NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Nanomaterials and Some Fibres. Lyon (FR): International Agency for Research on Cancer; 2017. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 111.)
4.1. Deposition, phagocytosis, translocation, retention, and clearance
4.1.1. Humans
No data were available to the Working Group.
4.1.2. Experimental animals
(a) Deposition
In male Wistar rats exposed by whole-body inhalation for 6 hours per day on 5 days per week to 0.37 mg/m3 of MWCNT (Nikkiso Co., Ltd; length, 1.1 µm; diameter, 63 nm) dispersed in an aqueous solution of 0.5 mg/mL Triton X-100 and atomized by a nebulizer into the exposure chamber (MWCNT aerosol comprised of approximately 70% of single fibres), lung deposition fractions of 0.18 or 0.2 were estimated from the measured mass of CNT in the lungs 3 days after the end of the 4-week experiment. The retained mass lung burdens were measured by X-ray diffraction or EC analysis at 3 days, 1 month, and 3 months after exposure, and the mass of MWCNT in the lungs 3 days after exposure was 68 and 76 µg/lung, as measured by the two methods, respectively (Oyabu et al., 2011).
A deposition fraction of 5.7% MWCNT was estimated in rats by Pauluhn (2010b) using data on the airborne size distribution (e.g. MMAD, ~3 µm; GSD, ~2) and the Multiple-Path Particle Dosimetry (MPPD) model 2 software (Anjilvel & Asgharian, 1995). NIOSH (2013) provided a comparison of the rat alveolar deposition fraction estimates from Pauluhn (2010b) using two different versions of the MPPD software (v. 2.0 and 2.1) (CIIT & RIVM, 2006; ARA, 2011) and density values of either 1 or 0.2 g/mL. Estimated deposition fractions were 0.046, 0.027, or 0.023 from MPPD 2.0 (density 1 g/mL), MPPD 2.1 (density 1 g/mL), or MPPD 2.1 (density 0.2 g/mL), respectively. The aerodynamic particle size used was 2.74 µm MMAD (GSD, 2.11) [middle of the three measures reported by Pauluhn (2010b)].
A 3-week inhalation study in male C57BL/6J mice exposed to 5 mg/m3 of MWCNT (Mitsui-7 [MWCNT-7]; Hodogaya Chemical Co.) for 5 hours per day for 12 days provided information to estimate the lung deposition fraction (Mercer et al., 2013a). [The Working Group noted that, although a mouse lung deposition fraction was not reported in Mercer et al. (2013a), it can be estimated (as shown below) to provide additional information to and enable comparisons with estimates from other animal studies on the inhalation of CNT. The Working Group also noted that estimation of the deposition fraction from the measured lung burden at the end of inhalation exposure would be underestimated by the amount of CNT that was cleared from the lungs during the exposure period.]
The average lung burden measured 1 day after the end of the 3-week inhalation exposure was 28.1 µg (Mercer et al., 2013a). The estimated deposition fraction can be estimated as:
Deposition fraction = total lung dose (mg)/exposure (h/d * d * min/h) * L/min * m3/L, or
0.0095 = 28.1/5*(5 * 12 * 60) * 0.165 * (1/1000)
where the total lung dose was measured 1 day (d) after the end of the 12-day inhalation exposure (Mercer et al., 2013a; Table 4.1) and the minute ventilation rate was 0.165 L/min (Shvedova et al., 2008). Shvedova et al. (2008) stated that mouse ventilation rates (including both tidal volumes and breathing rates) can be highly variable depending on how the values were measured. Using the EPA (1988, 2006) minute ventilation rate of 0.037 L/min in mice, the deposition fraction would be 0.042.
Table 4.1
Kinetics of carbon nanotubes in experimental animals.
Expressed as a percentage, the mouse lung deposition fractions estimated above were approximately 1% or 4% for MWCNT in Mercer et al. (2013a) (using a minute ventilation rate of either 0.165 or 0.037 L/min, respectively). In comparison, a mouse lung deposition fraction of 0.5% was reported for SWCNT by Shvedova et al. (2008), which was based on a mass mode aerodynamic diameter of 4.2 µm and estimation of the deposition fraction from Raabe et al. (1988) [Shvedova et al. (2008) used the estimated deposition fraction in mice to estimate the deposited lung dose in mice and the worker-equivalent lung dose.] The mass mode aerodynamic diameter of MWCNT was 1.3 µm (Mercer et al., 2013a) and the MMAD was 1.5 µm (GSD, 1.67) (Chen et al., 2012). [This comparison shows reasonably consistent estimated deposition fractions in mice inhaling CNT, given the differences in the measures of aerodynamic diameter and the uncertainty about mouse ventilation rates.]
A study of MWCNT in male Sprague-Dawley rats (age, 9–10 weeks) provided a comparison of the lung responses to exposure to three different forms of MWCNT, including original (O), purified (P), and carboxylic acid-functionalized (F), at similar estimated lung doses by nose-only inhalation or tracheal instillation (Silva et al., 2014). The O-MWCNT contained 4.49% nickel and 0.76% iron residual catalysts; P-MWCNT contained 1.8% nickel and 0.08% iron; while F-MWCNT contained no detectable levels of nickel or iron. The dimensions of these MWCNT were: outer diameter, 20–30 nm; inner diameter, 5–10 nm; and length, 10–30 μm. The MWCNT were aerosolized for inhalation by nebulization. The MMADs (GSD) for O-, P-, and F-MWCNT were 3.7 (2.5), 4.8 (2.9), and 3.3 (3.1) µm, respectively. Doses for tracheal instillation were 0, 10, 50, or 200 µg in a biocompatible dispersion medium. The single (6-hour) inhalation exposure at a concentration of approximately 30 mg/m3 was estimated to result in a deposited lung dose that was similar to or higher than that of the intratracheally administered dose of 200 µg (estimated by assuming an alveolar and tracheobronchial deposition fraction of 0.14 and a ventilation rate of 0.15 L/min: 30 mg/m3 × 0.15 L/min × 6 h × 60 min/h × 1 m3/1000 L × 0.14 × 1000 µg/1 mg = 227 µg). [The Working Group noted that the “Inhalation Exposure and Aerosol Characterization” section of the Methods in the publication reported a MWCNT aerosol concentration of 38 µg/L (equal to 38 mg/m3), which would result in a deposition of 287 µg MWCNT.]
(b) Phagocytosis
CNT have been observed in cells using confocal Raman microscopy (Romero et al., 2011) or TEM (Ryman-Rasmussen et al., 2009a). The possible mechanisms by which CNT can enter cells include diffusion or penetration through cell membranes (passive internalization) or endocytosis (active internalization) (Kunzmann et al., 2011; Ye et al., 2013), both of which may depend on the surface properties of the CNT and the activation state of the phagocytic cells. Four types of endocytosis have been reported (Ye et al., 2013): phagocytosis, clathrin-mediated endocytosis, caveolea-mediated endocytosis, and macrophage pinocytosis. The first three types have been studied in relation to CNT. Phagocytosis is the engulfment of foreign materials by macrophages, monocytes, and neutrophils, the primary purpose of which is considered to be the elimination of larger pathogens (bacteria and yeast) or cell debris. Larger CNT structures (e.g. > 400 nm) or agglomerates were recognized by phagocytes, while individual structures evaded phagocytosis (Antonelli et al., 2010; Ali-Boucetta & Kostarelos, 2013). Clathrin-mediated endocytosis involves the internalization of macromolecules by the inward budding of plasma membrane vesicles (with or without receptor- or ligand-specific binding) and many studies have reported the cell uptake of CNT by this mechanism (Ye et al., 2013). Caveolea-mediated endocytosis involves caveolar vesicles that are composed of cholesterol and sphingolipids. A CNT radius of 25 nm was estimated to be associated with a maximal rate of endocytosis (Jin et al., 2009), while a maximum length of 189 nm of DNA-wrapped SWCNT was effectively endocytosed by various cell lines (Becker et al., 2007).
The mechanisms of cell uptake also depend on the cell type encountered by the CNT (which also depends on the route of exposure). Macrophages in the pulmonary or interstitial regions of the lungs are capable of phagocytosing CNT, although the size and surface properties of CNT influence their ability to be recognized and phagocytosed by these cells. In the liver, Kupffer cells are the primary cellular site where CNT are observed. Functionalizations/modifications to the surface of CNT (e.g. covalently bonded functional groups or non-covalently bonded coatings) can also influence the cell uptake of CNT (Ali-Boucetta & Kostarelos, 2013).
In rats exposed by pharyngeal aspiration, the alveolar macrophage uptake of SWCNT (< 0.23% iron) was low (Shvedova et al., 2005). Morphological analysis showed that only 10% of the alveolar burden of SWCNT was located within the alveolar macrophages (Shvedova et al., 2005), while 90% of the dispersed SWCNT structures were observed to cross alveolar epithelial cells and enter the interstitium (Mercer et al., 2008). MWCNT were recognized more proficiently by alveolar macrophages; approximately 70% of MWCNT in the respiratory airways was taken up by alveolar macrophages, 8% migrated into the alveolar septa, and 22% was observed in granulomatous lesions (Mercer et al., 2010, 2011).
In an additional investigation of rats in a subchronic inhalation study (Ma-Hock et al., 2009), Treumann et al. (2013) examined ultra-thin lung tissue sections from two rats by TEM at the end of the 13-week exposure to 2.5 mg/m3 of MWCNT. MWCNT structures were observed in the alveolar macrophages within the cytoplasm and membrane-bound organelles (phagosomes) in the form of “large (> 2 µm) electron-dense clews of intermingled MWCNT” and irregularly shaped structures up to 100 nm in diameter; some MWCNT were observed free in the alveolar lumen. MWCNT were also observed in focal accumulations of phagocytic cells within the subpleural connective tissue.
In the study by Silva et al. (2014), the physico-chemical properties of MWCNT influenced their uptake, location, and structure within the alveolar macrophages (as observed by TEM and bright-field microscopy). Rats that inhaled F-MWCNT had significantly more alveolar macrophages containing MWCNT structures than rats that inhaled O-MWCNT or P-MWCNT (as observed in the bronchoalveolar lavage fluid [BALF]) on days 1 and 21 after exposure (P-MWCNT were obtained after the treatment of O-MWCNT with nitric acid and ethyldiamine tetra-acetate in acetic acid at pH 4 to remove residual metals and amorphous carbon). On day 1 after exposure, O-MWCNT and P-MWCNT were observed within the phagolysosomes of macrophages, while F-MWCNT were seen in the cytosol and also protruding the cell membrane. On day 21 after exposure, O- and P-MWCNT were no longer compartmentalized but were observed in the cytosol as larger focal agglomerates; the F-MWCNT (obtained by adding the P-MWCNT to a reaction chamber containing nitric acid and sulfuric acid) in the cytosol were smaller, dispersed aggregates. The acidic functional groups brought about by increasing the pH and the resulting increase in hydrophilicity were thought to reduce the toxicity of F-MWCNT by preventing phagolysosome permeability – and the subsequent release of lysosomal contents into the cytosol with the downstream activation of the nucleotide-binding oligomerization domain receptor (NLRP3) inflammasome – after F-MWCNT were taken up by the alveolar macrophages. Thus the uptake of F-MWCNT into macrophages did not appear to cause cell toxicity at the doses and observation time-points in this study (Silva et al., 2014).
(c) Translocation
Several studies have provided evidence that CNT can translocate from the lungs into the blood circulation. Adult CD-1 mice [sex unspecified] were exposed to untreated SWCNT (synthesized with an iron-cobalt/magnesium oxide catalyst) by nebulization. Acute exposures to a water aerosol containing CNT [concentration and dose unspecified] lasted 15 minutes. CNT structures were observed by Raman spectroscopy in blood samples taken from mice 24 hours after the inhalation of CNT. The quantity was not specified, but exceeded the detection limit of Raman spectroscopy. These CNT were observed as clusters with average diameters of several micrometres (Ingle et al., 2013). [The Working Group noted that smaller CNT clusters, if present, would have been below the detection threshold due to the qualitative nature of the Raman spectroscopy methods, which detect CNT in tissues but cannot provide quantitative dose measures.]
Evidence that CNT could translocate from the lungs of adult male Wistar albino rats (weighing 0.2–0.225 kg) after intratracheal administration of two types of MWCNT at a dose of 0.2, 1, or 5 mg/kg bw was reported by Reddy et al. (2010a). CNT were produced by electric arc (size, 90–150 nm; surface area, 197 mg2/g; crystallinity, hexagonal) or CVD (size, 60–80 nm; surface area, 252 mg2/g; crystallinity, cubic) and were dispersed in PBS plus Tween 80 solution then sonicated to prevent agglomeration before administration. Dose-dependent toxicity was observed in the liver and kidney of rats exposed to either type of MWCNT. Light micrographs of the liver tissue 1 day after instillation showed black pigments, but no quantitative data on CNT tissue doses were provided.
Inhaled MWCNT were observed in the subpleural wall and within the subpleural macrophages in groups of 10 male C57BL6 mice after a single 6-hour inhalation exposure to 1 or 30 mg/m3 of MWCNT (Helix, Inc.; MMAD, 164 or 183 nm, respectively; length, < 100 nm to > 10 µm; diameter, 10–50 nm) (Ryman-Rasmussen et al., 2009a). Carbon black (MMAD, 209 nm) at a concentration of 30 mg/m3 was used as a comparison material. Lung tissues were collected 1 day, 2 weeks, 6 weeks, or 14 weeks after exposure. The calculated deposited doses of CNT were 0.2 or 4 mg/kg at concentrations of 1 or 30 mg/m3 MWCNT, respectively (assuming a 10% deposition of the inhaled dose). The inhaled MWCNT were engulfed by macrophages, which migrated to the subpleural region. TEM images showed CNT within macrophages beneath the pleura. The authors hypothesized that activated macrophages containing MWCNT migrate through the pleural lymphatic drainage and stimulate the recruitment of mononuclear cells in the pleura (consistent with their previous finding (Ryman-Rasmussen et al., 2009b) that monocyte chemokine CCL2 was increased in mice after inhalation of MWCNT). Significant fibrosis (focal subpleural) was observed in mice 2 and 6 weeks after inhalation exposure to 30 mg/m3 of MWCNT, but not in mice exposed to 1 mg/m3 of MWCNT or 30 mg/m3 of carbon black. Aggregates of MWCNT in lung tissues were significantly elevated in mice inhaling 30 mg/m3 of MWCNT (but not carbon black or 1 mg/m3 of MWCNT). No quantitative data on the dose of MWCNT in the lung tissues were reported (Ryman-Rasmussen et al., 2009a).
Translocation to the pleura was observed in a study of male Fischer 344 rats exposed five times to MWCNT (0.5 mL of 500 µg/mL) by intrapulmonary spraying over a 9-day period (Xu et al., 2012). The total mass dose was 1.25 mg/rat. Two types of MWCNT were studied – MWCNT-N (Nikkiso Co., Ltd) and MWCNT-M (Mitsui-7; Mitsui Chemicals, Inc.) – in addition to crocidolite as a control. Pleural cavity lavage was used to examine the presence of MWCNT or crocidolite in the pleural cavity, and SEM was used to confirm the location of the MWCNT or crocidolite fibres in lung tissue sections. Both types of MWCNT and crocidolite fibres were found in the pleural cavity lavage cell pellets, mostly in macrophages. A few fibres were found in the intercellular space or on cell surfaces. In the tissue sections, both MWCNT and crocidolite were observed in the focal granulomatous lesions in alveoli and in alveolar macrophages. The MWCNT or crocidolite fibres were also found in the mediastinal lymph nodes, and a few were observed in liver sinusoid cells, blood vessel wall cells in the brain, renal tubular cells, and spleen sinus and macrophages. A few fibres were observed penetrating directly from the lungs to the pleural cavity through the visceral pleura, but no fibres were seen in the parietal pleura.
Mercer et al. (2013a) investigated the extrapulmonary transport of MWCNT in male C57BL/6 mice after inhalation exposure to 5 mg/m3 of MWCNT (Mitsui-7) for 5 hours per day for 12 days in a 3-week study [the same study as that reported in Mercer et al. (2013b) for disposition in the lungs]. The lung burden of MWCNT on day 1 after exposure was 28.1 µg (47 × 106 MWCNT fibres/µg). Optical sectioning through serial sections of the lung, liver, and kidney was carried out to measure the length of the single MWCNT in those organs on days 1 and 336 after the end of inhalation exposure (Mercer et al., 2013b). The amount of MWCNT in the tracheobronchial lymph nodes was determined as the volume density of MWCNT in the lymph nodes relative to the volume density of MWCNT in the lungs 1 day after exposure. The numbers of MWCNT fibres in the extrapulmonary organs, diaphragm, and chest wall were counted per unit area and converted to number per organ using morphometric methods. Enhanced-darkfield light microscopy imaging of CNT was performed on sections of the exposed lungs to identify CNT that would not otherwise be detected. Most of the MWCNT that translocated from the lungs were found in the tracheobronchial lymph nodes (1.08% on day 1 and 7.34% on day 336 after exposure, as a percentage of the lung burden on day 1 after exposure). The next highest extrapulmonary tissue burdens of MWCNT were reported in the liver (0.0028% on day 1 and 0.027% on day 336) and kidneys (0.0010% on day 1 and 0.0052% on day 336). Smaller amounts of MWCNT were detected in the heart, brain, chest wall, and diaphragm (with higher amounts at day 336 than at day 1 after exposure in all tissues except the chest wall). In the lungs, 54% of the MWCNT burden was agglomerated, while only singlet MWCNT were observed in the liver, kidney, heart, brain, chest wall, and diaphragm (Mercer et al., 2013a).
In an ex-vivo model, SWCNT (100 µg) were instilled into the airway of isolated perfused rat lung. The isolated perfused rat lung model retains the lung architecture but eliminates the systemic pharmacokinetics. The pulmonary translocation of SWCNT from the airways across the pulmonary barrier was less than 0.05% of the instilled dose after 90 minutes. A pharmacokinetic simulation estimated a cumulative pulmonary translocation from the rat lung of less than 0.15% over 14 days (Matthews et al., 2013).
The length of CNT that translocate from the lungs to the pleura (or were instilled therein in an experimental study) may influence their retention. Longer structures (> 5 µm) were retained in the pleura, while shorter structures were able to drain to the lymph nodes (Poland et al., 2008; Murphy et al., 2011); however, Kim et al. (2014) found a persistent presence in the pleura and lung parenchyma 90 days after subacute (28 days) inhalation exposure to short-length (330.18 ± 1.72 nm) MWCNT. Stomata are outlets in the parietal pleura through which lymphatic drainage occurs (Donaldson et al., 2010; Murphy et al., 2011). The maximum diameter of stomata in mice is 10 µm (Murphy et al., 2011). Using single-photon emission computed tomographic imaging, Murphy et al. (2011) reported that radiolabelled short CNT (length, 0.5–2 µm) were observed in the cranial mediastinal lymph nodes (two bilateral lymph nodes located lateral to the thymus) within 1 hour of intrapleural injection of 5 µg/mouse, and increased up to the end of observation 24 hours after the injection. Qualitatively, fewer long (length, > 15 µm) than short CNT were observed in the lymph nodes (Murphy et al., 2011).
The translocation of 14C-radiolabelled MWCNT from the lungs to other organs up to 1 year after pharyngeal aspiration of 20 µg CNT (suspended in 50 µL dispersion medium) was investigated in seven groups of 4 female Balb/c mice (age, 6 weeks). After dispersion, the mean length of the CNT was 3.9 μm (range, 500 nm–12 μm) and the mean diameter was approximately 40 nm (range, 10–150 nm). Time-points of examination were 1 and 7 days, and 1, 3, 6, 9, and 12 months after exposure. At 6 months after exposure, the average concentration of MWCNT in the lungs decreased to less than 10% of the administered dose, but increased at the last two time-points (to about 20% at 12 months after exposure). In contrast, the MWCNT concentration in the spleen and liver – which was detectable on day 1 after exposure – increased over time to approximately 0.1–0.2% of the administered dose in the spleen at 6–12 months after exposure and approximately 0.5–1% in the liver at the same time-points, although the liver had about half the mass concentration of MWCNT (µg/g) compared with the spleen (Czarny et al., 2014). [The Working Group noted that the authors reported that only half of the 20-µg dose administered was measured in the lungs on day 1 after exposure, and the initial lung dose was therefore adjusted to 10 µg; the remainder of the lung dose was considered to have probably been swallowed, thus reaching the stomach and gastrointestinal tract.]
A subsequent experiment on oral ingestion through the intra-oesophageal instillation of 50 µg of 14C-MWCNT showed that approximately 95% of the ingested MWCNT dose was measured in the gastrointestinal tract and faeces after 24 hours; no MWCNT were detected (by radioactive signal) after 4 days; and no MWCNT were detected in the spleen or liver tissue sections on 1, 7, and 30 days after gavage with MWCNT. This finding was considered by the authors to support the evidence that translocation of the MWCNT after pharyngeal aspiration occurred through the air–blood barrier (including crossing the epithelial cells of the airways or the alveoli) and not across the intestinal lining (Czarny et al., 2014).
(d) Retention
Retention refers to the temporal distribution of uncleared particles in the respiratory tract (Lioy et al., 1984). This section focuses on retention in the lungs and lung-associated tissues (i.e. lung parenchyma, pleura, and lung-associated lymph nodes) (see also Section 4.1.2 (c) for data on doses of CNT in extrapulmonary organs). Retention (or biopersistence) in the lungs is higher for inhaled particles that are poorly soluble and poorly cleared from the lungs (e.g. due to size, shape, surface reactivity, and/or to a high dose that exceeds clearance capacity).
The lung burden of MWCNT (Baytubes, a proprietary product; Bayer MaterialScience, Germany) was measured in male Wistar rats 1 day, and 17, 26, and 39 weeks after 13 weeks of inhalation exposure to 0.1, 0.4, 1.5, and 6 mg/m3 for 6 hours per day on 5 days per week. Tissue burdens of MWCNT (in the left lung lobe and in the lung-associated lymph nodes) were estimated from the measurements of residual cobalt tracer (0.115% matrix-bound). The dose deposited in the alveoli was calculated from the following information: concentration of cobalt (ng/L [air]) × minute ventilation rate (0.8 L/min/kg) [male rat control body weights: 231 and 369 g, at the beginning and end, respectively, of the 13-week exposure] × the alveolar deposition fraction (5.7%) × the cobalt fraction (%/100) (see also Section 4.1.2 (a) for more information on the estimated deposition fraction). The retained MWCNT dose (measured as µg cobalt tracer/lung) in the lungs decreased slowly during the 39-week period after exposure, while the MWCNT in the lung-associated lymph nodes increased after exposure in mice exposed to 1.5 or 6 mg/m3 (Pauluhn, 2010a).
The retention half-times at each concentration were calculated in Pauluhn (2010a) from the equation: dc/dt = a(1–kt) where k is the first-order elimination constant (calculated from the cobalt lung burden data 17, 26, and 39 weeks after exposure [although not reported]). The retention half-time (i.e. time to reduce the retained lung dose by half; also called the elimination half-time [t1/2]) was calculated from t1/2 = ln(2)/k. The retention t1/2 was 151, 350, 318, and 375 days at exposure concentrations of 0.1, 0.4, 1.5, and 6 mg/m3, respectively. [From the retention half-times [t1/2], the first-order rate constant k can be estimated as approximately 0.002 d-1 for the three higher concentrations and approximately 0.004 d-1 for the lowest concentration.] Pauluhn (2010a) noted that the levels of cobalt measured in the lungs at the lowest concentration (0.1 mg/m3) were in the range of the limit of quantification, indicating possible imprecision in the t1/2 estimate at that concentration. In comparison, the retention t1/2 for respirable particles in rats at non-overloading doses was approximately 60 days, indicating that the rat lung clearance rates of MWCNT (Baytubes) were reduced by several fold at all exposure concentrations.
Pauluhn (2010a) estimated the MWCNT particle volume lung dose as 107–325, 466–1413, 1192–3917, and 3961–12 002 nL/g of lung in rats exposed to 0.1, 0.4, 1.5, and 6 mg/m3, respectively (at a density of MWCNT of 0.1–0.3 g/cm3, “corrected for void space volume which is 1.43 times greater than the volume of the MWCNT themselves” (Brown et al., 2005)). In comparison, Pauluhn (2010a) quoted Morrow (1988, 1994), who found no significant difference in retention half-times between the control (unexposed) rats and the rats that had a particle volume lung dose of 100 nL/g of rat lung, and Oberdörster (1995), who observed a doubling of the retention half-times in rats with a particle volume lung dose of 1400 nL/g of lung. Pauluhn (2010a) interpreted these comparisons as indicating that overloading of lung clearance was “minimal to moderate” in rats at 0.1 and 0.4 mg/m3 of MWCNT while, at 1.5 and 6 mg/m3, clearance may have been completely impaired.
