The content of this book is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 Unported license. To view the terms and conditions of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 4th edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2022. doi: 10.1101/glycobiology.4e.22
This chapter describes the current knowledge of archaeal glycobiology. As in bacteria and eukaryotes, the archaeal cell surface is covered with glycans, which serve as an essential part of the cell wall polysaccharides and as a modification of lipids or surface proteins, as well as the major component of the extracellular matrix. Recent discoveries shed light on a tremendous structural and functional diversity of carbohydrates in this domain of life. In particular, the pathways of N-linked protein glycosylation, homologous to the eukaryotic N-glycosylation machinery, generate a wide variety of N-linked glycans in different archaeal species.
BACKGROUND
Based on Carl Woese's pioneering use of 16S ribosomal (r)RNA analysis, the Archaea were first recognized as a separate domain of life, distinct from either Bacteria or Eukarya. As the first Archaea identified were isolated from some of the most physically challenging environments on the planet, such as those defined by extremes in salinity, pH, or temperature, it was assumed that all Archaea were extremophiles. However, the discovery of Archaea in a variety of “normal” as well as “extreme” biological niches revealed that Archaea represent a major portion of the microbial population and that they play crucial roles in geochemical cycles on Earth. Furthermore, the discoveries of new archaeal lineages in the last decade, predominantly based on metagenomics studies, have led to extensive expansion and reconstruction of the archaeal phylogenetic tree. At present, attempts to cultivate these newly identified Archaea have rarely been successful. Nevertheless, the study of cultivated Archaea has led to many important discoveries.
Since Neuberger's discovery of protein glycosylation in the late 1930s (Chapter 1) and his description of the N-acetylglucosamine (GlcNAc)-β-asparagine linkage of a glycan to the modified protein, it became generally accepted that protein glycosylation was a process limited to eukaryotes. This long-held belief was challenged in 1976, when Mescher and Strominger showed that the surface (S)-layer glycoprotein of the archaeon Halobacterium salinarum was subject to both N- and O-glycosylation, thus offering the first example of a noneukaryotic glycoprotein. Today, it is clear that protein glycosylation is an almost universal trait of the Archaea.
The discovery that Archaea do not contain murein in their cell wall was one of the main arguments used to distinguish this group of prokaryotes from Bacteria. Indeed, at the time, cell wall composition was considered to be “the only useful phylogenetic criterion, other than direct molecular phylogenetic measurement” to distinguish between the two prokaryotic domains. Some methanogenic Archaea were, however, shown to include a distinct polymer termed pseudomurein (or pseudopeptidoglycan) in their cell wall, whereas other archaeal species were found to assemble cell walls based on different sugar-based polymers. Today, as more and more archaeal species are cultivated, it is becoming clear that Archaea present numerous variations in the composition of the cell surface. For instance, although many species seem to mainly rely on a cell envelope in which the cytoplasmic membrane is enclosed by a two-dimensional crystalline proteinaceous layer called the surface (S)-layer, strains surrounded by two membranes have been identified. Figure 22.1 summarizes current knowledge about archaeal cell surfaces.
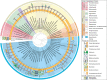
FIGURE 22.1.
Diversity of archaeal cell wall structures. The different cell wall components are shown on the right. In the inner circle, the number of loci encoding putative oligosaccharyltransferases in the respective genomes is shown.
THE ARCHAEAL CELL WALL
Similar to the Bacteria, there is no cell wall structure unique to all Archaea. However, like in Bacteria, there are building blocks that are found in different archaeal clades (Figure 22.1). Some of these cell wall components are very similar in structure to their bacterial counterparts, yet seem to be the product of convergent evolution. Other cell wall–generating processes seem to be homologous to pathways used for eukaryotic extracellular matrix assembly. The biophysical properties of the building blocks of the archaeal cell wall provide the basis for the ability of many archaeal species to thrive in extreme habitats.
