NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Occupational Exposures in Insecticide Application, and Some Pesticides. Lyon (FR): International Agency for Research on Cancer; 1991. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 53.)
This substance was considered by a previous Working Group in 1978 (IARC, 1979a). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the present evaluation.
1. Exposure Data
1.1. Chemical and physical data
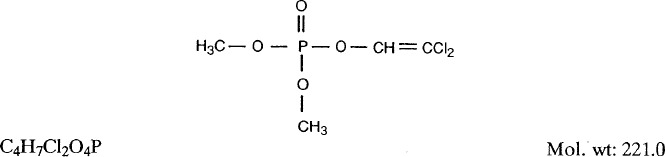
1.1.1. Synonyms, structural and molecular data
- Chem. Abstr. Serv. Reg. No.: 62-73-7
- Replaced CAS Reg. Nos.: 8023-22-1; 8072-21-7; 8072-39-7; 8076-16-2; 11095-17-3; 11096-21-2; 11111-31-2; 11126-72-0; 12772-40-6; 55819-32-4; 62139-95-1; 62655-59-8; 95828-55-0; 116788-91-1
- Chem. Abstr. Name: Phosphoric acid, 2,2-dichloroethenyl dimethyl ester
- IUPAC Systematic Name: 2,2-Dichlorovinyl dimethyl phosphate
- Synonyms: 2,2-Dichloroethenol, dimethyl phosphate; dimethyl dichlorovinyl phosphate; dimethyl 2,2-dichloroethenyl phosphate; dimethyl 2,2-dichlorovinyl phosphate; O,O-dimethyl 2,2-dichlorovinyl phosphate; 2,2-dichloroethenyl dimethyl phosphate; phosphoric acid, 2,2-dichlorovinyl dimethyl ester
1.1.2. Chemical and physical properties
From AMVAC Chemical Corp. (1986), otherwise specified
- (a) Description: Clear, colourless to pale yellow, almost odourless liquid
- (b) Boiling-point: 117°C at 10 mm Hg [1.33 kPa]
- (c) Melting-point: < −60 °C (AMVAC Chemical Corp., 1990)
- (d) Density: 1.422 at 25°C/4°C
- (e) Spectroscopy data: Infrared (prism [7721]; grating [44551P]) spectroscopy data have been reported (Sadtler Research Laboratories, 1980).
- (f) Solubility: Completely miscible with aromatic hydrocarbons, chlorinated hydro-carbons, alcohols, ketones and esters; slightly soluble in water (approx. 1%) and glycerine (approx. 0.5%); insoluble in kerosene and aliphatic hydrocarbons
- (g) Volatility: Vapour pressure, 0.012 mm Hg [1.6 x 10-3 kPa] at 20°C
- (h) Stability: Hydrolysed by water and readily decomposed by strong acids and bases; breaks down on standing in the presence of traces of moisture, with the formation of acidic products that catalyse further decomposition; corrosive to black iron and mild steel (WHO, 1989)
- (i) Octanol/water partition coefficient (P): log P, 1.47 (WHO, 1989)
- (j) Half-time in water: 301 min at pH 8; 462 min at pH 7; 2100 min at pH 6; 4620 min at pH 5.4 (Latif et al., 1984)
- (k) Conversion factor for airborne concentrations1: mg/m3 = 9.04 x ppm
1.1.3. Trade names, technical products and impurities
Some examples of trade names are: Atgard; Bibesol; Brevinyl; Canogard; Chlorvinphos; DDVP; Dedevap; Des; Dichlofos; Dichlorman; Dichlorovos; Divipan; ENT 20738; Equigard; Equigel; Estrosel; Estrosol; Fecama; Fekama; Insectigas D; Mopari; Nefrafos; Nerkol; Nogos; Novotox; Nuan; Nuvan; OKO; OMS 14; Panaplate; Phosvit; Prima U; SD 1750; Szklarniak; TAP 9VP; Task; Unifos; Unitox; Vapona; Vapona Insecticide; Vaponite; Vinylofos; Vinylophos; Winylophos
WHO (1985, 1989) specifications for technical-grade dichlorvos for public health use require a minimum purity of 97%; the value was previously 93% (WHO, 1967).
Dichlorvos is available in the USA as a technical-grade product with a minimal purity of 93 or 96% (AMVAC Chemical Corp., 1986, 1990). In the past, 2–4% epichlorohydrin (see IARC, 1987a) was added to stabilize the technical-grade product; other stabilizers may now be used in some products, but improved technology and purity has largely eliminated the need for them (WHO, 1989). Analysis of a sample of commercial-grade dichlorvos (produced in about 1970) showed the following constituents (%): dichlorvos, 95–97; dipterex (trichlorfon; 0,0-dimethyl 2,2,2-trichloro-l-hydroxyethylphosphonate) (see IARC, 1983a, 1987b), 1.5–3; 0,0-dimethyl 2-chlorovinyl phosphate, 0.4–0.7; 0,0-dimethyl methyl-phosphate, trace-0.1; 0,0,0-trimethyl phosphate, 0.3–0.8; and chloral (trichloro-acetaldehyde), 0.1–0.5 (Santodonato et al., 1985).
In the USA and Europe, registered formulations include dusts, granules, pellets/tablets, impregnated resin strips, emulsifiable concentrates, soluble concentrates, wettable powders and pressurized formulations (Royal Society of Chemistry, 1986; US Environmental Protection Agency, 1987). In the USSR, dichlorvos is formulated as emulsion concentrates pellets and aerosols (Izmerov, 1984). Dichlorvos is also formulated in combination with dimethoate, dinocap, fenchlorphos, fenitrothion, iodofenphos, lindane (see IARC 1987c) malathion (see IARC, 1983b, 1987d), methoxychlor (see IARC, 1979b, 1987e) phosalone, piperonyl butoxide (see IARC, 1983c, 1987f), pirimiphos-methyl, propoxur, tetrasul, pyrethrins and trichlorfon (Royal Society of Chemistry, 1986).
1.1.4. Analysis
Selected methods for the analysis of dichlorvos in various matrices are given in Table 1. Dichlorvos residues can be determined by gas chromatography; the same method can be used for product analysis. Alternative methods include infrared spectrometry and reaction with excess iodine, with estimation by titration (WHO, 1989). Several other methods in various media have been reviewed (Porter, 1964; Anon., 1972; Vevai, 1974; Worthing & Walker, 1987; WHO, 1989).
Table 1.
Methods for the analysis of dichlorvos.
1.2. Production and use
1.2.1. Production
Dichlorvos was first synthesized in the late 1940s (Tinker, 1972). It has been commercially manufactured and used throughout the world since 1961 (WHO, 1989). It was first found as a highly insecticidal impurity of trichlorfon (chlorophos) in 1955;trichlorfon is rapidly converted to dichlorvos at above pH 6 (Eto, 1974). Dichlorvos is manufactured by the dehydrochlorination of trichlorfon in aqueous alkali at 40–50 °C or by the reaction between chloral and trimethyl phosphite (WHO, 1989).
Dichlorvos is produced currently in Argentina, Brazil, Germany, India, Israel, Japan, the Republic of Korea, Mexico, the Netherlands, Spain, Sweden, Switzerland and the USA (Meister, 1990). The present worldwide production of dichlorvos is about 4000 tonnes per year (WHO, 1989). Worldwide production figures for 1984 were as follows (tonnes): eastern Europe, 220; western Europe, 300; Latin America, 400; south-east Asia, 500; USA, 500; Japan, 1100; Middle East, India and Pakistan, 1200 (WHO, 1989).
1.2.2. Use
Dichlorvos is a contact and stomach insecticide with fumigant and penetrant action. It is used as granules or impregnated resin to control internal and external parasites (especially fleas and ticks) in livestock and as aerosols or liquid sprays or as impregnated cellulose ceramic or resin strips to control insects in houses, buildings and outdoor areas (especially flies and mosquitos). It is also used to protect certain crops (and other plants) from insects in the field and in storage. Dichlorvos is not generally applied directly to soil, but it is added to water to control parasites in the case of intensive fish farming (WHO, 1989). It is used as an antihelminthic by incorporation in animal feeds (Worthing & Walker, 1987).
Worldwide, it is estimated that 60% is used in plant protection, 30% for public health and vector control and 10% to protect stored products (WHO, 1989).
In the USA, in 1971, 16 tonnes of dichlorvos were used on crops (mainly tobacco) and 1100 tonnes were used on livestock and livestock buildings; in 1976, 50 tonnes were used on crops and 390 on livestock. In 1975, 80% of the dichlorvos produced in the USA was formulated into polyvinyl chloride resin strips containing 20% by weight of dichlorvos which were used primarily in households. These strips were first marketed in 1967 to control flies and mosquitoes in the home; they were introduced earlier in dairy and poultry operations. Flea collars containing dichlorvos, for dogs and cats, have also been commercially marketed (Santodonato et al., 1985).
In the USA in 1980, the yearly agricultural usage of dichlorvos (active ingredient) was estimated as follows (tonnes): dairy cattle, 340; beef cattle, 30; hogs, 6; poultry, 2; and other livestock, 14; about 50 tonnes were used for treatment of tobacco. Overall, 680–1200 tonnes of dichlorvos were used for agricultural uses, 450 tonnes for public health and about 450 tonnes for household use (US Environmental Protection Agency, 1980). Less than 450 tonnes of dichlorvos (active ingredient) are believed to have been used in the USA in 1989.
In Finland, about 1500 kg (active ingredient) of dichlorvos were sold in 1988 (Agrochemical Producers' Association of Finland, 1989).
1.3. Occurrence
1.3.1. Air
Dichlorvos is degraded rapidly in air, the rate depending on humidity. The method of application is an important factor in determining its concentration in air (Gillett et al.,1972a).
Examples of indoor air concentrations resulting from household and public health use are shown in Table 2 (WHO, 1989).
Table 2.
Indoor air concentrations of dichlorvos following various application.
The highest exposure recorded in a vaporizer production plant and its packaging rooms was 3 mg/m3, with an average value of 0.7 mg/m3 (Menz et al., 1974). The mean concen-tration of dichlorvos in air did not exceed 0.5 ppb (0.005 mg/m3) in the office and insecticide storage rooms of commercial pest control buildings and 0.1 ppb (0.001 mg/m3) in vehicles (Wright & Leidy, 1980).
Dichlorvos was sprayed at 8 ml active ingredient/100 m3 in a unit used for mushroom cultures, and kept closed for 24 h. The air concentration decreased from 3.3 to 0.006 mg/m3 in 24 h. The unit was also treated with paper strips drenched in 50% dichlorvos formulations (40 ml/100 m3), which gave air concentrations of 0.38 and 0.024 mg/m3 at 3 and 24 h, respectively (Grübner, 1972).