Mercer et al. (2010) reported the distribution of MWCNT-7 (Mitsui & Co.) (diameter, 49 nm; length, 3.9 µm) in the lungs of male C57BL/6J mice exposed by pharyngeal aspiration to 10, 20, 40, and 80 µg of MWCNT or the vehicle. The distribution of MWCNT was determined in fixed lung sections using morphometric methods at 1, 7, 28, and 56 days after exposure. Field-emission SEM was used to detect and count the number of MWCNT fibre penetrations of three biological tissue barriers: the alveolar epithelium (alveolar penetrations), the alveolar epithelium immediately adjacent to the pleura (subpleural tissue), and the visceral pleural surface (intrapleural space). The number of penetrations per lung (into the subpleural tissue and intrapleural space) increased with increasing dose administered. On day 1 after exposure to 80 µg, 18% of the MWCNT was observed in the airways, 81% in the alveolar region, and 0.6% in the subpleural tissue. Within the alveolar region, 62% of the dose was inside alveolar macrophages on day 1 after exposure. MWCNT penetrations were observed most frequently in the alveolar macrophages, followed by alveolar type I epithelial cells, and less frequently in alveolar interstitial cells (typically observed as fibres passing through adjacent epithelial cells). MWCNT inside the cells were not confined to phagolysosomes and were observed to extend from the cell surface through the nuclei and other organelles. Alveolar type II epithelial cells (2% of the normal epithelial surface) were rarely found to be penetrated by MWCNT. In the airways, MWCNT were observed in the mucous layer above airway epithelial cells and in airway macrophages contained in the cilia-mucous lining layer; penetrations by MWCNT in the airways were rare. At the 20-µg dose, a total of 15 × 106 MWCNT penetrations were observed in the 11 × 106 alveolar type I epithelial cells in mouse lungs (Mercer et al., 2010).
The time course of this MWCNT in the intrapleural space showed a decrease from day 1 to day 7 after exposure (Mercer et al., 2010). This is consistent with a mechanism of shorter fibre clearance from the intrapleural space through the stomata (duct in the parietal pleura) to the lymphatic system (Donaldson et al., 2010; Murphy et al., 2011). However, the amount of MWCNT in the intrapleural space increased again at day 28 after exposure and remained elevated at 56 days after exposure. The lung burden of MWCNT may act as a reservoir to replenish MWCNT in the intrapleural space or that even shorter fibres (length, 3.9 μm) could begin to clog the ducts if they reach a sufficient level within the intrapleural space (Mercer et al., 2010).
Mercer et al. (2013b) provided quantitative data on the retention and distribution of MWCNT in the lung and associated tissues of male C57BL/6 mice after a 3-week exposure by inhalation for 5 hours per day for 12 days to 5 mg/m3 of MWCNT (Mitsui-7; mean length, 4.3 µm). The MWCNT lung burden was determined using a method reported by Elder et al. (2005). The lungs were removed after mice were killed on 1, 14, 28, 84, 168, or 336 days after exposure. Lung tissue was processed by digestion (in 25% potassium hydroxide/methanol (w/v)), centrifugation, and re-suspension of the pellet, and measurements of the optical density of the solution were compared with MWCNT standards that were processed in parallel with the lung samples. The mass of MWCNT in the lungs was determined from a standard curve. [Elder et al. (2005) reported that the limit of detection of this assay was 0.1 µg/mL of suspended solution.] Several imaging techniques (light microscopy, field emission SEM, and enhanced-darkfield microscopy) were used to observe and quantify the distribution of the MWCNT fibres in tissue sections of the lungs. MWCNT were counted in lung tissue sections using an enhanced-darkfield optical system; eight animals were analysed per group and counting was accomplished using an 11 × 11 (121 point) overlay grid pattern to ensure uniform sampling of the section. The number of fibres per MWCNT structure was also determined by enhanced-darkfield microscopy. MWCNT fibres were observed in the alveolar macrophages and alveolar interstitium, and penetrated the visceral pleura (Mercer et al., 2013b). On day 14 and later time-points after exposure, clusters of MWCNT were observed within the ridge of the first alveolar duct bifurcation [which is the primary site of particle deposition after inhalation exposures to particles and fibres, as reported previously by Brody & Roe (1983) and Chang et al. (1988)]. The MWCNT lung burden in mice measured on day 1 after exposure was 28.1 µg (1321 × 109; total fibre number estimate based on 47 million MWCNT fibres/µg [conversion reported in Chen et al. (2012)]) (Mercer et al., 2013b). Of this lung burden, 84% (23.6 μg) was found in the alveolar (pulmonary) region of the lungs and 16% (4.5 μg) in the airways. Similar distributions of MWCNT were observed in two previous studies of MWCNT by Porter et al. (2010, 2013) in mice exposed by pharyngeal aspiration or acute inhalation.
Within the alveolar region, 56% of the MWCNT lung burden was in alveolar macrophages on day 1 after exposure, 7% was in the alveolar airways, and 20% was in the alveolar tissue. These findings indicated a fairly rapid and substantial distribution of inhaled MWCNT to the lung interstitium. By day 1 after exposure, ~1.2% (0.34 µg) of the MWCNT lung burden was observed as single fibres in the pleural compartment (including the subpleural tissue and visceral pleura) (Mercer et al., 2013b).
At 336 days after exposure, 65% of the MWCNT lung burden (28.1 µg) on day 1 after exposure was retained in the lungs (18.2 µg), most of which (96%) was retained in the alveolar region (including 4.8% in subpleural tissue) and 4% (0.73 µg) of which was retained in the airways. The distribution of MWCNT in the lungs shifted from alveolar macrophages (3 times more than in lung tissue on day 1 after exposure) to the alveolar tissue, where the dose increased from 5.8 to 9.5 µg on days 1 and 168 after exposure, respectively. Thus, the alveolar interstitial lung burden increased as the MWCNT in the alveoli were cleared (Mercer et al., 2013b).
The number of larger or agglomerated MWCNT structures (> 4 fibres/MWCNT) decreased over time (from 53 to 25% of the lung burden on days 1 and 168 after exposure, respectively). The number of structures with 2, 3, or 4 fibres also decreased significantly. However, the percentage of single fibres in the MWCNT lung burden did not change significantly from days 1 to 168 after exposure. Thus, the MWCNT structures decreased in size, resulting in a relatively constant number of single MWCNT fibres in the lungs over time (Mercer et al., 2013b).
The mouse lung response on day 1 of this study after exposure to a lung dose of 28.1 µg MWCNT was an increase in the thickness (fibrillar collagen) of the alveolar connective tissue over time, with a 70% increase on day 336 after exposure (Mercer et al., 2013b). The translocation of MWCNT to extrapulmonary organs were described in Mercer et al. (2013a) (see also Section 4.1.2 (c)).
The lung burden of MWCNT (diameter, 90.7 nm; length, 5.7 µm; MMAD (GSD), 1.4–1.6 µm (2.3–3.0)) was measured in male and female Fischer 344 rats after 13 weeks of whole-body inhalation exposure for 6 hours per day on 5 days per week to concentrations of 0, 0.2, 1, and 5 mg/m3. Left lung tissues (0.18–0.36 g) were sampled from five rats in each MWCNT-exposed group. MWCNT was quantified using a technique in which a specific polycyclic aromatic hydrocarbon (benzo[g,h,i]perylene) serves as a marker of these MWCNT. The mass of MWCNT in the lungs of male and female rats increased in relation to the exposure concentration (Table 4.1) and was reported to be 1.4–1.6 times greater in the left lungs of males than in those of females (Kasai et al., 2015). [The Working Group noted that, when the measured MWCNT lung doses are normalized to the average control left lung weight (0.43 g, males; 0.32 g, females), the retained lung doses in males were similar (1.0–1.2 times the lung doses in females).]
In the study by Silva et al. (2014), the physico-chemical properties (metal content, hydrophilicity, and carboxylic acid functionalization) or route of exposure did not significantly influence the retention of MWCNT in the lungs of male Sprague-Dawley rats (as measured in the right caudal lung lobe by programmed thermal analysis). However, the findings suggested that instilled F-MWCNT were retained in the lungs to a greater extent than the same instilled dose of O-MWCNT or P-MWCNT. The retention of instilled F-MWCNT in the lungs was also greater, although not significantly, than the retention of a similar deposited dose of inhaled F-MWCNT (Silva et al., 2014).
(e) Clearance
The mechanisms of clearance depend on the initial site of particle deposition within the respiratory tract and on the physico-chemical properties of the particle (e.g. solubility and functionalization). Soluble particles can dissolve in alveolar lining fluid and then enter the blood or lymph (Dahl et al., 1991; ICRP, 1994; Schlesinger, 1995). Dissolution rates do not vary widely across species, because they are primarily determined by the physico-chemical properties of the material (Dahl et al., 1991). Clearance rates of poorly soluble particles, however, can fluctuate among species due to differences in the macrophage-mediated clearance from the alveolar region and the rates of mucociliary transport in the conducting airways (Snipes, 1989).
Inhaled CNT may be phagocytosed by macrophages and cleared from the lungs by the mucociliary escalator and swallowed (entering the gut). CNT that are not cleared from the lungs by macrophages may enter the epithelial cells that line the alveolar region of the lungs, where their fibres can be retained in the lung interstitium or pass into the lymph or blood circulation (Mercer et al., 2008; Ryman-Rasmussen et al., 2009a; Mercer et al., 2013a, b).
CNT that reach the blood circulation (either by translocation from the lungs or through direct intravenous administration) may be excreted from the body through either the renal (urine) or biliary pathway. Many SWCNT or MWCNT exceed the particle size threshold for renal excretion, particularly if agglomerated (Liu et al., 2008a), and thus accumulate in the liver where they undergo biliary excretion (Cherukuri et al., 2006). The type of surface functionalization can also strongly influence the biodistribution and elimination pathways (see also Section 4.1.2 (g)).
In a study of male Wistar rats that inhaled 0.37 mg/m3 of MWCNT for 6 hours per day on 5 days per week for 4 weeks, lung clearance was reported to be proportional to the amount in the lungs. The retention half-time (t1/2), defined as the time for the retained dose to be reduced by half, was estimated to be 51 or 54 days (based on the lung dose measured by X-ray diffraction or EC analysis, respectively) (Oyabu et al., 2011). [These retention half-time estimates are consistent with normal rat clearance rates reported in other studies, indicating that no reduction in the clearance rate (due to overloading of clearance mechanisms) occurred at the concentration and duration of exposure used in this study.]
In a study of the intratracheal instillation of 10 or 100 µg/mouse of pristine [as-produced] (mean length, 7.5 nm; mean diameter, 13.5 nm) or acid-treated (mean length, 400 nm; mean diameter, 15 µm) MWCNT in male C57BL/6 mice, both types of MWCNT were seen in the lymphatic system in the mediastinal lymph nodes. The acid-treated MWCNT (that contained fewer metal contaminants and were more hydrophilic) induced less severe acute lung inflammation than the pristine MWCNT (Kim et al., 2010). [No quantitative data on the dose were provided.]
No significant lung clearance was observed from day 1 to day 21 after intratracheal administration of O-, P-, or F-MWCNT to male Sprague-Dawley rats. MWCNT structures were observed inside the alveolar macrophages and polymorphonuclear leukocytes in BALF and in the airway cilia, suggesting some, but insignificant, MWCNT clearance (Silva et al. 2014).
(f) Biodegradation
The size, structure (wall number), and functionalization of CNT may influence their distribution in the body and their ability to pass into cells through cell membranes (Bianco et al., 2011). Much of the literature on the kinetics of CNT is motivated by the potential use of CNT as targeted medical delivery systems to specific tissues for therapeutic purposes (Ali-Boucetta & Kostarelos, 2013). SWCNT functionalized with polyethylene glycol (PEG) were more hydrophilic, had greater dispersibility in aqueous media than unfunctionalized SWCNT, and were excreted through biliary and renal pathways (Bhirde et al., 2010).
(i) In vitro and ex vivo
Pulmonary eosinophils from humans (in vitro) and mice (activated ex vivo) were shown to degrade SWCNT through an enzyme (eosinophil peroxidase, EPO) that is exocytosed when cells are activated (e.g. by the presence of CNT) and is one of the major oxidant-generating enzymes in the human lung. The EPO-catalysed oxidative biodegradation was assessed by TEM, ultraviolet-visible-near-infrared absorption spectroscopy, and Raman spectroscopy and was found to occur extracellularly (Andón et al., 2013).
Another study by Kagan et al. (2010) showed that polymorphonuclear leukocyte (neutrophil) myeloperoxidase (MPO) also catalysed the biodegradation of SWCNT, although the SWCNT in this study were pre-opsonized with immunoglobulins to increase the internalization efficiency by neutrophils. The difference in the mechanisms of degradation of the two cell types is due to neutrophils using MPO to kill bacteria inside the phagolysosome, while eosinophils use secreted EPO to kill larger extracellular organisms, such as parasites.
(ii) In vivo
The role of neutrophils or eosinophils in the biodegradation of CNT in vivo is unclear. In the lungs, CNT that are not cleared by alveolar macrophages may translocate into the lung interstitium and stimulate the development of fibrosis or translocate to distant sites and elicit systemic inflammatory and/or immunological responses (Mercer et al., 2008; Ryman-Rasmussen et al., 2009a).
Shvedova et al. (2012a) demonstrated the role of MPO, an abundant enzyme in inflammatory cells such as polymorphonuclear leukocytes (or neutrophils), in the clearance and retention of CNT in the lungs of mice by comparing the clearance of SWCNT in wild-type and MPO-deficient (knockout) C57Bl/6 mice given 40 µg/mouse by pharyngeal aspiration. The MPO-mediated biodegradation of SWCNT occurs through the oxidative modification or “cutting” of the SWCNT (resulting in oxidative defects in CNT that are detectable by Raman spectroscopy). A significant difference was observed in the clearance of SWCNT from the lungs in wild-type compared with MPO-knockout mice. The degradation of SWCNT (assessed by Raman spectroscopy) was significantly greater in wild-type mice and the volume of SWCNT aggregates per total lung volume (quantified in lung tissues by light microscopic imaging analysis) was significantly greater in MPO-knockout mice than in wild-type mice 28 days after exposure. [The Working Group noted that, consistent with the higher dose of SWCNT retained, the MPO-deficient mice showed a greater degree of fibrosis, as measured by higher collagen content, and a greater average thickness of the alveolar connective tissue in the lungs than wild-type mice; however, wild-type mice also showed significant fibrosis.]
(g) Biokinetics of bioengineered CNT administered by intravenous administration
Much of the literature on the biokinetics of CNT in the body involves studies on their potential use in biomedical applications. The route of exposure has been shown to influence the biodistribution of CNT in the body (Ali-Boucetta & Kostarelos, 2013), with the highest initial dose observed at the site of administration. In medical imaging or therapeutical applications, the route of exposure is typically the intravenous injection.
CNT injected intravenously accumulate in the liver and spleen, while CNT administered orally are found primarily in the stomach and intestines (Ali-Boucetta & Kostarelos, 2013).
Well dispersed short MWCNT (length < 500 nm) injected intravenously into mice were excreted rapidly through the kidneys (no nephrotoxicity observed), while longer MWCNT were retained in the spleen, lungs, and liver (resulting in hepatotoxicity) (Jain et al., 2011).
Surface modification was considered to be the most important factor influencing the biodistribution of CNT (Ali-Boucetta & Kostarelos, 2013). The two main types of functionalization are the coating (i.e. non-covalent surface modification) of SWCNT and the covalent functionalization of SWCNT or MWCNT.
The coatings that have been studied include surfactant Pluronic F108, Tween 80, and PEG phospholipid. The blood clearance of Pluronic F108-coated CNT injected intravenously into rabbits was rapid (half-life t1/2 < 1 h), which was attributed to the formation of SWCNT–protein complexes or SWCNT aggregates that accumulated primarily in the liver (Cherukuri et al., 2006). Tween 80-coated SWCNT were retained (for up to 28 days) in the liver, lungs, and spleen in injected mice (the CNT had a 13C-enriched backbone) (Yang et al., 2007). The circulation of PEGylated CNT in the blood was longer (half-life t1/2, 5 h) and was further extended (half-life t1/2, 12–22 hours) by increasing the branching of the PEG and liver uptake was reduced in injected mice (Liu et al., 2008a, 2011a; Prencipe et al., 2009). PEGylated CNT were eliminated through biliary excretion (over 2 months). Pristine, non-covalently functionalized SWNCT mostly accumulated in the liver.
The covalent functional groups that have been studied include hydroxyl, ammonium, glucosamine, and taurine (Ali-Boucetta & Kostarelos, 2013). Many of these studies used radiolabelled CNT. Higher degrees of functionalization facilitated the dispersion of individual CNT that led to predominantly urinary excretion (Singh et al., 2006; Lacerda et al., 2008a, b, c). However, MWCNT–taurine accumulated in the liver, heart, and lung (Deng et al., 2007).
Many of these studies reported qualitative estimates of the amount of CNT in various organs (e.g. by whole-body imaging). The techniques used showed the relative amount of CNT, although small amounts may have been missed due to limits of sensitivity. The most typically reported quantitative measure was the half-life t1/2 in blood circulation. While these studies provide valuable insights into the factors that influence the biodistribution of CNT, they focused on medical applications of CNT and thus have limited direct relevance to occupational or environmental exposures.
4.2. Physico-chemical properties associated with toxicity
The physico-chemical properties of CNT may be modulated by varying the method of synthesis, by applying modification processes after synthesis, and/or by the covalent functionalization of their external surface. A large variety of CNT forms may thus be produced that exhibit different features that influence their pathogenicity. CNT cannot be considered as a single well defined substance but as families of different materials, the number of which is growing dramatically.
Evidence has been found that the responses of cells to CNT are modulated by their physico-chemical properties. The variability of the CNT employed in different studies gives rise to the discrepancies observed in biological outcomes (Muller et al., 2005; Kagan et al., 2006; Elgrabli et al., 2008; Poland et al., 2008; Takagi et al., 2008; Ma-Hock et al., 2009; Sakamoto et al., 2009; Fubini et al., 2010, 2011).
Major studies on the effects of relevant physico-chemical characteristics on the adverse responses to CNT in various experimental models are summarized in Table 4.2.
Table 4.2
Physico-chemical properties of carbon nanotubes that are relevant to toxicity: summary of the most pertinent studies.
4.2.1. Crystal structure and defects
Purification and functionalization can induce defects in CNT and may modify or increase their toxicity. Nitric acid, which is involved in purification and functionalization, destroys SWCNT, resulting in the production of amorphous carbon and a reduction in the amount of the transition metal catalyst used in their production (Hu et al., 2003).
Perfectly crystalline CNT are formed only by hexagonal rings of sp2 hybridized carbons. However, the graphene layers contain a variable number and degree of defects that may arise directly from the process of synthesis or may be introduced or eliminated during treatments after synthesis (Galano et al., 2010). The CNT that are currently produced are far from perfect and may include various numbers and types of defect, such as non-hexagonal rings, atom vacancies (topological defects), carbon with sp3 hybridization, incomplete bonding, and oxygenated groups (Ebbesen & Takada, 1995; Charlier, 2002; Galano, 2010). After systematic variation of the physical and chemical features of a given MWCNT specimen with or without defects, genotoxicity in vitro and inflammogenicity and fibrogenicity in vivo (Muller et al., 2008a), but not carcinogenicity (Muller et al., 2009; see Section 3), were correlated with the presence of defects. [The Working Group noted that only a single type of defect (i.e. broken C–C bonding generated by grinding) was evaluated in these studies.]
Defects impart the potential to quench free radicals to both MWCNT and SWCNT (Galano, 2010). CNT retard the oxidation of polystyrene, polyethylene, polypropylene, and poly(vinylidene) fluoride due to their strong ability to accept radicals, which may interrupt chain propagation, leading to antioxidant effects in polymeric material (Watts et al., 2003). Pristine SWCNT were demonstrated to be powerful antioxidants (Lucente-Schultz et al., 2009) and a variety of modified CNT exhibited different defective sites.
4.2.2. Form and size
A fibre shape associated with high durability has been proposed as a critical factor in CNT-induced pleural toxicity and carcinogenicity (Donaldson et al., 2011 and references therein). Poland et al. (2008) reported an “asbestos-like” pathogenicity of long, rigid CNT in the induction of inflammation while tangled CNT were less potent. Similarly to short amphibole asbestos fibres, shorter CNT induced less inflammation. In addition to dimensions and shape, other physico-chemical features are involved in fibre toxicity, suggesting that the fibre paradigm is not the only mechanism (Jaurand et al., 2009; Sanchez et al., 2009; Fubini et al., 2011). The physico-chemical properties of asbestos fibres and CNT differ substantially, correlating with the marked differences in their chemical composition and structure (Fubini et al., 2010, 2011) which are illustrated in Table 4.3 (Fubini et al., 2011).
Table 4.3
Comparison of the major physico-chemical features of carbon nanotubes and asbestos.
(a) Length
Schinwald et al. (2012) reported that CNT over 4 μm in length are pathogenic to the pleura in mice and proposed a threshold length value (4–5 μm) for the induction of an acute inflammatory response in a mouse model. Pleural inflammation and fibrosis are induced only by long (> 10 μm) CNT after intraperitoneal (Kolosnjaj-Tabi et al., 2010) or intrapleural (Murphy et al., 2011) injection. The adverse effects of long (> 10 μm), rigid CNT were related to their physical interaction with cells resulting in incomplete internalization and “frustrated phagocytosis”, which activate an inflammatory response. Stomata (diameter, 3–10 μm) in the parietal pleura act as a “sieve” in drainage from the pleural space and fail to clear the long CNT (Murphy et al., 2011).
Manshian et al. (2013) investigated the role of the length of SWCNT in the induction of genotoxicity in human bronchial epithelial BEAS-2B and lymphoblastoid MCL-5 cells. SWCNT induced significant levels of chromosomal damage at subcytotoxic concentrations, the potency of which, according to the length of the SWCNT, was 400–800 nm > 5–30 μm > 1–3 μm. The authors hypothesized that surface area is an important determinant in cellular response, as well as the secondary structure of CNT under experimental conditions. In contrast, only SWCNT 1–3 μm in length were found to be mutagenic in mammalian cells (see Section 4.3).
(b) Thickness
A study of two MWCNT of similar length (< 5 μm) and surface reactivity but different diameter (9.4 and 70 nm) showed that thinner MWCNT appeared to be significantly more toxic than their thicker counterparts in vivo (rat lung) and in vitro (murine alveolar macrophages) (Fenoglio et al., 2012). Nagai et al. (2011) also reported an effect of the diameter of CNT on mesothelial toxicity and carcinogenicity in rats (see also Section 3). Short CNT with different diameters that had or had not been subjected to carboxyl surface functionalization were assessed for cytotoxicity in phagocytic and non-phagocytic cells. The role of oxidative stress was evaluated by assessing the intracellular glutathione (GSH) levels and protection by N-acetyl cysteine (NAC). CNT < 8 nm in diameter were more cytotoxic than CNT ≥ 20 nm in diameter and carboxylated CNT were more toxic than as-produced CNT. Protection by NAC was maximal for larger-diameter as-produced CNT and minimal for small-diameter carboxylated CNT. Thinner (diameter < 8 nm) CNT acted mainly through the disruption of membrane integrity, and CNT with a larger diameter mainly induced apoptotic changes (Fröhlich et al., 2013).
4.2.3. Surface reactivity
The variability in the toxicity elicited by CNT can mostly be ascribed to both differences in shape and modifications to the chemical composition/structure of the CNT employed in the different studies (Fubini et al., 2011 and references therein). Differences in surface state between asbestos and CNT (in contrast to asbestos, CNT quench radicals, are hydrophobic, and may be fully freed from metal impurities) suggest that these two fibrous materials might induce toxicity by different mechanisms (Fubini et al., 2011)
Physical and chemical properties are generally accepted to modulate the cell responses to CNT. The introduction of surface oxygenated functionalities increased the toxicity of CNT in some models (Bottini et al., 2006; Vittorio et al., 2009; Pietroiusti et al., 2011). In contrast, Cheng et al. (2008) reported that purified PEGylated SWCNT, although reversibly internalized and translocated into the nucleus, were non-genotoxic in mammalian cells in terms of cell-cycle distribution and mitosis after 5 days of continuous exposure, suggesting that intensive purification and functionalization improves the biocompatibility of CNT.
Li et al. (2013) reported the role of surface charge in determining the pulmonary fibrogenic effects of MWCNT. Anionic functionalization with carboxylate and PEG decreased pulmonary fibrogenic potential compared with as-prepared MWCNT; strong cationic functionalization with polyetherimide induced a greater degree of pulmonary fibrosis. Neutral and weakly cationic (sidewall amine) functionalized CNT had similar fibrogenic potential to as-produced CNT. The mechanism of these effects involves differences in the cellular uptake of MWCNT, lysosomal damage, and cathepsin B release in macrophages, associated with the activation of NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome (Li et al., 2013).