Archaeal Cell Wall Polysaccharides
Pseudomurein (Pseudopeptidoglycan)
Although pseudomurein was identified as a component of the cell wall early in the study of Archaea, it subsequently became clear that in terms of distribution, the occurrence of this structure was relatively limited. Pseudomurein shares structural similarities with bacterial murein yet presents significant differences (Figure 22.2). It usually consists of N-acetyl-L-talosaminuronic acid linked via a β1-3 linkage to N-acetyl-D-glucosamine, unlike murein, which contains alternating N–acetylmuramic acids linked via β1-4 linkage to GlcNAc. Moreover, the glycan strands of pseudomurein are cross-linked by peptides composed of L-amino acids (glutamic acid, alanine, and lysine), in contrast to the D-amino acids used in murein. Pseudomurein surrounds cells of all species belonging to the genus Methanopyrus and the order Methanobacteriales, which can, as in the case of Methanothermus fervidus, be bordered by an outer S-layer. Homologs of bacterial murein biosynthesis proteins (e.g., MurG or MraY) have been identified in these methanogens, although the functions of these enzymes have not yet been studied.

FIGURE 22.2.
The chemical structure of pseudomurein.
Glutaminylglycan
The cell wall of the highly halophilic and alkaliphilic genus Natronococcus (3.5 M salt and pH 9.5–10) consists of a glutamine polymer. In contrast to poly-γ-D-glutamyl polymers in the bacteria Bacillus, Sporosarcina, or Planococcus, the archaeal polymer is formed from L-glutamines linked via the γ-carboxylic group, yielding a chain of about 60 monomers. Also, in contrast to the bacterial polymer, the poly-γ-L-glutamine chain is glycosylated, containing two types of oligosaccharide. The first oligosaccharide consists of a GlcNAc pentasaccharide at the reducing end and multiple GalA residues at the nonreducing end. The second presents a GalNAc disaccharide at the reducing end and two Glc units at the nonreducing end.
Heteropolysaccharides
Halococcus morrhuae is an extreme halophile surrounded by an electron-dense 50- to 60-nm-thick cell wall composed of a complex, highly sulfated heterosaccharide consisting of glucosamine, galactosamine, gulosaminuronic acid, glucose, galactose, mannose, glucuronic acid, galacturonic acid, N-acetylated amino sugars, and sulfated subunits. Different heteropolysaccharides are thought to be connected via glycine bridges between the amino groups of the glucosamines and the carboxyl groups of the uronic residues. Although the building blocks of heteropolysaccharide have been suggested, biosynthesis of this cell wall structure has yet to be described.
Methanochondroitin
Individual cells of Methanosarcina rely on an S-layer as their cell wall. A cubic aggregate of four cells (sarcina) is covered by an additional rigid fibrillar polymer called methanochondroitin. Degradation of methanochondroitin results in the disaggregation of the cells, underlining that the matrix is responsible for maintenance of the aggregate. Methanochondroitin, which is similar to eukaryotic connective tissue chondroitin, is composed of a repeating trimer of uronic acid and two GalNAc residues. Yet unlike chondroitin, methanochondroitin is not sulfated. A pathway of methanochondroitin biosynthesis has been proposed based on activated precursors in Methanosarcina barkeri cell extracts. Methanosarcina species can further modify the methanochondroitin condition largely through the addition of glucose and galactose acids.
Lipoglycan
Members of the thermoacidophilic order Thermoplasmatales (pH 1–2 and 60°C), such as Ferroplasma acidophilum and Thermoplasma acidophilum, lack a rigid cell envelope. Such organisms thus display a pleomorphic shape, similar to mycoplasma. Stabilization of the cell is most likely realized by the oligosaccharide portions of lipoglycans and membrane-associated glycoproteins. The outwardly oriented glycan chains form a protective slime coat called the glycocalyx. A recent study of different T. acidophilum cell-surface glycoproteins identified an N-linked branched octosaccharide described below.
Archaeal Surface (S)-Layer Glycoproteins
The majority of characterized Archaea rely on a proteinaceous cell wall, the S-layer, comprising a regularly structured two-dimensional array based on a single protein species, the S-layer glycoprotein, or a limited number of proteins (Figure 22.1). In some archaeal species, the S-layer can be further supported by polysaccharides, by a second S-layer sheet, or by additional surface glycoproteins. For example, the S-layer of Methanospirillum hungatei is further enclosed by a tubular proteinaceous sheath. These sheaths form a paracrystalline structure based on a simple p2 lattice, which is distinct from that of the S-layer. Depending on the species, the proteinaceous sheaths can be glycosylated. Another example is the extremely halophilic Haloquadratum walsbyi. The unique square-shaped cells, with a thickness of only 0.1–0.5 µm, are surrounded by one or two S-layer sheets. An extremely large glycoprotein, termed halomucin, highly similar to mammalian mucin, is loosely connected to the S-layer. Halomucin is heavily glycosylated, containing more than 280 potential N-glycosylation sites (on average one site every 32 residues). This cell envelope is further enforced by analogues of halomucin, termed Hmu2 and Hmu3, and most likely by a poly-γ-glutamate capsule.
