NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants; Some Hair Dyes, Cosmetic Colourants, Industrial Dyestuffs and Aromatic Amines. Lyon (FR): International Agency for Research on Cancer; 1993. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 57.)

Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants; Some Hair Dyes, Cosmetic Colourants, Industrial Dyestuffs and Aromatic Amines.
Show details1. Exposure Data
1.1. Chemical and physical data
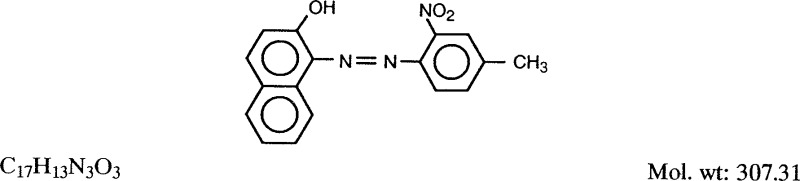
1.1.1. Synonyms, structural and molecular data
Chem. Abstr. Serv. Reg. No.: 2425-85-6
Replaces CAS Reg. No.: 12238-48-1; 12240-01-6; 12240-02-7; 39310-30-0
Chem. Abstr. Name: l-[(4-Methyl-2-nitrophenyl)azo]-2-naphthalenol
Colour Index No.: 12120
Synonyms: D&C Red No. 35; D and C Red No. 35; 1-(4-methyl-2-nitrophenylazo)-2-naphthol; 1-[(2-nitro-4-methylphenyl)azo]-2-naphthol; 1-(ortho-nitro-para-tolylazo)-2-naphthol; toluidine red
1.1.2. Chemical and physical properties
- (a) Description: Dark-red powder (Stubbs, 1973; Green, 1990)
- (b) Melting-point: 270–272 °C (Green, 1990)
- (c) Density: 1.37–1.50 g/cm3 (Stubbs, 1973)
- (d) Spectroscopy data: Infrared, ultraviolet and nuclear magnetic resonance spectral data have been reported (Pouchert, 1981; Green, 1990; Sadtler Research Laboratories, 1980, 1991; US National Toxicology Program, 1992).
- (e) Solubility: Slightly soluble in water (0.8 g/l), ethanol (0.7 g/l), ethylene glycol methyl ether (0.9 g/l), acetone and benzene; very soluble in mineral spirits, aromatic hydrocarbons and plasticizers (Stubbs, 1973; Green, 1990)
1.1.3. Trade names, technical products and impurities
Some trade names are: Accosperse Toluidine Red XL; ADC Toluidine Red B; Atlasol Spirit Red 3; CP Toluidine Toner A 2989; CP Toluidine Toner A 2990; CP Toluidine Toner Dark RS 3340; CP Toluidine Toner Deep X 1865; CP Toluidine Toner Light RS 3140; CP Toluidine Toner RT 6101; CP Toluidine Toner RT 6104; Calcotone Toluidine Red YP; Carnelio Helio Red; Chromatex Red J; Dainichi Permanent Red 4R; Deep Fastona Red; Duplex Toluidine Red L 20-3140; Eljon Fast Scarlet PV Extra; Eljon Fast Scarlet RN; Enialit Light Red RL; Fast Red A; Fast Red AB; Fast Red J; Fast Red JE; Fast Red R; Fastona Red B; Fastona Scarlet RL; Fastona Scarlet YS; Graphtol Red A 4RL; Hansa Red B; Hansa Red G; Hansa Scarlet RB; Hansa Scarlet RN; Hansa Scarlet RNC; Helio Fast Red BN; Helio Fast Red RL; Helio Fast Red RN; Helio Red RL; Helio Red Toner; Hispalit Fast Scarlet RN; Independence Red; Irgalite Fast Red P 4R; Irgalite Fast Scarlet RND; Irgalite Red PV 2; Irgalite Red RNPX; Irgalite Scarlet RB; Isol Fast Red HB; Isol Fast Red RN 2B; Isol Fast Red RN 2G; Isol Fast Red RNB; Isol Fast Red RNG; Isol Toluidine Red HB; Isol Toluidine Red RN 2B; Isol Toluidine Red RN 2G; Isol Toluidine Red RNB; Isol Toluidine Red RNG; Japan Red 221; Japan Red No. 221; Kromon Helio Fast Red; Kromon Helio Fast Red YS; Lake Red 4R; Lake Red 4RII; Lithol Fast Scarlet RN; Lutetia Fast Red 3R; Lutetia Fast Scarlet RF; Lutetia Fast Scarlet RJN; Monolite Fast Scarlet CA; Monolite Fast Scarlet GSA; Monolite