Following weekly 6-h applications, the maximum concentrations of dichlorvos observed in a large warehouse ranged from 2.4 to 7 mg/m3. The amount of dichlorvos dispensed per application was 25–59 mg/m3, which resulted in average air concentrations after eight applications of 4 mg/m3 (Gillenwater et al., 1971).
The work place concentration resulting from hot spraying of dichlorvos in six greenhouses at 0.4 ml/m3 was 7–24 mg/m3 (average, 16 mg/m3) (Wagner & Hoyer, 1975). Spraying of 12 glass and plastic greenhouses gave concentrations between 0.7 and 2.7 mg/m3 (average, 1.3 mg/m3). Field application by spraying resulted in air concentrations of 0.01–0.26 mg/m3 (average, 0.08 mg/m3) (as reported by WHO, 1989). The air concentration of dichlorvos in greenhouses immediately after spraying with 0.2–0.3% dichlorvos solutions was 1.2 mg/m3, which decreased to 0.01 mg/m3 within 24 h. Disturbing the plants resulted in an increase of 10–26% in the dichlorvos concentration in air (Zotov et al., 1977).
In a study designed to test the aeration period needed for safe reentry into a room following dichlorvos treatment with a pressurized home-fogger, the air levels after 30 min were below the industrial workplace permissible exposure level of 1 mg/m3. Without ventilation, 18 h were required to reach an acceptable level. Because of concern for the health of infants and elderly persons, the acceptable level for homes was established at 1/40 of the permissible exposure level. Rooms treated with this type of applicator and ventilated after treatment were considered safe for reentry after 10 h (as reported by WHO, 1989).
Monitoring of mushroom-growing houses in the USA gave air concentrations of 0.1 mg/m3; swabs of exposed surfaces revealed maximal residues of 0.026 µg/cm2 (as reported by WHO, 1989).
In houses treated for pest control with 230–330 g dichlorvos as an aerosol and 40–50 g as emulsion spray, the mean dichlorvos residue on surfaces was 0.24 µg/cm2 at the end of day 1 and decreased to 0.06 µg/cm2 by day 5 (Das et al., (1983).
1.3.2. Water
In water, dichlorvos is hydrolysed into dimethyl phosphoric acid and dichloroacetic acid (WHO, 1989).
1.3.3. Soil
Dichlorvos vaporized in a mushroom house to give 0.2–0.4 mg/m3 degraded rapidly in the moist loam soil, with only 37% remaining after 3 days. The amount of free dichloroacetaldehyde at that time was 4% (Hussey & Hughes, 1964).
Dichlorvos is degraded by microorganisms. Bacillus cereus utilizes it as a single carbon source. In soil columns perfused with an aqueous solution containing dichlorvos at 1 kg/litre, 30% of the loss of the compound was attributed to microbial action (Lamoreaux & Newland, 1978). Species of Pseudomonas derived from sewage converted dichlorvos to dichloroethanol, dichloroacetic acid and ethyl dichloroacetate (Lieberman & Alexander, 1983). Fungi, such as Trichoderma viride, also degrade dichlorvos into water-soluble metabolites (Matsumura & Boush, 1968).
1.3.4. Plants
Dichlorvos is rapidly lost from leaf surfaces by volatilization and hydrolysis, with a half-time of only a few hours. A small amount penetrates the waxy layers of plant tissues, where it may persist for longer (FAO/WHO, 1971).
In California, the estimated safe level of dislodgeable foliar dichlorvos from turf is 0.06 µg/cm2 (WHO, 1989). Studies by Goh et al. (1986a,b) found that dislodgeable foliar dichlorvos residues decreased rapidly after 2–6 h and were not detectable after 24–48 h.
1.3.5. Food
Data on residues in food commodities resulting from pre- and post-harvest treatment and from use on animals were summarized by FAO/WHO (1967, 1968, 1971, 1975).
In Canada, of 262 bovine and porcine fat samples analysed between 1973 and 1981, only one was contaminated with dichlorvos (Frank et al., 1983). In a national surveillence programme in Canada, 1984–85 to 1988–89, no residue was found in 898 samples of fruit, vegetables, meat or wine (Government of Canada, 1990).
Normally, dichlorvos residues present in food are destroyed by washing and cooking. Abbott et al. (1970) confirmed the absence of residues in a total-diet study in the United Kingdom, 1966–67, finding no dichlorvos in 462 samples. A total-diet study carried out in the USA from 1975 to 1976 gave similar results (Johnson et al., 1981).
Food, meals and unwrapped ready-to-eat foodstuffs exposed to dichlorvos from resin strips had mean residue levels of < 0.05 mg/kg (range, < 0.01−0.1 mg/kg) (Elgar et al., 1972a,b) and < 0.02 mg/kg (Collins & deVries, 1973). No residue of dichloroacetaldehydè (< 0.03 mg/kg) was detected in the ready-to-eat foodstuffs (Elgar et al., 1972b). Food and beverages exposed to air concentrations of 0.04−0.58 mg/m3 for 30 min contained dichlorvos residues of 0.005−0.5 mg/kg, except for margarine which had up to 1.7 mg/kg (Dale et al., 1973).
1.3.6. Occupational exposure
Mixed dermal and inhalation exposures were assessed for 13 professional pesticide applicators after a day's work spraying dichlorvos preparations. Absorbent pads recorded average exposures of 0.08 μ.g/cm2 on the back and 0.04 μg/cm2 on the chest. Levels found in respirator filters were 1.1 μg/cm2, in contrast to surface residues of 0.04−0.5 μg/cm2 measured at various sites around the treated houses. Although the men wore protective equipment, some absorption of dichlorvos occurred, as shown by the recovery of 0.32−1.4 μg dimethylphosphate from their urine (Das et al., 1983).
1.4. Regulations and guidelines
The FAO/WHO Joint Meeting on Pesticide Residues evaluated dichlorvos at its meetings in 1965, 1966, 1967, 1969, 1970, 1974 and 1977 (FAO/WHO, 1965, 1967, 1968, 1970, 1971, 1975, 1978). In 1966, the Meeting established an acceptable daily intake for humans of 0.004 mg/kg bw (FAO/WHO, 1967).
Maximum residue levels have been established by the Codex Alimentarius Commission for dichlorvos in or on the following agricultural commodities (in mg/kg): fruit (e.g., apples, peaches, pears, strawberries), 0.1; mushrooms and vegetables (except lettuce), 0.5; head lettuce, 1; cereal grains, coffee beans, dried lentils, dried soya beans and peanuts, 2; and cacao beans, 5. As such residues decline rapidly during storage and shipment, these limits are based on residues likely to be found at harvest (Codex Committee on Pesticide Residues 1990).
Maximum residue limits have also been established by the Codex Alimentarius Commission for dichlorvos in or on the following animal commodities (in mg/kg): milk, 0.02; eggs, goat meat, meat of cattle, pigs, sheep and poultry, 0.05, based on residues likely to be found at slaughter (Codex Committee on Pesticide Residues, 1990).
In the USSR, dichlorvos residues are not allowed in fishing areas; however, a level of 0.1 mg/1 was established for other surface waters (Izmerov, 1984).
National and regional pesticide residue limits for dichlorvos in foods are presented in Table 3.
Table 3.
National and regional pesticide residue limits for dichlorvos in foods.
Occupational exposure limits and guidelines for dichlorvos in some countries and regions are given in Table 4.
Table 4.
Occupational exposure limits and guidelines for dichlorvos.
2. Studies of Cancer in Humans
2.1. Case reports
In a case series, four children with aplastic anaemia and one with acute lymphoblastic leukaemia were reported by their parents to have been exposed at home to dichlorvos and propoxur (Reeves et al., 1981).
2.2. Case-control studies
In a case-control study of leukaemia in the USA(Brown et al., 1990), described in detail in the monograph on occupational exposure in spraying and application of insecticides (p. 68), significant excesses of leukaemia were noted among farmers who reported use of dichlorvos on animals (odds ratio, 2.0; 95% confidence interval [CI], 1.2–3.5). Risks were greater among those who had first used dichlorvos 20 or more years before diagnosis of leukaemia (odds ratio, 2.4; 95% CI, 1.1–5.4). The risks were greatest among farmers who used dichlorvos on animals on 10 or more days per year (odds ratio, 3.8; 95% CI, 1.0–14.8). The risk for leukaemia in this study was also associated with use of other agricultural pesticides, including crotoxyphos, famphur, pyrethrins, methoxychlor, nicotine and DDT, and it was not possible to evaluate exposure to dichlorvos in the absence of these other pesticides.
3. Studies of Cancer in Experimental Animals
The Working Group was aware of a study by Horn et al. (1987), which was of short duration and not considered informative for an evaluation.
3.1. Oral administration
3.1.1. Mouse
Groups of 50 male and 50 female B6C3F1 hybrid mice, five to seven weeks of age, were fed technical-grade dichlorvos (minimum purity, 94%) in the diet at initial doses of 1000 and 2000 mg/kg. After two weeks, the doses were reduced to 300 and 600 mg/kg of diet, respectively, due to severe toxicity, and treated animals were maintained at these dietary levels for 78 weeks followed by 12–14 weeks on dichlorvos-free diets, after which time (92–94 weeks) the animals were killed and necropsied. The measured time-weighted average doses were 318 and 635 mg/kg of diet, respectively. Groups of 10 male and 10 female mice that served as matched controls were maintained on dichlorvos-free diets for 92 weeks; further control data were obtained from pooled control animals (100 males and 80 females). In females, 13/50 low-dose animals died before week 90; survival to 90 weeks was greater than 84% in all other groups. Average weights of high-dose males and females were generally lower than those of the low-dose and control groups, but the differences did not exceed 10%. The only findings of note were two squamous-cell carcinomas of the oesophagus (in one low-dose male and one high-dose female), one papilloma of the oesophagus (in a high-dose female) and three cases of focal hyperplasia of the oesophageal epithelium (in three low-dose males) (US National Cancer Institute, 1977). [The Working Group noted the short duration of treatment.]
Groups of 50 male and 50 female B6C3F1 mice, eight weeks of age, were administered 0, 10 or 20 (males) and 0, 20 or 40 mg/kg bw (females) dichlorvos (99% pure) in corn oil by gavage per day on five days per week for 103 weeks. Survival was not affected by treatment. The incidence of squamous-cell papillomas of the forestomach was increased in males and females. A significant dose-response trend for the incidence of squamous-cell papillomas was seen in males (1/50 control, 1/50 low-dose, 5/50 high-dose; p = 0.032) and in females (5/49 control, 6/49 low-dose, 18/50 high-dose; p = 0.002). In females, the incidence in the high-dose group was significantly greater than that in controls (p = 0.004). Two of 50 high-dose females also had squamous-cell carcinomas (US National Toxicology Program, 1989).