Hamilton et al. (2013b) examined the consequences of surface carboxylation of MWCNT on bioactivity. Hydrochloric acid refluxing was used to purify raw “as-received” MWCNT by removing the amorphous carbon layer on their surface and reducing the metal impurities (e.g. nickel). The sidewall of raw and hydrochloric acid-purified MWCNT was further functionalized with the carboxyl moiety using nitric acid oxidation, a common approach that imparts the carboxyl functional group to the MWCNT. No structural damage was observed. Four distinct MWCNT were compared for their bioactivity: raw “as-received”, purified, carboxyl-terminated raw MWCNT, and carboxyl-terminated purified MWCNT. Raw and hydrochloric acid-purified MWCNT are poorly soluble in water. In contrast, after nitric acid oxidation, both carboxylated forms of MWCNT showed very good water solubility. Freshly isolated alveolar macrophages from C57Bl/6 mice were exposed to these nanomaterials to determine the effects of these modifications on cell viability and inflammasome activation, which was confirmed using inhibitors of cathepsin B and caspase-1. Purification slightly reduced cell toxicity and inflammasome activation compared with raw MWCNT. In contrast, functionalization of MWCNT with carboxyl groups dramatically reduced cytotoxicity and inflammasome activation. Similar results were seen in human monocytic THP-1 cells. All nanomaterials, regardless of modification, were taken up by alveolar macrophages. However, the manner in which the nanomaterials were processed within the cells differed. Purified MWCNT were taken up in large vacuoles or phagolysosomes and did not appear to be free in the cytoplasm. In contrast, the two functionalized MWCNT did not appear to be incorporated in large vacuoles, but were more evenly distributed in smaller phagolysosomal structures or free in the cytoplasm. The results confirmed that MWCNT activate NLRP3 inflammasome through a process that involves phagolysosomal permeabilization, the release of cathepsin B, and the activation of caspase-1 (Hamilton et al., 2013b).
Sager et al. (2014) investigated whether MWCNT (same nanomaterial as that used in Hamilton et al., 2013b) with different surface functionalities would exhibit different bioactivity profiles in vivo. Unmodified (bare) MWCNT and MWCNT that were surface functionalized with the carboxyl group (F-MWCNT) were instilled intratracheally into C57BL/6 mice. Mice were then examined for biomarkers of inflammation and injury, as well as histologically for the development of pulmonary disease as a function of dose and time. Biomarkers for pulmonary inflammation included cytokines (interleukin [IL]-1β, IL-18, and IL-33), profibrotic mediators, the presence of inflammatory cells (neutrophils), lysosomal release of cathepsin B, and markers of injury (albumin and lactate dehydrogenase [LDH]). The results showed that surface modification of the MWCNT by the addition of the carboxyl group significantly reduced bioactivity and pathogenicity. Bare MWCNT were more bioactive, causing more inflammation, lung pathology, and fibrosis than the F-MWCNT. This difference in bioactivity correlated with the activation of NLRP3 inflammasome (Sager et al., 2014).
(a) Generation of free radicals
Unlike other toxic particulates (e.g. asbestos), CNT modified by grinding to introduce structural defects have been reported not to generate but to quench free radicals in cell-free systems. This scavenging activity was eliminated in CNT that were fully divested of their defects (i.e. by heating at 2400 °C) (Fenoglio et al., 2008). CNT in composites (CNT-polymer) have been employed to preserve the polymeric matrix from oxidative degradation by their radical scavenging ability (Watts et al., 2003). The susceptibility of CNT to attack by radicals has been exploited to introduce functionalities at their surface (Ghiazza et al., 2014 and references therein). However, SWCNT with different iron contents displayed different redox activity in a cell-free model system, as revealed by the formation of ascorbate radicals resulting from ascorbate oxidation detected by electron paramagnetic resonance (Kagan et al., 2006). In the presence of zymosan-stimulated RAW 264.7 macrophages, non-purified iron-rich SWCNT were more effective in generating hydroxyl radicals (documented by electron paramagnetic resonance spin-trapping with 5,5-dimethyl-1-pyrroline-N-oxide) than purified SWCNT (Kagan et al., 2006). Exposure of immortalized human epidermal HaCaT keratinocytes to SWCNT induced oxidative stress, which was confirmed by the formation of free radical species, the accumulation of peroxidation products and thiobarbituric acid-reactive substances, the reduction of low-molecular-mass thiols and protein sulfhydryls, and a decrease in vitamin E and total antioxidant reserves in the cells. As-produced unrefined SWCNT contain up to 30% iron, and the authors hypothesized a Fenton-like reaction resulting in HO• generation, which increased in the presence of hydrogen peroxide and decreased in the presence of catalase (a hydrogen peroxide scavenger) or desferoxamine (a strong iron chelator) (Shvedova et al., 2003).
Whether CNT in cell-free media do not generate hydroxyl radicals and/or other reactive oxygen species (ROS) per se or whether what is hypothetically generated would immediately be quenched by defects is not clear (Fenoglio et al., 2006, 2008). Purified MWCNT scavenge hydroxyl radicals generated by different sources (Fenoglio et al., 2006). A local decrease in ROS was observed in vivo after intratracheal instillation of DWCNT in mice (Crouzier et al., 2010). However, CNT and other graphene materials have been reported to deplete the cellular antioxidant defences of cells by oxidizing GSH through a reaction with oxygen at the surface (Liu et al., 2011b). Therefore, CNT interact with the cellular antioxidant defence system in several ways. [The overall effect of CNT on the homeostasis of ROS in cells still needs to be clarified.]
(b) Bioavailability and biodeposition of metals
After synthesis, CNT generally contain amorphous carbon and metals – iron and other different redox active metals (e.g. cobalt, nickel, and molybdenum) – as a residue of the catalyst employed in their synthesis. The amounts are highly variable and may reach 20% in unpurified, as-produced CNT (see also Section 1). Metals may be present in different oxidation states as ions, clusters, or even organized in metal nanoparticles. The iron in CNT has been reported to be a mixture of α-Fe0, γ-Fe0, and carbide phases; much of the metal appears by TEM to be at least superficially encapsulated by carbon (Guo et al., 2007). Most of the iron is located within the tube, and is thus not readily accessible to target cells. The metal residues may be extracted from the CNT, e.g. by acid treatment, but often a fraction remains inside. Toxicologically significant amounts of iron can be mobilized from a diverse set of commercial nanotube samples in the presence of ascorbate and the chelating agent, ferrozine. This mobilized iron is redox active and induces single-strand breaks in plasmid DNA in the presence of ascorbate. Iron bioavailability is not fully suppressed by vendor “purification” and is sensitive to partial oxidation, mechanical stress, sample ageing, and intentional chelation (Guo et al., 2007). Because iron sealed within the graphene layers cannot be released in physiological media, the amount of bioavailable iron in CNT varies greatly from sample to sample and cannot be predicted from the total iron content (Guo et al., 2007, Fubini et al., 2011 and references therein).
Redox active metals associated with CNT (e.g. iron) have been reported to induce oxidative stress and toxicity (Kagan et al., 2006; Pulskamp et al., 2007). Clear evidence on the role of iron in the toxicity of CNT was obtained by showing that simple removal of most of the iron residues caused a remarkable decrease in the toxicity of SWCNT (Kagan et al., 2006) and MWCNT (Aldieri et al., 2013). Iron-rich SWCNT caused a significant loss of intracellular low-molecular-masss thiols (predominantly GSH) and accumulation of lipid hydroperoxides in both zymosan- and phorbol myristate acetate-stimulated RAW 264.7 macrophages (Kagan et al., 2006). Two MWCNT differing only in the presence or absence of iron were compared at dose ranges of 25–100 μg/cm2. While iron-rich MWCNT (50 μg/cm2) were significantly cytotoxic and genotoxic and induced a potent cellular oxidative stress response, iron-free MWCNT (50 μg/cm2) did not exert any of these adverse effects (Aldieri et al., 2013).
Complete elimination of any metal trace can be achieved only by heating CNT to a very high temperature (2400 °C) at which metal vapourizes. Lung toxicity in vivo but not genotoxicity in vitro induced by MWCNT was decreased, but not completely eliminated, by heating at 600 °C, when metals are fully vapourized but defects remain (Fenoglio et al., 2008; Muller et al., 2008a).
4.2.4. Fibre durability (leaching, dissolution, and breakage)
CNT are highly insoluble due to their graphitic structure (Lam et al., 2004) and they have been suggested to be as biopersistent as amphiboles (Sanchez et al., 2009). However, several studies reported that the carbon structure may be attacked and degraded, mainly by endogenous oxidants in biological simulation fluids or in vivo (Kagan et al., 2010; Shvedova et al., 2012a, b).
(a) In vivo
Using MPO-deficient mice, Shvedova et al. (2012a) showed that MPO contributes to the pulmonary oxidative biodegradation of SWCNT in vivo (see also Section 4.1.2 (f)).
(b) In vitro
Two different routes of attack and degradation of CNT by endogenous oxidants were reported in studies in vitro (see Section 4.1.2 (f)). One enzymatic route is through degradation by several peroxidases, such as MPO (Kagan et al., 2010), lactoperoxidase, and EPO (Shvedova et al., 2012b), while the second route follows non-enzymatic degradation when CNT are in contact with simulated phagolysosomal fluid (Liu et al., 2010). Degradation of SWCNT after incubation with human EPO and hydrogen peroxide has been reported; the biodegradation was greater in the presence of sodium bromide. However, neither EPO nor hydrogen peroxide alone caused SWCNT degradation (Andón et al., 2013).
Surface functionalization affects the biodegradability of CNT (Liu et al., 2010; Bianco et al., 2011). The rate of degradation is associated with both the degree of surface functional groups and the type of CNT; MWCNT are more resistant than SWCNT and thus require a longer time for degradation (Bianco et al., 2011).
4.2.5. Physico-chemical determinants of defined biological end-points
Because of the extreme variability of the features of CNT, the method to be adopted to associate a physico-chemical feature to a given effect in vivo is to modify one single property at a time of a well defined specimen of CNT and test all modified specimens using exactly the same procedure. Two typical examples of this type of procedure taken from Table 4.2 are highlighted below.
This approach showed clearly that a slight modification in cytotoxicity and inflammogenicity occurred after purification, while acute inflammogenicity (demonstrated by inflammasome activation in MWCNT in vitro and in vivo) was dramatically reduced, with a consequent reduction in pathogenicity after functionalization of the surface with carboxyl (Hamilton et al., 2013b; Sager et al., 2014).
Modification of MWCNT by progressive heating during which metals and defects are gradually eliminated (see Table 4.4) enabled an association of genotoxicity in vitro with defects and respiratory toxicity in vivo with both metals and defects (Fenoglio et al., 2008; Muller et al., 2008a).
Table 4.4
Example of an experimental mechanistic approach to evaluate specific physico-chemical determinants of biological activity for ground multiwalled carbon nanotubes.
4.3. Genetic and related effects
4.3.1. Humans
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
4.3.2. Experimental systems
(a) In vivo
(i) DNA damage
Investigations on the direct genotoxicity of CNT have focused on end-points measured by the comet assay and oxidatively generated DNA lesions. Table 4.5 lists the in-vivo studies that have assessed levels of DNA damage in rodent tissues after exposure to CNT.
Table 4.5
Studies of DNA damage and mutation in tissues of experimental animals exposed to carbon nanotubes in vivo.
Intratracheal instillation of SWCNT into mice (54 µg/animal) increased the levels of DNA strand breaks in BALF cells 3 hours after exposure (Jacobsen et al., 2009). Another study showed that a single intratracheal instillation of MWCNT (50 or 200 µg/mouse) was associated with increased levels of DNA strand breaks in lung tissues of mice 3 hours after exposure, and also documented increased levels of 8-oxodeoxyguanosine (8-oxodG) and lipid peroxidation-derived DNA lesions in lung tissues of mice 3–168 hours after exposure. However, the baseline level of 8-oxodG was 4.8 lesions/106 nucleotides (corresponding to 22 lesions/106 deoxyguanosine [dG]), indicating spurious oxidation of DNA during the processing or analysis of samples (Kato et al., 2013). Another study showed that pulmonary exposure to MWCNT once every 2 weeks for 24 weeks was associated with an increased level of 8-oxodG in lung tissues of rats [the detection method was not described and the basal level of 8-oxodG was very high (1.3 ng/µg DNA, corresponding to 7600 lesions/106 dG)] (Xu et al., 2014).
Nose-only inhalation of 0.17–0.96 mg/m3 of MWCNT for 6 hours per day on 5 days per week for 28 days was associated with increased levels of DNA strand breaks in the lung tissues of rats (Kim et al., 2014). A similar study by the same authors in which rats were exposed by whole-body inhalation of 0.16–0.94 mg/m3 of MWCNT for 6 hours per day for 5 days also showed increased levels of DNA strand breaks in lung tissues (Kim et al., 2012a). Weekly intratracheal instillations of 25.6 µg of MWCNT for 5 weeks were associated with elevated levels of DNA strand breaks in the lung tissues of mice, whereas unaltered levels of formamidopyrimidine glycosylase (FPG)-sensitive sites were found in the same tissues (Cao et al., 2014). Two intratracheal instillations of 0.5 mg/kg bw of SWCNT at an interval of 24 hours did not increase the level of DNA strand breaks or FPG-sensitive sites in mice 2 hours after the last injection (Vesterdal et al., 2014a). No difference in the levels of DNA strand breaks was observed in the lung tissues of rats after intratracheal instillation of a single dose of 0.2 or 1 mg/kg bw or of 0.04 or 0.2 mg/kg bw once per week for 5 weeks of MWCNT (Ema et al., 2013a) or SWCNT (Naya et al., 2012). Increased immunostaining of 8-oxodG was seen in the lung tissues of mice exposed to SWCNT by intratracheal instillation of 50 µg/mouse per week for 6 weeks (Inoue et al., 2010).
Intraperitoneal injection of 0.25–0.75 mg/kg bw of MWCNT once per day for 5 days resulted in increased levels of DNA strand breaks in the peripheral blood leukocytes of mice 24 hours after the last exposure (Patlolla et al., 2010). A single intraperitoneal injection of 2–10 mg/kg bw of MWCNT was also associated with an increased level of DNA strand breaks in the bone marrow cells of mice 3 hours after exposure (Ghosh et al., 2011).
Gastrointestinal exposure by gavage to 0.064 or 0.64 mg/kg bw of SWCNT in either saline suspension or corn oil was associated with increased levels of 8-oxodG in the liver and lung tissues of rats, whereas the same doses did not affect the level of 8-oxodG in colon mucosa cells (Folkmann et al., 2009).
(ii) Gene mutation
See Table 4.5
Inhalation exposure to 5 mg/m3 of SWCNT for 5 hours per day for 4 days enhanced mutation of the proto-oncogene K-ras in the lung of C57BL/6 mice. Mutations were found 1 day after the end of inhalation and progressed at 28 days (compared with sham-exposed controls, P = 0.045), but mutations were not increased after a single pharyngeal aspiration of 5–20 μg/mouse (Shvedova et al., 2008). One year after exposure, karyotypic changes were shown by micronuclei and multinucleated cells in type II pneumocytes (Shvedova et al., 2014). A study of the intratracheal instillation of 0.2 mg/mouse of MWCNT once per week for 4 weeks showed enhanced guanine phosphoribosyltransferase Gpt gene mutation frequencies in the lungs (Kato et al., 2013).
(iii) Chromosomal alterations
Table 4.6 lists the studies that have assessed chromosomal alterations (micronucleus formation and chromosomal aberration) in rodents after exposure to CNT.
Table 4.6
Studies of micronucleus frequency and chromosomal aberrations in cells of experimental animals exposed to carbon nanotubes in vivo.
Only one investigation examined CNT-induced chromosomal aberrations in rodents. In this study, Swiss-Webster mice (age, 6 weeks) received intraperitoneal injections of 0.25–0.75 mg/kg bw of native and acid-washed MWCNT (diameter, 12 nm; length, < 12 µm) once per day for 5 days. The bone marrow cells were prepared for cytogenetic analysis 24 hours after the exposure, which was associated with a dose-dependent increase in the levels of chromosome gaps, chromatid and isochromatid breaks, fragments, and structural rearrangements, including centromeric fusions and dicentric chromosomes (Patlolla et al., 2010).
Studies on the formation of micronuclei in experimental animals have mainly explored effects after non-pulmonary exposures, although one study in Wistar rats showed an increased frequency of micronuclei in type II pneumocytes isolated 3 days after intratracheal instillation of 0.5–2 mg/rat of MWCNT (Muller et al., 2008b). Oral exposure to 60–200 or 5–20 mg/kg bw of SWCNT once per day for 2 days did not affect the frequency of micronucleated polychromatic or immature erythrocytes in the bone marrow cells of ICR or CD-1 mice (Naya et al., 2011; Ema et al., 2013b). Intraperitoneal injection of 0.25–0.75 mg/kg bw of MWCNT once per day for 5 days was associated with an increased frequency of micronuclei in bone marrow cells in one study in Swiss-Webster mice (Patlolla et al., 2010). Intraperitoneal injection of 2–10 mg/kg bw of MWCNT in Swiss albino mice increased the frequency of micronuclei in bone marrow cells, whereas the percentage of polychromatic erythrocytes was unaltered (Ghosh et al., 2011). Another study showed no increase in the frequency of micronuclei and no alteration in the frequency of polychromatic erythrocytes in the bone marrow cells of ICR mice after a single intraperitoneal injection of 12.5–50 mg/kg bw of MWCNT (Kim et al., 2011).
(b) In vitro
(i) DNA damage
Studies that have assessed the levels of DNA damage in cell cultures after exposure to CNT are presented in Table 4.7. The neutral version of the comet assay showed unaltered levels of double-strand breaks in human alveolar basal epithelial adenocarcinoma A549 cells after exposure to MWCNT (Ju et al., 2014).
Table 4.7
Studies of DNA damage and mutation in experimental systems after exposure to carbon nanotubes in vitro.
Several studies have documented that exposure to SWCNT or MWCNT increased the levels of DNA strand breaks in human colon carcinoma tissue HT29 cells (Pelka et al., 2013), bronchial epithelial BEAS-2B cells (Lindberg et al., 2009, 2013), lung adenocarcinoma A549 cells (Karlsson et al., 2008; Cavallo et al., 2012), mesothelial cells (Pacurari et al., 2008b; Lindberg et al., 2013), human gingival fibroblasts (Cicchetti et al., 2011), Chinese hamster V79 fibroblasts and primary mouse embryo fibroblasts (Kisin et al., 2007, 2011; Yang et al., 2009), human lymphocytes (Ghosh et al., 2011), phytohaemagglutinin-stimulated human lymphocytes (Kim & Yu, 2014), murine macrophages (Migliore et al., 2010; Di Giorgio et al., 2011; Aldieri et al., 2013), human and rat kidney epithelial cells (Barillet et al., 2010; Kermanizadeh et al., 2013), and human hepatocytes (Kermanizadeh et al., 2012; Alarifi et al., 2014; Vesterdal et al., 2014b). Increased levels of DNA strand breaks were also observed in rat aortic endothelial and human lung adenocarcinoma A549 cells after exposure to CNT, but the statistical analysis appeared to have been based on the total number of comets from a single experiment rather than the mean values from independent experiments (Yamashita et al., 2010; Cheng et al., 2012). However, another study used all comets in the statistical analysis and showed no alteration in DNA strand breaks in human peripheral lymphocytes exposed to SWCNT (Zeni et al., 2008). [The Working Group noted the uncertainty that replicates were independent experiments.] Other studies have shown no alterations in the levels of DNA strand breaks in human lung adenocarcinoma A549 cells and human HaCaT keratinocytes after exposure to MWCNT (Thurnherr et al., 2011; McShan & Yu, 2014) or in FE1 MML mouse lung epithelial cells exposed to SWCNT (Jacobsen et al., 2008).
The protocol of the alkaline comet assay that measures DNA strand breaks can be extended using an additional DNA digestion step with DNA repair enzymes from bacterial or human cells. The bacterial enzymes include FPG and endonuclease III (ENDOIII). The FPG enzyme also cleaves DNA at ring-opened formamidopyrimidine lesions, including 2,6-diamino-4-hydroxy-5-formamidopyrimidine and 4,6-diamino-5-formamidopyrimidine. ENDOIII lesions comprise oxidized pyrimidines, such as uracil glycol, thymine glycol, 5-hydroxycytosine, and 5-hydroxyuracil. Results from these enzyme-modified comet assay measurements have been reported either as total sites (DNA strand breaks plus extra breaks generated by the enzyme) or enzyme-sensitive sites (breaks generated by the enzyme minus the basal level of DNA strand breaks).
Exposure to SWCNT increased the level of FPG-sensitive sites in FE1-MML mouse lung epithelial and human hepatoblastoma HepG2 cells (Jacobsen et al., 2008; Vesterdal et al., 2014b). Both SWCNT and MWCNT increased the level of ENDOIII- and FPG-sensitive sites in rat RAW 264.7 macrophages (Migliore et al., 2010), but the levels of FPG-sensitive sites in human lung adenocarcinoma A549 cells were unaltered after exposure to MWCNT, although the validity of this observation is questionable due to the lack of a positive control (Cavallo et al., 2012). Another study also found unaltered levels of FPG-sensitive sites in A549 cells after exposure to MWCNT (Karlsson et al., 2008). [The Working Group noted that the increased levels of FPG-sensitive sites were observed in cells after exposure to zinc oxide and copper oxide nanoparticles, indicating a reliable methodology for measuring oxidative damage to DNA.]
Exposure of human colon carcinoma cells to SWCNT did not increase the level of extra FPG sites (Pelka et al., 2013). Exposure to two different types of MWCNT increased the levels of total sites in human hepatoblastoma cells after treatment with FPG, whereas the FPG-modified assay generated fewer lesions compared with DNA strand breaks with one type of MWCNT (NM 402) but not with the other (NM 400) (Kermanizadeh et al., 2012). The same authors also showed increased levels of total sites in renal cells exposed to the same types of MWCNT, but the net level of FPG-sensitive sites did not appear to differ between exposed and unexposed cells (Kermanizadeh et al., 2013), indicating that the exposure to MWCNT was not associated with specific oxidative damage to DNA nucleobases but did seem to generate DNA strand breaks. Exposure of human HaCaT keratinocytes to MWCNT was associated with increased levels of total FPG sites (McShan & Yu, 2014).
Increased levels of lipid peroxidation product-derived 3-(2′-deoxy-β-D-erythropentofuranosyl)pyrimido[1,2-α]-purin-10(3H)-one] adducts [M1dG or N1N2malondialdehyde-2′-deoxyguanosine] were detected by immunoblot in human bronchial epithelial BEAS-2B and human pleural mesothelial Met-5A cells, after 48 hours of exposure to SWCNT but decreased levels 72 hours after exposure (Lindberg et al., 2013). No increase in the levels of 8-oxodG, measured by high-performance liquid chromatography with electrochemical detection, were observed in human pleural mesothelial Met-5A cells after exposure to SWCNT or MWCNT (Ogasawara et al., 2012). [The Working Group noted the uncertainty that replicates were independent experiments.] However, the baseline level of 8-oxodG (8 lesions/106 dG) was high, indicating spurious oxidation of DNA during the processing or analysis of samples (Ogasawara et al., 2012). [In keeping with the recommendations of the European Committee on Oxidative DNA Damage, reports with baseline levels of 8-oxodG higher than 5 lesions/106 dG in unexposed cells or animals should be interpreted with caution because of the risk of flawed methodology (ESCODD, 2003).] One study investigated oxidative damage to DNA in cells using antibody-based techniques, and showed increased levels of 8-oxodG by immunostaining in chicken lymphoid cells after exposure to MWCNT (Mohiuddin et al., 2014).
(ii) Gene mutations
Exposure of mouse embryonic stem cells to MWCNT increased the mutation frequency in the adenine phosphoribosyltransferase (Aprt) gene (Zhu et al. 2007). However, mutation frequency in the hypoxanthine-guanine phosphoribosyltransferase (Hgprt) gene was unaltered after Chinese hamster lung cells were exposed to MWCNT (Asakura et al., 2010). Increased levels of mutations in the Hgprt gene were observed in human lymphoblastic MCL-5 cells after exposure to SWCNT with a length of 1–3 µm, whereas shorter (0.4–0.8 µm) and longer (5–30 µm) nanotubes were not associated with mutagenicity (Manshian et al., 2013). Long-term exposure (24 days) of FE1-MutaTMMouse lung epithelial cells to SWCNT (length < 1 µm) did not increase the frequency of mutation in the cII gene (Jacobsen et al., 2008).
(iii) Micronucleus formation
The results on the induction of micronuclei in cultured cells after exposure to CNT have been conflicting. No difference between the distribution of studies showing an increased formation of micronuclei or a null effect was apparent, with regard to the use of the cytokinesis-block micronucleus protocol or other protocols to score micronuclei. Specific assay protocols have therefore not been highlighted in the descriptions of the findings in cell cultures. Table 4.8 lists the studies that have assessed chromosomal alterations in cell cultures after exposure to CNT.
Table 4.8
Studies of micronucleus frequency, chromosomal aberrations, and sister-chromatid exchange in experimental systems after exposure to carbon nanotubes in vitro.