PROTEIN GLYCOSYLATION IN ARCHAEA
The Diversity of N-Linked Glycans in Archaea
To date, glycoproteins from Archaea isolated from a wide range of habitats have been studied to various degrees of detail. Possibly reflecting the varied niches occupied by these organisms, their S-layer glycoproteins and other glycoproteins, such as archaellins and pilins, bear N-linked glycans that present wider diversity in terms of size; degree of branching; the identity of the linking sugar; modification of sugar components by amino acids, sulfate, and methyl groups; and the presence of unique sugars than reported to date in Bacteria or Eukarya. Currently defined archaeal N-linked glycans are depicted in Figure 22.3.

FIGURE 22.3.
The structural diversity of N- and O-linked glycans in Archaea. The structures of N-linked glycans found in the Euryarchaeota and Crenarchaeota are shown. The OTase (STT3 or AglB) within the endoplasmic reticulum (ER) or cytoplasmic membrane (right), (more...)
Delineated Pathways of Archaeal N-Glycosylation
The first reported archaeal N-glycosylated protein, the Halobacterium salinarum S-layer glycoprotein, was shown to be modified by two different N-linked oligosaccharides, a repeating sulfated pentasaccharide linked via N-glycosylamine to Asn-2 and a sulfated glycan linked by a glucose residue to 10 other Asn residues. The latter glycan is also N-linked to archaellins in this haloarchaeon. Efforts undertaken at the time aimed at deciphering the pathways responsible for the synthesis of these glycans relied solely on biochemical approaches because neither suitable genetic tools nor a genome sequence were available.
Despite these biochemical advances, delineation of archaeal N-glycosylation pathways had to wait until the genome age and the development of tools for the genetic manipulation of various species. Through the subsequent identification of homologs of eukaryotic and/or bacterial N-glycosylation pathway components, genome scanning for additional components, the generation of deletion strains, and characterization of reporter glycoproteins, agl (archaeal glycosylation) genes comprising archaeal N-glycosylation pathways have been identified in several Archaea, including halophilic, methanogenic, and thermophilic species.
Halophilic Euryarchaeota
In the last decade, progress in defining pathways of N-glycosylation has relied on Haloferax volcanii as a model organism. In Hfx. volcanii, a series of Agl proteins mediate the assembly and attachment of a pentasaccharide with the structure mannose-1,2-[methyl-O-4-]glucuronic acid-β1-4-galacturonic acid-α1-4-glucuronic acid-β1-4-glucose-β-Asn to the S-layer glycoprotein and archaellins (Figure 22.4). Acting at the cytoplasmic face of the plasma membrane, the glycosyltransferases AglJ, AglG, AglI, and AglE sequentially add the first four pentasaccharide residues onto a common Dol-P carrier, whereas AglD adds the final pentasaccharide residue, mannose, to a distinct Dol-P (Figure 22.3). Assembly of the Dol-P-linked tetrasaccharide also involves AglF, a glucose-1-phosphate uridyltransferase, AglM, a UDP-glucose dehydrogenase, AglP, a methyltransferase, and AglQ, a predicted isomerase. AglF and AglM have been shown to act in a sequential and coordinated manner in vitro, transforming glucose-1-phosphate into UDP-glucuronic acid. AglB, the archaeal oligosaccharyltransferase, transfers the lipid-linked tetrasaccharide to select Asn residues of target proteins. The final mannose residue is subsequently transferred from its Dol-P carrier to the protein-bound tetrasaccharide in a reaction requiring AglR, a protein that either serves as the Dol-P-mannose flippase or contributes to such activity, and AglS, a Dol-P-mannose mannosyltransferase. Interestingly, N-glycosylation of the S-layer protein is altered in response to a change in environmental conditions. When grown in low-salt medium, Hfx. volcanii alters the N-glycan structure in a site-specific manner.

