The content of this book is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 Unported license. To view the terms and conditions of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 4th edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2022. doi: 10.1101/glycobiology.4e.18
The glycosylation of macromolecules is a highly compartmentalized process. Most of the enzyme donor precursors are synthesized in the cytoplasm or nucleoplasm and transferred into the secretory pathway. There they are incorporated into the glycoproteins, glycolipids, or polysaccharides that are fated for delivery to the extracellular environment or organelles such as the lysosome. In some cases, the donor sugars are transferred to intermediate lipids presented at the cytoplasmic surface of secretory pathway membranes, and subsequently flipped into the secretory pathway for final incorporation into glycoconjugates. In other cases, polysaccharides are synthesized at the cytoplasmic surface of the plasma membrane and simultaneously translocated to the cell surface. Thus the cytoplasm has a key role in the assembly of glycans that are, however, destined to function outside of the cytoplasm. In addition, entirely different glycosyltransferases exist in the cytoplasm or nucleus to glycosylate proteins and lipids that remain to function in the cytoplasm or nucleoplasm. There is also evidence for cytoplasmic and nuclear glycoconjugates that have acquired secretory pathway–type glycans by unexplained mechanisms. The origin and role of these nucleocytoplasmic glycoconjugates, mainly glycoproteins, are the focus of this chapter. We begin with examples of monoglycosylation, including both endogenous processes and those associated with parasitism, and note that one form, O-linked β-N-acetylglucosamine (O-β-GlcNAc), is so prevalent that an entire chapter is devoted to it (Chapter 19). We then continue to complex glycans and glycosylation associated with mitochondria and chloroplasts and conclude with an assessment of nucleocytoplasmic carbohydrate binding proteins that may serve as glycan readers.
MONOGLYCOSYLATION OF NUCLEOCYTOPLASMIC PROTEINS
Eukaryotic Monoglycosylation
Monoglycosylation, the addition of a single monosaccharide to the hydroxyl or amide moieties of amino acid side-chains, was first discovered in the form O-β-GlcNAc and is now known as a widespread modification of thousands of nuclear mitochondrial and cytoplasmic proteins throughout the animal and plant kingdoms and some protists, fungi, and bacteria (see Chapter 19). The addition of O-GlcNAc is linked to the side-chains of Ser or Thr and is mediated by a highly conserved lineage of O-GlcNAc transferases (OGT), a nonmembrane bound enzyme that resides in the cytoplasm and nucleus. A splicing variant is also found in animal mitochondria in which O-GlcNAcylation is found on respiratory enzymes. A highly related enzyme lineage, termed O-fucosyltransferase (OFT), mediates the addition of O-fucose (O-Fuc) to dozens of nucleocytoplasmic proteins in plants, numerous protists, and possibly bacteria. The OFT was first discovered genetically in plants as Spy, in which it controls transcriptional processes. In the protist pathogen Toxoplasma gondii, OFT modifies numerous proteins involved in transcription, mRNA biogenesis, nuclear transport, and cell signaling. Recently, Greb1, derived from another lineage of enzymes, was shown to O-β-GlcNAcylate estrogen receptor-α and potentially other nuclear proteins, which if verified is an interesting example of convergent evolution. Paralogs of these enzymes have not been described in the well-studied Saccharomyces cerevisiae, but biochemical data suggests that this yeast modifies intracellular proteins with mannose (Man) and even di- and tri-Man. Furthermore, several reports suggest that select intracellular proteins of mammalian cells are modified by N-acetylgalactosamine (GalNAc). The significance of these intriguing latter examples awaits identification of the responsible glycosyltransferases and evidence for their roles in cells. Examples in which the glycosyltransferases are known are represented schematically in Figure 18.1A and summarized in Table 18.1.
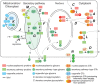
FIGURE 18.1.
Cellular topography of nucleocytoplasmic glycosylation. (A) Monoglycosylation (m) in the cytoplasm (e.g., O-GlcNAc and O-Fuc). (B) Complex (c) glycosylation in the cytoplasm by O-GlcNAc transferases (GTs) that extend the first sugar. (C) Cytoplasmic monoglycosylation (more...)
TABLE 18.1.