The assessment of micronucleus frequency in human lymphocytes after exposure to six different types of MWCNT did not show a monotonic concentration–response relationship, although one sample with a short fibre length (0.4 μm) gave statistically significant results at all concentrations tested and one other sample yielded increased micronucleus frequencies at a low concentration of 2.5 μg/mL. The diameter and length of the tubes could not explain the observed results and other structural differences, including surface area and transition metal content, might be implicated (Tavares et al., 2014). Observations from cultured lymphocytes indicated no effect on micronucleus formation after exposure to MWCNT (Szendi & Varga, 2008), whereas both MWCNT and SWCNT increased the frequency of micronuclei in another study in lymphocytes (Cveticanin et al., 2010). Exposure to SWCNT was also associated with an increased frequency of micronuclei in phytohaemagglutinin-stimulated human lymphocytes (Kim & Yu, 2014). Increased frequencies of micronuclei (Kato et al., 2013) or no increase in micronuclei (Thurnherr et al., 2011) were observed in human lung adenocarcinoma A549 cells after exposure to MWCNT. Exposure of human immortalized bronchial epithelial BEAS-2B cells to SWCNT yielded either a null effect (Lindberg et al., 2009, 2013) or an increased frequency of micronuclei (Manshian et al., 2013). Similarly, hamster lung V79 fibroblasts responded with unaltered micronucleus frequency (Kisin et al., 2007; Pelka et al., 2013) or increased micronucleus frequency (Asakura et al., 2010; Cicchetti et al., 2011; Kisin et al., 2011) after exposure to either SWCNT or MWCNT. Increased micronucleus frequencies were also observed in human breast epithelial MCF-7 and lung adenocarcinoma A549 cells, rat lung epithelial cells, mouse RAW 264.7 macrophages, and human B-lymphoblastoid MCL-5 cells after exposure to either MWCNT or SWCNT (Muller et al., 2008b; Di Giorgio et al., 2011; Kato et al., 2013; Manshian et al., 2013). A sample of MWCNT with a relatively short fibre length (0.7 µm) and low transition metal content (iron, 0.48%; cobalt, 0.49%) was used to study the impact of structural defects and metals content on the formation of micronuclei in rat lung epithelial cells. Ground MWCNT (producing structural defects) increased the micronucleus frequency, whereas heated (2400 °C) ground MWCNT (which ablates the structural defects and eliminates metals) did not (Muller et al., 2008a).
[Collectively, cell culture studies document the ability of MWCNT and SWCNT to increase the frequency of micronuclei in proliferating cells, although substantial differences in effects were seen between studies, possibly originating from differences in cell types, characteristics of the CNT, dispersion protocols, and assay conditions.]
(iv) Chromosomal aberrations
Table 4.8 lists in-vitro investigations in which established cell lines were exposed to SWCNT and MWCNT.
Increased chromosome breakage and aneuploid cells were demonstrated in mouse macrophage RAW 264.7 cell lines, Chinese hamster lung (CHL/IU) cell lines, primary human respiratory epithelial SAEC cell lines, and human bronchial epithelial BEAS-2B cells (Sargent et al., 2009, 2012a; Asakura et al., 2010; Di Giorgio et al., 2011; Siegrist et al., 2014). Other investigations in immortalized Chinese hamster lung fibroblasts and Chinese hamster ovary cells did not show increased aneuploidy or chromosomal aberrations after exposure to SWCNT (Naya et al., 2011; Ema et al., 2013b) or MWCNT (Kim et al., 2011). Both SWCNT and MWCNT increased chromosome and chromatid breakage in phytohaemagglutinin-stimulated human lymphocytes (Catalán et al., 2012).
Chromosome breakage and translocations between chromosomes were observed in an immortalized mouse macrophage RAW 264.7 cell line after exposure to 10 µg/mL of SWCNT or MWCNT. The modal number of the macrophage cell line karyotype was 40 chromosomes; however, the mean number of chromosomes per cell after exposure to either SWCNT or MWCNT was 20–60 with no distinct modal number, indicating a high degree of aneuploidy in the original cell line (Di Giorgio et al., 2011). Asakura et al. (2010) demonstrated an 8–34-fold increase in polyploidy in Chinese hamster lung cells treated with MWCNT (diameter, 88 nm; length, 5 µm). The authors of both studies attributed the increase in polyploid cells to a failure of cytokinesis (Asakura et al., 2010; Di Giorgio et al., 2011).
Chromosome breakage and errors in chromosome number were observed in cultured primary human respiratory epithelial cells after exposure to either SWCNT or MWCNT determined by analysis of chromosomes spreads or fluorescence in situ hybridization. The analysis of cultured primary human respiratory cells exposed to SWCNT demonstrated significantly increased aneuploidy, which was due to an equal number of gains and losses of chromosomes, while MWCNT-exposed cells had a significantly greater number of chromosomal gains than losses, indicating polyploidy (Sargent et al., 2009, 2012a, b; Siegrist et al., 2014).
[Collectively, in-vitro investigations in immortalized and primary cells documented the ability of CNT to increase the frequency of chromosomal damage and aneuploidy in proliferating cells. Similar to the results from studies of micronucleus frequency after exposure to CNT, substantial effect differences between studies were found, possibly originating from differences in cell types, characteristics of CNT, dispersion protocols, and assay conditions.]
(v) Alterations in the mitotic spindle, cell cycle, and sister-chromatid exchange
The data that demonstrated chromosomal damage and errors in chromosomes after in-vitro exposure to either SWCNT or MWCNT (see Table 4.8) suggested an alteration in the integrity of the mitotic spindle, which was investigated by exposure to SWCNT (diameter, 1.0 nm) or MWCNT (diameter, 10–20 nm). The exposure to 1-nm SWCNT resulted in mitotic spindles with multiple poles (Sargent et al., 2009, 2012a), while cells treated with 10–20-nm MWCNT had mitotic spindles with one pole (Sargent et al., 2012b; Siegrist et al., 2014). Three-dimensional reconstructions of 0.1-μm optical sections showed CNT integrated with microtubules, DNA and within the centrosome structure. Further analysis by confocal microscopy and TEM demonstrated fragmented centrosomes after exposure to either SWCNT or MWCNT (Sargent et al., 2009, 2012a, b; Siegrist et al., 2014). The mitotic disruption associated with SWCNT treatment resulted in a G2/M block in the cell cycle while MWCNT treatment was associated with a block in G1/S (Sargent et al., 2009, 2012b; Siegrist et al., 2014). [When mammalian cells are exposed to agents that cause a block in the S-phase, the DNA is repaired by homologous recombination. The increased recombination between sister chromatids can be observed by the incorporation of 5-bromodeoxyuridine. The increase in sister-chromatid exchange suggests genotoxicity (Pfuhler et al., 2013).] The observation of increased sister-chromatid exchange in Chinese hamster ovary AA8 cells after exposure to MWCNT 90 nm in diameter further suggests a block in the S-phase (Kato et al., 2013).
[To date, four studies have shown CNT-induced mitotic spindle and cell-cycle disruption and three investigations demonstrated CNT-mediated centrosome disruption. These investigations documented the ability of CNT to disrupt the mitotic spindle, fragment the centrosome, and cause a block in the cell cycle in cultured cells.]
(vi) Mutation in bacteria
See Table 4.9
Table 4.9
Studies of mutation in bacteria exposed to carbon nanotubes.
The mutagenic effect of MWCNT was evaluated in the bacterial reverse mutation assay (Ames test) in Salmonella typhimurium TA98 and TA100 and in Escherichia coli WP2uvrA in the presence and in absence of a metabolic activation system. MWCNT did not produce mutagenic effects at any concentration tested. In S. typhimurium TA98 in absence of metabolic activation, a reduction in the number of spontaneous revertant colonies was observed at concentrations ranging from 0.13 to 9.0 μg/plate, which was not concentration-dependent. In this bacterial strain, spontaneous mutational DNA damage is reverted to wild-type by specific mechanisms of frameshift (Di Sotto et al., 2009).
Kim et al. (2011) studied high-aspect-ratio (diameter, 10–15 nm; length, ≈10 nm) and low-aspect-ratio (diameter, 10–15 nm; length, ≈150 nm) MWCNT. Neither the high- nor the low-aspect-ratio MWCNT induced genotoxicity in the bacterial reverse mutation test in Salmonella typhimurium TA98, TA100, TA1535 and TA1537, and in Escherichia coli WP2uvrA in the presence and in absence of a metabolic activation system.
4.4. Other mechanisms of carcinogenesis
No published studies concerning the health effects of CNT in exposed humans were available to the Working Group. The body of relevant literature primarily comprises in-vivo studies in experimental animals, in-vitro studies using human cell lines, and a limited number of studies of occupational exposure (see Section 1, Table 1.5).
There were no studies in humans exposed to CNT only. However, four studies have been published in which several biological end-points of an occupational cohort (in Taiwan, China) exposed to engineered nanomaterials (n = 241) were compared with those of an unexposed control group (n = 196). Among the population exposed to engineered nanomaterials, a subgroup of workers (n = 57) was exposed to CNT originating from three facilities that used CNT and one facility that used and produced CNT. [The Working Group noted that the number of subjects in each individual study varied, probably due to missing data on specific end-points or follow-up. With the exception of the study describing the results on fractional exhaled nitric oxide, the studies did not report results separately for the CNT-exposed population.] Wu et al. (2014) described an increase in fractional exhaled nitric oxide in workers exposed to nanomaterials, that was limited to the population exposed to titanium dioxide (β = 0.351, SE = 0.166; P = 0.035). Results among CNT-exposed workers (n = 57) were null (β = 0.045, SE = 0.124; P = 0.715). Liou et al. (2012) studied approximately the same population and measured antioxidant enzyme activities, markers of inflammation and oxidative damage, cardiovascular biomarkers, genotoxicity, lung function, and neurobehavioural functions. In a cross-sectional evaluation, associations were found with significantly lower antioxidant enzyme activity (i.e. superoxide dismutase [SOD]), elevated markers of cardiovascular disease (i.e. fibrinogen and intercellular adhesion molecule-1), and reduced neurobehavioural function. No specific analyses for the CNT-exposed population (n = 52) were presented. In a longitudinal analysis of a subpopulation of the same population with a follow-up of 6 months, Liao et al. (2014a) reported a significant association between exposure to engineered nanomaterials and an increase in antioxidant enzymes (SOD and GSH peroxidase [GPx]) and cardiovascular markers (vascular cell adhesion molecule and paraoxonase) among exposed workers compared with control workers over the follow-up period. The results were not presented by subtype of engineered nanomaterials. Liao et al. (2014b) studied the same exposed population for work-related symptoms and diseases and reported a significant worsening of allergic dermatitis among workers exposed to engineered nanomaterial. No specific results for workers exposed to CNT were presented.
[The Working Group noted that the exposure assessments of Liao et al. (2014a, b), Liou et al. (2012), and Wu et al. (2014) were based on the control-banding approach of Paik et al. (2008). The exposure scores were based on both an estimate of nano-toxicity and the expected probability of exposure. The selection of controls for the above studies was not clearly described, although confounding factors did not seem to differ between the exposed and unexposed workers except for gender and level of education.]
4.4.1. Inflammasome activation
(a) Human cells in vitro
An interlaboratory validation study confirmed the extracellular release of IL-1β from the human THP-1 macrophage cell line exposed to as-produced MWCNT (Cheap Tubes Inc., Brattleboro, VT, USA) at non-cytotoxic doses (Xia et al., 2013). A comprehensive analysis of surface functionalization of the as-produced MWCNT (Li et al., 2013), and the dispersion of MWCNT in bovine serum albumin or by the triblock copolymer Pluronic F108 (Wang et al., 2012a) showed that surface charge, chemical functionalization, and dispersal state were important determinants of inflammasome activation and release of IL-1β from THP-1 macrophages. Anionic functionalization (carboxylate or PEG) decreased, cationic functionalization (polyetherimide) increased (Li et al., 2013), and dispersion (using Pluronic F108) prevented (Wang et al., 2012a) the release of IL-1β from THP-1 macrophages .
Platelet-derived growth factor (PDGF), in combination with transforming growth factor (TGF)-β, activates the “epithelial-mesenchymal trophic unit” in the lungs resulting in collagen deposition and fibrosis (reviewed in Bonner et al., 2013). These reciprocal interactions of cytokines and growth factors, initiated by inflammasome activation and the release of IL-1β from macrophages, were repeated in transwell co-cultures of human THP-1 macrophages and immortalized human BEAS-2B lung epithelial cells exposed to as-produced or cationic functionalized MWCNT (Wang et al., 2012a; Li et al., 2013).
Hamilton et al. (2013b) used the same as-produced MWCNT with variable diameters and lengths to assess the role of dimensions in the release of IL-1β from human THP-1 macrophages or murine primary alveolar macrophages. MWCNT with a greater diameter (30–50 nm) or length (10–30 μm) were more potent in inducing the release of IL-1β than shorter (length < 2 μm) or thinner (diameter, 10–20 nm) MWCNT. These investigators also studied a panel of nine MWCNT (including the as-produced sample from Cheap Tubes) and compared their potency for inflammasome activation and IL-1β and IL-18 release in primary murine alveolar macrophages co-stimulated with 20 ng/mL of lipopolysaccharide (LPS). Linear regression analysis demonstrated a significant correlation between the nickel content of the MWCNT samples and the release of IL-1β (Hamilton et al., 2012). Removal of nickel contaminants from the as-produced MWCNT sample slightly decreased the release of IL-1β in this in-vitro assay (Hamilton et al., 2013a). Haniu et al. (2011) confirmed that another commercial MWCNT sample (VGCF; Showa Denko, Tokyo, Japan) also induced IL-1β as well as the release of tumour necrosis factor (TNF)-α from human THP-1 macrophages. Inflammasome activation and IL-1β and IL-18 release was also induced by CNT (80% DWCNT; DWCNT, 0.1–100 μm in length) synthesized by CVD in human peripheral blood monocytes primed with LPS (Meunier et al., 2012).
(b) Experimental systems in vivo
See Table 4.10
Table 4.10
Studies of persistent inflammation, granulomatosis, and fibrosis in experimental animals after exposure to carbon nanotubes.
(i) Inhalation
Three 13-week studies of two MWCNT and one CNF in Wistar rats showed that exposure to MWCNT induced persistent inflammation. In the studies of Ma-Hock et al. (2009) and Pauluhn (2010b), these inflammatory responses to MWCNT were observed in the lungs of males and females. The response to CNF was observed at high concentration (Delorme et al., 2012). The minimum concentrations that induced persistent or moderate inflammation were 2.5 mg/m3 and 1.5 mg/m3 of MWCNT and 25 mg/m3 of CNF (Ma-Hock et al., 2009; Pauluhn, 2010b).
Histopathological analysis of the 13-week inhalation study of MWCNT of Ma-Hock et al. (2009) demonstrated focal granuloma formation and the accumulation of subpleural cells in a dose-dependent manner. Masson’s stain established the presence of collagen within the sites of granuloma formation, while a reticulin fibre index further confirmed a dose-dependent increase in collagen within the alveolar walls (Treumann et al., 2013).
Two 4-week studies of SWCNT and MWCNT in Wistar rats showed evidence of transient (not persistent) inflammation. The maximum concentration that did not induce significant inflammation was 0.13 mg/m3 of SWCNT and 0.37 mg/m3 of MWCNT (Morimoto et al., 2012a, b).
A 4-week study of MWCNT (length, 330.18 nm; diameter, 10–15 nm) in Sprague-Dawley rats with a 90-day recovery period showed no statistically significant difference in the levels of inflammatory cytokines, bronchoalveolar cell distribution, or markers in the BALF or in histopathology (Kim et al., 2014).
Exposure to MWCNT for 6 hours provided evidence of persistent inflammation at high concentrations in male rats (Ellinger-Ziegelbauer & Pauluhn, 2009).
Kasai et al. (2015) reported a dose-independent increase in inflammatory parameters after exposure of male rats to a lower dose of MWCNT (0.2 mg/m3). In male mice, subpleural fibrosis increased 2 and 6 weeks after inhalation of MWCNT (Ryman-Rasmussen et al., 2009a).
(ii) Intratracheal instillation
Fifteen studies of intratracheal instillation in rats and pharyngeal aspiration in mice have been reported (Lam et al., 2004; Warheit et al., 2004; Muller et al., 2005, 2008b; Shvedova et al., 2005; Aiso et al., 2010; Cesta et al., 2010; Han et al., 2010; Kobayashi et al., 2010, 2011; Mercer et al., 2011; Porter et al., 2010; Morimoto et al., 2012b, c; Murray et al., 2012; Sager et al., 2013; Fujita et al., 2015), most of which revealed that exposure to SWCNT and MWCNT led to persistent inflammation in the lung. In contrast, several studies in rats and mice revealed that exposure to SWCNT and MWCNT led to transient responses in the lung. [From the above studies, the Working Group considered that the pulmonary responses of rats and mice to SWCNT and MWCNT did not differ significantly.]
(c) Experimental systems in vitro
CNT, as well as asbestos fibres and poorly soluble crystalline particles (IARC, 2012), have been shown to induce inflammation, as assessed by the release of pro-inflammatory mediators (reviewed in Boyles et al., 2014). Two hypotheses have been proposed for the pro-inflammatory effects of high-aspect-ratio nanoparticles, including CNT and asbestos fibres: (1) frustrated phagocytosis; and (2) inflammasome activation (see Fig. 4.1). Frustrated phagocytosis is elicited in response to high-aspect-ratio, fibrous nanoparticles longer than ~15 μm that cannot be completely phagocytized by macrophages, resulting in their impaired clearance from the lungs and pleural linings and persistent inflammation accompanied by the prolonged release of ROS, pro-inflammatory mediators, and proteases (Johnston et al., 2010). Inflammasome activation triggered by lysosomal damage after the phagocytosis of crystalline minerals (e.g. silica, asbestos fibres) or CNT is the second mechanism that leads to the secretion of the pro-inflammatory mediators, IL-1β and IL-18 (Biswas et al., 2011; Palomäki et al., 2011). These two mechanisms are not exclusive and Hamilton et al. (2009) proposed that all high-aspect-ratio nanomaterials can induce frustrated phagocytosis and inflammasome activation similarly to asbestos fibres. The experimental evidence for inflammasome activation and the release of pro-inflammatory mediators based on in-vivo and in-vitro studies is summarized below.
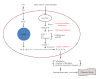
Fig. 4.1
Inflammasome activation and inflammation induced by nanoparticles, including carbon nanotubes
Functionalized SWCNT produced by the HiPCO procedure (Unidym Inc., Sunnyvale, CA, USA) were evaluated for inflammasome activation and the release of IL-1β in LPS-primed, immortalized, bone marrow-derived murine macrophages (Yang et al., 2013). Oxidized SWCNT increased the release of IL-1β while benzoic acid functionalization decreased the release of pro-inflammatory cytokines.
See also Hamilton et al. (2013a); discussed in Section 4.4.1 (b).
4.4.2. Release of cytokines, chemokines, and growth factors
(a) Exposed humans
Markers of inflammation and oxidative stress were monitored among workers handling engineered nanomaterials (Liao et al., 2014a, b). No effects were reported for IL-6 and IL-6 receptors, but depression of antioxidant enzymes was found among these workers (Liou et al., 2012).
(b) Human cells in vitro
A panel of well-characterized MWCNT was investigated for their ability to stimulate cytokine release from human THP-1 macrophages and the immortalized human mesothelial Met-5A cell line. Only long CNT samples (mean length, 13–36 µm) at a sublethal dose of 5 μg/mL induced the release of TNF-α, IL-1β, and IL-6 from macrophages but not from mesothelial cells after 24 hours (Murphy et al., 2012). Immortalized human lung epithelial BEAS-2B cells were exposed to 30 µg/mL of highly purified (10 minutes at 37 °C) MWCNT (HTT2800 (see Haniu et al., 2010); diameter, 100–150 nm; length, 10–20 μm) and assessed for the release of TNF-α, IL-1β, IL-6, IL-8, IL-10, and IL-12 after 24 hours; only the release of IL-6 and IL-8 was detected (Tsukahara & Haniu, 2011). A dynamic cell growth model designed to mimic expansion and contraction during normal breathing was established using human lung adenocarcinoma A549 cells. Two samples of SWCNT (Cheap Tubes) were tested for the release of the cytokine, IL-8: a short CNT (diameter, 1–2 nm; length, 0.5–2 μm) and a long CNT (diameter, 1–2 nm; length, 5–30 μm). Only the long CNT induced the release of IL-8 after 24–72 hours and the levels were significantly higher in the dynamic cell growth model compared with static growth conditions (Patel & Kwon, 2013). A triple co-culture model of human lung epithelial 16HBE14o cells, primary human blood-derived dendritic cells, and primary human blood-derived macrophages in transwell cultures was used to evaluate the release of TNF-α and IL-8 after exposure to MWCNT (3 or 30 µg/mL) for 24 hours. As-produced and carboxylated MWCNT synthesized by CVD (Chengdu Carbon Nanomaterials R&D Center, Sichuan, China) were pre-coated with Curosurf 120 (porcine lung surfactant). Pre-coated, as-produced, and carboxylated MWCNT elicited the release of both TNF-α and IL-8 in this model system (Gasser et al., 2012). Transwell co-cultures of human THP-1 macrophages and immortalized human lung epithelial BEAS-2B cells exposed to MWCNT released the profibrotic mediators, TGF-β1 and PDGF (Wang et al., 2012a; Li et al., 2013).
[The use of in-vitro human or animal cell systems does not represent physiological routes of exposure for humans. The doses used in the in-vitro studies should be relevant to those to which humans are exposed, and the doses used in in-vitro studies may lead to mechanisms that differ from those that arise from the actual exposure concentrations of humans. Thus, interpretation of the data, including those on cytotoxicity and mechanisms, obtained from in-vitro studies should be evaluated cautiously.]
(c) Experimental systems in vivo
Several studies have investigated the pulmonary effects of CNT after inhalation, intratracheal or intranasal instillation, and pharyngeal aspiration. The results of these studies are summarized and the characteristics of the CNT investigated are described in Table 4.11.
Table 4.11
Studies of pulmonary effects in experimental animals exposed to carbon nanotubes in vivo.
Inflammatory responses were assessed after the inhalation of MWCNT (Ma-Hock et al., 2009; Kim et al., 2014; Kasai et al., 2015). Ma-Hock et al. (2009) performed a 5-day range-finding inhalation study to select test concentrations for a 90-day inhalation toxicity study with MWCNT (Nanocyl NC 7000). Groups of male Wistar rats were exposed by head/nose-only inhalation to an aerosol of MWCNT dust for 6 hours per day on 5 consecutive days at target concentrations of 0, 2, 8, and 32 mg/m3. Treatment-related increases in BALF total cell counts (due to a significant increase in polymorphonuclear neutrophils), total protein content, and enzyme activities were observed in all treated groups 3 days after the last exposure. At the end of the 24-day recovery period, the same pattern of BALF findings was found. In the animals exposed to 2 mg/m3 (the lowest concentration), slight recovery was observed; protein content and N-acetyl glucosaminidase activity returned to control levels, but the other parameters were still significantly increased. Kasai et al. (2015) conducted a 13-week study in male and female Fischer 344 rats exposed by whole-body inhalation to MWCNT (Hodogaya Chemical Co., Ltd, Tokyo, Japan) at concentrations of 0, 0.2, 1, and 5 mg/m3 using a generation and exposure system based on the cyclone sieve method. In the BALF analyses, inflammatory parameters were increased in a concentration-dependent manner in both sexes from the lowest dose upwards (Kasai et al., 2015). A 4-week inhalation study of MWCNT (length, 330.18 nm; diameter, 10–15 nm) in Sprague-Dawley rats with a recovery period of up to 90 days showed that the levels of inflammatory cytokines (TNF-α, TGF-β, IL-1, IL-2, IL-4, IL-5, IL-10, IL-12, and interferon (IFN)-γ) and inflammatory proteins (albumin, total protein, and LDH) in the BALF did not differ significantly (Kim et al., 2014). No local pulmonary effects were observed in C57BL/6 mice exposed to a mixture of MWCNT and graphitic nanofibres (Mitchell et al., 2007).
Intratracheal or intranasal instillation and pharyngeal aspiration are not physiological routes of exposure for humans but have been used in mice and rats to investigate the potential pulmonary and systemic toxicity of high concentrations of CNT.
Biological responses to MWCNT (Mitsui & Co. Ltd) were assessed in male rats after a single intratracheal instillation (0.04–1 mg/kg bw) (Kobayashi et al., 2010). Transient pulmonary inflammatory responses were observed in the lungs of the rats exposed to 1 mg/kg bw of MWCNT. However, the levels of cytokines in BALF did not change significantly at any time-point (3, 7, 28, or 91 days after exposure).
Pulmonary and systemic responses were assessed in male rats after the intratracheal instillation of highly pure, well dispersed, and well-characterized SWCNT. The numbers of BALF inflammatory cells (neutrophils, macrophages, lymphocytes, and eosinophils) were increased in a dose-dependent manner. LDH values and the protein contents in BALF were significantly greater in the groups exposed to doses of SWCNT of 0.2 mg/kg bw and higher compared with those in the control group up to 3 months after instillation. Only small differences were observed between the SWCNT-exposed groups and the control group for the cytokines IL-1α, IL-2, IL-4, IL-10, granulocyte macrophage colony-stimulating factor, IFN-γ, and TNF-α at any of the time-points, but significant increases were observed for IL-1β and IL-6 at several time-points (Kobayashi et al., 2011).
Intratracheal instillation of 0.5 mg of SWCNT into male ICR mice induced alveolar macrophage activation, various chronic inflammatory responses, and severe pulmonary granuloma formation. Affymetrix microarrays were used to investigate the molecular effects on the macrophages exposed to SWCNT. A biological pathway analysis, a literature survey, and experimental validation suggested that the uptake of SWCNT into the macrophages can activate various transcription factors, such as nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1), and that this leads to oxidative stress, the release of pro-inflammatory cytokines, the recruitment of leukocytes, the induction of protective and anti-apoptotic gene expression, and the activation of T-cells. [The resulting innate and adaptive immune responses may explain the chronic pulmonary inflammation and granuloma formation in vivo caused by SWCNT (Chou et al., 2008).]