FIGURE 22.4.
The pathway of N-glycosylation in Haloferax volcanii. The oligosaccharide is assembled on a dolichol phosphate lipid carrier, translocated across the plasma membrane, and transferred to a target protein by the AglB oligosaccharyltransferase. The N-linked (more...)
Methanogenic Euryarchaeota
Mass spectrometry studies elucidated the glycan N-linked to archaellins of Methanococcus voltae strain PS. GlcNAc, the linking sugar, is connected to a diacetylated glucuronic acid, in turn linked to an acetylated mannuronic acid modified by a threonine at the C-6 position (β-ManpNAcA6Thr-(1-4)-β-Glc-pNAc3NAcA-(1-3)-β-GlcpNAc), although archaellins from other versions of M. voltae strain PS presented an N-glycan bearing an additional mass of either 220 or 260 Da at the nonreducing end, likely representing an additional sugar. As with Hfx. volcanii, the identification of M. voltae N-glycosylation pathway components initially relied on gene deletion and subsequent analysis of the N-linked glycans generated in mutant strain. As such, the oligosaccharyltransferase AglB and the glycosyltransferase AglA, responsible for transfer of the third sugar of the glycan, were discovered. The same strategy was later used to identify AglC and AglK, glycosyltransferases proposed to be involved in the biosynthesis or transfer of the second sugar. A genetic approach also assigned AglH responsibility for adding the linking sugar GlcNAc to the lipid carrier on which the N-linked glycan is assembled. Although aglH could not be deleted in M. voltae, it was able to complement a conditional lethal mutation in the alg7 gene of Saccharomyces cerevisiae. Alg7, sharing 25% identity with M. voltae AglH, catalyzes the conversion of UDP-GlcNAc and Dol-P to UMP and Dol-PP-GlcNAc in the eukaryotic N-glycosylation process. Additional insight into M. voltae N-glycosylation has come from in vitro studies. In contrast to the earlier genetics-based studies showing AglH to be the first glycosyltransferase of the pathway, a bacterially expressed version of the enzyme was not able to add GlcNAc to Dol-P. On the other hand, purified AglK catalyzed the formation of Dol-P-GlcNAc from Dol-P and UDP-GlcNAc. The seeming disagreement between the genetics and biochemical results concerning AglH and AglK functions remains to be solved.
Methanococcus maripaludis has become an important model for genetic and structural research on N-glycosylation in the methanogens. In M. maripaludis, archaellins are modified by an N-linked tetrasaccharide similar to its M. voltae counterpart. In the M. maripaludis glycan, the linking sugar is GalNAc and not the GlcNAc used by M. voltae. The second sugar in the M. maripaludis glycan is a diacetylated glucuronic acid, as in M. voltae. Although the third sugar is a modified mannuronic acid with a threonine attached at the C-6 position in both organisms, there is an additional acetamidino group added at position C-3 of the M. maripaludis glycan. The fourth and terminal sugar of the M. maripaludis glycan is a novel sugar, (5S)-2-acetamido-2,4-dideoxy-5-O-methyl-α-L-erythro-hexos-5-ulo-1,5-pyranose. It was later reported that the major M. maripaludis pilin is modified by the same N-linked tetrasaccharide bearing an extra hexose branching from the linking GalNAc subunit. The pathway used for N-glycosylation of M. mariplaudis archaellin has been largely delineated. The process seemingly starts with the addition of UDP-GalNAc to Dol-P by an unidentified glycosyltransferase. Like Hfx. volcanii Dol-P, M. mariplaudis Dol-P includes two saturated isoprenes, likely at the α and ω positions. The AglO, AglA, and AglL glycosyltransferases add the next three nucleotide-activated sugars, respectively. AglU adds the threonine moiety to sugar three, apparently only following the addition of the fourth sugar by the glycosyltransferase AglL. AglV then methylates sugar four. The Dol-P-bound tetrasaccharide is then flipped across the membrane by an unidentified flippase, where AglB transfers the lipid-linked glycan to target Asn residues.