Examples of nuclear or cytoplasmic glycosylation events
Prokaryotic Monoglycosylation
Highly conserved homologs of OGT and OFT are found in numerous bacterial genomes, although studies of their functional roles are essentially nonexistent. An example of bacterial monoglycosylation that leads to a known function comes from the EF-P protein, the bacterial homolog of eukaryotic elongation initiation factor 5a. In some bacteria, EF-P suppresses translational stalling by a mechanism that involves oxidation of a critical lysyl residue, and its eukaryotic ortholog is regulated by hypusylation. A recent phylogenetic analysis of EF-P sequences revealed a subset of enzymes with an Arg in place of Lys, and a coevolving gene that was subsequently identified by biochemistry and mass spectrometry as an argininyl rhamnosyltransferase. Assembly of the Rha-Arg linkage activates EF-P and is required for pathogenicity of Pseudomonas, a Gram-negative, opportunistic human pathogen, and a number of other bacteria. The discovery of this linkage by a phylogenetic approach suggests that more noncanonical examples of glycosylation are yet to be discovered.
A directed proteomics search for glycoproteins in the Gram-positive intestinal bacterium Lactobacillus plantarum, biased only because potential glycopeptides were affinity enriched with the lectin wheat germ agglutinin (WGA), identified several glycoproteins with cytoplasmic localization and functions: the molecular chaperone DnaK, the PdhC E2 subunit of the pyruvate dehydrogenase complex, the DNA translocase FtsK1, the signal recognition particle receptor FtsY, FtsZ involved in cell division, and others of unknown function. Peptides from these proteins were modified with single HexNAc residues attached to Ser residues. Some of the sites were variably modified, hinting at a novel regulatory process, but nothing is known about the mechanism of this glycosylation.
Bacterial Glycosyltransferase Toxins
Pathogenic bacteria have developed a wide array of toxin and effector virulence factors that also monoglycosylate host-cell proteins (Figure 18.1C). By virtue of their glycosyltransferase domains, these impair the host's cytoplasmic or nuclear machinery and disrupt host-cell immune response. For example, small cytoplasmic G-proteins (GTP-binding proteins) of the Rho family are involved in regulating the cytoskeleton. Certain toxins from anaerobic bacteria were found to contain retaining glycosyltransferase activities that inhibit G-proteins by attaching a glycosyl moiety to a threonine residue (Thr-37) in their GTP-binding sites. These secreted toxins show the remarkable ability of translocating across the surface membrane into the cytoplasm of mammalian target cells. The enterotoxins from Clostridium difficile (ToxA) and Clostridium sordellii are α-glucosyltransferases from CAZy family GT44 that use UDP-glucose (UDP-Glc) as the donor. In contrast, a similar toxin from Clostridium novyi is an O-αGlcNAc transferase that uses UDP-GlcNAc as the donor. The C. novyi toxin has no primary sequence relationship to host glycosyltransferases. A distantly related toxin from Legionella pneumophila, also delivered by a type IV secretion system, installs an αGlc residue on elongation factor 1A in a broad range of eukaryotic host cells. The target residue, Ser53, is located in the G domain near the switch-1 region of the GTPase, and glucosylation inhibits its activity in vitro and in vivo. The recent discovery of a new effector glucosyltransferase, LtpM, shows that this is an expanding field of glycosyltransferase discovery. Interestingly, an unrelated toxin from Yersinia, which enters host cells via a phage tail-derived translocation system, was found to be an αGlcNAc-transferase that modifies Tyr-34 of RhoA. Typically, the sugar–amino acid linkage is not recognized by endogenous glycosidases, allowing the pathogen to irreversibly alter the function of the target—typically a GTPase domain.
Other Gram-negative bacteria use a type 3 secretion system to inject virulence factors that mediate a different form of monoglycosylation: GlcNAcα-Arg. This novel reaction is catalyzed by NleB-related glycosyltransferases that promote host-cell longevity during infection. NleB and NleB orthologs target arginine side-chains within death domains of death receptors and associated proteins TRADD, FADD, RIPK1, and TNFR1. Importantly, these factors all work to attenuate NF-κB signaling to dampen host-cell antibacterial and inflammatory responses. Furthermore, various components of glucose metabolism such as GAPDH (glyceraldehyde 3-phosphate dehydrogenase) and HIF-1α are also targeted to reduced NF-κB signaling. Although these examples involve monoglycosylation mediated by exogenous glycosyltransferases (see Table 18.1), their existence reinforces the potential impact of glycosylation as a regulatory mechanism, and the diversity of glycosylation events that probably remain to be discovered.