Pulmonary and systemic immune responses induced by intratracheal instillation of 5, 20, and 50 mg/kg bw of MWCNT (Sigma-Aldrich, St. Louis, MO, USA (Cat. No. 659258)) into male mice were investigated (Park et al., 2009). Total numbers of immune cells in BALF were significantly increased in the treated groups and the distribution of neutrophils was elevated on day 1 after instillation. Pro-inflammatory cytokines (IL-1, TNF-α, IL-6, IL-4, IL-5, IL-10, IL-12, and IFN-γ) were also increased in a dose-dependent manner in both BALF and blood. The highest levels of the cytokines were seen on day 1 after instillation and then decreased. The levels of T-helper (Th) 2-type cytokines (IL-4, IL-5, and IL-10) in the treated group were higher than those of the Th1-type cytokines (IL-12 and IFN-γ).
Male Swiss mice were intranasally instilled with 1.5 mg/kg bw of CNT (DWCNT, 80%; SWCNT, 20%). Local oxidative perturbations were investigated using electronic spin resonance (ESR) spin trapping experiments, and systemic inflammation was assessed by measuring the plasma concentrations of TNF-α, IL-1α, IL-1β, IL-6, insulin-like growth factor 1, leptin, granulocyte-colony stimulating factor, and vascular endothelial growth factor (Crouzier et al., 2010). Examination of the lungs and the elevation of pro-inflammatory cytokines in the plasma (leptin and IL-6 at 6 h) confirmed the induction of an inflammatory response, which was accompanied by a decrease in the local oxidative stress.
A dose–response and time-course study of MWCNT (Mitsui & Co. Ltd) was conducted in male mice exposed by pharyngeal aspiration. Examination of the BALF demonstrated that pulmonary inflammation and damage were dose-dependent and peaked at 7 days after exposure. By 56 days after exposure, markers of pulmonary inflammation and damage began returning to control levels, except in mice exposed to the 40-μg MWCNT dose which still had significantly higher levels than vehicle controls (Porter et al., 2010).
Pharyngeal aspiration of SWCNT (CNI, Inc.) by female C57BL/6 mice elicited unusual pulmonary effects that combined robust but acute inflammation with early-onset, progressive fibrosis and granulomas. A dose-dependent increase in LDH and γ-glutamyl transferase activities was found in the BALF, together with an accumulation of 4-hydroxynonenal [an oxidative biomarker] and the depletion of GSH in the lungs. An early accumulation of neutrophils, followed by lymphocyte and macrophage influx, was accompanied by an early elevation of pro-inflammatory cytokines (TNF-α and IL-1β) followed by fibrogenic TGF-β1 (Shvedova et al., 2005).
Female C57BL/6 mice were maintained on vitamin E-sufficient or vitamin E-deficient diets and were exposed by aspiration to SWCNT (CNI, Inc.) to explore and compare their pulmonary inflammatory reactions. The vitamin E-deficient diet caused a 90-fold depletion of α-tocopherol in the lung tissue and resulted in a significant decline of other antioxidants (reduced GSH and ascorbate) as well as an accumulation of lipid peroxidation products. A greater decrease in pulmonary antioxidants was detected in SWCNT-treated vitamin E-deficient mice compared with controls. The lower levels of antioxidants in vitamin E-deficient mice were associated with a higher sensitivity to SWCNT-induced acute inflammation (increases in the total number of inflammatory cells, the number of polymorphonuclear leukocytes, the release of LDH, total protein content, and the levels of pro-inflammatory cytokines, TNF-α and IL-6) and enhanced profibrotic responses (elevation of TGF-β and collagen deposition). Exposure to SWCNT also markedly shifted the ratio of cleaved to full-length extracellular SOD (Shvedova et al., 2007).
In female C57BL/6 mice, the inhalation of stable and uniform dispersions of 5 mg/m3 of unpurified SWCNT (CNI, Inc.; iron, 17.7% wt) for 5 hours per day for 4 days was compared with the pharyngeal aspiration of varying doses (5–20 g/mouse) of the same SWCNT. Overall, the outcomes of inhalation exposure to respirable SWCNT were very similar to those of pharyngeal exposure, both of which led to pulmonary toxicity. However, inhalation of SWCNT was more effective than aspiration in causing inflammatory response, oxidative stress, collagen deposition, and fibrosis, as well as mutations at the K-ras gene locus in the lung (Shvedova et al., 2008).
(d) Experimental systems in vitro
No data were available to the Working Group
4.4.3. Immune effects
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
In in-vitro test systems, macrophages and other relevant mammalian cells are frequently used as test cells for nanomaterials because they are primarily responsible for surveillance in the body. However, they are highly reactive with endotoxins and the distinction between the response to endotoxins and that to nanomaterials is difficult to make. Consequently, contamination with endotoxins confounds the result of tests in vitro. A preliminary examination for endotoxins is therefore required to minimize contamination or confirm an insignificant level in the test sample. Their quantification is also important for an adequate interpretation of data obtained in in-vitro biological test systems (ISO, 2010e).
Exposure to CNT may alter innate immune responses by triggering the complement system, the clearance of apoptotic cells by macrophages, and the induction of adaptive immune responses (reviewed by Andersen et al., 2012). Different responses have been reported for as-produced versus functionalized or coated CNT, and deliberate surface modifications have been attempted to enhance biocompatibility for drug delivery applications (Hamad et al., 2010; Moghimi et al., 2010). The complement system is present in the lining fluid of the lung, and inhaled particles and fibres have been shown to induce complement-generating chemotactic activity that correlates with macrophage accumulation in vivo (Warheit et al., 1985, 1988). Direct binding of as-produced CNT to Clq protein, which leads to the classical pathway of complement activation, has been described in some studies while other investigators reported that complement proteins were bound to CNT but were not activated (Ling et al., 2011). The macrophage-mediated clearance of apoptotic cells is important for the regulation of immune responses and the suppression of macrophage function, and may lead to impaired clearance of particle-laden neutrophils from the lungs (Wiethoff et al., 2003) and chronic inflammation (Witasp et al., 2008). Human peripheral blood monocyte-derived macrophages exposed to purified SWCNT (CNI, Inc.) at non-toxic doses impaired the chemotaxis and phagocytosis of apoptotic target cells – human Jurkat T lymphoblastic leukaemia cells (Witasp et al., 2009). In an in-vitro three-dimensional model of granuloma formation, three commercial samples of MWCNT (MWCNT-7, Mitsui & Co. Ltd; other MWCNT, MER Corp., AZ, USA) or crocidolite asbestos fibres (UICC) altered the phenotype with the co-expression of pro-inflammatory (M1) and profibrotic (M2) markers of murine bone marrow-derived macrophages after 7–14 days (Sanchez et al., 2011).
Exposure of innate immune cells or lymphocytes to CNT in vitro may also impair the presentation of antigens and the activation of lymphocytes with variable results depending on the physical properties and surface functionalization of the CNT tested (Andersen et al., 2012). For example, carboxylated MWCNT have been reported to enhance cytokine secretion by purified human peripheral blood lymphocytes and stimulate lymphocyte-mediated tumour cell cytotoxicity (Sun et al., 2011), while amino-functionalized or oxidized MWCNT activated human monocytes and natural killer cells (Delogu et al., 2012). Purified samples (SES Research) of short SWCNT (length, 1–5 μm) and short MWCNT (length, 1–2 μm) caused minimal activation of antigen-presenting cells in vitro in contrast to titanium dioxide (rutile) or zinc nanoparticles (Palomäki et al., 2010).
(c) Experimental systems in vivo
See Table 4.11
[The Working Group noted that, as in the case of in-vitro experiments on immune effects, the number of published in-vivo studies on CNT is too limited to draw any general conclusions, and the wide variation in the CNT used impedes any comparison of the reports from different studies.]
The pulmonary and systemic immune responses of male C57BL/6 mice to the inhalation of MWCNT were assessed (Mitchell et al., 2007). Analysis by TEM revealed that the material used was a mixture of MWCNT and graphitic nanofibres (Lison & Muller, 2008; McDonald & Mitchell, 2008). After whole-body inhalation for 14 days, MWCNT were engulfed by alveolar macrophages and were distributed throughout the lung. However, no increases in inflammatory cell infiltration were found and no inflammation, granuloma formation, fibrosis, or tissue injury occurred up to the highest dose (5.3 mg/m3) tested. Despite the lack of local pulmonary effects, systemic immunity was affected at all concentrations tested. The measurement of immune function in spleen-derived cells showed a suppressed T-cell-dependent antibody response, a decreased proliferation of T-cells after mitogen stimulation, and altered natural killer cell killing. These results were accompanied by increased gene expression of indicators of oxidative stress and altered immune function [nicotinamide adenine dinucleotide phosphate (NADPH) dehydrogenase quinone 1 and IL-10] in the spleen, but not in the lung. Immune suppression persisted for up to 30 days after exposure (Mitchell et al., 2007). A follow-up study investigated the mechanism of the suppressed systemic immune function. Mice exposed to a dose of 1 mg/m3 of MWCNT by whole-body inhalation presented suppressed immune function, which involved the activation of cyclooxygenase enzymes in the spleen in response to a signal from the lung. Inhaled MWCNT were shown to activate the release of TGF-β in the lung, which was postulated to have a direct effect on prostaglandin production in spleen cells, leading to immune suppression. However, to induce this altered systemic immunity, an additional [yet unknown] signalling mechanism from the lung would be necessary because not all observed systemic effects could be explained by this pathway (Mitchell et al., 2009).
Based on the results from these two studies (Mitchell et al., 2007, 2009), Aschberger et al. (2010) concluded that systemic immune effects are related to relatively short-term exposures to MWCNT. [The Working Group noted that the translocation of CNT from the lung does not appear to be necessary for such effects, although further investigation is required to confirm this hypothesis.]
In the study conducted by Park et al. (2009), distributions of B-cells in the spleen and blood were significantly increased on day 1 after intratracheal instillation of MWCNT into ICR mice, indicating that Th2-type cytokines had activated B-cells and caused them to proliferate. Together with the increased number of B-cells, granuloma formation in the lung tissue and the production of immunoglobulin (Ig) E were also observed with an intensity that was dependent on the dose of MWCNT instilled. [The Working Group noted that this study suggested that MWCNT may induce allergic responses in mice through B-cell activation and the production of IgE.]
(d) Experimental systems in vitro
Murine bone marrow-derived dendritic cells exposed to purified SWCNT (CNI, Inc.) in vitro and co-cultured with splenic T lymphocytes suppressed T-cell proliferation (Tkach et al., 2011). SWCNT (Chengdu Organic Chemicals Co., Ltd) also suppressed lymphocyte proliferation in co-cultures of primary murine peritoneal macrophages and T lymphocytes activated by concanavalin A (Dong et al., 2012).
4.4.4. Apoptosis
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
Several mechanisms have been shown to cause cell death in target cells exposed to engineered nanoparticles in vitro, including apoptosis, necrosis, and autophagic cell death (reviewed in Andón & Fadeel, 2013). In general, acute exposure to high concentrations (~30–400 µg/mL) of engineered nanoparticles can cause mitochondrial injury, increased intracellular generation of ROS, impaired adenosine triphosphate synthesis, and lysosomal damage leading to cell death. Therefore, the selection of doses of CNT for short-term in-vitro toxicity testing is problematic. Ideally, the doses should reflect the mass dose retained in workers exposed to CNT expressed as dose per alveolar epithelial cell surface area (Gangwal et al., 2011).
For a lifetime exposure to an airborne concentration of 1 mg/m3 over 45 years, the relevant in-vitro dose would be ~50–70 μg/mL. However, short-term in-vitro toxicity testing is usually conducted after 24 hours of exposure and the equivalent dose for a 24-hour exposure of workers to an airborne concentration of 1 mg/m3 would be ~0.2–0.6 μg/mL, which is two orders of magnitude lower, and doses > 50 μg/mL have been considered to be an “extraordinary high concentration” for use as a bolus dose in short-term in-vitro toxicity assays (Oberdörster, 2012).
The mechanistic pathways leading to cell death vary depending on the dose as well as the physical and chemical characteristics of the nanoparticles and the target cell type (see Section 4.2). Additional caveats in short-term in-vitro toxicity studies include other variables in experimental design (Table 4.12) and the variability and purity of the sample (Table 4.13).
Table 4.12
Limitations of in-vitro assays for nanoparticle-induced toxicity.
Table 4.13
Minimal criteria required for the interpretation of in-vitro nanotoxicology assays.
Various mechanisms have been proposed for the induction of cell death by CNT, including direct membrane damage, intracellular generation of ROS, and destabilization of lysosomal membranes. Thin, rigid MWCNT (Mitsui & Co., Ltd) directly penetrated human mesothelial cells and induced the depletion of adenosine triphosphate and cell death at a dose of 5 μg/cm2 after 4 days (Nagai et al., 2011). In normal and malignant human mesothelial cell lines, exposure to MWCNT prepared by the CVD process (Mitsui & Co., Ltd) induced low levels of intracellular ROS and induced apoptosis at doses ≥ 50 μg/cm2 after 24 hours (Pacurari et al., 2008a, b). A commercial sample of carboxylated SWCNT (Sigma-Aldrich) induced autophagic cell death in the human lung adenocarcinoma A549 cell line at a dose of 1 mg/mL after 24 hours (Liu et al., 2011).
(c) Experimental systems in vivo
After the intratracheal instillation of 0, 1, 10, or 100 μg of MWCNT (dispersed with albumin) into rats, inflammation, apoptosis, fibrosis, respiratory function, and granuloma formation were assessed after 1, 7, 30, 90, and 180 days. The results were obtained by plethysmography, soluble collagen quantification, quantitative real-time polymerase chain reaction (qRT–PCR), luminex measurement of cytokine expression, and histopathological examination. Only evidence of apoptosis of the alveolar macrophages was shown (Elgrabli et al., 2008).
(d) Experimental systems in vitro
High-aspect-ratio fibrous nanomaterials, including MWCNT, have been shown to cause direct plasma membrane penetration and increased permeability in the murine J774.1 (Hirano et al., 2008) and RAW 264 (Shimizu et al., 2013) macrophage cell lines after exposure to ~100 μg/mL. Acid-functionalized SWCNT synthesized by the CVD process (Chengdu Organic Chemicals Co.) induced autophagy and cell death in primary murine peritoneal macrophages at doses of 10–50 μg/mL after 24 hours (Wan et al., 2013).
4.4.5. Activation of intracellular signalling pathways
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
In addition to inflammasome activation, other intracellular signalling pathways have been shown to be activated by the exposure of macrophages in vitro to CNT.
In human THP-1 macrophages, MWCNT (XNRI, Bussan Nanotech Research, Ibaraki, Japan) at a dose of 10 μg/mL for 24 hours induced the release of IL-1β via the activation of the Rho-kinase pathway (Kanno et al., 2014). In human lung fibroblast (IMR-90 or CRL-1490) cell lines, exposure to MWCNT prepared by the CVD method or SWCNT (CNI, Inc.) prepared by the HiPCO method activated the mitogen-activated protein kinase (MAPK)/p38 pathway at low doses ≤ 5 μg/mL, leading to the upregulation of pro-inflammatory gene expression and collagen deposition (Ding et al., 2005; Azad et al., 2013). In normal and malignant human mesothelial cell lines, MWCNT (Mitsui & Co., Ltd) induced the activation of the MAPK/p38 pathway at a dose of 25 μg/cm2 after 30–120 minutes (Pacurari et al., 2008a). Exposure of normal and malignant human mesothelial cell lines to unpurified SWCNT (National Institute of Standards and Technology, Gaithersburg, MD, USA) induced the intracellular generation of ROS and activation of the AP-1 and NF-κB pathways at a dose of 25 μg/cm2 after 1–4 hours (Pacurari et al., 2008b).
(c) Experimental systems in vivo
Some studies revealed that a Th2-associated response to CNT is activated through both adaptive and innate immune responses. In studies of MWCNT (NanoTechLabs, Inc., Yadkinville, USA)-exposed mice, the expression of IL-33 was accompanied by lung dysfunction and the upregulation of Th2-associated cytokines, such as IL-5 and eotaxin (Katwa et al., 2012; Beamer et al., 2013). The exposure of mice to SWCNT or MWCNT produced a dose-dependent increase in ovalbumin (OVA)-specific IgE and IgG1 in the serum (Nygaard et al., 2009).
With regard to signal transduction, the expression of four genes (coiled-coil domain containing-99, muscle segment homeobox gene-2, nitric oxide synthase-2, and wingless-type inhibitory factor-1) among 63 genes in the lung of mice exposed to MWCNT (Mitsui & Co. Ltd) was altered at two time-points, as determined by a quantitative PCR assay (Pacurari et al., 2011). In a study in mice, exposure to semi-SWCNT [semi-conductive components of SWCNT] (metal content approximately 10% wt, selectively separated from a mixture of metallic and semi-metallic SWCNT) induced phosphorylation of the signal transducer and activator of transcription-3, which forms part of the Janus kinase/signal transducers and activators of transcription signalling cascade, at two time-points (Park et al., 2011a). With regard to transcription factors, cFos mRNA levels in whole blood were increased in SWCNT-exposed mice (Erdely et al., 2009). In another study in mice, although the dose used for intratracheal instillation was excessive (1 mg/mouse, 10 mg/kg bw), NF-κB and AP-1 transcription factors were activated in the lung after exposure to SWCNT (Chou et al., 2008; Zhang et al., 2013).
(d) Experimental systems in vitro
The MAPK/extracellular signal-regulated kinase 1,2 pathway was upregulated in the murine macrophage RAW 264.7 cell line exposed to MWCNT (Helix Material Solutions, Inc., Richardson, TX, USA) at doses ≥ 50 μg/mL for 24 hours, resulting in an increased expression of cyclooxygenase-2 and inducible nitric oxide synthase (Lee et al., 2012). The exposure of murine epidermal JB6P+ cells to partially purified SWCNT produced by the HiPCO process (CNI, Inc.) at doses > 60 μg/mL for 24 hours, activated the NF-κB pathway dose-dependently (Murray et al., 2009). Exposure of Chinese hamster ovary K1 cells (transfected with an NF-κB reporter gene construct) to MWCNT (XNRI, Bussan Nanotech Research) prepared by the CVD process at doses between 1 and 10 μg/mL for 20 hours upregulated the NF-κB pathway (Hirano et al., 2010).
4.4.6. Resistance to apoptosis
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
No data were available to the Working Group.
(c) Experimental systems in vivo
The formation of large tumours from injected SWCNT-transformed cells (which were also reported to be resistant to apoptosis due to a low level of p53 phosphorylation in an in-vitro study) was observed in immunodeficient mice (Wang et al., 2011a). The expression of LC3B and the autophagy-related proteins p62 and Beclin-1 was upregulated and the expression of proliferating cell nuclear antigen was also elevated in mice exposed intratracheally to 100 µg of MWCNT (Yu et al., 2013). In another study, the expression of anti-apoptotic genes, such as cIAP2, SOD2, and A20, was induced in mouse lung by an [excessive] dose of SWCNT (1 mg/mouse) (Chou et al., 2008).
(d) Experimental systems in vitro
No data were available to the Working Group.
4.4.7. Cell proliferation
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
Human cell lines were exposed to titanium dioxide nanobelts or purified MWCNT (Cheap Tubes, Inc.) at doses of 10 or 100 μg/mL for 1 and 24 hours. The target cell lines included human THP-1-derived macrophages and primary cultures of small airway epithelial cells. Total RNA was isolated and used for microarray analysis using Human Genome U133A 2.0 GeneChips (Affymetrix, Sana Clara, CA, USA). An analysis of the Global proteomics was conducted using liquid chromatography tandem mass spectrometry on tryptic digested cell lysates. The short exposure elicited similar proteomic responses to titanium dioxide nanobelts and MWCNT with different patterns of expression in various cell types. THP-1 macrophages showed the most significant transcriptional responses in 272 genes after 24 hours of exposure to MWCNT with unique patterns of gene expression in pathways related to cell-cycle regulation and cell proliferation (MYC and CDK1), as well as anti-apoptosis (survivin). Genes involved in the Sp1/AhR-dependent stress response were downregulated by exposure to MWCNT (Tilton et al., 2014).
In a study in human lung small airway epithelial cells exposed to SWCNT or MWCNT, occupationally relevant concentrations of CNT produced a neoplastic-like transformation phenotype depicted by increased cell proliferation, anchorage-independent growth, invasion, and angiogenesis (Wang et al., 2014).
(c) Experimental systems in vivo
(i) Bronchiolar and alveolar epithelial cells
Some, but not all, studies provided evidence of the proliferation of bronchiolar and alveolar epithelial cells.
In a 13-week inhalation study, exposure to CNF (VGCF-H) induced cell proliferation in the terminal bronchioles, alveolar ducts, and subpleural region of the respiratory tract in the lungs of male and female rats; however, this proliferation was not persistent and was absent in the subpleural region in females 3 months after exposure (Delorme et al., 2012).
Two studies of exposure by intratracheal instillation of rats to MWCNT (Roda et al., 2011) and mice to SWCNT (Murray et al., 2012) were performed. Exposure to as-produced or functionalized MWCNT induced proliferation of the alveolar and bronchiolar epithelial cells and alveolar macrophages in rats (Roda et al., 2011). Exposure to SWCNT decreased the proliferation of splenic T cells in mice (Murray et al., 2012). In another study in rats (Warheit et al., 2004), intratracheal instillation of SWCNT did not induce the proliferation of lung parenchymal cells assessed by 5-bromo-2-deoxyuridine.
(ii) Other cells
The proliferation of T-cells was induced in mice exposed to MWCNT in one study (Grecco et al., 2011) but was decreased in another study (Murray et al., 2012).
Exposure of mice to SWCNT increased the occurrence of epithelial-derived fibroblasts. The aberrant activation of TGF-β/p-Smad2 or β-catenin was postulated to induce epithelial–mesenchymal transition during SWCNT-induced fibrosis (Chang et al., 2012). In rats, MWCNT induced visceral mesothelial cell proliferation, (assessed by proliferating cell nuclear antigen immunostaining), accompanied by increases in the number of macrophages and of the concentration of protein in the pleural lavage (Xu et al., 2012).
(d) Experimental systems in vitro
Murine lung epithelial FE1 cells are a spontaneously immortalized cell line isolated from a MutaTM Mouse (Søs Poulsen et al., 2013). These cells were exposed to 12.5, 25, or 100 μg/mL of MWCNT (Mitsui-7; MWCNT-XNRI-7, lot 05072001K28, Hadoga Chemical Industry, Japan) suspended by sonication in cell culture medium. After 24 hours, the cells were harvested and total RNA was extracted for microarray analysis (Agilent 8 × 66K oligonucleotide microarrays); selected genes were verified using qRT–PCR. A total of 565 genes were differentially expressed at all concentrations. Classification of gene ontology revealed that most of the differentially expressed genes were involved in cell proliferation. At the highest exposure concentration, differentially expressed genes were related to cell death, cell-cycle arrest, oxidation reduction, and other metabolic pathways. Genes involved in fibrosis, cholesterol biosynthesis, GSH-mediated detoxification, and aryl hydrocarbon receptor signalling molecular canonical pathways were downregulated.
4.4.8. Granuloma formation and fibrosis
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
No data were available to the Working Group.
(c) Experimental systems in vivo
See Table 4.10
(i) Inhalation
Two 13-week studies of MWCNT in Wistar rats provided evidence of granulomatous inflammation and fibrosis in the lungs of male and female rats. The minimum concentrations of MWCNT that induced the persistent or slight fibrotic responses were 0.5 mg/m3 (Pauluhn, 2010b) and 0.4 mg/m3 (Treumann et al., 2013), respectively.
Two 4-week studies of MWCNT and SWCNT in Wistar rats showed no evidence of fibrosis in the lung at maximal concentrations of 0.37 mg/m3 of MWCNT and 0.13 mg/m3 of SWCNT (Morimoto et al., 2012a, b).
Two 6-hour exposure studies to MWCNT provided evidence of persistent fibrosis at high concentrations (241 and 30 mg/m3) in male rats (Ellinger-Ziegelbauer & Pauluhn, 2009) and mice (Ryman-Rasmussen et al., 2009a).
(ii) Intratracheal instillation
Fifteen studies of intratracheal instillation in rats and pharyngeal aspiration in mice have been reported (Lam et al., 2004; Muller et al., 2005; Warheit et al., 2004; Shvedova et al., 2005; Aiso et al., 2010; Cesta et al., 2010; Han et al., 2010; Kobayashi et al., 2010, 2011; Porter et al., 2010; Mercer et al., 2011; Morimoto et al., 2012b, c; Murray et al., 2012; Sager et al., 2013; Fujita et al., 2015). Most of the studies revealed that exposure to SWCNT and MWCNT resulted in persistent or progressive fibrosis in the lung (Muller et al., 2005; Shvedova et al., 2005; Aiso et al., 2010; Cesta et al., 2010; Porter et al., 2010; Mercer et al., 2011; Murray et al., 2012; Sager et al., 2013), whereas some studies in rats and one study in mice demonstrated transient or minimal fibrosis in the lung (Kobayashi et al., 2010, 2011; Morimoto et al., 2012b, c). In a long-term study, the formation of granuloma in the lungs disappeared over time (Fujita et al., 2015). [No significant differences in pulmonary responses were observed between rats and mice.]
(iii) Intraperitoneal injection
Exposure to long MWCNT led to granulomatous inflammation in the peritoneal cavity but exposure to tangled MWCNT induced weak or slight responses (Poland et al., 2008). In another study, MWCNT did not induce sustained inflammatory responses (Muller et al., 2009).