Fast Scarlet RB; Monolite Fast Scarlet RBA; Monolite Fast Scarlet RN; Monolite Fast Scarlet RNA Monolite Fast Scarlet RNV; Monolite Fast Scarlet RT; No. 2 Forthfast Scarlet; Oralith Red P 4R; Permanent Red 4R; Pigment Red 3; Pigment Red RL; Pigment Scarlet; Pigment Scarlet B; Pigment Scarlet N; Pigment Scarlet R; Polymo Red FGN; Recolite Fast Red RBL; Recolite Fast Red RL; Recolite Fast Red RYL; Sanyo Scarlet Pure; Sanyo Scarlet Pure No. 1000; Scarlet Pigment RN; Segnale Light Red 2B; Segnale Light Red B; Segnale Light Red BR; Segnale Light Red C 4R; Segnale Light Red RL; Seikafast Red 4R4016; Siegle Red 1; Siegle Red B; Siegle Red BB; Silogomma Red RLL; Silosol Red RBN; Silosol Red RN; Siloton Red BRLL; Siloton Red RLL; Symuler Fast Red 4R100; Symuler Fast Scarlet 4R; Syton Fast Scarlet RB; Syton Fast Scarlet RD; Syton Fast Scarlet RN; Tertropigment Red HAB; Tertropigment Scarlet LRN; Toluidine Red 10451; Toluidine Red 3B; Toluidine Red 4R; Toluidine Red BFB; Toluidine Red BFGG; Toluidine Red D 28-3930; Toluidine Red Light; Toluidine Red M 20-3785; Toluidine Red R; Toluidine Red RT 115; Toluidine Red Toner; Toluidine Red XL 20-3050; Toluidine Toner; Toluidine Toner Dark 5040; Toluidine Toner HR-X2700; Toluidine Toner HR-X 2741; Toluidine Toner Keep HR-X 2742; Toluidine Toner L 20-3300; Toluidine Toner RT 252; Versal Scarlet PRNL; Versal Scarlet RNL; Vulcafor Scarlet A
Technical grades of CI Pigment Red 3 are available commercially with purities ranging up to > 97%. One impurity that has been reported is 1-[(4-methoxy-2-nitrophenyl)azo]-2-naphthalenol (BASF Corp., 1991; Aldrich Chemical Co., 1992; US National Toxicology Program, 1992).
1.1.4. Analysis
No data were available to the Working Group.
1.2. Production and use
1.2.1. Production
CI Pigment Red 3 was first prepared in 1905. It is manufactured by diazotization of 2-nitro-para-toluidine and coupling the resultant diazonium salt with 2-naphthol (Stubbs, 1973; Society of Dyers and Colourists, 1982; Green, 1990).
Approximate US production of CI Pigment Red 3 was 1430 tonnes in 1950,930 tonnes in 1960, 780 tonnes in 1970, 754 tonnes in 1975, 460 tonnes in 1980, 380 tonnes in 1985 and 350 tonnes in 1990 (Stubbs, 1973; US International Trade Commission, 1977, 1981, 1986, 1991).
1.2.2. Use
CI Pigment Red 3 is one of the most widely used of all red pigments because of its bright scarlet hue, high tinctorial strength and good stability to acids, alkalis and light. Some limitation is imposed by its relatively poor fastness to organic solvents. It is widely used in paints and printing inks. Other uses have included synthetic resin lacquers and leather finishes, inks for foil and tinplate printing, paper coating and dyeing, wallpaper, linoleum, carbon papers, typewriter ribbons, artists' materials and textile printing. It has been used in rubber, plastics and cement (Society of Dyers and Colourists, 1971; Stubbs, 1973; Green, 1990).
1.3. Occurrence
1.3.1. Natural occurrence
CI Pigment Red 3 is not known to occur as a natural product.
1.3.2. Occupational exposure
No data were available to the Working Group.