Glycosylation of DNA
Although the focus of this chapter is on nuclear and cytoplasmic glycoproteins, it should be noted that specific bases in DNA have long been known to be a target of bacteriophage-encoded glycosyltransferases. Modification of T4-phage DNA hydroxymethylcytosine residues by βGlcTs or αGlcTs (Table 18.1) aids in the distinction of native and foreign DNA, rendering them resistant to host restriction enzyme digestion and impairing a subset of CRISPR/Cas systems. The use of Glc disaccharide, arabinose, and other sugar modifications of bases suggests that the full diversity of sugar modifications remains to be described. A related modification occurs in a protist, Trypanosoma brucei, in the form of base J. Using a mechanism resembling Skp1 glycosylation (vide infra), DNA thymidine residues are initially hydroxylated by an O2-dependent nonheme dioxygenase generating hydroxymethyl moieties suitable for glycosylation by a novel nuclear β-glucosyltransferase, JGT, a CAZy GT2 glycosyltransferase (Table 18.1). Base J influences Pol II termination by recruiting a complex containing protein phosphatase 1 to dephosphorylate RNA polymerase II. Disruption in complex formation affects polycistronic transcription termination in the wider group of kinetoplastids.
COMPLEX GLYCOSYLATION OF NUCLEOCYTOPLASMIC PROTEINS
Complex glycans, consisting of more than a single sugar, have also been reported in the nucleocytoplasmic compartment (Figure 18.1B). The better understood examples are described here, together with evidence for glycosylation associated with two organelles that reside in the cytoplasm: mitochondria and chloroplasts. Less well described candidates are addressed in a subsequent section.
Hydroxyproline-Linked Skp1 Glycans
The Skp1 glycoprotein, which bears a single linear pentasaccharide, was discovered in the nucleus and cytoplasm of a free-living soil amoeba, the cellular slime mold Dictyostelium discoideum. Skp1 is an adaptor in SCF (Skp1/Cullin1/F-box protein)-type E3-ubiquitin ligase complexes that function in the cytoplasm and nucleus of all eukaryotes. There they mediate the polyubiquitination and eventual proteasomal degradation of hundreds of proteins involved in cell cycle regulation, transcription, and signal transduction. Skp1 is subject to O2-dependent hydroxylation at Pro143, which generates the substrate for five glycosyltransferase reactions. Based on mass spectrometric, sequential exoglycosidase treatments, and nuclear magnetic resonance (NMR) studies (Chapter 50), the glycan consists of a core trisaccharide equivalent to the type 1 blood group H structure, Fucα1-2Galβ1-3GlcNAc1α-, substituted at the 3-position of Fuc by a Galα1,3Galα- disaccharide (Figure 18.2B). In most other protists that modify Skp1, including the apicomplexan parasite T. gondii, a similar core trisaccharide is capped by a Galα1-3Glcα1- disaccharide (Figure 18.2A). The linkage of this glycan to protein, its localization in the cytoplasm, and the structures themselves are currently unique. Because the Pro residue is conserved in the Skp1 genes of plants, invertebrates, and unicellular eukaryotes, and gene sequences related to those of known Skp1 modification enzymes (see below) are present in the genomes of many protists, this modification appears to be widespread in aerobic unicellular eukaryotes and pathogenic fungi.
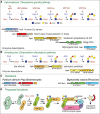
FIGURE 18.2.
Mechanism of glycosylation of Skp1 in the cytoplasm of protists. Glycosylation is enabled by prior hydroxylation of a single Pro-residue by the action of a cytoplasmic, O2-dependent prolyl 4(trans)-hydroxylase that is homologous to the HIFα prolyl (more...)
The glycosyltransferases are all NDP-sugar-dependent and colocalize with Skp1 in the cytoplasm or nucleus (Figure 18.2A-C; Table 18.1). The Skp1 αGlcNAc-transferase (EC 2.4.1.229), which adds the first sugar, is related to the Thr/Ser polypeptide αGalNAc-transferases that initiate mucin-type O-glycosylation in the Golgi of animals, and therefore forms an anomeric linkage opposite to that of the aforementioned OGT (Chapter 19). However, unlike known polypeptide αGalNAc-transferases (Chapters 6 and 10), the Skp1 αGlcNAc-transferase lacks an amino-terminal signal anchor, consistent with its biochemical fractionation as a soluble cytoplasmic protein. Thus, the mechanism of initiation of Skp1 glycosylation is like that of mucin-type domains of secretory proteins, except that a different N-acetylhexosamine (GlcNAc vs. GalNAc) is attached to a distinct hydroxyamino acid (Hyp vs. Thr or Ser) in the cytoplasm versus the Golgi lumen. Additions of the second (βGal) and the third (α-L-Fuc) sugars are catalyzed by separate domains of the same soluble protein, PgtA. The β3-galactosyltransferase catalytic domain is most like bacterial lipopolysaccharide and capsular glycosyltransferases, which are also cytoplasmic, suggesting that cytoplasmic glycosylation in Dictyostelium has its evolutionary origins in bacterial glycolipid synthesis. The β-galactosyltransferase catalytic domain belongs to the inverting CAZy GT2 family, which includes numerous glycosyltransferases with catalytic domains exposed to the cytoplasm. In Dictyostelium, the fourth and fifth sugars are added by the two-domain glycosyltransferase AgtA, in which the α3-galactosyltransferase domain is fused to a β-propeller-like domain, which has a second function that may involve constitutive Skp1 sequestration activity. The catalytic domain is related to a large number of plant Golgi glycosyltransferases implicated in pectin biosynthesis and here catalyzes two successive additions. In contrast, most protists employ separate glycosyltransferases with independent evolutionary origins to catalyze the completion of the glycan (Figure 18.2A). In Toxoplasma and Pythium ultimum, a crop plant pathogen and agent for human pythiosus, addition of the αGlc residue is carried out by a CAZy GT31 enzyme most related to Golgi glycosyltransferases that extend mannose chains in yeast, and the terminal αGal residue is added by Gat1, a CAZy GT8 enzyme that is most related to the enzyme discussed below, glycogenin. Finally, in other protists, gene fusions consolidate the first two or final two enzymes as separate domains of the same protein (Figure 18.2C), which may facilitate efficient extension of the glycan to its final length.