(d) Experimental systems in vitro
No data were available to the Working Group.
4.4.9. Alterations in DNA damage-induced response pathways
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
See Table 4.14
Table 4.14
Studies of impaired DNA repair in human cells exposed to carbon nanotubes in vitro.
Human cells were used to study the effect of SWCNT on the expression of stress-response genes. The target cells included primary normal human bronchial epithelial cells, diseased human bronchial epithelial cells from asthma patients or from patients with chronic obstructive pulmonary disease, lung adenocarcinoma A549 cells and pharyngeal carcinoma FaDu cells. Cells were exposed to 0.1 or 1.0 mg/mL of SWCNT (Meijo Nano Carbon Co., Ltd, Nagoya, Japan) for 6 hours. A PCR array was conducted to examine 84 stress-response genes. Expression levels of 11 stress-response genes, including ERCC1 encoding a DNA repair enzyme, were downregulated more than twofold after exposure to SWCNT. Other genes belonging to the inflammatory responses, IL-6 and TNF-α, were significantly downregulated in normal human bronchial epithelial cells indicating that inflammatory cytokines were not activated under these conditions (Hitoshi et al., 2012).
Protein expression was investigated in human monoblastic leukaemia U937 cells exposed to MWCNT (100 µg/mL) that had been thermally treated at 1800 °C or 2800 °C. An analysis of proteomics was performed after two-dimensional electrophoresis and protein identification by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. The expression of proteins involved in stress responses and DNA repair (such as DNA mismatch repair protein Msh2 and DNA damage-binding protein 1) was enhanced, suggesting the induction of DNA repair; however, the efficiency of repair was not evaluated (Haniu et al., 2010).
Gene and protein expression was studied in three human cell lines exposed to two types of high-aspect-ratio nanoparticles: MWCNT and titanium dioxide nanobelts that are known to exert low and high toxicity, respectively, in other cell systems. The sizes of the MWCNT and titanium dioxide nanobelts were 375 ± 23 nm and 1590 ± 126 nm in RPMI medium and 458 ± 16 nm and 634 ± 86 nm in DMEM medium, respectively. The cell lines were human THP-1 cells, primary small airway epithelial cells, and Caco-2/HT29-MTX co-cultures. The Caco-2 is a malignant human intestinal epithelial cell line and HT29-MTX is a goblet cell line. This co-culture mimics the intestinal epithelium. The cells were exposed to 10 or 100 µg/mL for 1 and 24 hours and 3 and 24 hours for transcriptomic and proteomic analyses, respectively. Titanium dioxide nanobelts were more cytotoxic than MWCNT, but the toxicity was low at early time-points. THP-1 cells were the most responsive and showed time-, concentration-, and particle type-dependent responses; the highest responses occurred at 100 µg/mL. Fewer genes were differentially expressed between untreated and treated cells in the other cell types. Cells responded to these nanoparticles by differentially regulating both common and unique sets of biological processes. In this study, the results were analysed by a comparison of the changes in regulatory pathways. When comparing signatures induced by titanium dioxide nanobelts and MWCNT in THP-1 cells, three pathways were upregulated specifically by MWCNT and not by titanium dioxide nanobelts: DNA damage checkpoint, DNA strand break repair, and cytoskeleton spindle microtubules. These results were consistent with others that demonstrated DNA damage and mitotic perturbations caused by MWCNT (Tilton et al., 2014). [Globally, these data showed common differential expression across the cell types and common pathways in response to exposure to titanium dioxide nanobelts or MWCNT, possibly linked to a common mechanism. However, they also showed cell-specific responses and particle-specific effects, thus addressing the question of target cell specificity.]
(c) Experimental systems in vivo and in vitro
See Table 4.15
Table 4.15
Studies of impaired DNA repair in tissues of experimental animals exposed to carbon nanotubes in vivo or in vitro.
Female Fischer 344 rats received a single intragastric dose of SWCNT (Thomas Swan and Co. Ltd, Consett, United Kingdom) or C60 fullerenes by gavage (0.064 or 0.64 mg/kg bw). Both C60 fullerenes (highest dose only) and SWCNT significantly enhanced the 8-oxodG level in the liver and lung tissues of rats in comparison with controls. SWCNT did not produce a significant increase in the gene expression of the DNA repair enzyme 8-oxoguanine DNA glycosylase in the liver. DNA repair activity was assessed in the liver using substrate nuclei containing 8-oxodG, and the level of 8-oxoguanine DNA glycosylase was not significantly altered (Folkmann et al., 2009).
In RAW 264.7 macrophages, SWCNT (Chengdu Organic Chemicals Co., Ltd) downregulated several genes involved in the DNA repair process (Dong et al., 2012).
The assessment of MWCNT genotoxicity in rats (Kim et al., 2012a, 2014) and mice (Ghosh et al., 2011) showed a persistence of DNA damage up to 90 days after exposure. [The findings may suggest a low or lack of DNA repair.]
Pregnant heterozygous p53 mice (p53+/-) received an intravenous injection of MWCNT (200 µL, 2 mg/kg bw). DNA integrity was assessed using a long PCR assay. In general, DNA damage was found in the fetuses and in placental cells, with an enhancement of the mRNA expression of bax and p21. Nonetheless, DNA damage was higher in p53−/− and p53+/- fetuses than in p53+/+ fetuses and neither Bax nor p21 expression levels were modified in p53−/− fetuses, in agreement with a defective DNA repair system in these mice cells due to the absence of p53 (Huang et al., 2014). [These results were consistent with the induction of p53-dependent apoptosis in this test system, and showed that the repair of DNA damage and apoptosis are dependent on the p53 status resulting from the intravenous injection of MWCNT into mice. They did not directly demonstrate that DNA repair was impaired by MWCNT, but showed an impaired response in p53-deficient mice.]
4.4.10. Depletion of antioxidants
As described previously, CNT have been shown to generate (or catalyse the formation of) ROS directly; however, this does not exclude a secondary generation of ROS by target cells in vitro or in vivo after exposure to CNT (Stone et al., 2009; Fubini et al., 2010, 2011).
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
See Table 4.16
Table 4.16
Depletion of antioxidants in cultured cells exposed to carbon nanotubes.
(i) SWCNT
The effects of SWCNT (NASA-JSC, TX, USA) were determined in cell cultures of immortalized human epidermal HaCaT keratinocytes. The generation of HO• was observed in HaCaT keratinocytes exposed to SWCNT at 0.24 mg/mL, using the ESR spin trapping technique. Both GSH and the antioxidant levels of the HaCaT keratinocytes were decreased at doses of 0.06, 0.12, and 0.24 mg/mL. In parallel, a significant increase in the accumulation of lipid peroxidation products (thiobarbituric acid-reactive substances) was seen in cells exposed to SWCNT (Shvedova et al., 2003).
Human BJ foreskin cells were exposed to SWCNT (Sigma) dispersed in dimethyl formamide. ROS production was determined using the 2,7-dichlorofluorescein diacetate assay, and was induced by exposure to SWCNT at doses of 6, 8, and 10 μg/mL after 3 hours of incubation. Antioxidant defences were assessed in BJ cells co-incubated with exogenous antioxidants, NAC and GSH, in the presence or absence of SWCNT (6 μg/mL). Exogenous NAC and GSH decreased the induction of ROS by SWCNT approximately two- and 2.5-fold, respectively (Sarkar et al., 2007). [These results suggested that compromised cellular antioxidant defences may be responsible for the generation of excess ROS in response to SWCNT in BJ foreskin cells.]
The effects of SWCNT were investigated in human macrophage-like cells differentiated from a human monocytic leukaemia THP-1 cell line. Affymetrix microarrays were performed to investigate the changes in gene expression after exposure to SWCNT. Cells were exposed to 0.05 µg/mL of SWCNT for 24 hours, which resulted in an increased expression of SOD2; the levels of catalase, GPx1, GSH reductase, GSH synthetase, and NADPH-dependent oxidase were not altered (Chou et al., 2008).
Human hepatoma HepG2 cells were exposed to purified HiPCO SWCNT (Unidym) produced by the CVD process or to graphene. Proteins were extracted and their profile analysed using the iTRAQ-coupled two-dimensional liquid chromatography tandem mass spectrometry approach. Peptides and proteins were identified automatically using the Spectrum Proteomics Workbench software. Protein ratios were determined in treated and untreated control HepG2 cells. Only quantification data on proteins with relative changes in expression of > 1.25 or < 0.8 were considered. After exposure to 1 µg/mL for 48 hours, 37 differentially expressed proteins were found in cells exposed to SWCNT or to graphene. Differentially expressed proteins involved in metabolic pathways, redox regulation, and cytoskeletal dynamics were identified. The antioxidant protein SOD2 was downregulated (Yuan et al., 2011).
Human colon adenocarcinoma Caco-2 cells were exposed to 0, 5, 10, 50, 100, 500, and 1000 µg/mL of F-SWCNT (Sigma-Aldrich, Madrid, Spain). The production of ROS and biomarkers of oxidation were quantified, including lipid peroxidation, generation of ROS, GSH levels, and SOD, GPx, GSH reductase, and catalase activities. ROS generation was increased at a dose of 100 µg/mL and lipid peroxidation at a dose of 50 µg/mL. Catalase activity increased at doses up to 500 µg/mL, then significantly decreased at 1000 µg/mL. SOD activity was also increased at doses up to 100 µg/mL. GPx activity was enhanced at the highest doses and GSH reductase at a dose of 1000 µg/mL. GSH level was depleted at all doses tested (significant at 1000 μg/mL) (Pichardo et al., 2012). [These results showed the induction of antioxidant defences in response to exposure to F-SWCNT and an increase in lipid peroxidation products, possibly causing toxic effects.]
Intracellular generation of ROS and expression profiles of oxidative stress response genes were assessed in human bronchial epithelial BEAS-2B cells exposed to SWCNT. Three SWCNT samples were tested (diameter, 1–2 nm ; length, 400–800 nm, 1–3 µm or 5–30 µm). Crocidolite asbestos fibres were used as positive control. Gene expression profiles were studied using a pathway-specific RT-PCR array (SuperArray) comprising primers for 84 oxidative stress and antioxidant defence pathway genes. All SWCNT samples and crocidolite asbestos fibres induced the formation of micronuclei; the generation of ROS was detected in cells exposed to the two shortest SWCNT samples and crocidolite asbestos fibres. The 1–3-µm SWCNT sample upregulated the expression of several genes: epoxide hydrolase 2, surfactant protein D, and neutrophil cytosolic factor 1. Few genes showed differential expression with the other SWCNT samples; however, 400–800-nm SWCNT induced the upregulation of three proteins: copper chaperone for SOD, metallothionein 3, and nitric oxide synthase 2. Differentially expressed genes common to all SWCNT included the upregulation of titin and copper chaperone for SOD and downregulation of the GPxs, GPX4 and GPX7, and the peroxidase cytoglobin that may be involved in protection against oxidative stress; neutrophil cytosolic factor 1 (required for the activation of latent NADPH oxidase, which is necessary for superoxide anion production) was also upregulated by all samples (Manshian et al., 2013).
The effects of SWCNT dispersed in sodium cholate on total GSH levels was evaluated in the human colon carcinoma HT29 cell line. Nine doses of SWCNT were tested (from 0.01 ng/mL to 0.2 µg/mL). Exposure to SWCNT in a dose range of 0.0001 µg/mL to 0.01 μg/mL increased the intracellular level of total GSH (significant at 0.001 and 0.01 μg/mL) (Pelka et al., 2013).
(ii) MWCNT
Human epidermal keratinocytes were exposed to MWCNT manufactured using a microwave plasma-enhanced CVD system. After exposure to 0.4 mg/mL for 24 and 48 hours, proteins were extracted and analysed by two-dimensional gel electrophoresis and mass spectrometry. Of these, 152 were observed to be differentially expressed and to be associated with several pathways: metabolism, cell signalling, stress, the expression of cytoskeletal components, and vesicular trafficking. Among them, SOD2 protein was decreased by 1.4- and 1.9-fold at 24 and 48 hours, respectively, in comparison with untreated cells (Witzmann & Monteiro-Riviere, 2006).
MWCNT produced by an electric arc process using graphite as a source (MWCNT1) and by CVD using methane as the hydrocarbon (MWCNT2) were obtained from the Centre for Environment, Institute of Science and Technology, JNTU, Hyderabad, India. Cytotoxicity and oxidative stress were studied in the human embryonic kidney HEK 293 cell line. Cellular levels of reduced GSH and MDA content were measured to assess lipid peroxidation. Exposure of HEK 293 cells to MWCNT (10–100 µg/mL) for 48 hours resulted in concentration-dependent cytotoxicity, increased levels of MDA, and decreased intracellular levels of GSH (Reddy et al., 2010b). [These findings suggested that MWCNT induced oxidative stress and cytotoxicity in these target cells.]
Oxidative stress was studied after exposure to MWCNT of the human monocytic THP-1 cell line differentiated into macrophages using phorbol myristate acetate. Three types of MWCNT were used: long straight MWCNT (length, approximately 50 µm; diameter, 20–100 nm; NT1); micron-sized aggregated MWCNT (relatively straight MWCNT; diameter, approximately 150 nm; NT2); and MWCNT with an aggregated entangled structure (individual diameter, approximately 20 nm; NT3). Control particles were carbon black 260 nm in diameter, carbon black 14 nm in diameter and long fibre amosite asbestos (length > 5 μm; diameter < 200 nm). Investigations included the assessment of gene expression using RT-PCR (GSH S-transferase [GST]pi and haeme oxidase 1[HO-1]), expression of HO-1 protein (enzyme-linked immunosorbent assay) and GST activity. After exposure of THP-1 cells to 62.5 µg/mL of particles for 4 hours, GSTpi expression was not modified after any treatment; HO-1 expression was enhanced in cells exposed to NT1 (statistically significant) and NT2 (not statistically significant), and reduced in cells exposed to NT3, 260-nm carbon black and long fibre amosite asbestos. No significant difference from the control was observed with any of the treatments for either GST activity or HO-1 protein expression. However, an enhancement of the Nrf2 protein expression was found in the nucleus of cells treated with NT1, which was eradicated by the addition of antioxidants (Brown et al., 2010). [This study suggested that the activation of the antioxidant response pathway is mediated by the antioxidant response element and Nrf2.]
Human umbilical vein endothelial cells were exposed for 2 hours to 0, 5, and 20 µg/mL of MWCNT (from Dr F. Chen, Lawrence Berkeley National Laboratory, Berkeley, CA) synthesized using a CVD method. Lipid peroxidation and antioxidant defences were determined by the quantification of MDA, and the activity of SOD and GPx. These cells were also incubated with the antioxidant NAC to explore the role of ROS in the induction of cell injury. Cellular ROS levels were significantly increased by exposure to 20 µg/mL of MWCNT. The activities of SOD and GPx were enhanced at the lowest concentrations but reduced at 20 µg/mL. When human umbilical vein endothelial cells were pretreated with NAC before exposure to MWCNT, ROS production was reduced compared with the groups exposed to MWCNT only, and cytotoxicity and DNA damage (DNA breakage by quantification of γH2AX foci) were also reduced (Guo et al., 2011). [These results showed that depletion of antioxidants was associated, at least partially, with cytotoxicity and DNA damage.]
Cellular levels of GSH were determined in the human embryonic kidney HEK 293 cell line exposed to four MWCNT with different dimensions (CNM1, CNM2, CNM4, and CNM3; size: 100–800, 200–500, 150–750, and 230–1700 nm, respectively) from the Centre for Environment (Institute of Science and Technology, JNTU, Hyderabad). In-vitro exposure of HEK 293 cells to 3–300 µg/mL of these MWCNT for 48 hours produced cytotoxicity and oxidative stress in a concentration-dependent manner. Increased lipid peroxidation (measured by MDA content) and decreased intracellular GSH levels were observed at concentrations of 30 and 100 µg/mL (Rama Narsimha Reddy et al., 2011).
Human lung adenocarcinoma epithelial A549 cells were exposed to MWCNT (provided by Professor D.G. Weiss, Department of Biological Sciences, Institute of Cell Biology and Biosystems Technology, Rostock University, Germany) at 0.5–100 µg/mL for 6–72 hours. Apoptotic cells were detected after exposure to 50 µg/mL for 72 hours. Significant ROS production was found at doses of 10 and 50 µg/mL, which was not due to mitochondrial activity. Lipid peroxidation was determined using a Lipid Peroxidation Assay Kit, and GSH levels determined using a colorimetric assay. Increased lipid peroxidation was significant at all concentrations of MWCNT after 24 hours of treatment, and both intracellular GSH levels and catalase activity were significantly reduced at a dose of 50 µg/mL (Srivastava et al., 2011).
The effects of two types of MWCNT – NM 400 (Nanocyl; diameter, 30 nm; length, 5 μm) and NM 402 (Arkema Graphistrength C100 ; diameter, 30 nm; length, 20 μm) – were assessed in the human hepatoblastoma C3A cell line. Intracellular ROS generation was measured using the 2,7-dichlorofluorescein diacetate assay, and GSH was quantified by the reaction of sulfhydryl groups with the fluorescent substrate ortho-phthalaldehyde. Both samples of MWCNT induced the generation of ROS and decreased total GSH and cellular GSH content after 24 hours. Pretreatment with the antioxidant, Trolox, prevented MWCNT-induced production of ROS. Cytotoxicity was also reduced in C3A cells pretreated with Trolox before exposure to MWCNT (Kermanizadeh et al., 2012).
The intracellular level of GSH was quantified in telomerase-immortalized human keratinocytes (N-hTERT) exposed to 100 µg/mL of MWCNT (NanocylTM NC7000 MWCNT from Nanocyl, produced by catalytic CVD) for 30 minutes or 24 hours. Oxidative stress was assessed using 2,7-dichlorofluorescein oxidation. MWCNT stirred or sonicated in water were marked by increased dichlorofluorescein fluorescence, suggesting an increased intracellular generation of ROS. In contrast, MWCNT sonicated in dispersants (HPC, Fagron, or Pluronic F108) showed no significant effects. The addition of the antioxidant, Trolox, a hydrophilic analogue of the lipophilic antioxidant vitamin E, prevented 2,7-dichlorofluorescein oxidation. Moreover, GSH was significantly decreased compared with untreated controls (Vankoningsloo et al., 2012).
(c) Experimental systems in vivo
See Table 4.17
Table 4.17
Depletion of antioxidants in experimental animals exposed to carbon nanotubes.
(i) SWCNT
C57BL/6 mice were maintained on vitamin E-sufficient or vitamin E-deficient diets and were exposed to SWCNT (CNI, Inc.) by pharyngeal aspiration. Antioxidant levels were determined in lung homogenates 28 days after exposure to 40 μg/mouse of SWCNT. Treatment with SWCNT induced greater increase in lipid peroxidation products and greater decrease in GSH levels in mice fed a vitamin E-deficient diet than in those fed basal diet, showing that SWCNT produced antioxidant depletion which was associated with a higher sensitivity to SWCNT-induced acute inflammation (Shvedova et al., 2007).
C57BL/6 mice were exposed to 5 mg/m3 of SWCNT in inhalation chambers for 5 hours per day for 4 days. The level of oxidative damage produced was measured in the lung homogenates. GSH levels were significantly depleted, and the level of lipid peroxidation products – measured as malondialdehyde (MDA) – showed a significant accumulation compared with controls 7 and 28 days after exposure. Total antioxidant capacity was reduced 1 and 7 days after treatment, but returned to the control level by 28 days after exposure (Shvedova et al., 2008).
SKH-1 immune competent hairless mice were exposed to SWCNT (CNI, Inc.) by daily skin application at doses of 40, 80, or 160 µg/mouse for 5 days. A reduction in GSH levels was found in skin homogenates of mice treated with the highest dose, but no change was found with the other doses (Murray et al., 2009).
Male BALB/c mice were exposed to 5 µg/g bw of aerosolized SWCNT (diameter, 1–2 nm; length, 0.5–2.0 µm; from Aldrich) in PBS or to PBS only, for 20 minutes per day on 7 consecutive days in a nose-only exposure system. The animals were killed at the end of the exposure period and lung tissues were collected. The intracellular levels of MDA and ROS, and the activities of SOD, catalase, and GPx were measured. Apoptosis was assessed by the measurement of caspase-3 and -8 activities in lung homogenates. MPO activity was measured in the BALF. MPO activity was higher in the BALF from SWCNT-exposed mice compared with controls. ROS and MDA levels were significantly higher in the lungs of SWCNT-exposed mice compared with controls, and SOD, catalase, and GPx were reduced. In parallel, apoptosis was demonstrated by the enhancement of caspase-3 and -8 activities (Ravichandran et al., 2011).
(ii) MWCNT
The antioxidant status of rat serum was evaluated after intratracheal instillation of MWCNT into male Wistar albino rats. Two MWCNT samples were used (from the Centre for Environment, Institute of Science and Technology, JNTU, Hyderabad), produced either by the electric arc process using graphite as a source or CVD using methane as the hydrocarbon. The rats received a single dose of 0.2, 1, or 5 mg/kg bw of MWCNT or quartz-crystalline silica particles (positive control). Blood samples were collected at 1, 7, 30, and 90 days after the instillation. Antioxidant capacity was determined by the measurement of GSH, a lipid peroxidation product (MDA), and SOD and catalase activities. Both MWCNT induced a significant dose-dependent depletion of GSH levels, decrease in SOD activity, and a transient dose-dependent decrease in catalase activity. Similarly, the amount of MDA was increased by both MWCNT in a dose-dependent manner 1 day after instillation and later decreased. Total antioxidant capacity, assessed by the ability to scavenge the free radical α,α-diphenyl-β-picryl hydrazyl, was decreased after exposure to MWCNT (Reddy et al., 2011). [These results indicated a reduction in antioxidant defence mechanisms after an instillation of MWCNT.]
Wistar rats received a single intraperitoneal injection of 270 mg/L of MWCNT (exterior diameter, 15–25 nm; interior diameter, 10–15 nm; surface, 88 m2/g) synthesized by the CVD technique and functionalized with single-strand DNA. The level of GSH was measured in the plasma and liver 1, 3, 6, 24, 48, and 144 hours later. A significant decrease in the level of GSH was observed in the plasma at all timepoints after exposure, and after 3 and 24 hours (but not after 48 or 144 hours) in the liver. This result was consistent with a depletion of antioxidants by this single-strand DNA–MWCNT sample. The GSH level returned to normal within 6 days. The activity of manganese SOD (SOD2) in the liver was decreased after 1, 24, and 48 hours, but not after 144 hours (Clichici et al., 2012). [These results could be consistent with a decrease in antioxidant defence after exposure to this single strand DNA–MWCNT sample.]
In a transplacental study carried out on mouse embryo fibroblasts from fetuses of p53+/- heterozygous mice, the treatment of dams with MWCNT and an antioxidant, NAC, abolished the MWCNT-only induced DNA breakage observed in these cells (Huang et al., 2014). [This result was consistent with the generation of ROS in cells exposed to MWCNT.]
Male BALB/c mice were exposed daily to 5 µg/g bw of aerosolized MWCNT (diameter, 20–50 nm; length, 6–13 nm; from Sigma) in PBS or to PBS only for 20 minutes on 7 consecutive days, in a nose-only exposure system. Animals were killed at the end of the exposure period and lung tissues were collected. The intracellular levels of MDA and ROS, and the activities of SOD, catalase, and GPx were measured. Apoptosis was assessed by the measurement of caspase-3 and -8 activities in lung homogenates. MPO activity was measured in the BALF. MPO activity was higher in the BALF from MWCNT-exposed mice compared with controls. ROS and MDA levels were significantly higher in the lungs of MWCNT-exposed mice compared with controls, and SOD, catalase, and GPx were reduced. In parallel, apoptosis was demonstrated by the enhancement of caspase-3 and -8 activities (Ravichandran et al., 2011).
(c) Experimental systems in vitro
See Table 4.16
(i) SWCNT
Rat lung epithelial cell cultures were exposed to SWCNT (Sigma Chemical Co.). The levels of ROS (2,7-dichlorofluoroscein diacetate assay), GSH content, and the levels of SOD1 and SOD2 antioxidant enzymes were quantified. The results showed the production of ROS in a concentration-dependent manner. GSH levels were decreased in cells treated with 10 μg/mL for 6 hours. Expression of SOD1 and SOD2 proteins was decreased after 24 hours in comparison with control cells (Sharma et al., 2007). [Globally, exposure to SWCNT induced oxidative stress and depletion of antioxidants.]
Rat adrenal gland pheochromocytoma PC12 cells were exposed to SWCNT (diameter, 1–2 nm; length, ~20 µm) (Beijing Nachen Technology & Development Co. Ltd, Beijing, China) at concentrations of 5–600 µg/mL for 24 and 48 hours. Exposure to SWCNT induced mitochondrial membrane damage, the formation of ROS, and increased levels of the lipid peroxidation product MDA. GSH levels, and activities of SOD, GPx, and catalase were decreased at cytotoxic concentrations in a concentration-dependent manner (Wang et al., 2011b). [These findings revealed that SWCNT induced oxidative stress in these target cells.]