The US Environmental Protection Agency, the American Textile Manufacturers Institute and the Ecological and Toxicological Association of the Dyestuffs Manufacturing Industry conducted a joint survey in 1986–87 to estimate airborne concentrations of dye dust in dye weighing rooms of industrial plants where powder dyes are used in the dyeing and printing of textiles. The survey was based on a sample of 24 sites chosen at random from among textile plants where powder dyes are weighed. Although CI Pigment Red 3 was not among the dyes included in the survey, the results are considered to be representative of dye dust levels during weighing of this type of powder dye. The mean airborne concentration of active colourant in the plants monitored was estimated to be 0.085 mg/m3 (US Environmental Protection Agency, 1990).
On the basis of a survey conducted in the USA between 1981 and 1983, the US National Institute for Occupational Safety and Health estimated that a total of 51 931 workers, including 11 615 women, were potentially exposed to CI Pigment Red 3 in 57 industries (US National Library of Medicine, 1992).
1.4. Regulations and guidelines
CI Pigment Red 3 is allowed for use exclusively in cosmetic products intended to come into contact only briefly with the skin (Commission of the European Communities, 1976, 1986, 1990).
CI Pigment Red 3 (D&C Red No. 35) was cancelled for use in foods, drugs or cosmetics in the USA in 1966 (US Food and Drug Administration, 1992).
2. Studies of Cancer in Humans
No data were available to the Working Group.
3. Studies of Cancer in Experimental Animals
3.1. Oral administration
3.1.1. Mouse
Groups of 50 male and 50 female B6C3F1 mice, six weeks old, were fed 0,12 500,25 000 or 50 000 mg/kg of diet (ppm) CI Pigment Red 3 (purity > 97%) in the diet for 103 weeks. At the end of the experiment (110 weeks), 33/50 control, 28/50 low-dose, 31/50 mid-dose and 33/50 high-dose males, and 39/50 control, 37/50 low-dose, 31/50 mid-dose and 25/50 high-dose females (p < 0.001) were still alive. Renal-cell adenomas occurred at a significantly higher incidence in high-dose male mice than in controls (control, 0/50; low-dose, 0/50; mid-dose, 0/50; high-dose, 6/50; p = 0.017, logistic regression test); and follicular-cell adenoma of the thyroid gland occurred with a positive trend in male mice (control, 0/50; low-dose, 0/49; mid-dose, 1/50; high-dose, 5/50; p = 0.001, logistic regression trend test) (US National Toxicology Program 1992).
3.1.2. Rat
Groups of 50 male and 50 female Fischer 344/N rats, six weeks old, were fed 0, 6000, 12 500 or 25 000 ppm (mg/kg) CI Pigment Red 3 (purity > 97%) in the diet for 103 weeks. At the end of the experiment (110 weeks), 28/50 control, 40/50 low-dose, 28/50 mid-dose and 20/50 high-dose males, and 32/50 control, 41/50 low-dose, 39/50 mid-dose and 40/50 high-dose females were still alive. In male rats, the incidence of benign adrenal phaeochromo-cytomas was significantly increased in the mid- and high-dose groups compared to controls (control, 22/50; low-dose, 29/50; mid-dose, 35/50; high-dose, 34/50; p = 0.004, logistic regression trend test). In female rats, hepatocellular adenomas occurred with a positive trend, with a significantly greater incidence in the high-dose group than in the control group (control, 0/50; low-dose, 0/50; mid-dose, 1/50; high-dose, 10/50: p = 0.001, logistic regression test) (US National Toxicology Program, 1992).
4. Other Relevant Data
4.1. Absorption, distribution, metabolism and excretion
4.1.1. Humans
No data were available to the Working Group.
4.1.2. Experimental systems
The absorption of CI Pigment Red 3 (purity, 94.7 %) was studied in male Fischer 344 rats, seven to eight weeks of age, given the compound once at 11.8 mg/kg bw suspended in corn oil by gavage. Gut contents, faeces and various tissues were extracted 1, 4, 24 and 48 h after dosing and the amount of parent compound present was determined. None was found in blood, liver or kidneys, and it was concluded that the compound is not absorbed from the intestinal tract. Recovery after 48 h was 72.4% of the dose, suggesting that the compound may be partly degraded by intestinal bacteria. [The Working Group noted that absorption of cleavage products or metabolites was not analysed.]
4.2. Toxic effects
4.2.1. Humans
CI Pigment Red 3 has been associated with contact dermatitis following cosmetic use (Sugai et al., 1977).
