef]
- Spence L, Asmussen G (2003). Spectral enhancement of leucocrystal violet-treated footwear impression evidence in blood. Forensic Sci Int. 132(2):117–24. 10.1016/S0379-0738(03)00003-3 [PubMed: 12711191] [CrossRef]
- Tao Y, Chen D, Chao X, Yu H, Yuanhu P, Liu Z, et al. (2011). Simulataneous determination of malachite green, gentian violet and their leuco-metabolites in shrimp and salmon by liquid chromatography tandem mass spectrometry with accelerated solvent extraction and auto solid-phase clean-up. Food Control. 22(8):1246–52. 10.1016/j.foodcont.2011.01.025 [CrossRef]
- Tarbin JA, Chan D, Stubbings G, Sharman M (2008). Multiresidue determination of triarylmethane and phenothiazine dyes in fish tissues by LC-MS/MS. Anal Chim Acta. 625(2):188–94. 10.1016/j.aca.2008.07.018 [PubMed: 18724993] [CrossRef]
- Thomas SM, MacPhee DG (1984). Crystal violet: a direct-acting frameshift mutagen whose mutagenicity is enhanced by mammalian metabolism. Mutat Res. 140(4):165–7. 10.1016/0165-7992(84)90071-X [PubMed: 6472325] [CrossRef]
- Thompson HC Jr, Rushing LG, Gehring T, Lochmann R (1999). Persistence of gentian violet and leucogentian violet in channel catfish (Ictalurus punctatus) muscle after water-borne exposure. J Chromatogr B Biomed Sci Appl. 723(1–2):287–91. 10.1016/S0378-4347(98)00536-2 [PubMed: 10080657] [CrossRef]
- Tiwari KK, Mundhara GL, Rai MK, Gupta VK (2006). A simple and sensitive analytical method for the determination of antimony in environmental and biological samples. Anal Sci. 22(2):259–62. 10.2116/analsci.22.259 [PubMed: 16512419] [CrossRef]
- Tkaczyk A, Mitrowska K, Posyniak A (2020). Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci Total Environ. 717:137222. 10.1016/j.scitotenv.2020.137222 [PubMed: 32084689] [CrossRef]
- United States Pharmacopeia (2014). Gentian violet. Safety data sheet. United States Pharmacopeia. Available from: https://static
.usp.org /pdf/EN/referenceStandards /msds/1290002.pdf, accessed 13 May 2021. - US EPA (2020a). ToxCast/Tox21 for gentian violet. DSSTox Substance. Distributed Structure-Searchable Toxicity (DSSTox) database. United States Environmental Protection Agency. Available from: https://comptox
.epa.gov /dashboard/dsstoxdb /results?search=DTXSID5020653#invitrodb-bioassays-toxcast-tox21. - US EPA (2020b). ToxCast/Tox21 for malachite green. DSSTox Substance. Distributed Structure-Searchable Toxicity (DSSTox) database. United States Environmental Protection Agency. Available from: https://comptox
.epa.gov /dashboard/dsstoxdb /results?search=569-64-2#invitrodb-bioassays-toxcast-tox21. - US EPA (2020c). ToxCast/Tox21 for malachite green oxalate. DSSTox Substance. Distributed Structure-Searchable Toxicity (DSSTox) database. United States Environmental Protection Agency. Available from: https://comptox
.epa.gov /dashboard/dsstoxdb /results?search=DTXSID6025513#invitrodb-bioassays-toxcast-tox21. - US EPA (2020d). ToxCast/Tox21 for leucomalachite green. DSSTox Substance. Distributed Structure-Searchable Toxicity (DSSTox) database. United States Environmental Protection Agency. Available from: https://comptox
.epa.gov /dashboard/dsstoxdb /results?search=DTXSID7031531#invitrodb-bioassays-toxcast-tox21. - US EPA (2021). CompTox Chemicals Dashboard [online database]. United States Environmental Protection Agency. Available from: https://comptox
.epa.gov/dashboard/, accessed 24 February 2021. - US FDA (2007). CFR - Code of Federal Regulations Title 21. Food and drugs. Chapter I--Food and Drug Administration, Department of Health and Human Services. Subchapter E--Animal drugs, feeds, and related products. Part 589--Substances prohibited from use in animal food or feed. Subpart B--Listing of specific substances prohibited from use in animal food or feed. Sec. 589.1000 Gentian violet. Available from: https://www
.accessdata .fda.gov/scripts/cdrh /cfdocs/cfcfr/CFRSearch .cfm?fr=589.1000/. - US FDA (2020). Substances added to food (formerly EAFUS). Silver Spring (MD), USA: United States Food and Drug Administration. Available from: https://www
.cfsanappsexternal .fda.gov/scripts/fdcc/index .cfm?set =FoodSubstances&sort =Sortterm_ID&order =ASC&startrow =1&type =basic&search=. - US FDA (2021). Inventory of effective food contact substance (FCS) notifications. Silver Spring (MD), USA: United States Food and Drug Administration. Available from: https://www
.cfsanappsexternal .fda.gov/scripts/fdcc/index .cfm?set=FCN. - Verdon E, Andersen WC (2017). Certain dyes as pharmacologically active substances in fish farming and other aquaculture products. In: Kay JF, MacNeil JD, Wang J, editors. Chemical analysis of non-antimicrobial veterinary drug residues in food. 1st ed. John Wiley & Sons, Inc.; pp. 497–548.