The effects of SWCNT (diameter, 1–2 nm; length, ~20 µm; Beijing Nachen Technology & Development Co. Ltd) were studied in rat adrenal gland pheochromocytoma PC12 cells. The activities of catalase, SOD, and GPx and the GSH content were determined 24 and 48 hours after exposure to 50 µg/mL. The generation of ROS was enhanced in SWCNT-treated PC12 cells, but the level of the lipid peroxidation product, MDA, did not appear to be elevated. The activities of SOD, catalase, and GPx were all decreased (Wang et al., 2012b).
Oxidative stress was assessed in RAW 264.7 macrophages exposed to SWCNT produced by the HiPCO disproportionation technique, with iron carbonyl as the iron-containing catalyst precursor (CNI, Inc.). The SWCNT used were unpurified (iron, 26.0 wt%) or purified (iron, 0.23 wt%) to determine the effects of iron. Specific free radical intermediates produced by RAW 264.7 cells exposed to 0.12–0.5 mg/mL for 1–2 hours were determined using electron paramagnetic resonance spectroscopy. Neither purified nor unpurified SWCNT induced the intracellular production of superoxide radicals or nitric oxide in RAW 264.7 macrophages. The production of radicals was observed when RAW 264.7 cells were stimulated with zymosan (0.25 mg/mL), an agent known to activate the generation of ROS in macrophages. Under these conditions, HO• production was enhanced in zymosan-treated cells, and unpurified iron-rich SWCNT were more potent than purified SWCNT. Lipid peroxidation assessed by MDA levels was enhanced and GSH content was decreased in zymosan-stimulated RAW 264.7 macrophages. The addition of SWCNT lowered both lipid peroxidation and GSH content in comparison with zymosan-stimulated macrophages (Kagan et al., 2006).
Murine epidermal JB6 P+ cells were exposed to SWCNT (CNI, Inc.) produced by the HiPCO disproportionation process. A significant concentration-dependent decrease in GSH content was observed after a 24-hour incubation of JB6 P+ cells with 0.06 mg/mL, 0.12 mg/mL, and 0.24 mg/mL of partially purified SWCNT. Exposure to unpurified (iron, 30 wt%) SWCNT induced a greater reduction in GSH than exposure to partially purified (iron, 0.23 wt%) SWCNT (Murray et al., 2009).
Primary mouse embryo fibroblasts were exposed to various manufactured nanoparticles: SWCNT (diameter, 8 nm; length < 5 μm), carbon black, silicon dioxide, and zinc oxide. Intracellular generation of ROS, GSH and MDA levels, and SOD activity were determined after exposure to particle suspensions at doses of 5, 10, 20, 50, and 100 μg/mL for 24 hours. ROS production was enhanced by all particles in a concentration-dependent manner up to 50 μg/mL. Intracellular GSH levels decreased dose-dependently and the activity of SOD was decreased in treated fibroblasts in comparison with untreated control cells. Lipid peroxidation was significantly enhanced in cells exposed to SWCNT at a dose of 100 μg/mL only. In this study, SWCNT exhibited greater genotoxicity than zinc oxide nanoparticles, although zinc oxide induced more oxidative stress (Yang et al., 2009).
(ii) MWCNT
Two types of MWCNT – MWCNT1 (diameter, 10–20 nm; average length, 2 µm) and MWCNT2 (diameter, 40–100 nm; average length, 10 µm) – were studied in the C6 rat glioma cell line with regard to effects on MDA levels and SOD activity. Exposure of C6 rat glioma cells to MWCNT (25–400 µg/mL) for 24 hours resulted in an increased level of oxidative stress, and MWCNT1 was more cytotoxic than MWCNT2. MDA levels increased significantly after treatment with 100 µg/mL of both MWCNT1 and MWCNT2 compared with those in untreated controls. SOD activity was decreased by both MWCNTs (Han et al., 2012).
4.4.11. Activation of oncogenes and inactivation of tumour-suppressor genes
The expression of an important number of oncogenes and tumour-suppressor genes has been analysed in CNT-exposed experimental animals. Overall, most of these genes had different expression levels compared with unexposed control animals.
Lists of oncogenes, tumour-suppressor genes and cancer genes are available in the supplementary tables in Vogelstein et al. (2013).
(a) Exposed humans
No data were available to the Working Group.
(b) Human cells in vitro
See Table 4.18
Table 4.18
Expression of genes and proteins in human cells and experimental systems exposed to carbon nanotubes in vitro.
(i) SWCNT
Cell-cycle regulation was investigated using microarray analysis in human embryo kidney HEK 293 cells exposed to 25 µg/mL of SWCNT (Carbon Nanotechnologies, Inc.) for 48 hours. Under these experimental conditions, cell viability was approximately 84%. Exposure to SWCNT was associated with the induction of apoptosis and cell-cycle control genes, and several oncogenes or tumour-suppressor genes were either downregulated (e.g. CDK2, CDK4, CDK6), or upregulated (e.g. CDKN2A, TP53), consistent with the activation of an apoptotic response and cell-cycle arrest (Cui et al., 2005). [A discrepancy was noted by the Working Group between the text and Table 2 in Cui et al. (2005) regarding the up- or downregulation of TP53.]
Human BJ foreskin CRL-2522 cells were exposed to 0 (control) or 6 μg/mL of SWCNT (Sigma) in dimethylformamide vehicle for 24 hours. Gene expression was assessed using a Stress and Toxicity Array (Super Array, Frederick, MD, USA) and was altered in 96 genes in SWCNT-treated cells compared with controls; 28 of these genes showed significant upregulation, with a ratio ranging from 1.5 to 3. The altered genes were involved in several pathways – apoptosis, xenobiotic metabolism, DNA repair, and oxidative stress – and may represent potential oncogenes or tumour-suppressor genes (i.e. DNAJB4, ATM, CCNC) (Sarkar et al., 2007). [These genes play a role in the response to stress, DNA repair and apoptosis, and cell-cycle progression. Their activation in SWCNT-exposed cells was not indicative of damage to these cells but signified the activation of defence mechanisms by BJ cells.]
The effects of SWCNT (CNI, Inc.) produced by the HiPCO technique were evaluated in human bronchial BEAS-2B cells using the Human Apoptosis Array (R&D Biosystems) which detects the 35 most common apoptosis-regulatory proteins by immunoblotting. The cells were continuously exposed to a subcytotoxic concentration (0.02 μg/cm2) of SWCNT in culture and were passaged weekly. After 24 weeks of exposure, SWCNT-treated cells showed the morphological features of malignant transformation. Transformed SWCNT-exposed cells exhibited differential expression of apoptosis-related proteins compared with controls. A differential expression of the phosphorylated forms of p53 was observed which was lower in SWCNT-treated cells than in untreated cells. [Because the phosphorylation of p53 is an indicator of the activation of the p53 tumour suppressor, these results suggested a loss of p53 activity.] (Wang et al., 2011a). [This SWCNT sample can be assumed to have impaired the apoptotic potential of p53. However, these results should be interpreted with caution because BEAS-2B cells are immortalized with SV40 viral oncoproteins and express large SV40 T-antigen, a protein that binds to and inactivates p53 protein.]
Toxicity (cell growth, ROS production, DNA damage assessed by the comet and micronucleus assays, and p53 induction) of SWCNT dispersed in sodium cholate was studied in the human colon carcinoma HT29 cell line. Eight doses from 0.05 ng/mL to 0.2 µg/mL were tested. Phosphorylation of the tumour-suppressor protein p53 was investigated in exposed and untreated control cells using Western blot analysis. After 3 and 24 hours of exposure, the phospho-p53 protein was induced at concentrations ≥ 5 ng/mL. A decline was observed at higher concentrations (0.1 and 0.2 µg/mL) (Pelka et al., 2013). [These results are consistent with the activation of DNA repair at subcytotoxic doses.]
Human mesothelial cells were continuously exposed to SWCNT synthesized by HiPCO (CNI, Inc.) at concentrations of 0.02, 0.06, or 0.2 μg/cm2 for 2 months. Expression of the HRAS oncogene was assessed using HRAS protein analysis by Western blot and the activation of downstream signalling of the HRAS pathway. In parallel, phenotypical changes characteristic of neoplastic transformation were studied (cell growth in soft agar and invasion capability). Increased HRAS protein expression and activation of the ERK1/2 pathway were found to be associated with more neoplastic phenotypes. SWCNT enhanced the expression of a potential oncogenic protein (AKT) and downregulated expression of genes (TWIST and SNAI1) known to be involved in the epithelial–mesenchymal transition process (Lohcharoenkal et al., 2014).
(ii) MWCNT
A whole genome expression array (GeneChip® assay) was performed using human skin HSF42 fibroblasts and human embryonic lung IMR-90 fibroblasts exposed for 48 hours to 0.6 and 6 µg/mL of multiwalled carbon nano-onions and 0.06 and 0.6 µg/mL of MWCNT synthesized by the CVD method. Numerous genes showed changes in expression after treatment with the different particles. Similar to multiwalled carbon nano-onions, exposure to MWCNT upregulated the expression of genes involved in pathways related to cellular transport, metabolism, cell-cycle regulation, and stress response, but no evidence of oncogene activation or tumour-suppressor gene inactivation was found (Ding et al., 2005).
Human lung adenocarcinoma A549 cells were exposed to 1–50 µg/mL of MWCNT (provided by Professor D.G. Weiss, Department of Biological Sciences, Institute of Cell Biology and Biosystems Technology, Rostock University, Germany) for 3, 6, 12, 24, and 48 hours. Gene expression was analysed using semiquantitative PCR (RT-PCR). mRNA expression of TP53 and CDKN1A (that encode p53 and p21Cip1/Waf1, respectively) and the apoptotic gene BAX, was increased in comparison with untreated cells at doses of 10 and 50 µg/mL, and the expression of the anti-apoptotic and potential oncogene BCL2 was decreased. Protein levels were determined using Western blot analysis and confirmed differential mRNA expression. Apoptotic cells were detected after exposure to a dose of 50 µg/mL of MWCNT for 72 hours (Srivastava et al., 2011). [These results were consistent with a change in the expression of tumour-suppressor genes/oncogenes related to the induction of apoptosis.]
Normal human bronchial epithelial cells were exposed to MWCNT or crocidolite asbestos fibres at doses of 0.01–0.1% for 24 or 48 hours. Gene expression was investigated using the Whole Human Genome Microarray (44 K) (Agilent Technology). A total of 1201 and 1252 genes were upregulated and 1977 and 1542 genes were downregulated by both asbestos and MWCNT after 6 and 24 hours of exposure, respectively. These lists were compared with a list of genes known to be deregulated in human malignant mesothelioma or human lung cancers, using a data mining database (GeneCards). The authors found 12 and 22 genes modulated by exposure to both MWCNT and crocidolite in malignant mesothelioma and lung cancers, respectively, some of which were oncogenes and known or potential tumour-suppressor genes. One tumour-suppressor gene – CDKN2A – was downregulated 24 hours after exposure to each of the particles; and CTGF was upregulated similarly to human lung cancers. In addition, the expression of the BCL2 oncogene was enhanced in comparison with control cells, similarly to both malignant mesothelioma and lung cancers (Kim et al., 2012b). [These results demonstrated that exposure to MWCNT in vitro (i) downregulated or upregulated some tumour-suppressor genes and oncogenes, respectively, and (ii) modified the expression of cancer genes also found to be deregulated in human lung cancers and malignant mesotheliomas with similar effects produced by exposure to crocidolite asbestos.]
A transcriptomic analysis was performed in telomerase-immortalized human keratinocytes (N-hTERT) exposed to MWCNT (NanocylTM NC7000 MWCNT; from Nanocyl) produced by catalytic CVD. Cells were exposed to a dose of 100 µg/mL for 24 hours and mRNA expression was investigated using small-scale transcriptomic TaqMan low density array profiling (TLDA, Applied Biosystems). The relative expression levels of 46 mRNAs in treated cells compared with untreated cells were reported. Expression of the BCL2 oncogene was increased after exposure to water-sonicated MWCNT (Vankoningsloo et al., 2012). [The authors did not discuss the statistical analysis of these results.]
Carboxylated MWCNT were studied in human embryonic kidney epithelial HEK 293 cells, mouse mesenchymal stem C2C12 cells, and human neuroblastoma NB1691 cells. The expression of cell-cycle regulatory proteins was analysed using Western blot and mRNA expression was assessed using RT-PCR after exposure to 100 μg/mL of MWCNT for 24 hours. Expression of the protein p21, encoded by CDKN1A (a potential tumour-suppressor gene), was enhanced in MWCNT-exposed proliferating C2C12, HEK 293, and NB1691 cells. This was associated with an increased expression of the unphosphorylated form of pRb, concordant with cell-cycle downregulation. The expression of CDKN1A (an inhibitor of cell-cycle progression) was also enhanced at the transcriptional level as assessed using RT-PCR. Interestingly, expression of the p53 protein was not found to be enhanced, consistent with the absence of apoptosis (Zhang & Yan, 2012). [These results suggested the p53-independent induction of p21 in this experimental model.]
(c) Experimental systems in vivo
See Table 4.19
Table 4.19
Activation of oncogenes and inactivation of tumour-suppressor genes in experimental animals exposed to carbon nanotubes.
(i) SWCNT
C57BL/6 mice were exposed to 5 mg/m3 of SWCNT by inhalation for 5 hours per day for 4 days, or to 10 µg of SWCNT delivered by pharyngeal aspiration, after which DNA was isolated from lung sections. Three different types of mutation were detected in the K-ras gene after inhalation; two at codon 12, one of the most common mutation sites in human lung cancer, and one double mutation at codons 12 and 8. Pharyngeal aspiration did not significantly enhance K-ras gene mutations (Shvedova et al., 2008).
ICR mice were exposed to 100 µg/kg bw of SWCNT (ASP-100F from Hanhwa Nanotech, Republic of Korea) delivered by intratracheal instillation. The lungs were harvested 1, 7, 14, and 28 days after injection, and proteins were extracted from the lung tissue and analysed using Western blots. The expression of p53 protein was enhanced as early as 1 day after exposure (Park et al., 2011a). [The authors did not discuss the origin of the cell or the mechanism responsible for increased p53 protein expression.]
Exposure of male ICR mice to 100 µg/kg bw of SWCNT (metal content, approximately 10% wt; diameter, 1.2 nm; length, 2–10 µm; ASP-100F, Hanhwa Nanotech) by intratracheal instillation resulted in the modification of the expression of several proteins assessed by Western blot. One, 7, and 14 days after exposure, the expression of p53, cyclooxygenase 2, and caspase-3 proteins was increased in the lungs of exposed mice in comparison with controls, then decreased after 28 days (Park et al., 2011b). [These findings were consistent with an increase in the expression of the p53 tumour-suppressor gene that is related to the induction of apoptosis.]
The effects of SWCNT (diameter, 1.2 nm; length, 2–10 µm; ASP-100 F, Hanhwa Nanotech) were studied in CCR5+/+ (wild-type) and CCR5−/− (knockout) mice exposed to a dose of 100 µg/kg bw delivered by intratracheal instillation. CCR5 is a chemokine receptor that plays a role in inflammatory responses. The cell cycle was analysed to determine the expression of apoptosis-related proteins, and p21Cip1/Waf1, cyclin D1 (ccd1), and TGF-β in the lungs 7 days after instillation. The expression of apoptosis-related proteins – caspase-9, caspase-3, and cleaved poly(ADP-ribose) polymerase – and phospho-p53 protein was more markedly increased in the lung tissue of knockout mice than in that of wild-type mice. The expression of other proteins – p21Cip1/Waf1 and ccd1 (both potential oncogenes) – was also increased in knockout mice (Park et al., 2013). [These results were consistent with SWCNT-induced apoptosis, but also showed that the expression of some known or potential oncogenes (Cdkn1a and Ccnd1 encoding p21Cip1/Waf1 and ccd1, respectively) can be altered in the lungs of mice exposed to SWCNT.]
(ii) MWCNT
Male C57BL/6J mice were exposed to 10, 20, 40, or 80 μg of MWCNT (MWCNT-7, lot # 05072001K28, from Mitsui & Co.) or vehicle by pharyngeal aspiration for 7 or 56 days. Total RNA was extracted from frozen lung and quantified using qRT-PCR. A total of 63 genes were investigated, 47 of which were selected from previous studies that had identified gene expression signatures of human non-small cell lung cancers, determined using genome-wide DNA microarray analyses as being potentially associated with lung cancer risk, and 16 of which were hallmarks of cancer signalling pathways. At 7 and 56 days after exposure, a set of seven and 11 genes, respectively, showed differential expression in the lungs of mice exposed to MWCNT compared with the vehicle-treated control group. Among these, Wif1 (a gene functioning as a tumour-suppression gene that has been found to be epigenetically silenced in various cancers) was downregulated and an oncogene, Bcl-2, was also downregulated. Four genes from these two subsets of genes showing significant differential mRNA expression at both time-points were either upregulated (Ccdc99, Msx2, and Nos2) or downregulated (Wif1) (Pacurari et al., 2011). [These results demonstrated that exposure to this sample of MWCNT could modify the expression of genes that have been shown to be prognostic biomarkers in human lung cancers, including persistent downregulation of a putative tumour-suppressor gene.]
C57BL/6J mice were exposed to 0 (vehicle control), 10, 20, 40, or 80 μg of MWCNT (MWCNT-7, lot # 05072001K28; from Mitsui & Co.) by pharyngeal aspiration. RNA extracted 1, 7, 28, and 56 days after exposure was analysed for gene expression profiling using Agilent Mouse Whole Genome Arrays (Agilent, Santa Clara, CA). Selected genes showed significant changes at a minimum of two time-points and with a more than 1.5-fold change at all doses, and were significant in the linear model for dose or interaction of time and dose. The authors compared the list of differentially expressed genes from the microarray gene expression data with two published studies on microarray profiles in human lung carcinomas. In treated mice, 24 genes were consistently differentially expressed. From data at 56 days after exposure, 38 genes were selected as being associated with cancer. When matched in human genomes using gene symbols, 16 and 35 genes were found to predict the risk and prognosis of human lung cancer from data obtained at all time-points and at 56 days, respectively. Among the proteins encoded by the list of 35 genes with differential expression induced by exposure to MWCNT, several were implicated in lung cancer development, including two potential oncogenes – BCL3 and EGFR. However, both genes were downregulated after exposure to MWCNT (Guo et al., 2012).
Microarray gene expression profiling was investigated using RNA isolated from the lungs of male C57BL/6J mice exposed to 0 (vehicle control), 10, 20, 40, or 80 μg of MWCNT (MWCNT-7, lot #05072001K28; from Mitsui & Co.) delivered by pharyngeal aspiration for 1, 7, 28, or 56 days. The authors applied a novel computational model to generate genome-wide mRNA expression profiles that correlated with histopathological analysis of mouse lungs, focusing on inflammatory and fibrosis pathways identified using Ingenuity Pathway identification. Twenty-three genes were found to be involved in both MWCNT-induced inflammation and fibrosis – 67 in inflammation and 69 in fibrosis. Two of these genes are potential oncogenes; egfr was downregulated and junb was overexpressed across all days at most doses, possibly in relation to persistent inflammation (Snyder-Talkington et al., 2013a).
The expression of Tgfβ1 was measured in spontaneously hypertensive male rats exposed to PBS (control) or 0.6 mg/rat of short (0.5–2 μm) or long (20–50 μm) unpurified MWCNT (Nanotech Port, Chengdu, China) suspended in PBS by non-surgical intratracheal instillation once per day for two consecutive days. Tgfβ1 expression was evaluated by immunohistochemistry on the lung tissue sections and by qRT-PCR analysis. mRNA expression of other genes involved in the TGF-β Smad signalling pathway was also measured. [Several genes – Tgfbr2, Smad2, and Smad3 – are potential tumour-suppressor genes.] TGF-β1 protein expression was detected in lung macrophages and near the bronchiolar epithelium in response to MWCNT; the expression of both Tgfβ1 and Tgfbr2 genes was increased after 7 days of exposure (other times tested: 1 and 30 days). Additional results suggested that the TGF-β/Smad signalling pathway was upregulated only in rats exposed to long MWCNT (Wang et al., 2013).
Pregnant heterozygous p53+/− mice received an intravenous injection of 2 mg/kg bw of MWCNT. Exposure to MWCNT induced mRNA expression of two tumour-suppressor genes – Cdkn1a (encoding p21Cip1) and Bax – in p53+/+ fetuses, but to a lesser extent in p53+/− and p53−/− mice (Huang et al., 2014). [These results suggested that exposure to MWCNT triggers apoptosis in mice, a process decreased or inactivated in p53-deficient mice, depending on their p53 status.]
Pulmonary responses of C57BL/6 mice after exposure to MWCNT (Mitsui 7) were compared with in-vitro studies using cultured lung epithelial FE1 cells at the global transcriptomic level. Mice were exposed by intratracheal instillation to doses of 18, 54, and 162 μg/mouse of MWCNT, and lung samples were collected 24 hours after exposure. Microarray analyses were performed using Agilent 8 × 66K oligonucleotide microarrays, and gene expression was analysed using the gene ontology classifications of all of the differentially expressed genes. After in-vivo exposure, several pathways were commonly (more than one dose) or uniquely (one dose) affected. Referring to human orthologous genes, expression of some oncogenes was upregulated (Aurka and Bcl3). Downregulated genes also included known or potential oncogenes (Wnt1, Myb, and Dnaja4) (Søs Poulsen et al., 2013). [When comparing in-vivo and in-vitro models, most of the genes associated with exposure to MWCNT involved the same pathways, but the number of differentially expressed genes, in comparison with untreated mice, was higher in vivo than in vitro, which was at least partly linked to the multicellular versus unicellular nature of these model systems.]
(d) Experimental systems in vitro
See Table 4.18
(i) SWCNT
Murine monocytic RAW 264.7 cells were exposed to 0, 1, 10 or 50 µg/mL of acid-functionalized SWCNT (AF-SWCNT) for 24 hours. Gene expression profiles were analysed using cDNA microarrays. Based on the criteria of significance (P < 0.001 and fold change > 2), differentially expressed genes were identified at a dose of 10 µg/mL. A total of 130 genes were differentially expressed: 126 were underexpressed and four were overexpressed. Among these genes, MYC (oncogene) mRNA expression was upregulated in AF-SWCNT-treated RAW 264.7 cells in comparison with controls, confirmed using RT-PCR analyses. Several genes involved in DNA repair were downregulated, including XPA, XRCC1, XRCC4, and CHEK1 (potential tumour-suppressor genes). Globally, AF-SWCNT altered the expression of genes related to ribosome function, mitochondrial function, inflammatory response, cell cycle/apoptosis, and the proteasome pathway (Dong et al., 2012). [These results showed that AF-SWCNT may downregulate tumour-suppressor genes involved in the repair of DNA damage and stimulate the expression of oncogenes in RAW 264.7 cells.]
(ii) MWCNT
Rat lung epithelial RL 65 cells exposed to MWCNT (diameter, 6–13 nm; length, 2.5–20 µm; Sigma-Aldrich) showed increased levels of p53, p21Cip1/Waf1, and bax protein expression after 12 hours of exposure to 5 µg/mL, probably related to the induction of apoptosis (Ravichandran et al., 2010).
SWCNT (outside diameter, 1–2 nm; length, ~20 µm; Beijing Nachen Technology & Development Co. Ltd) were studied in rat adrenal gland pheochromocytoma PC12 cells. After 24 and 48 hours of exposure to 50 µg/mL of SWCNT, the expression of proteins involved in apoptosis – Bcl-2, an oncogenic protein, and bax – was determined using flow cytometry. Bcl-2 expression was decreased and bax protein and caspase-3 activity were increased in comparison with control cells, consistent with the induction of apoptosis in SWCNT-treated PC12 cells (Wang et al., 2012b).
Mouse embryonic J11 stem cells were exposed to MWCNT (Tsinghua and Nanfeng Chemical Group Cooperation, China) and the DNA damage response induced was analysed by measuring p53 protein expression levels. The expression of p53 protein was observed within 2 hours of exposure, and increased proportionally with the dose (5 and 100 µg/mL). Phosphorylation of p53 protein was assessed using the phospho-specific antibody to p53-Ser-23 and confirmed the activation of the p53 DNA damage-induced response pathway (Zhu et al., 2007). [Increased p53 protein expression suggests that MWCNT could cause DNA damage.]
The expression of TGFβ1, a tumour-suppressor gene that might also be an oncogene, was assessed in a co-culture of the mouse leukaemic monocyte macrophage RAW 264.7 cell line and the mouse embryonic fibroblast NIH 3T3 cell line, using RT-PCR analysis. RAW 264.7 cells seeded in the bottom well were first exposed to short (length, 0.5–2 µm) or long (20–50 µm) MWCNT (15 μg/mL) for 24 hours, and then NIH 3T3 cells that had attached on the top of the insert for 24 hours were co-cultured with RAW 264.7 for another 24 hours. mRNA expression of TGF-β1 was more upregulated by exposure to long MWCNT in comparison with short MWCNT. In parallel, more TGF-β1 protein was expressed in co-cultures exposed to long MWCNT than those exposed to short MWCNT (Wang et al., 2013).