4.2.2. Experimental systems
CI Pigment Red 3 (purity greater than 97%) was tested for toxicity in groups of five (14-day study) and 10 male and 10 female Fischer 344 rats and B6C3F1 mice in a range-finding study for carcinogenicity testing (Morgan et al., 1989; US National Toxicology Program, 1992). The compound was mixed into the feed at concentrations of 0,6000,12 500, 25 000,50 000 or 100 000 ppm (mg/kg) and administered for 14 days, or at concentrations of 0,3000, 6000,12 500, 25 000 or 50 000 ppm (mg/kg) and administered for 90 days. Female rats gained less weight than controls with all doses in both studies. The liver weight:body weight ratio increased significantly in male and female rats with all doses in both studies, and the kidney weight:body weight ratio in all males except the 6000 ppm group in the 90-day study. Body weight gains of mice treated for 90 days did not differ from those of controls. The haematocrit and haemoglobin concentrations were decreased in a dose-related manner in rats after 14 and 90 days, and reticulocytosis occurred at dose levels of 25 000 and 100 000 after 14 days and at all dose levels after 90 days in both males and females. These changes were observed to a lesser extent in mice. Histological lesions were seen in both species, which consisted of haematopoietic cell proliferation in the bone marrow, spleen, liver and kidney in the 90-day study.
4.3. Reproductive and prenatal effects
No data were available to the Working Group.
4.4. Genetic and related effects
4.4.1. Humans
No data were available to the Working Group.
4.4.2. Experimental systems (see also Table 1 and Appendices 1 and 2)
Table 1
Genetic and related effects of CI Pigment Red 3.
CI Pigment Red 3 was not mutagenic to Salmonella typhimurium, except in the presence of an exogenous metabolic system from hamster (but not rat) liver, when it was weakly mutagenic at precipitating doses. It did not induce sister chromatid exchange or chromosomal aberrations in cultured Chinese hamster ovary cells.
5. Summary of Data Reported and Evaluation
5.1. Exposure data
CI Pigment Red 3, one of the most widely used red pigments, is found in paints, inks, plastics, rubber and textiles.
5.2. Human carcinogenicity data
No data were available to the Working Group.
5.3. Animal carcinogenicity data
CI Pigment Red 3 was tested for carcinogenicity by administration in the diet in one study in mice and in one study in rats. In male mice, it induced follicular-cell adenomas of the thyroid and renal-cell adenomas. There was an increased incidence of adrenal phaeochro-mocytomas in male rats and of hepatocellular adenomas in female rats.
5.4. Other relevant data
CI Pigment Red 3 did not induce gene mutation in bacteria or sister chromatid exchange or chromosomal aberrations in cultured mammalian cells.
5.5. Evaluation1
There is inadequate evidence in humans for the carcinogenicity of CI Pigment Red 3.
There is limited evidence in experimental animals for the carcinogenicity of CI Pigment Red 3.
Overall evaluation
CI Pigment Red 3 cannot be classified as to its carcinogenicity to humans (Group 3).
6. References
- Aldrich Chemical Co. (1992) Aldrich Catalog/Handbook of Fine Chemicals 1992–1993, Milwaukee, WI, p. 1203.
- BASF Corp. Material Safety Data Sheet: S1CO Red NB L 3740 (Pigment Red 3), Parsippany, NJ Commission of the European Communities (1976) Council Directive 76/768/EEC of 27 July 1976. Off. J. Eur. Commun. 1991;L262:169–200.
- Commission of the European Communities. Commission Directive 86/179/EEC of 28 February 1986 (Seventh Adaptation to Technical Progress of Council Directive 76/768/EEC of 27 July 1976). Off. J. Eur. Commun. 1986;L138:40.
- Commission of the European Communities. Proposal for a Council Directive on the approximation of the laws of the Member States relating to cosmetic products. Off. J. Eur. Commun. 1990;C322:29–77.
- El Dareer S.M., Tillery K.F., Hill D.L. Investigations on the disposition of oral doses of some water-insoluble pigments. Bull. environ. Contam. Toxicol. 1984;32:171–174. [PubMed: 6704550]
- Green, F.J. (1990) The Sigma-Aldrich Handbook of Stains, Dyes and Indicators, Milwaukee, WI, Aldrich Chemical Co., p. 716.
- Miyagoshi M., Hayakawa Y., Nagayama T. Studies on the mutagenicity of cosmetic azodyes. Eisei Kagaku (J. hyg. Chem.). 1983;29:212–220.