Dictyostelium cells aggregate when starved and the resulting multicellular slug differentiates to form a fruiting body with stress-tolerant spores. This process is sensitive to the level of O2, which is thought to help the cells determine whether they are below or above ground in their native soil environment. Considerable biochemical, genetic, and physiological evidence supports the model that O2 levels regulate the rate of Skp1 hydroxylation, which in turn controls the rate of glycosylation. Glycosylation regulates the relative representation of different F-box protein substrate receptors in the Skp1 interactome (Figure 18.2D), by a mechanism that most likely controls the conformational profile of Skp1 rather than recognition by a glycan receptor. This may differentially regulates the lifetime of proteins that critically control or execute developmental progression in response to starvation.
Glycogenin
A second well-studied example is glycogen, a large branched homopolysaccharide used as a short-term storage form of glucose in bacteria, yeast, and animals. Glucose is added to and removed from the nonreducing termini per the availability of the sugar donor UDP-Glc and the nutritional needs of the cell. Glycogen stores in the liver are important in maintaining glucose homeostasis in the blood. Glycogen is assembled initially from a linear oligoglucoside that is extended by glycogen synthase and rearranged by the so-called branching enzyme. The initial oligoglucose is assembled by another enzyme, glycogenin, which attaches the first sugar to itself in an uncommon Glcα1-Tyr linkage, at Tyr-195 in human glycogenin-1. Thus, every glycogen molecule, which can contain up to 105 Glc residues and 12 generations of branchpoints, is thought to have a single glycogenin protein molecule at its nonreducing end. Therefore, glycogen is a glycoprotein whose glycosylation is initiated by glycogenin, extended by glycogen synthase, and modified by branching enzyme. Free glucose-1-PO4 is released, as needed, by the actions of debranching enzyme and glycogen phosphorylase.
Glycogenin naturally assembles as a homodimer under cellular conditions. Evidence indicates that the first Glc is transferred to the acceptor hydroxyl on Tyr across subunits, and extended up to a chain length of ∼10 by mixed inter- and intrasubunit additions of αGlc residues to the 4-position of underlying Glc residues. Interestingly, glycogenin shows triphasic kinetics in which there is a priming step typically with UDP-Glc; however, UDP-Gal can form this initial bond. After priming there is a short extension step again with some flexibility with substrate sugars, and finally a refining step catalyzed by the adjacent glycogenin that shows strong substrate specificity for UDG-Glc. Despite the in vitro evidence, aberrant levels of glycogen are still formed in mice lacking glycogenin-1 and its paralog glycogenin-2. Mutations in glycogenin-1 leading to decreased enzymatic function or loss of the protein cause glycogen storage disease type XV characterized by the presence of polyglucosan bodies of abnormally structured glycogen that cannot be digested by amylase. Interestingly, these polyglucosan bodies are derived from aberrant glycogen formation by glycogen synthase. These mutations contribute to respiratory distress, limb-girdle muscular dystrophy–like symptoms, and progressive weakness. However, patients do not show hypoglycemia, hepatomegaly, or hyperlipidemia as with other glycogen storage diseases. Crystallographic and mutational evidence for formation of a complex between glycogenin and glycogen synthase that affects glycogen synthase activity in vitro and in vivo offers a potential explanation for the mutational effects. Thus, levels of glycogenin may operate together with the better understood hormonally controlled mechanisms of glycogen formation that involve enzyme phosphorylation/dephosphorylation and that regulate glycogen elongation.