Thermophilic Crenarchaeota
Studies on the N-glycosylation process in Crenarchaeota have focused on the thermoacidophilic archaeon Sulfolobus acidocaldarius. In S. acidocaldarius, the S-layer glycoprotein, archaellin, and cytochrome b558/566 are modified by an N-linked hexasaccharide with the structure (Man-α1-6)(Man-α1-4)(Glc-β1-4-Qui6S-β1-3)GlcNAcβ1-4GlcNAc-β-Asn. This glycan is unusual as it contains the typical eukaryotic chitobiose core and a sulfoquinovose, a sugar generally found only in photosynthetic membranes of plants and phototrophic bacteria. Biosynthesis of the N-linked glycan begins with the transfer of GlcNAc-phosphate, derived from a nucleotide-activated precursor, onto an unusually short and highly saturated Dol-PP lipid carrier by AglH, a UDP-GlcNAc-1-P: Dol-P-GlcNAc-1-P transferase. Information concerning addition of the second and third sugars is lacking. However, Agl3 converts UDP-glucose and sodium sulphite into UDP-sulfoquinovose, which is subsequently added to Dol-PP-bound trisaccharide by an unknown glycosyltransferase. In the final steps of N-linked glycan assembly, the terminal mannose and glucose moieties are added, with Agl16, a soluble glycosyltransferase, adding the final glucose. A so-far unidentified flippase translocates the Dol-PP-bound hexasaccharide across the membrane, where AglB transfers the glycan to target protein Asn residues. In contrast to Hfx. volcanii, M. voltae, and M. maripaludis, aglB is essential in S. acidocaldarius.
The Diversity of Archaeal O-Linked Glycans
In comparison to N-glycan biosynthesis, relatively little is known of how archaeal O-glycans are assembled. The O-glycans of four archaeal species have been characterized to a limited extent (Figure 22.3). The only published report on archaeal O-glycan biosynthesis revealed that Haloarcula hispanica requires Dol-P-Glc as a sugar donor for the assembly of the N-linked glucose-α-(1,2)-[sulfoquinovosamine-β-(1,6)-]galactose trisaccharide and the O-linked glucose-α-(1,4)-galactose disaccharide.
PHYSIOLOGICAL ROLES OF ARCHAEAL GLYCOSYLATION
N-glycosylation has been considered as assisting Archaea to cope with the challenges of the extreme environments they often occupy. For instance, enhanced surface charge in the face of hypersaline conditions and hence increased solubility was offered as an explanation for the high sulfated sugar content of N-linked glycans decorating the Hbt. salinarum S-layer glycoprotein relative to its Hfx. volcanii counterpart, given the higher salinity of the locale in which the former lives. In other instances, it is not clear how a given N-glycosylation profile contributes to life in harsh surroundings. In Hfx. volcanii, however, N-glycosylation may provide cells with the ability to respond to changes in the surrounding salinity. As noted above, the N-glycosylation profile of the S-layer glycoprotein differs in cells grown in 3.4 or 1.75 m NaCl-containing medium (Figure 22.3). Modified glycosylation in response to environmental conditions has also been reported in the case of M. hungatei, in which archaellins are only modified in a low phosphate-containing medium. Furthermore, a fully assembled N-glycan has been shown to be required for a broad range of biological functions, including motility and species-specific cell-cell recognition.
Extracellular Polysaccharides
Archaeal biofilms have been identified in a large variety of habitats (e.g., at low and high temperatures), as well as under acidic, alkaline, and high-salt conditions. It is proposed that Archaea, especially those that require interaction with other species, synthesize biofilms to support these cell–cell interactions. Detailed analyses of the structure and composition of extracellular polymeric substances (EPSs) of archaeal biofilms are limited. The sugar composition of a few EPSs has been analyzed by lectin binding assays. Such studies revealed that in Sulfolobales species, for instance, the EPS mainly consists of glucose, galactose, mannose, and N-acetyl-D-glucosamine.
ACKNOWLEDGMENTS
The authors acknowledge contributions to previous versions of this chapter by Jeffrey D. Esko, Tamara L. Doering, and the late Christian R.H. Raetz and appreciate helpful comments and suggestions from Ramya Chakravarthy and Debra Mohnen.