3.1.2. Rat
Groups of 50 male and 50 female Osborne-Mendel rats, five to seven weeks of age, were fed diets containing 150 or 1000 mg/kg of diet technical-grade dichlorvos (minimum purity, 94%); due to severe toxicity, the high-dose was reduced to 300 mg/kg of diet after three weeks. Both groups were treated for 80 weeks and were maintained for a further 30 weeks on a dichlorvos-free diet. Time-weighted average doses were 150 and 326 mg/kg of diet, respectively. Groups of 10 males and 10 females served as matched controls and groups of 60 animals of each sex as pooled controls. Weight gain was consistently lower in high-dose groups than in low-dose and control groups. No significant difference in survival was observed between treated and control groups at 105 weeks. The incidence of malignant fibrous histiocytomas in male rats showed a statistically significant trend (pooled control 2/58; low-dose, 4/48; high-dose, 8/50;p = 0.018); a histiocytoma occurred in 1/10 matched male controls (US National Cancer Institute, 1977). [The Working Group noted the short duration of treatment].
Technical-grade dichlorvos (97% purity) was administered by gavage in water to 70 male and 70 female rats (inbred strain BD IX/Bln), six to eight weeks of age, at a dose of 0.1 mg per animal twice a week; or to 99 male and 99 female rats at a dose of 0.1 mg per animal three times a week for 60 weeks. Groups of 59 male and 60 female rats served as vehicle controls. Animals were killed 111 weeks after the beginning of treatment. There was no difference in median survival times between treated and control animals. Forestomach papillomas were observed in two males and in one female that received 0.3 mg dichlorvos. One male and one female rat receiving 0.3 mg had two and five papillomas of the urinary bladder, respectively (Horn et al., 1988). [The Working Group noted the short duration of exposure.]
Groups of 50 male and 50 female Fischer 344/N rats, seven weeks of age were administered 0, 4 or 8 mg/kg bw dichlorvos (99% pure) per day in corn oil by gavage on five days per week for 103 weeks. Survival was 31/50 control, 25/50 low-dose and 24/50 high-dose males and 31/50 control, 26/50 low-dose and 24/50 high-dose females; body weight gain was not affected by administration of dichlorvos. The incidence of acinar-cell adenomas of the pancreas was increased in treated males (16/50 control, 25/49 low-dose and 30/50 high-dose; p < 0.001 for trend). Further examination of horizontal sections of all pancreases revealed increases of reduced statistical significance (25/50,30/50 and 33/50; [p for trend = < 0.05]). There were also more male rats with multiple adenomas in the treated groups than among controls (2/50, 7/49 and 13/50). Mononuclear-cell leukaemia occurred with a significant dose-response trend in male rats (p = 0.011), and the incidence in each of the treated groups was significantly greater than that in controls (11/50 control, 20/50 low-dose and 21/50 high-dose males). In females, fibroadenomas and adenomas of the mammary gland occurred with a significant dose-response trend (p = 0.028), and the incidence in both treated groups was significantly greater than that in controls (9/50 control, 19/50 low-dose and 17/50 high-dose females). Two female rats in the control group and two in the low-dose group had carcinomas of the mammary gland (US National Toxicology Program, 1989).
3.2. Inhalation and/or intratracheal administration
Rat: Groups of 50 male and 50 female Carworth Farm E strain rats, five weeks of age, were exposed continuously to atmospheres containing 0 (control), 0.05, 0.5 or 5 mg/m3 technical-grade dichlorvos (purity, > 97%) for 104 weeks. The mean values for the entire test period were 0.05, 0.48 and 4.7 mg/m3 [range ± 20%]. All treated groups showed decreased weight gain compared with controls, especially in the high-dose group The numbers of males surviving at 99–102 weeks were 11/50 control, 21/50 low-dose, 15/50 mid-dose and 32/50 high-dose; survival in females at 104 weeks was 22/47 control, 27/47 low-dose, 26/47 mid-dose and 34/47 high-dose. Complete necropsy and histopathology were performed on 20–32% of males and 22–38% of females, reducing the effective numbers of animals per group to between 10 and 18. No significant increase in tumour incidence couldbe attributed to treatment (Blair et al., 1976). [The Working Group noted the small numbers of animals submitted for complete necropsy.]
Studies of cancer in experimental animals are summarized in Table 5.
Table 5.
Studies of cancer in experimental animals.
4. Other Relevant Data
The toxicity of dichlorvos has been reviewed (FAO/WHO, 1965, 1967, 1968, 1971; Anon., 1974; FAO/WHO, 1978; WHO, 1989).
4.1. Absorption, distribution, metabolism and excretion
4.1.1. Humans
Dichlorvos is rapidly hydrolysed in human blood (half-time, 7–11 min), and no unchanged dichlorvos was found (detection limit, 0.1 μg/g) in blood samples taken 1 min after cessation of inhalation by two male volunteers exposed to 0.25 mg/m3 for 10 h or to 0.7 mg/m3 for 20 h (Blair et al., 1975).
A human volunteer who ingested 5 mg [14C-vinyl]-dichlorvos excreted radiolabel at a rate similar to that seen after comparable oral dosing of rats, mice and hamsters, except that the output of 14CO2 was somewhat greater. The urinary metabolites were tentatively identified as demethyldichlorvos, urea and hippuric acid (Hutson & Hoadley, 1972a).
More recently, it was established that dimethylphosphate is a metabolite in the urine of workers occupationally exposed to dichlorvos (Das et al., 1983).
4.1.2. Experimental systems
The metabolism and disposition of dichlorvos have been reviewed (Wright et al., 1979).
Metabolic disposition studies using radiolabelled dichlorvos have been reported in rats, mice, hamsters, pigs and humans. Labelling at different sites (e.g., 14C-methyl, 14C-vinyl, 36Cl-chlorovinyl, 32P-phosphate) has enabled specific pathways to be traced (Hutson et al., 1971; Hutson & Hoadley, 1972a,b; Page et al., 1972; Potter et al.„ 1973; Blair et al., 1975). Furthermore, metabolic disposition has been determined after both inhalation exposure and oral administration (including slow-release polyvinyl chloride-pelleted dose forms).
There are two main metabolic pathways for dichlorvos: (1) ester hydrolysis of the PO-vinyl group to yield dimethylphosphate and dichloroacetaldehyde and (2) oxidative O-demethylation to demethyldichlorvos and formaldehyde. An alternative pathway for O-demethylation involves conjugation with glutathione (Dicowsky & Morello, 1971; Hutson et al., 1971; Hutson & Hoadley, 1972a,b; Page et al., 1972; Potter et al.„ 1973; Blair et al., 1975). Hydrolysis of the O-demethylated metabolite yields methylphosphate and, eventually, phosphoric acid and methanol (WHO, 1989). Radiolabel from [14C-methyl] and [14C-vinyl]-dichlorvos is ultimately incorporated into CO2 (e.g., 39% of an oral dose of [14C-vinyl]-dichlorvos over four days in rats) and enters the 1 and 2-carbon metabolic pools, resulting in the labelling of amino acids, proteins and purines. This labelling may confound the interpretation of studies of tissue disposition and urinary excretion if the chemical specificity and source of the radiolabel are not determined.
Patterns of urinary metabolites indicate that metabolic clearance varies very little by species or route. The hydrolysis pathway generally predominates over the O-demethylation pathway, although the latter is more prominent in mice.
Hydrolytic metabolism of dichlorvos to dimethylphosphate and dichloroacetaldehyde, which in turn is rapidly reduced to dichloroethanol and conjugated with glucuronic acid, is so rapid that the half-time for the reaction in vivo has not been determined with any accuracy. In vitro, the half-time for blood-catalysed hydrolysis ranges from 2 min in rabbits to 30 min in rats. In human blood, the half-time is approximately 10 min, and the Km for the reaction has been estimated to be approximately 3 μM. For this reason, unchanged dichlorvos is detected in blood only at relatively high dose rates (Blair et al., 1975).
4.2. Toxic effects
4.2.1. Humans
The adverse effects of dichlorvos in humans have been reviewed (Cavagna & Vigliani, 1970; Gillett et al., 1972a,b; Hayes, 1982). Depression of plasma cholinesterase is the most sensitive indicator of exposure to dichlorvos but is not necessarily an indicator of toxicity. At higher dose levels, red blood cell cholinesterase may also be affected.
Dichlorvos was administered in the form of slow-release polyvinyl resin formulation pellets as single doses (1–32 mg/kg bw) to 107 men and as repeated doses (1–32 mg/kg bwper day for 2–7 days; 1–16 mg/kg bw per day for up to three weeks) to 38 men. Maximal plasma cholinesterase depression occurred at approximately 6 mg/kg bw (single dose) and 1 mg/kg bw per day (repeated dose over three weeks). The single-dose threshold for plasma cholinesterase depression was approximately 1–3 mg/kg bw. Red blood cell cholinesterase activity was depressed at doses approximately four-fold higher. While the incidence of transient gastrointestinal and central nervous system-related subjective effects which accompanied the cholinesterase depression was relatively low at the lowest dose rates, they were sufficiently adverse to cause subjects given repeated doses of 8–32 mg/kg bw per day to withdraw from the study (Slomka & Hine, 1981).
Airborne levels of dichlorvos which cause slight to moderate cholinesterase depression have been reported to be 0.7 mg/m3 average over one year in factory workers producing dichlorvos vaporizers (Menz et al., 1974) and 0.1 mg/m3 for 24 h per day in children and adults hospitalized for various periods in wards provided with dichlorvos-impregnated plastic strips. Plasma (but not red blood cell) cholinesterase levels were slightly depressed in 11 hospitalized babies exposed to air levels of over 0.1 mg/m3 for 24 h per day, but children of 2–7 years were not affected at the same exposure level for 16 h per day (Cavagna et al., 1969). As reported in an abstract, neither plasma nor red blood cell cholinesterase depression was found in 22 newborn babies when the average air levels of dichlorvos were reported to be up to 0.159 mg/m3 (Vigliani, 1971).
Lethal exposures to dichlorvos have been reported in connection with accidental splashing of a concentrated formulation, coupled with failure to wash the material off (Hayes, 1982). A case of systemic poisoning resulted from an accident in which dichlorvos spray leaked down a man's back (Bisby & Simpson, 1975). Another accidental incident of skin contact resulted in symptomatic effects followed by the development of a persistent contact dermatitis (Mathias, 1983).
4.2.2. Experimental systems
The toxicology of dichlorvos in experimental animals has been reviewed (Attfield & Webster, 1966; Gillett et al., 1972a,b; Anon., 1974; Wright et al., 1979).
Dichlorvos is acutely neurotoxic by virtue of its ability to inhibit brain cholinesterase. The acute oral LD50 in rats was cited as 56–80 (Durham et al., 1957) and 25-30 mg/kg bw (Ben-Dyke et al., 1970) and that in mice as 140–275 mg/kg bw(Anon., 1974; Holmstedt et al., 1978). The oral LD50 of dichlorvos in young pigs was 157 mg/kg bw; no death occurred in animals administered up to 100 mg/kg of a polyvinyl chloride formulation of dichlorvos (Stanton et al., 1979). The large range cited for the dermal LD50 (75–900 mg/kg bw) in rats suggests that skin absorption is vehicle-dependent (Jones et al., 1968).