A comparison of the in-vivo pulmonary responses of C57BL/6 mice to MWCNT (Mitsui 7) with the in-vitro response of lung epithelial FE1 cells (a spontaneously immortalized lung epithelial cell line derived from a normal healthy MutaTM Mouse) was made at the global transcriptomic level (Søs Poulsen et al., 2013). This cell line retains key endogenous metabolic capacity and intact p53 signalling pathways, and expresses both type I and type II alveolar phenotypes (Berndt-Weis et al., 2009). FE1 cells were exposed to 12.5, 25, or 100 μg/mL of MWCNT for 24 hours. Microarray analyses were performed using Agilent 8 × 66K oligonucleotide microarrays and gene expression was analysed using the gene ontology classification of differentially expressed genes. After in-vivo exposure, several pathways were commonly (several doses) or uniquely (one dose) affected. In FE1 cells in vitro, genes commonly affected included pathways involving aryl hydrocarbon receptor signalling, GSH-mediated detoxification, acute phase response signalling, and the nuclear factor (erythroid-derived 2)-like 2-mediated oxidative stress response. Among the upregulated genes, several were known or potential oncogenes or genes involved in cancer, including Jun, Ddit3, Hmga2, Ctgf, Runx1, and Fosl1, some of which may also have tumour-suppressor functions in specific models. Among the downregulated genes, several were also known or potential tumour-suppressor genes or genes involved in cancer, such as Pdgfrl, Id4, Cdkn2c, Cdkn2d (p19), Tgfβ2, Gstm2, and Gstt1, some of which may also have oncogenic functions in specific systems (Pdgfra and Cdkn2d). In-vitro data showed a high degree of overlap across the exposure groups, with some exceptions at the highest concentration (Søs Poulsen et al., 2013). [When comparing the two in-vitro and in-vivo models after exposure to MWCNT, most of the genes were associated with the same pathways, but the number of differentially expressed genes was lower in vitro and in vivo in comparison with untreated mice, which was at least partly linked to the multicellular versus unicellular nature of the systems.]
(e) Acellular systems in vitro
The generation of radicals by MWCNT was studied in an acellular system. MWCNT were synthesized by the decomposition of ethylene on an alumina support doped with a cobalt–iron catalyst mixture and purified by subsequent treatment with sodium hydroxide. The potential of MWCNT to release free radicals in aqueous suspensions was thus monitored by ESR spectroscopy, using 5,5-dimethyl-1-pyrroline-N-oxide as a trapping agent. A suspension of 5 mg of MWCNT did not generate oxygen or carbon-centred free radicals in the presence of hydrogen peroxide or formate, respectively. In contrast, MWCNT were able to scavenge radicals in the presence of an external source of hydoxyl radicals, •OH, or superoxide radicals, O2•- (Fenoglio et al., 2006). [Although not formally demonstrated, it is possible that MWCNT might protect against antioxidant depletion.]
The ability of various types of MWCNT to generate/scavenge radical formation was studied in both cell-free systems and human bronchial BEAS-2B cells. Printex 90 carbon black, crocidolite asbestos, and glass wool were also used. Hydrogen peroxide-induced free radical formation was determined by ESR. All CNM were found to scavenge the induction of •OH, but the presence of bovine serum albumin abolished •OH production in some samples. In addition to a scavenging effect, two types of long, needle-like MWCNT (average diameter, > 74 and 64.2 nm; average length, 5.7 and 4.0 μm, respectively) induced the dose-dependent formation of a unique, as yet unidentified radical in both the absence and presence of cells, which also coincided with cytotoxicity. The ability of MWCNT to protect against oxidant formation also depended on the composition of the medium (Nymark et al., 2014).
4.5. Susceptible populations
See Table 4.20
Table 4.20
Susceptibility to cancer in experimental animals exposed to carbon nanotubes.
No data on human populations were available to the Working Group. One study was carried out in transgenic animals with increased susceptibility to carcinogenic substances (Takanashi et al., 2012). Several experimental studies focused on the possible aggravation of airway disease and the effects of CNT on pulmonary vessels using models of asthma in mice.
Studies of genes related to inflammation in genetically deficient mice are also reported below. Although not related to cancer, these studies are summarized in relation to their pertinence to inflammatory processes.
4.5.1. Modification of risks for cancer of the lung
(a) MWCNT
The effects of MWCNT on allergic airway inflammation were studied in four groups of ICR mice that received intratracheal injections of vehicle, MWCNT (50 μg/animal; one of two types: Bussan Nanotech Research and SES Research), OVA, and OVA+MWCNT. Biological parameters were measured in the BALF (cellularity), lungs (histology, protein levels of cytokines related to allergic inflammation in lung homogenates and BALF), and serum (Ig levels). MWCNT exacerbated murine allergic airway inflammation, as demonstrated by an aggravation of allergen-induced airway inflammation and an increased number of goblet cells in the bronchial epithelium, and exhibited adjuvant activity for allergen-specific IgG1 and IgE. OVA+MWCNT amplified the lung levels of Th2 cytokines (e.g. IL-4, IL-5, and IL-13) and chemokines (e.g. thymus- and activation-regulated chemokine and macrophage-derived chemokine) compared with OVA (Inoue et al., 2009).
The effects of the inhalation of MWCNT on airway fibrosis were investigated in normal and OVA-sensitized mice with allergic asthma. Quantitative morphometry showed significant airway fibrosis in OVA-sensitized mice 14 days after exposure to MWCNT but not in mice treated with OVA or MWCNT alone. The levels of inflammatory factors in the BALF differed according to the exposure: IL-13 and TGF-β1 were elevated in OVA-sensitized mice while PDGF-AA was elevated in MWCNT-treated mice, suggesting that the airway fibrosis resulting from the combined effect of OVA and MWCNT required PDGF (a fibroblast mitogen) and TGF-β1 that stimulates collagen production (Ryman-Rasmussen et al., 2009b). [These findings indicated that individuals with pre-existing allergic inflammation may be susceptible to airway fibrosis from inhaled MWCNT.]
Whether sensitization by MWCNT (30 μL of 0.01, 0.1, or 1 mg/mL) and OVA (30 μL of 2.5 mg/mL) (combined) promotes an allergic asthmatic response was examined in mice. An increase in airway resistance was observed in the groups treated with OVA + 0.1 or 1 mg/mL of MWCNT compared with controls and those treated with OVA or MWCNT alone. In OVA + 1-mg/mL MWCNT-treated mice, the concentration of pro-inflammatory cytokines (IL-4, IL-5, IL-13, and IL-17) was increased in lung tissues and that of the anaphylatoxin C3a in the BALF. OVA-specific IgE, IgG1, and IgG2a were increased in the serum of mice sensitized with OVA and MWCNT (Mizutani et al., 2012).
The effects of MWCNT on the systemic immune response, airway inflammation, and remodelling induced by house dust mites (HDM) was investigated in BALB/cByJ mice. MWCNT increased the systemic immune response (significantly enhanced levels of specific and total IgG1 in the serum of HDM+MWCNT-treated mice compared with control mice and mice treated with the highest dose of HDM), airway inflammation (significantly enhanced number of eosinophils, neutrophils, and lymphocytes in the BALF of HDM+MWCNT-treated mice compared with control mice and mice treated with the highest dose of HDM), mucus production, and fibrotic response in a dose-dependent manner, as demonstrated by histological analyses of the lungs (Ronzani et al., 2014). [HDM are the most frequent allergens associated with asthma to date; using this model of asthma in mice, exposure to MWCNT was found to aggravate allergen-induced systemic immune responses, as well as airway inflammation and remodelling.]
The instillation of CNT has been shown to induce granulomatous changes and a study was performed to determine whether peroxisome proliferator-activated receptor gamma (PPARγ) deficiency would enhance granuloma formation after exposure to MWCNT (Huizar et al., 2013). PPARγ is a transcription factor that acts as anegative regulator of genes linked to inflammatory events. The alveolar macrophages of healthy individuals constitutively express PPARγ but PPARγ is deficient in the alveolar macrophages of patients with severe sarcoidosis, a granulomatous disease. PPARγ was therefore hypothesized to play a role in the formation of MWCNT-induced granulomas. Wild-type and macrophage-specific PPARγ knockout C57BL/6 mice received oropharyngeal instillations of 100 μg of MWCNT. The expression and activity of PPARγ by alveolar macrophages were significantly reduced in MWCNT-treated wild-type mice bearing granulomas. Granuloma formation was more extensive in MWCNT-treated macrophage-specific PPARγ knockout mice than in wild-type mice. PPARγ knockout mice exposed to MWCNT also demonstrated an elevated expression of pro-inflammatory cytokines in the lung tissues, laser-microdissected lung granulomas, and BALF cells. [These data suggested that PPARγ deficiency may promote inflammation and granuloma formation.]
Wild-type or cyclooxygenase 2 knockout mice were sensitized to OVA to induce allergic airway inflammation before exposure to 4 mg/kg bw of MWCNT by oropharyngeal aspiration. Exposure to MWCNT significantly increased OVA-induced lung inflammation and mucus-cell metaplasia in knockout mice compared with wild-type mice. Allergen-induced cytokines involved in Th2, Th1, and Th17 inflammatory responses were significantly enhanced in MWCNT-treated knockout but not in wild-type mice (Sayers et al., 2013).
MWCNT were implanted subcutaneously into transgenic rasH2 mice that overexpress the c-Ha-ras oncogene and are highly sensitive to carcinogens. Carbon black and N-methyl-N-nitrosourea were used as controls. No tumour developed in MWCNT-treated mice. In the carbon black-treated group, one mouse had a haemangioma in the spleen and another had an adenoma in the lung. Neoplasms developed in all mice in the N-methyl-N-nitrosourea-treated group but in none of the solvent-treated group (Takanashi et al., 2012). [These results showed that carcinogen-sensitive rasH2 mice did not develop neoplasms after subcutaneous implantation of MWCNT under these experimental conditions.]
(b) SWCNT
OVA-sensitized rats were exposed to SWCNT by intratracheal instillation. SWCNT exacerbated OVA-induced allergic asthma and this exacerbation was counteracted by the concurrent administration of vitamin E (Li et al., 2014).
The effects of SWCNT on allergic airway inflammation was studied in four groups of ICR mice that received intratracheal instillations of vehicle, SWCNT (50 μg/animal), OVA, and OVA+SWCNT. Two types of SWCNT were administered: one type ranged from 0.8 to 1.2 nm in diameter and 100 to 1000 nm in length and contained < 35% (by weight) iron; the other type (SES Research) was formed in the arc process and ranged from 1.2 to 2 nm in diameter and 1 to 15 μm in length. Both types of SWCNT contained up to 75% nanotubes (the remaining material consisted of amorphous carbon and other carbon nanoparticles) and were autoclaved at 250 °C for 2 hours before use. SWCNT aggravated allergen-induced pulmonary inflammation with mucus hyperplasia. OVA+SWCNT enhanced the protein levels of Th cytokines and chemokines related to allergy in the lung and exhibited adjuvant activity for allergen-specific IgG1 and IgE compared with OVA alone. OVA+SWCNT-treated mice also had enhanced oxidative stress-related biomarkers in the airways (Inoue et al., 2010). [These results were consistent with an exacerbation of allergic airway inflammation in mice via the enhanced activation of Th immunity and increased oxidative stress.]
The effects of SWCNT were investigated in wild-type and Ccr5 (a C-C chemokine receptor predominantly expressed on T-cells, macrophages, dendritic cells, and microglia, which plays an important role in inflammatory responses to infections) knockout mice. A comparison of wild-type and knockout mice exposed to SWCNT showed a significant decrease in the levels of neutrophils and an increase in the expression of apoptosis-related proteins, TGF-βl, and mesothelin in knockout mice. Histopathological lesions were also observed more frequently in knockout mice. The concentrations of the pro-inflammatory cytokines IL-6, IL-13, and IL-17 in BALF were significantly higher in knockout than in wild-type mice, but the levels of IL-1β, IL-10, and IFN-γ were similar in both models. The authors suggested that Ccr5 deficiency delays the resolution of inflammatory responses triggered by SWCNT and shifts the inflammatory response for SWCNT clearance from a Th1-type to a Th2-type (Park et al., 2013).
Nanoparticles have been reported to produce respiratory damage associated with adverse cardiovascular effects. To evaluate the effects of SWCNT on the progression of atherosclerosis, apolipoprotein E knockout (ApoE–/–) C57BL/6 mice were fed normal or atherogenic diets and were exposed by intrapharyngeal instillation to SWCNT. ApoE–/– mice lack ApoE, a high-affinity ligand for lipoprotein receptors, and consequently have elevated plasma levels of cholesterol and triglycerides and develop atherosclerotic plaques. Exposure to SWCNT did not modify the lipid profiles of ApoE–/– mice but induced accelerated plaque formation in mice fed an atherogenic diet. This response was accompanied by increased mitochondrial DNA damage but not inflammation (Li et al., 2007b). [These findings suggested that ApoE deficiency may enhance sensitivity to SWCNT.]
4.6. Mechanistic considerations
4.6.1. Physical and chemical properties associated with biological activity
See Fig. 4.2
Fig. 4.2
Physical and chemical properties of carbon nanotubes associated with biological activity
The physico-chemical properties of CNT may be modulated by their production method, by applying post-synthesis modification (purification), and/or by covalent functionalization of their external surface. The resulting large variety of CNT, their different features and their impact on biological activity and pathogenicity are reviewed in Section 4.2 and summarized in Table 4.2 and Fig. 4.2.
4.6.2. Deposition, biopersistence, translocation, and associated end-points
See Table 4.21
Table 4.21
Studies of the kinetics of MWCNT or SWCNTa in vivo: deposition, biopersistence, translocation, and associated end-points.
The lung interstitium and pleura were the target tissues for the carcinogenic (see Section 3), inflammogenic, and fibrotic effects that have been reported to be associated with exposure to MWCNT in rats and mice.
The biokinetic factors that relate to the mechanisms of carcinogenicity are those that influence the dose to the target tissue. These factors include the particle characteristics that determine the efficiency of their deposition in the respiratory tract, their clearance or retention, and their potential for translocation to distal sites. Airborne CNT include inhalable (capable of depositing in any region of the respiratory tract; 50% cut size, 10 µm) or respirable size particles (capable of depositing in the pulmonary or alveolar region of the lungs where gas exchange occurs; 3 and 5 µm for adults and children, respectively) (Brown et al., 2013). Particles that are deposited in the pulmonary region can be cleared from the lungs by alveolar macrophages, and those that are not cleared have the potential to translocate beyond the lungs.
CNT of respirable size have been shown to be deposited in the lungs of rats and mice exposed by inhalation, with estimated pulmonary deposition fractions of approximately 1–4% for SWCNT or MWCNT in mice (Shvedova et al., 2008; Mercer et al., 2013a) and approximately 5–20% for MWCNT in rats (Pauluhn, 2010b; Oyabu et al., 2011). Estimated human pulmonary deposition fractions for MWCNT or SWCNT studied in rodents were approximately 8 to 10% (NIOSH, 2013).
CNT can enter cells by passive internalization (diffusion or penetration of the cell membrane) or active internalization (phagocytosis or other types of endocytosis) (Kunzmann et al., 2011; Ye et al., 2013). The mechanisms of cell uptake depend on the surface properties of the CNT, the cell type encountered and its activation state. SWCNT uptake into alveolar macrophages was low (10% of alveolar burden in mice) (Shvedova et al., 2005) and 90% of dispersed SWCNT structures were observed in the lung interstitium (Mercer et al., 2008). More effective uptake of MWCNT has been observed (Mercer et al., 2010, 2011; Treumann et al., 2013). F-MWCNT significantly increased the alveolar macrophage uptake in comparison with O- or P-MWCNT (Silva et al., 2014).
CNT translocated from the lungs of mice and were observed in blood samples (Ingle et al., 2013). Two sizes of MWCNT (diameter, 60–80 nm or 90–150 nm) were observed as black pigments in liver tissue 1 day after intratracheal administration; dose-dependent toxicity and necrosis were observed in the liver and kidney (Reddy et al., 2010a). MWCNT seen by TEM were located in alveolar macrophages in the subpleural region, where focal subpleural fibrosis was also observed 2 weeks after inhalation exposure of 30 mg/m3 in mice (Ryman-Rasmussen et al., 2009a). MWCNT administered to rats by intrapulmonary spraying were observed to penetrate directly from the lungs to the pleural cavity through the visceral pleura, where visceral pleural cell proliferation was apparent at the end of the 9-day exposure (Xu et al., 2012). MWCNT (as-produced, CM-100; diameter, ~10–15 nm; length, ~20 µm) were observed in the pleura 28 days after a 90-day exposure by inhalation in rats, and DNA damage was observed (by the comet assay) up to 90 days after exposure (Kim et al., 2014). [The Working Group noted the short length of the aerosol generated.]
The numbers of MWCNT in the lungs and other organs were quantified after a 12-day exposure of mice to 5 mg/m3 for 5 hours per day; most of the MWCNT in the lungs were agglomerated, but only singlet MWCNT structures (average length, 6.9 µm) were observed in the liver, kidney, heart, brain, chest wall, and diaphragm (Mercer et al., 2013a, b). Rapid translocation of MWCNT occurred and 0.6% of the dose administered by pharyngeal aspiration was seen in the subpleura of mice 1 day after exposure (Mercer et al., 2010). 14C-Radiolabelled MWCNT administered to mice by pharyngeal aspiration was detected in the spleen and liver 1 day after exposure, increasing to 0.1–1% of the administered dose by 6–12 months after exposure, while the lung dose decreased to 10–20% of the administered dose over that time (Czarny et al., 2014).
The length and rigidity of the MWCNT influenced their clearance from the pleura after intrapleural injection; mice given the longer structures (mean length, 13 µm) developed significant inflammation and fibrosis of the parietal pleura compared with those given the shorter MWCNT (length, 0.5–5 µm) (Murphy et al., 2011).
The rat lung retention rate of short MWCNT (geometric mean length, 1.1 µm; GSD, 2.7) was similar to that for respirable poorly soluble spherical particles, with a retention half-time of approximately 50 days after inhalation exposure to 0.37 mg/m3 of MWCNT (Oyabu et al., 2011). The rat lung retention half-times were greater for another MWCNT (Baytubes; MMAD, ~3 µm; GSD, ~2), ranging from 151 to 375 days in rats exposed to inhalation concentrations ranging from 0.1 to 6 mg/ mg/m3 (Pauluhn, 2010a).
4.6.3. Persistent inflammation, granuloma formation, fibrosis, and pleural end-points
The studies on the toxicity of CNT in vivo are summarized in Table 4.22, in which the types of CNT and biological end-points are identified. Acute or persistent pulmonary inflammation (Fig. 4.3), pulmonary granuloma, fibrosis, and pleural end-points with well-defined effects were observed in the studies of MWCNT, SWCNT, and other CNT. Regardless of the number of walls or extent of purification, significant dose–response relationships were observed for these pulmonary end-points.
Table 4.22
Summary of results for end-points related to persistent inflammation, granuloma formation, fibrosis, and pleural end-points after exposure to carbon nanotubes in vivo.
Fig. 4.3
Persistent inflammation and exposure to carbon nanotubes
Occupational exposures to CNT may be to various types and forms of CNT that vary with respect to purity, especially in the content and bioavailability of metal catalyst residues. In general, MWCNT and SWCNT used in their “as-produced” or pure or purified forms produce a marked acute inflammatory response in the lungs after inhalation/aspiration. There is some evidence that “fully purified” MWCNT showed less severe responses than “as-produced” or partially purified MWCNT. Repeated exposure to CNT by inhalation/aspiration induces a persistent inflammatory response with concomitant focal granuloma formation and co-localization of fibrosis in a dose-dependent fashion. Even acute exposure to MWCNT can lead to their translocation to the pleura with subpleural cellular infiltration, collagen deposition, and pleural (mesothelial) cell hyperplasia.
4.6.4. Genotoxicity
See Table 4.23, Table 4.24, and Table 4.25
Table 4.23
Summary of results for end-points related to genotoxicity, gene expression, and cellular transformation after exposure to carbon nanotubes in vitro.
Table 4.24
Summary of results for end-points related to genotoxicity and gene expression after exposure to carbon nanotubes in vivo.
Table 4.25
Overall summary of results for genetic and related end-points in studies of exposure to carbon nanotubes in vivo and in vitro.
[The Working Group recognized the difficulties in evaluating the results of studies of genotoxicity due to the lack of standardized methods for genotoxicity testing, and variations in sample preparations and characterization of CNT.]
The Working Group did not identify any studies on genotoxicity end-points in presumed target tissues, surrogate cells (peripheral blood leukocytes), or matrices (e.g. urine) in humans with well-defined exposure to CNT and therefore regarded the observations in cultured human cells as being the most relevant with regard to supporting mechanistic evidence for carcinogenicity. In particular, both MWCNT and SWCNT induced aneuploidy in primary or immortalized human airway epithelial cells (Sargent et al., 2009, 2012a). This mechanism, which is described as a physical interference between CNT and the mitotic apparatus or fragmentation of the centrosome, is considered to be relevant for (airway) exposure of humans in vivo. These observations of chromosomal damage are supported by positive findings for SWCNT in cultured primary human lymphocytes (Catalán et al., 2012) and for MWCNT in the bronchial epithelial BEAS-2B cell line (Siegrist et al., 2014). Further supporting evidence in six out of eight studies showed an increased frequency of micronuclei in human cell lines after exposure to either SWCNT or MWCNT (Muller et al., 2008b; Cveticanin et al., 2010; Cicchetti et al., 2011; Thurnherr et al., 2011; Lindberg et al., 2013; Manshian et al., 2013; Kim & Yu, 2014; Tavares et al., 2014). Studies that gave negative results investigated the effects of pure MWCNT (length, 2–5 μm; diameter, 6–26 nm; 0.4% iron) (Thurnherr et al., 2011) and SWCNT (length, 1–5 µm; diameter, < 2 nm; impurities not reported) (Lindberg et al., 2013) that did not appear to differ from samples that caused the formation of micronuclei. In addition, one study showed that only two out of six MWCNT samples generated micronuclei, although they did not have overtly different physico-chemical characteristics compared with non-genotoxic samples (Tavares et al., 2014).
The strongest evidence of mutagenesis derives from animal studies that showed increased levels of guanine phosphoribosyltransferase (Gpt) mutations in the lung tissues of mice after intratracheal exposure to MWCNT (Kato et al., 2013) and of K-Ras mutations after inhalation exposure to SWCNT (Shvedova et al., 2008, 2014). The results for mutagenesis in cultured cells have been negative, including one study in human lymphoblastoid MCL-5 cells (Manshian et al., 2013). Genotoxicity studies have provided information on the mechanisms of genomic instability generated by CNT (Fig. 4.4). Studies of DNA damage – essentially DNA strand breaks and oxidatively damaged DNA measured by the comet assay – in cultured human cells have shown genotoxicity after exposure to either MWCNT or SWCNT. Increased levels of DNA strand breaks in the lungs of rodents after pulmonary exposure to either MWCNT or SWCNT were found in four studies (Kim et al., 2012a; Kato et al., 2013; Cao et al., 2014; Kim et al., 2014) while no increase was found in three studies (Naya et al., 2012; Ema et al., 2013a; Vesterdal et al., 2014a); intraperitoneal injection of MWCNT yielded positive results in two studies (Patlolla et al., 2010; Ghosh et al., 2011). No data were available regarding the relationship between the characteristics of CNT and their ability to generate DNA damage in human cultured cells and organs of exposed animals. [These observations indicate that the mechanisms of genotoxicity involve chromosomal aberrations and oxidative stress, although a formal assessment of the inhibition of DNA damage through supplementation with antioxidants in CNT-exposed cells has not been pursued.] This mechanism of DNA damage is known to occur in human cells after exposure to particulate matter. Two human mesothelial (pleural Met-5A and peritoneal LP-9) cell lines showed features of morphological transformation and H-RAS expression after continuous exposure to SWCNT (Lohcharoenkal et al., 2014).

Fig. 4.4
Mechanisms of genomic instability generated by carbon nanotubes
Pulmonary exposure to MWCNT and SWCNT had no effect on oxidative DNA damage (i.e. FPG-sensitive sites) in studies that mainly focused on cardiovascular effects in atherosclerosis-prone (ApoE−/− knockout) mice, but the administered doses were low (maximal dose of 1 mg/kg bw as two intratracheal instillations (Vesterdal et al., 2014a) and 25.6 µg/mouse per week (Cao et al., 2014)). [Therefore, these studies cannot rule out the possibility that DNA damage is generated by oxidative stress in pulmonary tissues after airway exposure to MWCNT and SWCNT.] One study showed increased levels of pro-mutagenic 8-oxodG lesions in both lung and liver tissues after gastrointestinal administration of low doses (0.064 and 0.64 mg/kg bw) of SWCNT (Folkmann et al., 2009). [This study suggests the involvement of a genotoxic mechanism arising as a consequence of oxidative stress, although it is impossible to distinguish between direct and indirect genotoxic mechanisms.]
The MWCNT and SWCNT investigated originated from different manufacturing processes, leading to substantial differences in dimensions and residual transition metal content. The available literature supports the conclusion that exposure to a range of different MWCNT (including Mitsui-7) and SWCNT can generate DNA strand breaks, oxidized DNA nucleobases, micronuclei, and chromosomal aberrations in animal and human cells through various mechanisms according to the type of CNT material. Overall, there is strong evidence that a genotoxic mechanism in human cells leads to carcinogenesis after exposure to both MWCNT and SWCNT.
- Mechanistic and Other Relevant Data - Some Nanomaterials and Some FibresMechanistic and Other Relevant Data - Some Nanomaterials and Some Fibres
Your browsing activity is empty.
Activity recording is turned off.
See more...