Glycogenin-like proteins are found in a wide range of plants, animals, and free-living single-celled eukaryotes. The Escherichia coli homolog is incapable of autoglucosylation. The most closely related sequence homolog in alveolates and stramenopile protists, which lack glycogenin, modifies Skp1 (vide supra). Glycogenin is a GT-A superfamily member from CAZy family GT8 (Carbohydrate-Active enZYmes sequence database) (Chapter 52), and more distantly related glycosyltransferases include bacterial lipopolysaccharide (LPS) glucosyl and galactosyl transferases and galactinol synthases—all cytoplasmic as for glycogenin—as well as other glycosyltransferases in the eukaryotic Golgi. The eukaryotic parasite Leishmania assembles mannogen, a polymer of β2-linked mannose polymers, instead of glucose polymers, but a protein primer is not involved for the cytoplasmically localized mannogen synthase. Interestingly, a novel Glc-Arg linkage has been described in a plant protein potentially associated with the synthesis of starch, which is related to glycogen, but this protein has no apparent sequence similarity to glycogenin. The function of this and the glycogenin-like proteins deserve further investigation for their potential to mediate other cytoplasmic glycosylation events.
Mitochondrial and Chloroplast Glycosylation
Mitochondria and chloroplasts are independently replicating organelles that reside in the cytoplasmic compartment. Because of their evolutionary origin from bacteria, which are now known to have substantial glycosylation capacity, these organelles can be expected to possess glycosylation machinery. Indeed, there is evidence for peptidoglycan-like networks between the inner and outer membranes of chloroplasts of certain algae. In other algae, glycosyltransferase MDR1 (mitochondrion dividing-ring 1), an integral membrane protein with a CAZy GT8 glycosyltransferase domain, assembles the mitochondrial dividing ring using α-linked polyglucose nanofilaments that have been visualized between the two membranes. A similar process occurs in chloroplasts, and the conservation of these genes suggests that this might be a heretofore unrecognized example of organelle glycosylation for many eukaryotes.
More recent cell biological studies emphasize intracellular connections between mitochondria and rough endoplasmic reticulum (rER) elements of the secretory pathway. This might explain a pioneering observation that two nuclear-encoded mitochondrial glycoproteins appear to be conventionally N-glycosylated in the rER based on pulse-chase labeling studies and susceptibility to N-glycanase (Figure 18.1J). Indeed, lectin-binding studies suggest that mitochondria contain complex glycoconjugates. In Ewing's sarcomas, the plasma membrane protein MRP-1 (multidrug resistance-associated protein 1) is glycosylated and localized to the mitochondrial outer membrane and could play a role in drug resistance. Several plant chloroplast proteins, including carbonic anhydrase-1, α-amylase, and pyrophosphatase/phosphodiesterase in “higher” plants and Glx, Syn, MutS in diatoms, might be transported from the rER as N-glycosylated proteins (Table 18.1). The presence of N-glycans highlights their potential biosynthetic origin in the conventional secretory pathway, and how these glycoproteins transit to these organelles warrants further investigation.
As noted above, mammalian mitochondria import OGT that O-GlcNAcylates some of their interior proteins. In addition, mono- and digalactolipids (MGDG: 1,2-diacyl-3-O-[β-D-Gal]-sn-glycerol and DGDG: 1,2-diacyl-3-O-[α-D-Gal-(1,6)-O-β-D-Gal]-sn-glycerol) are prominent lipids in chloroplast thylakoid membranes, and MGDG is essential for photosystem function (Chapter 24). Their synthesis is controlled by UDP-Gal-dependent galactosyltransferases residing in the inner and outer layer membranes of the chloroplast (Figure 18.1K). Under conditions of phosphate deprivation, the synthesis of these glycolipids is dramatically increased, becoming up to 70 mol% of glycerolipids. The glycolipids replace conventional phospholipids such as phosphatidylcholine in a variety of other cellular membranes including vacuoles, mitochondria, and the plasma membrane (Table 18.1). DGDG almost exclusively resides in the cytoplasmic leaflet of these membranes and oriented toward the cytoplasm, which is opposite of the conventional orientation of glycolipids as known from animal and yeast cells. Variants containing sulfoquinovose and GlcUA, which conserve the negative charge, are also produced. Little is known about the trafficking of these glycolipids. Given the functional importance of DGDG for cells based on genetic studies, it will be interesting to understand how the asymmetric distribution contributes to cytoplasmic functionality of the cellular membranes.