Exposures after which cholinesterase depression was the only discernible toxic effect include two-year inhalation exposure of rats to 0.5–5 mg/m3 (Blair et al., 1976), 90-day feeding of 0.4–70 mg/kg bw per day to rats (effects observed at 3.5 mg/kg per day and above; Durham et al., 1957), administration for 30 days of 1–16 mg/kg bw per day in polyvinyl chloride pellets to pigs (Stanton et al., 1979) and administration for 10–21 days of 10–80 mg/kg bw per day in polyvinyl chloride pellets to rhesus monkeys (Hass et al., 1972).
Dichlorvos has been ascribed only a slight risk of causing delayed neuropathy, because doses that inhibit neuropathy target esterase and result in ataxia in hens exceed the LD50 by several fold; protection with atropine is required if the test is to be completed (Johnson 1978; Caroldi & Lotti, 1981; Johnson, 1981).
Both humoral immune response and cell-mediated immunity were inhibited in rabbits treated orally with dichlorvos for five days a week for up to five to six weeks at high dose rates (0.31–2.5 mg/kg bw: 2.5–20% of the LD50; Dési et al., 1978, 1980). Immunosuppression was also observed in mice given 120 mg/kg bw orally, but the authors commented that this phenomenon, seen with other organophosphonates and the cholinomimetic compound, arecoline, may be secondary to a profound cholinergic stimulation (Casale et al., 1983).
The diurnal rhythm of the pituitary/adrenal axis was altered in rats given 2 ppm (mg/1) dichlorvos in the drinking-water for two weeks (approximate intake, 0.3 mg/kg bw per day), causing changes in plasma adrenocorticotrophic hormone levels and adrenal cholesterol ester concentrations. While adrenocorticotrophic hormone secretion is believed to be acetylcholine-sensitive, there was no detectable change in cholinesterase activity (Civen et al., 1980).
Reactions with macromolecules: Dichlorvos is a phosphorylating and alkylating agent (Wright et al., 1979). 4-Nitrobenzylpyridine is alkylated by dichlorvos (half-time, 28 min) more slowly than methyl methanesulfonate (half-time, 9.6 min). Metabolites of dichlorvos did not react with 4-nitrobenzylpyridine in this system (Bedford & Robinson, 1972). The relative reactivity of dichlorvos toward 4-nitrobenzylpyridine and acetylcholinesterase was greatly in favour of esterase phosphorylation (WHO, 1989), indicating that dichlorvos-associated methylati ón of DNA purines may not be as important in vivo as the esterase phosphorylation reaction (Wright et al., 1979; Wooder et al., 1977).
There may appear to be some conflict between this conclusion and the detection of radiolabeled N-7-methylated guanine in mouse urine following administration of [14C-methyl]- or [3H-methyI]-dichlorvos (24–90 µCi intraperitoneally or an estimated 8.5–11 µCi by inhalation) (Wennerberg & Löfroth, 1974). Since, however, methylated purines occur naturally in urine and [14C-methyl]-dichlorvos metabolites enter the 1- and 2-carbon metabolic pool, it has been suggested that the mechanism of methylation may be indirect (Wooder & Wright, 1981). No N-7-guanine methylation was found in the DNA of lung, liver, heart, brain, testes or spleen of 20 rats exposed to [14C-methyl]-dichlorvos by inhalation at 0.064 μg/I for 12 h (estimated total dose, 6 μg; specific activity, 113 μCi/mmol; resulting in a DNA detection limit of 0.000001% of the dose) (Wooder et al., 1977).
Segerbäck and Ehrenberg (1981) also concluded that the likelihood of DNA methylation after dosing with dichlorvos in vivo is extremely small. Their estimate of the amount of DNA methylation in mice after intraperitoneal dosing with 1.9 μmol/kg bw [0.42 mg/kg] is of the order of 8 x 10-13 mol methyl per gram of DNA.
4.3. Reproductive and developmental effects
4.3.1. Humans
No data were available to the Working Group.
4.3.2. Experimental systems
Female Sherman rats were treated intraperitoneally with dichlorvos at 15 mg/kg bw in peanut oil on day 11 of gestation. No difference was noted in weight gain, number of fetuses per litter, number of resorptions per pregnant rat or weight of the fetuses or placentae on day 20 of gestation. Three omphalocoeles occurred among 41 offspring in the treated group, but no malformation was noted among controls (Kimbrough & Gaines, 1968).
No adverse developmental effect was observed in CF-1 mice administered the maximal tolerated dose by gavage on days 6–15 of gestation or in New Zealand rabbits administered 60 and 5 mg/kg bw per day on days 6–18 of gestation or by inhalation at 4 mg/m3 for 7 h per day (Schwetz et al., 1979).
Pregnant rabbits were treated [route not given] with dichlorvos at a dose of 6 mg/kg bw per day for the last 10 days of gestation. Light-microscopic examination of the brains of six pups from treated and six from untreated dams sacrificed at birth revealed no alteration in brain morphology; electron microscopic examination suggested 'immaturity' or delay in brain development in the treated animals. Synaptic junctions quantified in the motor cortex using electron microscopy were considered to be immature (Dambska et al., 1979). [The Working Group noted the lack of adequate controls and the poor description of the study.]
Carworth E rats and Dutch rabbits were exposed to dichlorvos in air at concentrations of up to 6.25 mg/m3 and 4 mg/m3, respectively, for 23 h per day on seven days per week from the day of mating until the end of gestation. These treatments produced a dose-dependent decrease in plasma, red cell and brain cholinesterase activity in both species but had no effect on the number of pregnancies, the number of resorptions, the number of fetal deaths, litter size or fetal weight in rats or rabbits (Thorpe et al., 1972).
In pregnant sows fed a polyvinyl chloride formulation of dichlorvos at doses of 5 or 25 mg/kg bw per day for the last 30 days of gestation, no alteration in reproductive performance was observed. Plasma and red cell cholinesterase activities and, at the high dose, myometrial acetylcholinesterase activity were decreased in the sows; the rhomb-encephalic acetylcholinesterase level was increased in fetuses (Stanton et al., 1979).
A series of early studies reported in abstracts also examined reproductive and developmental effects. No effect on reproduction or development was seen in more than 6000 offspring of male and female rats treated for three generations with dichlorvos in feed at doses of up to 500 ppm [mg/kg] (Witherup et al., 1971). In rabbits treated orally with a polyvinyl chloride formulation of dichlorvos, maternal toxicity was seen at 34 mg/kg; no alteration was observed in reproductive or developmental parameters at doses not associated with maternal toxicity (Vogin et al., 1971). No effect on reproduction or development was seen over two generations in male and female swine treated for 37 months at doses in the feed of up to 500 ppm [mg/kg] (Collins et al., 1971).
4.4. Genetic and related effects (see also Table 6 and Appendices 1 and 2)
Table 6.
Genetic and related effects of dichlorvos.
4.4.1. Humans
No data were available to the Working Group.
4.4.2. Experimental systems
The genetic activity of dichlorvos has been reviewed (Ramel et al., 1980).
In bacteria, dichlorvos bound covalently to DNA, RNA and protein and caused DNA damage and point mutations. Bacterial mutagenicity was reduced in the presence of liver preparations. Dichlorvos induced gene conversion, mutation and aneuploidy in yeast and fungi, and mutation, chromosomal aberrations and micronucleus formation in plants. In Drosophila melanogaster, chromosomal aberrations but not sex-linked recessive lethal mutation were induced. Autosomal lethal and polygenic viability mutations were induced in D. melanogaster by treatment over multiple generations. [The Working Group considered that these tests are not well validated.] In mammalian cells in vitro, dichlorvos caused DNA strand breaks, mutation, sister chromatid exchange, chromosomal aberrations and cell transformation. In human cells in vitro, it induced unscheduled DNA synthesis but neither chromosomal aberrations nor sister chromatid exchange.
No significant response was observed in vivo in any of the mammalian tests used for the induction of unscheduled DNA synthesis, sister chromatid exchange, micronucleus formation, chromosomal aberrations or dominant lethal mutation.
5. Summary of Data Reported and Evaluation
5.1. Exposure data
Dichlorvos has been used widely as an insecticide since 1961 to control internal and external parasites in livestock and domestic animals, to control insects in houses, and in crop protection.
Dichlorvos has been formulated for use as dusts, granules, pellets/tablets, impregnated resin strips and concentrates.
Household and public health uses represent the main sources of human exposure to dichlorvos. Exposure may also occur during its production and application.
5.2. Carcinogenicity in humans
One case-control study of leukaemia in the USA found an association with use of dichlorvos on animals; there were few exposed subjects, and they had potential exposure to many pesticides.
5.3. Carcinogenicity in experimental animals
Dichlorvos was tested for carcinogenicity by oral administration in two experiments in mice and in three experiments in rats. A few rare oesophageal squamous-cell tumours were found in mice treated with dichlorvos in the diet. A dose-related increase in the incidence of squamous-cell tumours (mainly papillomas) was noted in the forestomachs of mice that received dichlorvos in corn oil by gavage. In rats that received dichlorvos in water by gavage, a few squamous-cell papillomas of the forestomach were seen. In rats that received dichlorvos in corn oil by gavage, a dose-related increase in the incidence of mononuclear-cell leukaemia and an increased incidence of pancreatic acinar-cell adenomas were observed in males.
5.4. Other relevant data
A variety of studies in several species did not demonstrate developmental toxicity due to dichlorvos.
In vitro, dichlorvos phosphorylates esterases to a greater extent than it methylates nucleophiles; the likelihood of DNA methylation in vivo is extremely small.
Immunosuppression has been noted after short-term administration of high doses of dichlorvos which are associated with profound cholinergic hyperstimulation.
No data were available on the genetic and related effects of dichlorvos in humans.
Dichlorvos was not shown to have genetic activity in various assays in mammals in vivo. It induced gene mutation and chromosomal damage in cultured mammalian cells-and in insects, plants, fungi, yeast and bacteria.
5.5. Evaluation1
There is inadequate evidence in humans for the carcinogenicity of dichlorvos.
There is sufficient evidence in experimental animals for the carcinogenicity of dichlorvos.
Overall evaluation
Dichlorvos is possibly carcinogenic to humans (Group 2B).
6. References
- Abbott D.C., Crisp S., Tarrant K.R., Tatton J.O'G. 1970Pesticide residues in the total diet in England and Wales, 1966–1967. III. Organophosphorus pesticide residues in the total diet Pestic Sci. 110−13.
- Adler B., Braun R., Schöneich J., Böhme H. Repair-defective mutants of Proteus mirabilis as a prescreening system for the detection of potential carcinogens. Biol. Zbl. 1976;95:463–469.