- Morgan D.L., Jameson C.W., Mennear J.H., Prejean J.D. 14-Day and 90-day toxicity studies of CI Pigment Red 3 in Fischer 344 rats and B6C3F1 mice. Food Chem. Toxicol. 1989;27:793–800. [PubMed: 2606409]
- Mortelmans K., Haworth S., Lawlor T., Speck W., Tainer B., Zeiger E. Salmonella mutagenicity tests II. Results from the testing of 270 chemicals. Environ. Mutag. 1986;8(7):1–119. [PubMed: 3516675]
- Pouchert, C.J. (1981) The Aldrich Library of Infrared Spectra, 3rd ed., Milwaukee, WI, Aldrich Chemical Co., p. 1456.
- Sadtler Research Laboratories (1980) Sadtler Standard Spectra, 1980 Cumulative Index, Philadelphia, PA.
- Sadtler Research Laboratories (1991) Sadtler Standard Spectra, 1981–1991 Supplementary Index, Philadelphia, PA.
- Society of Dyers and Colourists (1971) Colour Index, 3rd ed., Vol. 3, Bradford, Yorkshire, pp. 3298– 3299.
- Society of Dyers and Colourists (1982) Colour Index, 3rd ed., Pigments and Solvent Dyes, Bradford, Yorkshire, p. 284.
- Stubbs, D.H. (1973) Toluidine, para and chlornitraniline reds. In: Patton, T.C., ed., Pigment Handbook, Vol. 1, Properties and Economics, New York, John Wiley & Sons, pp. 461–472.
- Sugai T., Takahashi Y., Takagi T. Pigmented cosmetic dermatitis and coal tar dyes. Contact Derm. 1977;3:249–256. [PubMed: 589997]
- US Environmental Protection Agency (1990) Textile Dye Weighing Monitoring Study (EPA Report No. EPA-560/5-90-009, Main Report and Site Visit Reports), Washington DC, Office of Toxic Substances.
- US Food and Drug Administration. 1992Cancellation of certificates. US Code fed. Regul. 21, pp. 366–369.part 81.30.
- US International Trade Commission (1977) Synthetic Organic Chemicals, US Production and Sales, 1975 (USITC Publication 804), Washington DC, US Government Printing Office, p. 79.
- US International Trade Commission (1981) Synthetic Organic Chemicals, US Production and Sales, 1980 (USITC Publication 1183), Washington DC, US Government Printing Office, p. 103.
- US International Trade Commission (1986) Synthetic Organic Chemicals, US Production and Sales, 1985 (USITC Publication 1892), Washington DC, US Government Printing Office, p. 87.
- US International Trade Commission (1991) Synthetic Organic Chemicals, US Production and Sales, 1990 (USITC Publication 2470), Washington DC, US Government Printing Office, p. 5–2.
- US National Library of Medicine (1992) Registry of Toxic Effects of Chemical Substances (RTECS No. QK4247000), Bethesda, MD.
- US National Toxicology Program (1992) Toxicology and Carcinogenesis Studies of CI Pigment Red 3 (CAS No. 2425-85-6) in F344/N Rats and B6C3F1 Mice (Feed Studies) (NTP TR 407; NIH Publ. No. 92–3138), Research Triangle Park, NC, US Department of Health and Human Services. [PubMed: 12621523]
Footnotes
- 1
For definition of the italicized terms, see Preamble, pp. 26–30.
- PubMedLinks to PubMed
- Review CI Acid Red 114.[IARC Monogr Eval Carcinog Risk...]Review CI Acid Red 114.. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:247-57.
- Review D&C Red No. 9 (CI Pigment Red 53:1).[IARC Monogr Eval Carcinog Risk...]Review D&C Red No. 9 (CI Pigment Red 53:1).. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:203-12.
- Review CI Direct Blue 15.[IARC Monogr Eval Carcinog Risk...]Review CI Direct Blue 15.. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:235-45.
- Direct Blue 6.[IARC Monogr Eval Carcinog Risk...]Direct Blue 6.. IARC Monogr Eval Carcinog Risk Chem Hum. 1982 May; 29:311-20.
- Review para-Chloroaniline.[IARC Monogr Eval Carcinog Risk...]Review para-Chloroaniline.. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:305-21.
- CI PIGMENT RED 3 - Occupational Exposures of Hairdressers and Barbers and Person...CI PIGMENT RED 3 - Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants; Some Hair Dyes, Cosmetic Colourants, Industrial Dyestuffs and Aromatic Amines
Your browsing activity is empty.
Activity recording is turned off.
See more...