POSSIBILITIES OF “CONVENTIONAL” SECRETORY-TYPE GLYCANS ON NUCLEOCYTOPLASMIC GLYCOPROTEINS
N-Glycan Types
The above examples describe well-documented examples from both prokaryotes and eukaryote of bona fide glycoproteins that function in the nucleus or cytoplasm and are glycosylated by residential nucleocytoplasmic glucosyltransferases (GTs). This section addresses other potential sources of nucleocytoplasmic glycoproteins. As suggested by the examples below, these proteins might be glycosylated by glycosyltransferases normally associated with the rER or Golgi, either by transient occurrence of the glycoprotein in the secretory pathway, or translocation of the secretory pathway glycosyltransferases into the nucleocytoplasmic compartment.
A sizable fraction of nascent proteins fail quality control of folding and assembly in the rER (Chapter 39) and are retrotranslocated to the cytoplasm as a part of ERAD (ER-associated degradation), as depicted in Figure 18.1H. Most of these are N-glycosylated and normally processed by a cytoplasmic N-glycanase (NGLY1) or an endo-β-N-acetylglucosaminidase (ENGase) to remove their N-glycans before their degradation by the 26S-proteasome or an autophagic vacuole. There are also N-glycan-dependent E3 ubiquitin ligases that assemble polyubiquitin chains that serve as a signal for proteasomal degradation. Interestingly, NGLY1 is essential for mitochondrial turnover via mitophagy, although evidence suggests it functions via a role other than deglycosylating mitochondrial proteins. If these processes do not operate on a given protein, because the N-glycan is modified in such a way that it is not recognized or is sequestered in a protein complex or the nucleus, then it may accumulate in the cytoplasm and potentially execute a novel function that might depend on its ER-derived glycan. In addition, there is evidence that endocytosed proteins, such as cholera toxin, can access the rER via retrograde transport and then enter the cytoplasm via retrotranslocation or a related process.
Other studies suggest that glycoproteins conventionally secreted or transported to the plasma membrane have the potential to later move to the nucleus (Figure 18.1I). This includes cytokines, growth factors, and sometimes their transmembrane receptors, some of which have been reported to exert direct effects on transcription. For example, fragments of the neural cell adhesion molecule (NCAM), which is modified by polysialic acid, appear to be internalized into cells in which they differentially impact transcriptional programs according to their polysialic acid content. These findings have been considered controversial because the mechanism of such translocation remains mysterious.
Different approaches support the occurrence of nucleus-associated glycoproteins. The sialic acid–specific lectin, Sambucus nigra agglutinin (SNA), was shown to bind to several proteins on the cytoplasmic face of the nuclear envelope, including two major nucleoporins: p62 and p180. Prior sialidase treatment blocked binding of SNA to these nuclear pore proteins and, based on peptide-N-glycosidase sensitivity, the sialic acids on p180 appeared to be on N-linked glycans. Furthermore, SNA blocked nuclear protein import in neuroblastoma cells, suggesting that the sialic acids might have functional importance on the nuclear pore proteins. Other notable reports of nuclear glycoproteins include a heat-shock-like nuclear chaperone protein with GlcNAc-binding activity, CBP70, which has been suggested to be N-glycosylated. A subpopulation of nuclear prion proteins may be N-glycosylated and possibly associated with the GlcNAc-binding lectin.
N-glycosylation of cytoplasmic proteins or protein domains has also been proposed. For example, the α-subunit of the dog kidney sodium pump (Na+, K+-ATPase), a transmembrane protein, was reported to contain traditional N-linked glycans with terminal GlcNAc residues in its cytosolic domain. This conclusion was based on the enzymatic attachment of radioactive Gal to GlcNAc residues by galactosyltransferase labeling of permeabilized right-side-out membrane vesicles. Peptide-N-glycosidase sensitivity of the radiolabeled products suggested that the acceptors are N-linked glycans (Chapters 9 and 50). However, this and other claims will be considered provocative until they are confirmed by direct site mapping of the putative glycans. Based on conventional principles of the separate cellular compartmentalization of the N-glycosylation pathway and cytoplasmic and nuclear proteins (not within the nuclear envelope, which is an extension of the rER) (Figure 18.1) and known pathways of translocation of proteins across membranes, there is not a simple explanation for how these modifications are acquired.
Finally, findings of cytoplasmic glycosylation in pathological tissues, such as tau N-glycosylation during neurodegeneration, raise the possibility that compartmental breakdowns result in the exposure of cytoplasmic proteins to normally latent Golgi glycosyltransferases.