- Agrochemical Producers' Association of Finland. 1988 Finnish pesticide sales. AGROW. 1989;97:11–12.
- Amer S.M., Ali E.M. Cytological effects of pesticides. XVII. Effect of the insecticide dichlorvos on root-mitosis of Vicia faba. Cytologia. 1986;51:21–25.
- American Conference of Governmental Industrial Hygienists (1989) Threshold Limit Values and Biological Exposure Indices for 1989–1990, Cincinnati, OH, p. 20.
- AMVAC Chemical Corp. (1986) Product Data Sheet: DDVP Technical, Los Angeles, CA.
- AMVAC Chemical Corp. (1990) Material Safety Data Sheet: DDVP Technical Grade, Los Angeles, CA.
- Anon. (1972) Vapona® insecticide Anal. Methods pestic. plant growth Regul 6, 529–533.
- Anon. (1974) Studies on dichlorvos Food Cosmet. Toxicol. 28, 765–772.
- Aquilina G., Benigni R., Bignami M., Calcagnile A., Dogliotti E., Falcone E., Carere A. Genotoxic activity of dichlorvos, trichlorfon and dichloroacetaldehyde. Pestic. Sci. 1984;15:439–442.
- Attfield, J.G. & Webster, D.A. (1966) Dichlorvos. Chem. Ind., 12 February, pp. 272–278.
- Bedford C.T, Robinson J. The alkylating properties of organophosphates. Xenobiotica. 1972;2:307–337. [PubMed: 4565008]
- Ben-Dyke R., Sanderson D.M., Noakes D.N. Acute toxicity data for pesticides. World Rev.Pest Control. 1970;9:119–127.
- Bhan A.K., Kaul B.L. Cytotoxic activity of dichlorvos in barley. Indian J. exp. Biol. 1975;13:403–405.
- Bisby J. A., Simpson G.R. An unusual presentation of systemic organophosphate poisoning.Med. J. Aust. 1975;2:394–395. [PubMed: 127108]
- Blair D., Hoadley E.C., Hutson D.H. The distribution of dichlorvos in the tissues of mammals after its inhalation or intravenous administration. Toxicol. appl. Pharmacol. 1975;31:243–253. [PubMed: 1129796]
- Blair D., Dix K.M., Hunt P.F., Thorpe E., Stevenson D.E., Walker A.I.T. Dichlorvos-a 2-year inhalation carcinogenesis study in rats. Arch. Toxicol. 1976;35:281–294. [PubMed: 989297]
- Braun R., Schöneich J., Weissflog L., Dedek W. Activity of organophosphorus insecticides in bacterial tests for mutagenicity and DNA repair—direct alkylation vs metabolic activation and breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos, dimethyl dichlorvos and dimethyl vinylbutonate. Chem.-biol. Interactions. 1982;39:339–350. [PubMed: 7074710]
- Breau A.P., Mitchell W.M., Swinson J., Field L. Mutagenic and cell transformation activities of representative phosphorothioate esters in vitro. J. Toxicol. environ. Health. 1985;16:403–413. [PubMed: 4087308]
- Bridges B.A. On the detection of volatile liquid mutagens with bacteria: experiments with dichlorvos and epichlorhydrin. Mutat. Res. 1978;54:367–371. [PubMed: 368620]
- Bridges B.A., Mottershead R.P., Green M.H.L., Gray W.J.H. Mutagenicity of dichlorvos and methyl methanesulfonate for Escherichia coli WP2 and some derivatives deficient in DNA repair. Mutat. Res. 1973;19:295–303. [PubMed: 4356893]
- Brown L.M., Blair A., Gibson R., Everett G.D., Cantor K.P., Schuman L.M., Burmeister L.F, Van Lier S.F., Dick F. Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer. 1990;50:6585–6591. [PubMed: 2208120]
- Buselmaier W, Röhrborn G., Propping P. Mutagenicity investigations with pesticides in the host-mediated assay and dominant lethal test in the mouse (Ger.). Biol. Zbl. 1972;91:311–325.
- Byeon W.-H., Hyun H.H., Lee S.Y. Mutagenicity of pesticides in the Salmonella/microsome system (Korean). Korean J. Microbiol. 1976;14:128–134.
- Carere A., Ortali VA., Cardamone G., Morpurgo G. Mutagenicity of dichlorvos and other structurally related pesticides in Salmonella and Streptomyces. Chem.-biol. Interactions. 1978;22:297–308. [PubMed: 699179]
- Caroldi S., Lotti M. Delayed neurotoxicity caused by a single massive dose of dichlorvos to adult hens. Toxicol. Lett. 1981;9:157–159. [PubMed: 7302988]
- Casale G.P, Cohen S.D., DiCapua R.A. The effects of organophosphate-induced cholinergic stimulation on the antibody response to sheep erythrocytes in inbred mice. Toxicol. appl. Pharmacol. 1983;68:198–205. [PubMed: 6857660]
- Cavagna G., Vigliani E.C. Problems of health and safety when using Vapona as an insecticide in domestic quarters (Fr.). Med. Lav. 1970;61:409–423. [PubMed: 5503896]
- Cavagna G., Locati G., Vigliani E.C. Clinical effects of exposure to DDVP (Vapona) insecticide in hospital wards. Arch, environ. Health. 1969;19:112–123. [PubMed: 5785969]
- Choi E.U., Kim Y.K., Roh J.K. Genetic toxicity of pesticides used in Korea on Salmonella typhimurium and Saccharomyces cerevisiae. Environ. Mutagen. Carcinogens. 1985;5:11–18.
- Civen M., Leeb J.E., Wishnow R.M., Wolfsen A., Morin R.J. Effects of low level administration of dichlorvos on adrenocorticotrophic hormone secretion, adrenal cholesteryl ester and steroid metabolism. Biochem. Pharmacol. 1980;29:635–641. [PubMed: 6245657]
- Codex Committee on Pesticide Residues (1990) Guide to Codex Maximum Limits for Pesticide Residues, Part 2 (CAC/PR 2—1990; CCPR Pesticide Classification No. 120), The Hague.
- Collins R.D., deVries D.M. Air concentrations and food residues from use of Shell's No-Pest® insecticide strip. Bull, environ. Contam. Toxicol. 1973;9:227–233. [PubMed: 4780726]
- Collins J.A., Schooley M.A., Singh V.K. The effect of dietary dichlorvos on swine reproduction and viability of their offspring (Abstract No 41). Toxicol. appl. Pharmacol. 1971;19:377.
- Cook, W.A., ed. (1987) Occupational Exposure Limits—Worldwide, Washington DC, American Industrial Hygiene Association, pp. 120, 136, 181.
- Dale W.E., Miles J.W., Weathers D.B. Measurements of residues of dichlorvos absorbed by food exposed during disinfection of aircraft. J. agric. Food Chem. 1973;21:858–860. [PubMed: 4733379]
- Dambska M., Iwanowski L., Kozlowski P. The effect of transplacental intoxication with dichlorvos on the development of cerebral cortex in newborn rabbits. Neuropathol. Pol. 1979;17:571–576. [PubMed: 514520]
- Das Y.T, Taskar P.K., Brown H.D., Chattopadhyay S.K. Exposure of professional pest control operator to dichlorvos (DDVP) and residue on house structures. Toxikol. Lett. 1983;17:95–99. [PubMed: 6623514]
- Dean B.J. The mutagenic effects of organophosphorus pesticides on microorganisms. Arch. Toxicol. 1972a;30:67–74. [PubMed: 4566919]
- Dean B.J. The effect of dichlorvos on cultured human lymphocytes. Arch. Toxikol. 1972b;30:75–78. [PubMed: 4674981]
- Dean B.J., Blair D. Dominant lethal assay in female mice after oral dosing with dichlorvos or exposure to atmospheres containing dichlorvos. Mutat. Res. 1976;40:67–72. [PubMed: 1250254]
- Dean B.J., Thorpe E. Cytogenetic studies with dichlorvos in mice and Chinese hamsters. Arch. Toxikol. 1972a;30:39–49. [PubMed: 4646173]
- Dean B.J., Thorpe E. Studies with dichlorvos vapour in dominant lethal mutation tests in mice. Arch. Toxikol. 1972b;30:51–59. [PubMed: 4646174]
- Dean B.J., Doak S.M.A., Funnell J. Genetic studies with dichlorvos in the host-mediated assay and in liquid medium using Saccharomyces cerevisiae. Arch. Toxikol. 1972;30:61–66. [PubMed: 4566918]
- Degraeve N., Chollet M.-C., Moutschen J. Cytogenetic and genetic effects of subchronic treatments with organophosphorus insecticides. Arch. Toxicol. 1984a;56:66–67. [PubMed: 6517715]
- Degraeve N., Chollet M.-C., Moutschen J. Cytogenetic effects induced by organophosphorus pesticides in mouse spermatocytes. Toxicol. Lett. 1984b;21:315–319. [PubMed: 6740720]
- Dési I., Varga L., Farkas I. Studies on the immunosuppressive effect of organochlorine and organophosphoric pesticides in subacute experiments. J. Hyg. Epidemiol. Microbiol. Immunol. 1978;22:115–122. [PubMed: 82575]
- Dési, I., Varga, L. & Farkas, I. (1980) The effect of DDVP, an organophosphorus pesticide on the humoral and cell-mediated immunity of rabbits. Arch. Toxicol., Suppl. 4, 171–174. [PubMed: 6933898]
- Deutsche Forschungsgemeinschaft (1989) Maximum Concentrations at the Workplace and Biological Tolerance Values for Working Materials. 1989 (Report No. XXV), Weinheim, VCH Verlagsgesellschaft, p. 32 (in German)
- Devadas N., Rajam M.V., Subhash K. Comparative mutagenicity of four organophosphorus insecticides in meiotic system of red pepper. Cytologia. 1986;51:645–653.
- Dicowsky L., Morello A. Glutathione-dependent degradation of 2,2-dichlorovinyl dimethyl phosphate (DDVP) by the rat. Life Sci. 1971;10:1031–1037. [PubMed: 5130706]
- Durham W.F., Gaines T.B., McCauley R.H. Jr, Sedlak VA., Mattson A.M., Hayes W.J. Jr. Studies on the toxicity of 0,0-dimethyl-2,2-dichlorovinyl phosphate (DDVP). Arch. ind. Health. 1957;15:340–349. [PubMed: 13410153]
- Dyer K.F., Hanna P.J. Comparative mutagenic activity and toxicity of triethylphosphate and dichlorvos in bacteria and Drosophila. Mutat. Res. 1973;21:175–177. [PubMed: 4200711]
- Dzwonkowska A., Hübner H. Induction of chromosomal aberrations in the Syrian hamster by insecticides tested in vivo. Arch. Toxicol. 1986;58:152–156. [PubMed: 3964078]
- Elgar K.E., Mathews B.L., Bosio P. Dichlorvos residues in food arising from the domestic use of dichlorvos PVC strips. Pestic. Sci. 1972a;3:601–607.