- Wakelin LPG, Adams A, Hunter C, Waring MJ (1981). Interaction of crystal violet with nucleic acids. Biochemistry. 20(20):5779–87. 10.1021/bi00523a021 [PubMed: 6170329] [CrossRef]
- Wang Y, Liao K, Huang X, Yuan D (2015). Simultaneous determination of malachite green, crystal violet and their leuco-metabolites in aquaculture water samples using monolithic fiber based solid-phase microextraction coupled with high performance liquid chromatography. Anal Methods. 7(19):8138–45. 10.1039/C5AY01611H [CrossRef]
- Watford S, Edwards S, Angrish M, Judson RS, Paul Friedman K (2019). Progress in data interoperability to support computational toxicology and chemical safety evaluation. Toxicol Appl Pharmacol. 380:114707. 10.1016/j.taap.2019.114707 [PMC free article: PMC7705611] [PubMed: 31404555] [CrossRef]
- Watson C, Calabretto H (2007). Comprehensive review of conventional and non-conventional methods of management of recurrent vulvovaginal candidiasis. Aust N Z J Obstet Gynaecol. 47(4):262–72. 10.1111/j.1479-828X.2007.00736.x [PubMed: 17627679] [CrossRef]
- WHO (2014a). Gentian violet. Residue evaluation of certain veterinary drugs. Seventy-eighth report of the Joint FAO/WHO Expert Committee on Food Additives. FAO JECFA Monogr. 15:39–59.
- WHO (2014b). Gentian violet. Toxicological evaluation of certain veterinary drug residues in food. Prepared by the seventy-eighth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA). WHO Food Additives Series 69. Geneva, Switzerland: World Health Organization. Available from: https://inchem
.org/documents /jecfa/jecmono/v69je01.pdf - Xiao X, Chen C, Deng J, Wu J, He K, Xiang Z, et al. (2020). Analysis of trace malachite green, crystal violet, and their metabolites in zebrafish by surface-coated probe nanoelectrospray ionization mass spectrometry. Talanta. 217:121064. 10.1016/j.talanta.2020.121064 [PubMed: 32498869] [CrossRef]
- Xu YJ, Tian XH, Zhang XZ, Gong XH, Liu HH, Zhang HJ, et al. (2012). Simultaneous determination of malachite green, crystal violet, methylene blue and the metabolite residues in aquatic products by ultra-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J Chromatogr Sci. 50(7):591–7. 10.1093/chromsci/bms054 [PubMed: 22542891] [CrossRef]
- Zhang Z, Zhou K, Bu YQ, Shan ZJ, Liu JF, Wu XY, et al. (2012). Determination of malachite green and crystal violet in environmental water using temperature-controlled ionic liquid dispersive liquid–liquid microextraction coupled with high performance liquid chromatography. Anal Methods. 4(2):429–33. 10.1039/C2AY05665H [CrossRef]
- Gentian Violet and Leucogentian Violet - Gentian Violet, Leucogentian Violet, Ma...Gentian Violet and Leucogentian Violet - Gentian Violet, Leucogentian Violet, Malachite Green, Leucomalachite Green, and CI Direct Blue 218
Your browsing activity is empty.
Activity recording is turned off.
See more...