Secretory Pathway O-Glycan Types
Glycosylation that is more reminiscent of secretory pathway–type O-glycans has also been reported. In plants, a nuclear pore–associated protein is recognized by the lectin WGA and could be labeled using tritiated UDP-Gal and β4-galactosyltransferase, which is specific for nonreducing terminal GlcNAc. The glycan, with an approximate size of five sugars, was released by mild alkaline degradation consistent with β-elimination from a Thr or Ser residue. The identity of the glycosyltransferases is unknown. More recently, evidence was presented for animal nuclear proteins, including lamins, ribonucleoproteins, and estrogen receptor-α, that are O-glycosylated by αGalNAc (Tn antigen) or core 1 O-glycans Galβ1,3-GalNAc-. Further data show that several intracellular proteins including p53 are enriched by the lectin VVA indicative of modification with O-GalNAc. Another potential example of a cytoplasmic glycan comes from a study on purified mammalian cytokeratin, which was reported to contain GalNAc and bind lectins that recognize α1,3-linked GalNAc in an N-acetylgalactosaminidase-sensitive fashion.
A distinct example of glycosylation has been described on parafusin, a cytoplasmic protein from the phosphoglucomutase family, in several eukaryotes. Evidence suggested that this protein possesses a Glcα1-PO4-Man- linkage, and data were provided for glucose phosphotransferase and Glc-1-phosphate phosphodiesterase activities in cytosolic extracts. However, little is known about the enzymes that catalyze these modifications or the specific structures themselves.
Secretory Pathway Glycosyltransferases in the Nucleus?
A possible mechanism for the occurrence of nuclear O-glycans in the nucleus is if the enzymes that are normally in the secretory pathway gain access to the nucleus or cytoplasm (Figure 18.1F). For example, several reports describe glycosyltransferase activities in highly purified preparations of rat liver nuclei judged to be >99% pure by marker enzyme analysis. These studies documented that incorporation of GlcNAc was inhibited by low concentrations of the antibiotic tunicamycin (an inhibitor of formation of the N-glycan precursor), and later studies showed the direct transfer of chitobiose (GlcNAcβ1-4GlcNAc) from chitobiosyl-dolichol to endogenous nuclear acceptors by these mammalian nuclear preparations, suggesting a novel pathway of N-glycosylation. The products of these in vitro reactions were validated by their sensitivity to peptide N-glycosidase F and hydrazinolysis but insensitivity to alkali-induced β-elimination (Chapter 50). Supporting these observations, recent data suggests that both the core GalNAcT1 and GalNAcT2 can localize to the nucleus. Similar studies have documented the presence of nuclear mannosyltransferases. The finding that CMP (cytidine 5′-monophosphate)-sialic acid is synthesized in the mammalian nucleus offers further support for the concept of nuclear glycosyltransferases. Although these studies are provocative, they must also be interpreted with caution. The rER, which is the widely accepted site of N-glycosylation (Chapter 9), is functionally contiguous with the outer nuclear envelope, which also can fold into the interior. Even a minor contamination of nuclear envelope could lead to misinterpretation of enzyme assays. Also, it is very difficult to purify nuclei such that other cellular components do not nonspecifically adhere to the otherwise “pure” nuclei during their preparation. Given these potential problems, widespread acceptance of the existence of these nuclear glycosyltransferases must await independent confirmation by alternative methods.
Glycosaminoglycans
Another class of glycans traditionally thought to reside at the cell surface and the extracellular matrix are the glycosaminoglycans (or GAGs) (Chapter 17). Early studies provided evidence of GAGs in purified nuclei, and soon thereafter studies on sea urchin embryos indicated developmentally regulated effects of stage-specific heparan sulfate (HS) on transcription that were not observed for chondroitin sulfates or hyaluronan (Chapter 16). Given recent advances in the tools to study proteoglycans, these potentially exciting observations warrant a critical reexamination. Years later, subcellular fractionation of rat hepatocytes radiolabeled with 35SO4 revealed substantial enrichment of specific fragments of HS (after cleavage) in their nuclear fractions. Given the unique nature of these structures, it is hard to conceive how they could be derived by contamination from cell surface HS molecules, which lack these structures. Subsequent pulse-chase studies suggested that exogenous or cell surface HS is taken into the nucleus and modified to these unique nuclear molecular species. In comparison, others found dermatan sulfates, but not HSs, associated with the nucleus of a different cell type. Given the ability of HSs to influence gene transcription in vitro, these studies could eventually prove highly significant. However, all of the known enzymes involved in the biosynthesis of glycosaminoglycans have their active sites in luminal compartments (Chapter 17), and pathways for nuclear uptake of such large and negatively charged molecules have not been described.