- Elgar K.E., Mathews B.L., Bosio P. Vapona strips in shops: residues in foodstuffs. Environ.Qual. Safi. 1972b;1:217–221.
- Epstein S.S., Arnold E., Andrea J., Bass W., Bishop Y. Detection of chemical mutagens by the dominant lethal assay in the mouse. Toxicol. appl. Pharmacol. 1972;23:288–325. [PubMed: 5074577]
- Eto, M. (1974) Organophosphorus Pesticides: Organic and Biological Chemistry, Cleveland, OH, CRC Press, Inc., p. 235.
- Fahrig R. Evidence of a genetic action of organophosphorus insecticides (Ger.). Naturwissenschaften. 1973;60:50–51. [PubMed: 4573870]
- Fahrig R. The effect of dose and time on the induction of genetic alterations in Saccharomyces cerevisiae by aminoacridines in the presence and absence of visible light irradiation in comparison with the dose-effect-curves of mutagens with other type of action. Mol. gen. Genet. 1976;144:131–140. [PubMed: 775287]
- FAO/WHO (1965) Evaluation of the Toxicity of Pesticide Residues in Food (FAO Meeting Report,No. PL/1965/10/1; WHO/Food Add./27.65), Rome.
- FAO/WHO (1967) Evaluation of Some Pesticide Residues in Food (FAO/PL:CP/15; WHO/Food Add./67.32), Rome.
- FAO/WHO (1968) 1967 Evaluations of Some Pesticide Residues in Food (FAO/PL:1967/M/ll/l;WHO/Food Add./68.30), Rome.
- FAO/WHO (1970) 1969 Evaluations of Some Pesticide Residues in Food (FAO/PL/1969/M/17/1;WHO/Food Add./70.38), Rome.
- FAO/WHO (1971) 1970 Evaluations of Some Pesticide Residues in Food (AGP: 1970/M/12/1;WHO/Food Add./71.42), Rome.
- FAO/WHO (1975) 1974 Evaluations of Some Pesticide Residues in Food (WHO Pesticide Residues Series No. 4), Geneva.
- FAO/WHO (1978) Pesticide Residues in Food: 1977 Evaluations (FAO Plant Production and Protection Paper 10 Sup.), Rome.
- Frank R., Braun H.E., Fleming G. Organochlorine and organophosphorus residues in fat of bovine and porcine carcasses marketed in Ontario, Canada from 1969 to 1981. J. Food Prot. 1983;46:893–900. [PubMed: 30921839]
- Gillenwater H.B., Harein P.K., Loy E.W. Jr, Thompson J.F., Laudani H., Eason G. Dichlorvos applied as a vapor in a warehouse containing packaged foods. J. stored Prod. Res. 1971;7:45–56.
- Gillett J.W., Harr J.R., Lindstrom F.T., Mount D.A., St Clair A.D., Weber L.J. Evaluation of human health hazards on use of dichlorvos (DDVP), especially in resin strips. Residue Rev. 1972a;44:115–159. [PubMed: 4576326]
- Gillett J.W, Harr J.R., St Clair A.D., Weber L.J. Comment on the distinction between hazard and safety in evaluation of human health hazards on use of dichlorvos, especially in resin strips. Residue Rev. 1972b;44:161–184. [PubMed: 4570403]
- Gilot-Delhalle J., Colizzi A., Moutschen J., Moutschen-Dahmen M. Mutagenicity of some organophosphorus compounds at the ade6 locus of Schizosaccharomyces pombe. Mutat. Res. 1983;117:139–148. [PubMed: 6835257]
- Goh K.S., Edmiston S., Maddy K.T, Margetich S. Dissipation of dislodgeable foliar residue for chlorpyrifos and dichlorvos treated lawn: implication for safe entry Bull environ Contam. Toxicol. 1986a;37:33–40. [PubMed: 2424528]
- Goh K.S., Edmiston S., Maddy K.T., Meinders D.D., Margetich S. Dissipation of dislodgeable foliar residue of chlorpyrifos and dichlorvos on turf. Bull environ contam Toxicol. 1986b;37:27–32. [PubMed: 2424527]
- Gold R.E., Holcslaw T., Tupy D., Ballard J.B. Dermal and respiratory exposure to applicators and occupants of residences treated with dichlorvos (DDVP). J. econ. Entomol. 1984;77:430–436. [PubMed: 6747084]
- Government of Canada (1990) Report on National Surveillance Data from 1984/85 to 1988/89, Ottawa.
- Green M.H.L., Medcalf A.S.C., Arlett C.F., Harcourt S.A., Lehmann A.R. DNA strand breakage caused by dichlorvos, methyl methanesulfonate and iodoacetamide in Escherichia coli and cultured Chinese hamster cells. Mutat. Res. 1974;24:365–378. [PubMed: 4369937]
- Green M.H L., Muriel W.J., Bridges B.A. Use of a simplified fluctuation test to detect low levels of mutagens. Mutat. Res. 1976;38:33–42. [PubMed: 768758]
- Griffin D.E III, Hill W.E. In vitro breakage of plasmid DNA by mutagens and pesticides. Mutat. Res. 1978;52:161–169. [PubMed: 368611]
- Grübner P. Residue problems in the use of phosphoric acid ester insecticides in mushroom cultures (Ger.). Nachrichtenbl. Pflanzenshutzdienst. 1972;26:245–247.
- Gupta A.K., Singh J. Dichlorvos (DDVP) induced breaks in the salivary gland chromosomes of Drosophila melanogaster. Curr. Sci. 1974;43:661–662.
- Hanna P.J., Dyer K.F. Mutagenicity of organophosphorus compounds in bacteria and Drosophda. Mutat. Res. 1975;28:405–420. [PubMed: 806014]
- Hass D.K, Collins J.A., Kodama J.K. Effects of orally administered dichlorvos in rhesus monkeys. J. Am. vet. Med. Assoc. 1972;161:714–719. [PubMed: 4626784]
- Hayes, W.J. (1982) Pesticides Studied in Man, Baltimore, MD, Williams & Wilkins, pp. 348–351.
- Health and Welfare Canada (1990) National Pesticide Residue Limits in Foods, Ottawa, Bureau of Chemical Safety, Food Directorate, Health Protection Branch.
- Holmstedt B., Nordgren I., Sandoz M., Sundwall A. Metrifonate. Summary of toxicological and pharmacological information available. Arch. Toxicol. 1978;41:3–29. [PubMed: 363095]
- Horn K.-H., Teichmann B., Schramm T. Investigation of dichlorvos (DDVP). I. Testing of dichlorvos for carcinogenic activity in mice (Ger.). Arch. Geschwulstforsch. 1987;57:353–360. [PubMed: 3689102]
- Horn K.-H., Teichmann B., Schramm T., Nischan P. Investigation of dichlorvos (DDVP). II. Testing of dichlorvos for carcinogenic activity in rats (Ger.). Arch. Geschwulstforsch. 1988;58:1–9. [PubMed: 3369918]
- Houk VS., DeMarini D.M. Induction of prophage lambda by chlorinated pesticides. Mutat.Res. 1987;182:193–201. [PubMed: 2956515]
- Hussey N.W., Hughes J.T. Investigations on the use of dichlorvos in the control of the mushroom phorid, Megaselia halterata (Wood). Ann. appl. Biol. 1964;54:129–139.
- Hutson D.H, Hoadley E.C. The comparative metabolism of [14C-vinyl]dichlorvos in animals and man. Arch. Toxikol. 1972a;30:9–18. [PubMed: 4646175]
- Hutson D.H., Hoadley E.C. The metabolism of [14C-methyl]dichlorvos in the rat and the mouse. Xenobiotica. 1972b;2:107–116. [PubMed: 4560365]
- Hutson D.H., Hoadley E.C., Pickering B.A. The metabolic fate of [vinyl-l-14C]dichlorvos in the rat after oral and inhalational exposure. Xenobiotica. 1971;6:593–611. [PubMed: 5173023]
- IARC (1979a) ¡ARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 20, Some Halogenated Hydrocarbons, Lyon, pp. 97–127. [PubMed: 397180]
- IARC (1979b) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 20, Some Halogenated Hydrocarbons, Lyon, pp. 259–281. [PubMed: 397167]
- IARC (1983a) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 30, Miscellaneous Pesticides, Lyon, pp. 207–231. [PubMed: 6578181]
- IARC (1983b) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 30, Miscellaneous Pesticides, Lyon, pp. 103–129. [PubMed: 6578176]
- IARC (1983c) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 30, Miscellaneous Pesticides, Lyon, pp. 183–195. [PubMed: 6578179]
- IARC (1987a) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, pp.202–203. [PubMed: 3482203]
- IARC (1987b) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, p. 73. [PubMed: 3482203]
- IARC (1987c) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, pp.220–222. [PubMed: 3482203]
- IARC (1987d) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, p. 65. [PubMed: 3482203]
- IARC (1987e) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, p. 66. [PubMed: 3482203]
- IARC (1987f) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, Lyon, p. 70. [PubMed: 3482203]
- Ishidate M. Jr, Sofuni T, Yoshikawa K. Chromosomal aberration tests in vitro as a primaryscreening tool for environmental mutagens and/or carcinogens. Gann Monogr. Cancer Res. 1981;27:95–108.
- Izmerov, N.P., ed. (1984) International Register of Potentially Toxic Chemicals, Scientific Reviews of Soviet Literature on Toxicity and Hazards of Chemicals: DDVP (Issue 79), Moscow, Centre of International Projects, United Nations Environment Programme.
- Jayasuriya V.U. de S., Ratnayake W.E. Screening of some pesticides on Drosophila melanogaster for toxic and genetic effects. Drosophila Inf. Serv. 1973;50:184–186.
- Johnson M.K. The anomalous behaviour of dimethyl phosphates in the biochemical test for delayed neurotoxicity. Arch. Toxicol. 1978;41:107–110. [PubMed: 718419]
- Johnson M.K. Delayed neurotoxicity-do trichlorphon and/or dichlorvos cause delayed neuropathy in man or in test animals? Acta pharmacol. toxicol. 1981;49 (Suppl. V):87–98. [PubMed: 7344417]
- Johnson R.D., Manske D.D., Podrebarac D.S. Pesticide, metal and other chemical residues in adult total diet samples. XII. August 1975-July 1976. Pestic. Monit. J. 1981;15:54–69. [PubMed: 7279595]
- Jones K.H., Sanderson D.M., Noakes D.N. Acute toxicity data for pesticides (1968) World Rev. Pest. Control. 1968;7:135–143.
- Kawachi, T, Komatsu, T., Kada, T, Ishidate, M., Sasaki, M., Sugiyama, T. & Tazima, Y. (1980) Results of recent studies on the relevance of various short-term screening tests in Japan In: Williams G.M., Kroes, R., Waaijers, H.W. & van de Poll, K.W., eds, The Predictive Value of Short-term Screening Tests in Carcinogenicity Evaluation, Amsterdam, Elsevier/North-Holland Biomedical Press, pp. 253–267.