FURTHER READING
- Sumper M. 1987. Halobacterial glycoprotein biosynthesis. Biochim Biophys Acta 906: 69–79. doi:10.1016/0304-4157(87)90005-0 [PubMed: 2882779] [CrossRef]
- Lechner J, Wieland F. 1989. Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem 58: 173–194. doi:10.1146/annurev.bi.58.070189.001133 [PubMed: 2673008] [CrossRef]
- Kandler O, Konig H. 1998. Cell wall polymers in Archaea (Archaebacteria). Cell Mol Life Sci 54: 305–308. doi:10.1007/s000180050156 [PubMed: 9614965] [CrossRef]
- Schäffer C, Messner P. 2001. Glycobiology of surface layer proteins. Biochimie 83: 591–599. doi:10.1016/s0300-9084(01)01299-8 [PubMed: 11522387] [CrossRef]
- Albers SV, Meyer BH. 2011. The archaeal cell envelope. Nat Rev Microbiol 9: 414–426. doi:10.1038/nrmicro2576 [PubMed: 21572458] [CrossRef]
- Visweswaran GR, Dijkstra BW, Kok J. 2011. Murein and pseudomurein cell wall binding domains of Bacteria and Archaea—a comparative view. Appl Microbiol Biotechnol 92: 921–928. doi:10.1007/s00253-011-3637-0 [PMC free article: PMC3210951] [PubMed: 22012341] [CrossRef]
- Eichler J. 2013. Extreme sweetness: protein glycosylation in Archaea. Nat Rev Microbiol 11: 151–156. doi:10.1038/nrmicro2957 [PubMed: 23353769] [CrossRef]
- Larkin A, Chang MM, Whitworth GE, Imperiali B. 2013. Biochemical evidence for an alternate pathway in N-linked glycoprotein biosynthesis. Nat Chem Biol 9: 367–373. doi:10.1038/nchembio.1249 [PMC free article: PMC3661703] [PubMed: 23624439] [CrossRef]
- Jarrell KF, Ding Y, Meyer BH, Albers SV, Kaminski L, Eichler J. 2014. N-linked glycosylation in Archaea: a structural, functional, and genetic analysis. Microbiol Mol Biol Rev 78: 304–341. doi:10.1128/mmbr.00052-13 [PMC free article: PMC4054257] [PubMed: 24847024] [CrossRef]
- Klingl A. 2014. S-layer and cytoplasmic membrane—exceptions from the typical archaeal cell wall with a focus on double membranes. Front Microbiol 5: 624. doi:10.3389/fmicb.2014.00624 [PMC free article: PMC4243693] [PubMed: 25505452] [CrossRef]
- van Wolferen M, Orell A, Albers SV. 2018. Archaeal biofilm formation. Nat Rev Microbiol 16: 699–713. doi:10.1038/s41579-018-0058-4 [PubMed: 30097647] [CrossRef]
- Review Archaea.[Essentials of Glycobiology. 2015]Review Archaea.Albers S, Eichler J, Aebi M. Essentials of Glycobiology. 2015
- Review N-glycosylation in Archaea-New roles for an ancient posttranslational modification.[Mol Microbiol. 2020]Review N-glycosylation in Archaea-New roles for an ancient posttranslational modification.Eichler J. Mol Microbiol. 2020 Nov; 114(5):735-741. Epub 2020 Jul 26.
- Review Protein glycosylation in Archaea: sweet and extreme.[Glycobiology. 2010]Review Protein glycosylation in Archaea: sweet and extreme.Calo D, Kaminski L, Eichler J. Glycobiology. 2010 Sep; 20(9):1065-76. Epub 2010 Apr 5.
- Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea.[Mol Microbiol. 2006]Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea.Chaban B, Voisin S, Kelly J, Logan SM, Jarrell KF. Mol Microbiol. 2006 Jul; 61(1):259-68.
- Review Hot and sweet: protein glycosylation in Crenarchaeota.[Biochem Soc Trans. 2013]Review Hot and sweet: protein glycosylation in Crenarchaeota.Meyer BH, Albers SV. Biochem Soc Trans. 2013 Feb 1; 41(1):384-92.
- Archaea - Essentials of GlycobiologyArchaea - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