- Kimbrough R.D., Gaines T.B. Effect of organic phosphorus compounds and alkylating agents on the rat fetus. Arch. environ. Health. 1968;16:805–808. [PubMed: 5654550]
- Kligerman A.D., Erexson G.L., Wilmer J.L. Induction of sister-chromatid exchange (SCE)and cell-cycle inhibition in mouse peripheral blood B lymphocytes exposed to mutagenic carcinogens in vivo. Mutat. Res. 1985;157:181–187. [PubMed: 3875033]
- Kramers P.G.N., Knaap A.G.A.C. Absence of a mutagenic effect after feeding dichlorvos to larvae of Drosophila melanogaster. Mutat. Res. 1978;57:103–105. [PubMed: 417243]
- Kurinnyi A.I. Comparative study of the cytogenetic effect of certain organophosphorus pesticides (Russ.). Genetika. 1975;11:64–69. [PubMed: 1225752]
- Lamoreaux R.J., Newland L.W. The fate of dichlorvos in soil. Chemosphere. 1978;10:807–814.
- Latif S., Haken J.K., Wainwright M.S. Gas chromatographic analysis of insecticidal preparations using carbon dioxide propellants. J Chromatogr. 1984;287:77–84.
- Lawley P.D., Shah S.A., Orr D.J. Methylation of nucleic acids by 2,2-dichlorovinyl dimethyl phosphate (dichlorvos, DDVP). Chem.-biol. Interactions. 1974;8:171–182. [PubMed: 4595853]
- Lieberman M.T, Alexander M. Microbial and nonenzymatic steps in the decomposition of dichlorvos. J. agric. Food Chem. 1983;31:265–167.
- Lin S.Y., Lee T.C, Cheng C.S., Wang T.C. Cytotoxicity, sister-chromatid exchange, chromosome aberration and transformation induced by 2,2-dichlorovinyl-O,O-dimethvl phosphate. Mutat. Res. 1988;206:439–445. [PubMed: 3205263]
- Löfroth G. Alkylation of DNA by dichlorvos. Naturwissenschaften. 1970;57:393–394. [PubMed: 5447862]
- Löfroth G. The mutagenicity of dichloroacetaldehyde. Z. Naturforsch. 1978;33:783–785. [PubMed: 153665]
- Löfroth G., Kim C., Hussain S. Alkylating property of 2,2-dichlorovinyl dimethyl phosphate: a disregarded hazard. EMS News Lett. 1969;2:21–26.
- Ma T.-H., Harris M.M., Anderson VA., Ahmed I., Mohammad K., Bare J.L, Lin G. Tradescantia-micronucleus (Trad-MCN) tests on 140 health-related agents. Mutat.Res. 1984;138:157–167. [PubMed: 6392874]
- Marcos R., Andreu H., Velásquez A., Xamena N., Creus A. Induction of polygenic mutations affecting viability in Drosophila after dichlorvos and malathion treatments. Genét.Ibér. 1989;41:147–159.
- Mathias C.G.T. Persistent contact dermatitis from the insecticide dichlorvos. Contact Derm. 1983;9:217–218. [PubMed: 6861485]
- Matsumura F., Boush G.M. Degradation of insecticides by a soil fungus Trichoderma viride. J. econ. Entomol. 1968;61:610–612. [PubMed: 5659010]
- Meister, R.T., ed. (1990) Farm Chemicals Handbook '90, Willoughby, OH, Meister Publishing Co., pp.C91-C92.
- Menz M., Luetkemeier H., Sachsse K. Long-term exposure of factory workers to dichlorvos (DDVP) insecticide. Arch, environ. Health. 1974;28:72–76. [PubMed: 4809916]
- Mirsalis J.C., Tyson C.K., Steinmetz K.L., Loh E.K., Hamilton C.M., Bakke J.P., Spalding J.W. Measurement of unscheduled DNA synthesis and S-phase synthesis in rodent hepatocytes following in vivo treatment: testing of 24 compounds. Environ, mol. Mutagenesis. 1989;14:155–164. [PubMed: 2792091]
- Mohn G. 5-Methyltryptophan resistance mutations in Escherichia coli K-12. Mutagenic activity of monofunctional alkylating agents including organophosphorus insecticides. Mutat.Res. 1973;20:7–15. [PubMed: 4357387]
- Moriya M., Kato K., Shirasu Y. Effects of cysteine and a liver metabolic activation system on the activities of mutagenic pesticides. Mutat. Res. 1978;57:259–263. [PubMed: 351392]
- Moriya M., Ohta T., Watanabe K., Miyazawa T., Kato K., Shirasu Y. Further mutagenicity studies on pesticides in bacterial reversion assay systems. Mutat. Res. 1983;116:185–216. [PubMed: 6339892]
- Morpurgo G., Aulicino E, Bignami M., Conti L., Velcich A., Montalenti S.G. Relationship between structure and mutagenicity of dichlorvos and other pesticides. Accad. naz. Lincei Rend.Cl. Sci. fis. mat. not. 1977;62:692–701.
- Morpurgo G., Bellincampi D., Gualandi G., Baldinelli L., Crescenzi O.S. Analysis of mitotic nondisjunction with Aspergillus nidulans. Environ. Health Perspect. 1979;31:81–95. [PMC free article: PMC1637643] [PubMed: 387402]
- Moutschen-Dahmen J., Moutschen-Dahmen M., Degraeve N. Metrifonate and dichlorvos: cytogenetic investigations. Acta pharmacol. toxicol. 1981;49:29–39. [PubMed: 7344409]
- Nagy Z., Mile I., Antoni F. The mutagenic effect of pesticides on Escherichia coli WP2 try” Acta microbiol. acad. sci. hung. 1975;22:309–314. [PubMed: 1098404]
- Nicholas A.H., Vienne M., Van den Berghe H. Sister chromatid exchange frequencies in cultured human cells exposed to an organophosphorus insecticide: dichlorvos. Toxicol. Lett. 1978;2:271–275.
- Nishio A., Uyeki E.M. Induction of sister chromatid exchanges in Chinese hamster ovary cells by organophosphate insecticides and their oxygen analogs. J. Toxicol. environ. Health. 1981;8:939–946. [PubMed: 7338954]
- Page A.C., Loeffler J.E., Hendrickson H.R., Huston C.K, DeVries D.M. Metabolic fate of dichlorvos in swine. Arch. Toxikol. 1972;30:19–27. [PubMed: 4646170]
- Paik S.G., Lee S.Y. Genetic effects of pesticides in the mammalian cells. I. Induction of micronucleus. Korean J. Zool. 1977;20:19–28.
- Panda B.B., Sharma C.B.S.R. Organophosphate induced chlorophyl mutations in Hordeum vulgare. Theor. appl. Genet. 1979;55:253–255. [PubMed: 24306772]
- Perocco P., Fini A. Damage by dichlorvos of human lymphocyte DNA. Tumori. 1980;66:425–430. [PubMed: 7414708]
- Porter, P.E. (1964) Vapona insecticide (DDVP). In: Zweig, G., ed., Analytical Methods for Pesticides, Plant Growth Regulators, and Food Additives, Vol. II, Insecticides, New York, Academic Press pp 561–579.
- Potter J.C., Boyer A.C., Marxmiller R.L., Young R., Loeffler J.E. Radioisotopic residues and residues of dichlorvos and its metabolites in pregnant sows and their progeny dosed with dichlorvos-14C or dichIovos-36Cl formulated as PVC pellets. J. agrie. Food Chem. 1973;21:734–738. [PubMed: 4718944]
- Ramel C., Drake J., Sugimura T. ICPEMC Publication No. 5. An evaluation of the genetic toxicity of dichlorvos. Mutat. Res. 1980;76:297–309. [PubMed: 6782471]
- Rao B.V, Sharma C.B.S.R., Rao B.G.S. Cytological effects of organophosphorus insecticides on Allium cepa root-meristems. Cytologia. 1987;52:365–371.
- Reeves J.D., Driggers D.A., Kiley VA. Household insecticide associated aplastic anaemia and acute leukaemia in children. Lancet. 1981;ii:300–301. [PubMed: 6114336]
- Rosenkranz H.S. Preferential effect of dichlorvos (Vapona) on bacteria deficient in DNA polymerase. Cancer Res. 1973;33:458–459. [PubMed: 4570270]
- Royal Society of Chemistry (1986) European Directory of Agrochemical Products, Vol. 3, Insecticides,Acaricides, Nematicides, Cambridge, pp. 198–212.
- Sadtler Research Laboratories (1980) The Sadtler Standard Spectra, 1980, Cumulative Index Philadelphia, PA.
- Santodonato, J., Bosch, S., Meylan, W., Becker, J. & Neal, M. (1985) Monograph on Human Exposure to Chemicals in the Workplace: Dichlorvos (US NTIS PB86-148343), Washington DC, US National Technical Information Service.
- Sasaki M., Sugimura K., Yoshida M.A., Abe S. Cytogenetic effects of 60 chemicals on cultured human and Chinese hamster cells. Kromosomo II. 1980;20:574–584.
- Schairer, L.A., Van't Hof, J., Hayes, C.G., Burton, R.M. & de Serres, F.J. (1978) Measurement of biological activity of ambient air mixtures using a mobile laboratory for in situ exposures: preliminary results from the Tradescantia plant test system. In: Waters, M.D., Nesnow, S., Huisingh, J.L., Sandhu, S.S. & Claxton, L., eds, Application of Short-term Bioassays in the Fractionation and Analysis of Complex Environmental Mixtures (EPA-600/9-78-027), Research Triangle Park, NC, US Environmental Protection Agency, pp. 421–440.
- Schwetz B.A., Ioset H.D., Leong B.K.J., Staples R.E. Teratogenic potential of dichlorvos given by inhalation and gavage to mice and rabbits. Teratology. 1979;20:383–388. [PubMed: 542893]
- Segerbäck D. Estimation of genetic risks of alkylating agents V Methylation of DNA in the mouse by DDVP (2,2-dichlorovinyl dimethyl phosphate). Hereditas. 1981;94:73–76. [PubMed: 7216827]
- Segerbäck D., Ehrenberg L. Alkylating properties of dichlorvos (DDVP). Acta Pharmacol toxicol. 1981;49 (Suppl. V):56–66. [PubMed: 7344412]
- Sharma, C.B.S.R., Panda, B.B., Behera, B.N. & Rao, R.N. (1983) Progeny testing in barley for monitoring environmental mutagens. In: Sinha, R.P. & Sinha, U., eds, Current Approaches in Cytogenetics, Patna, Delhi, Spectrum Publishing, pp. 245––255.
- Shirasu Y., Moriya M., Kato K., Furuhashi A., Kada T. Mutagenicity screening of pesticides m the microbial system. Mutat. Res. 1976;40:19–30. [PubMed: 814455]
- Shirasu Y., Moriya M., Tezuka H., Teramoto S., Ohta T, Inoue T. Mutagenicity of pesticides. Environ. Sci. Res. 1984;31:617–624.
- Singh R.M., Singh A.K., Singh R.B., Singh J., Singh B.D. Chlorophyll mutations induced by seed treatment with certain insecticides in barley Hordeum vulgare. Indian J exp Biol. 1980;18:1396–1397.
- Slomka M.B., Hine C.H. Clinical pharmacology of dichlorvos. Acta pharmacol. toxicol. 1981;49 (Suppl. V):105–108. [PubMed: 7344402]
- Sobeis F.H., Todd N.K. Absence of a mutagenic effect of dichlorvos in Drosophila melanogaster. Mutat. Res. 1979;67:89–92. [PubMed: 111118]
- Stanton H.C., Albert J.R., Mersmann H.J. Studies on the pharmacology and safety of dichlorvos in pigs and pregnant sows. Am. J. vet. Res. 1979;40:315–320. [PubMed: 475081]
- Tezuka H., Ando N, Suzuki R., Terahata M., Moriya M., Shirasu Y. Sister-chromatid exchanges and chromosomal aberrations in cultured Chinese hamster cells treated with pesticides positive in microbial reversion assays. Mutat. Res. 1980;78:177–191. [PubMed: 7393245]
- Thorpe E., Wilson A.B., Dix K.M., Blair D. Teratological studies with dichlorvos vapour in rabbits and rats. Arch. Toxicol. 1972;30:29–38. [PubMed: 4646172]
- Tinker J. The Vapona dossier. New Sci. 1972;53:489–492.
- Tu A., Hallowell W, Pallotta S., Sivak A., Lubet R.A., Curren R.D., Avery M.D., Jones C, Sedita B.A., Huberman E., Tennant R., Spalding J., Kouri R.E. An interlaboratory comparison of transformation in Syrian hamster embryo cells with model and coded chemicals. Environ. Mutagenesis. 1986;8:77–98. [PubMed: 3943499]
- US Environmental Protection Agency (1980) Preliminary Quantitative Usage Analysis of DDVP, Washington DC, Office of Pesticide Programs.
- US Environmental Protection Agency (1986) Method 8140. Organophosphorus pesticides. In: Test Methods for Evaluating Solid Waste—Physical/Chemical Methods, 3rd ed. (US EPA Publ. No. SW-846), Washington DC, Office of Solid Waste and Emergency Response.
- US Environmental Protection Agency (1987) Guidance for the Reregistration of Pesticide Products Containing DDVP as the Active Ingredient, Washington DC, Office of Pesticide Programs, p. 132.
- US Environmental Protection Agency (1988a) Method TO-10. Method for the determination of organochlorine pesticides in ambient air using low volume polyurethane foam (PUF) sampling with gas chromatography/electron capture detector (GC/ECD). In: Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air (US EPA Report No. EPA-600/4-89-017; US NTIS PB90-116989), Research Triangle Park, NC, Atmospheric Research and Exposure Assessment Laboratory.
- US Environmental Protection Agency (1988b) Method 507. Determination of nitrogen- and phosphorus-containing pesticides in water by gas chromatography with a nitrogen-phosphorus detector. In: Methods for the Determination of Organic Compounds in Drinking Water (USA EPA Report No. EPA-600/4-88-039; US NTIS PB89-220461), Cincinnati, OH, Environmental Monitoring Systems Laboratory, pp. 143–170.
- US Environmental Protection Agency 1989a2,2-Dichlorovinyl dimethyl phosphate; tolerances for residues. Part 180—Tolerances and exemptions from tolerances for pesticide chemicals in or on raw agricultural commodities US Code fed. Regui, Title 40Part 180.235, pp. 370–371.
- US Environmental Protection Agency 1989b2,2-Dichlorovinyl dimethyl phosphate. Part 185—Tolerances for pesticides in food US Code fed. Regul, Title 40Part 185.1900, p. 476.
- US Food and Drug Administration (1989a) Naled. In: Pesticide Analytical Manual, Vol. II, Methods Which Detect Multiple Residues, Washington DC, US Department of Health and Human Services.
- US Food and Drug Administration (1989b) 2,2-Dichlorovinyl dimethyl phosphate. In: Pesticide Analytical Manual, Vol. II, Methods Which Detect Multiple Residues, US Department of Health and Human Services.
- US National Cancer Institute (1977) Bioassay of Dichiorvos for Possible Carcinogenicity (Carcinogenesis Technical Report Series No- 10; DHEW Publ. No. (NIH) 77-810), Washington DC, US Government Printing Office.
- US National Institute for Occupational Safety and Health (1979) NIOSH Manual of Analytical Methods, Vol. 5, Dichlorvos P&CAM 295 (DHEW (NIOSH) Publ. No. 79-141) 2nd ed., Cincinnati, OH, US Department of Health, Education, and Welfare, pp 295-1-295-10.
- US National Toxicology Program (1989) Toxicology and Carcinogenesis Studies of Dichlorvos (CAS No.62-73-7) in F344/N Rats and B6C3F1 Mice (Gavage studies) (NTP Technical Report 342; NTH Publ. No. 89-2598), Research Triangle Park, NC. [PubMed: 12724783]
- US Occupational Safety and Health Administration (1989) Air contaminants-permissible exposure limits. US Code fed. Regul., Title 29, Part 1910.1000.
- Vevai E.J. Know your pesticide, its salient points and uses in pest control. 5. DichIorvos. Pesticides. 1974;8:15–23.
- Vigliani E.C. Exposure of newborn babies to Vapona® insecticide (Abstract No 48) Toxicol appl. Pharmacol. 1971;19:379–380.
- Vogin E.E., Carson S., Slomka M.B. Teratology studies with dichlorvos in rabbits (Abstract No.42). Toxicol appl pharmacol. 1971;19:377–378.
- Voogd C.E., Jacobs J.J.J.A.A., van der Stel J.J. On the mutagenic action of dichlorvos. Mutat. Res. 1972;16:413–416. [PubMed: 4563676]
- Wagner R., Hoyer J. Methods employed in determining workplace concentrations and occupational hygienic conditions during and after hot spraying of pesticides in greenhouses (Ger.). Z. ges. Hyg. 1975;21:18–20.
- Wennerberg R., Löfroth G. 1974Formation of 7-methylguanine by dichlorvos in bacteria and mice Chem.-biol. Interactions 8339−348. [PubMed: 4600775]
- WHO (1967) Specifications for Pesticides Used in Public Health, Geneva, pp. 69–75.
- WHO (1985) Specifications for Pesticides Used in Public Health, 6th ed., Geneva, pp. 163–168.
- WHO (1989) Dichlorvos (Environmental Health Criteria 79), Geneva.
- Wild D. Chemical induction of streptomycin-resistant mutations in Escherichia coli: dose and mutagenic effects of dichlorvos and methyl methanesulfonate. Mutat. Res. 1973;19:33–41. [PubMed: 4366325]
- Williams, S., ed. (1984) Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed., Washington DC, Association of Official Analytical Chemists, pp 126–127.
- Witherup S., Jolley W.J., Stemmer K., Pfitzer E.A. Chronic toxicity studies with 2,2-dichlorovinyl dimethyl phosphate (DDVP) in dogs and rats including observations on rat reproduction (Abstract No. 40). Toxicol. appl. Pharmacol. 1971;19:377.
- Wooder M.F., Wright A.S. Alkylation of DNA by organophosphorus pesticides. Acta Pharmacol. toxicol. 1981;49 (Suppl. V):51–55. [PubMed: 7344411]
- Wooder M.F., Wright A.S., King L.J. In vivo alkylation studies with dichiorvos at practical use concentrations. Chem.-biol. Interactions. 1977;19:25–46. [PubMed: 922973]
- Worthing, C.R. & Walker, S.B., eds (1987) The Pesticide Manual—A World Compendium, 8th ed,Thornton Heath, British Crop Protection Council, pp. 269–270.
- Wright C.G., Leidy R.B. 1980Air samples in vehicles and buildings turn up only very low levels of organic phosphate insecticides Pest Control 4822, 24, 26, 68.
- Wright A.S., Hutson D.H., Wooder M.F. The chemical and biochemical reactivity of dichlorvos. Arch. Toxicol. 1979;42:1–18. [PubMed: 378182]
- Wyrobek A.J., Bruce WR. Chemical induction of sperm abnormalities in mice. Proc. natl Acad. Sci. USA. 1975;72:4425–4429. [PMC free article: PMC388734] [PubMed: 1060122]
- Zeiger E., Anderson B., Haworth S., Lawlor T, Mortelmans K. Salmonella mutagenicity tests: iv Results from the testing of 300 chemicals. Environ. moL. Mutagenesis. 1988;11 (Suppl. 12):1–158. [PubMed: 3277844]
- Zotov VM., Svirin J.N., Prucakova RM. The development of health regulations for occupational exposure in greenhouses treated with chemical pesticides (Russ.). Gig. Tr. prof. Zabol. 1977;3:49–50. [PubMed: 852740]
Footnotes
- 1
Calculated from: mg/m3 = (molecular weight/24.45)×ppm, assuming standard temperature (25°C) and pressure (760 mm Hg [101.3 kPa])
- 1
For definition of the italicized terms, see Preamble, pp. 26−28.
- Review Fenvalerate.[IARC Monogr Eval Carcinog Risk...]Review Fenvalerate.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:309-28.
- Review Trifluralin.[IARC Monogr Eval Carcinog Risk...]Review Trifluralin.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:515-34.
- Review Permethrin.[IARC Monogr Eval Carcinog Risk...]Review Permethrin.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:329-49.
- Review Atrazine.[IARC Monogr Eval Carcinog Risk...]Review Atrazine.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:441-66.
- Review Captafol.[IARC Monogr Eval Carcinog Risk...]Review Captafol.. IARC Monogr Eval Carcinog Risks Hum. 1991; 53:353-69.
- Dichlorvos - Occupational Exposures in Insecticide Application, and Some Pestici...Dichlorvos - Occupational Exposures in Insecticide Application, and Some Pesticides
- Occupational exposures in spraying and application of insecticides - Occupationa...Occupational exposures in spraying and application of insecticides - Occupational Exposures in Insecticide Application, and Some Pesticides
- Simazine - Occupational Exposures in Insecticide Application, and Some Pesticide...Simazine - Occupational Exposures in Insecticide Application, and Some Pesticides
- SUMMARY TABLES OF GENETIC AND RELATED EFFECTS - Occupational Exposures in Insect...SUMMARY TABLES OF GENETIC AND RELATED EFFECTS - Occupational Exposures in Insecticide Application, and Some Pesticides
- P11137-4 (1)Identical Protein Groups
Your browsing activity is empty.
Activity recording is turned off.
See more...