Using another approach, immunocytochemical studies probing for a GPI-anchored HS proteoglycan (PG), glypican, have provided strong evidence for the nuclear accumulation of this PG in addition to its traditionally accepted presence at the cell surface. Although the mechanism of nuclear compartmentalization is not known, the glypican polypeptide harbors a nuclear localization sequence that is functional when grafted onto a neutral carrier expressed in the cytoplasm. In a separate study, overexpression of a chondroitin sulfate proteoglycan core protein in cultured cells resulted in accumulation of the core polypeptide in the cytoplasm and nucleus in addition to the secretory pathway, indicating, as has been suggested for other proteins, the possibility of dual compartmentalization. However, although these studies provide an explanation for how proteoglycan core proteins might accumulate in the nucleus, it is difficult to imagine how they have been modified by the glycosaminoglycan synthases residing in the Golgi lumen.
Cytochemical studies using naturally occurring proteins with high-affinity binding to hyaluronic acid have been used to document accumulation of hyaluronic acid in the nuclei of cultured cells. In response to inflammatory conditions, such as hypoglycemia, cells appear to synthesize hyaluronan in intracellular compartments. After cell division, the intracellular hyaluronan is extruded outside cells in cables that recruit inflammatory cells initiating a stress response. Mechanistically, this is likely caused by a redistribution of the hyaluronan synthases to intracellular membranes from the plasma membrane, across which hyaluronic acid is normally extruded (Figure 18.1N). Notably, hyaluronic acid–binding proteins naturally occur within the nucleus raising the possibility of a physiological role for nuclear hyaluronan via interaction with these hyaluronic acid–binding proteins.
VALIDATED CYTOPLASMIC GLYCOSYLTRANSFERASES WHOSE TARGETS ARE EXPORTED FROM THE CELL
A striking example of the assembly of complex glycans in the cytoplasm comes from the PBCV-1 viruses that infect the alga Paramecium. Its major capsid protein, MCP or VP54, is synthesized, posttranslationally modified, and incorporated into viral structures within the cytoplasm of the host. X-ray diffraction analysis shows that VP54 possesses four sites for unconventional Asn-linked-glycosylation, which do not match the canonical N-glycosylation sequon used for N-glycosylation in the eukaryotic secretory pathway, and the linkage sugar is βGlc. A major glycoform consists of a branched, partially methylated decasaccharide containing seven different sugars, including D-Glc, D-Gal, D-Man, D-Xyl, L-Fuc, L-Ara, D-Rha, L-Rha, and dimethylated L-Rha. The glycan is assembled stepwise by virally encoded, sugar nucleotide-dependent, cytoplasmic glycosyltransferases (Table 18.1). Glycosylation of MCP is at least partially virally encoded, and indeed the genome of PBCV-1 contains seven sequences predicted to encode cytoplasmically localized glycosyltransferases that lack targeting sequences for the secretory pathway. Some of these enzymes appear to have multiple enzymatic domains, as observed in the Skp1 glycosylation pathway. The structure is polymorphic between viral lineages and, because not all linkages have been assigned to predictable glycosyltransferase sequences, it is possible that new classes of glycosyltransferase genes are yet to be discovered. Because PBCV-1 eventually lyses its host, the product glycoprotein is expected to function outside of the alga (Figure 18.1L). The MCP pathway elegantly demonstrates the potential of the cytoplasmic compartment to mediate complex glycan assembly that, however, does not ultimately appear to contribute a cytoplasmic or nuclear function.
Protein glycosylation is becoming increasingly recognized as a common modification in bacteria with up to 50% of surface proteins predicted to bear glycans through N-, O-, S-, and C-linkages (Chapter 21). In comparison to eukaryotes, protein glycans appear to vary more among bacterial species. A substantial fraction of protein glycosylation occurs directly on the cytoplasmic surface of the cell membrane prior to export to the surface (Figure 18.1O). In Gram-positive bacteria, the serine-rich repeat protein (SRRP), a bacterial adhesin, is modified with GlcNAc residues by the combined action of GftA/B. Depending on the strain, this modification can be extended into longer structures containing GlcNAc or glucose/galactose residues. These glycoproteins are exported by an accessory secretion (SecA2/Y2) system and the glycan modification appears to play a role in modulating the adhesive properties of the bacterium. Haemophilus influenzae also modifies high molecular weight adhesions 1 (HMW1) prior to export. HMW1 is modified at some 31 asparagine residues, predominantly in the N-X-S/T amino acid motif, with mono- or dihexose modifications containing glucose and galactose. Glycosylation is initiated and extended by HMW1C (NGT), an enzyme with homology to the O-GlcNAc transferase. Of note, HMW1C uses UDP-Glucose and UDP-Galactose to initiate and extend glycans in a stepwise manner. Glycosylation of HMW1 protects against premature degradation and promotes tethering to the bacterial cell surface. Recent studies suggest that closely related proteins in Actinobacillus pleuropneumoniae, Kingella kingae, and Aggregatibacter aphrophilus are also glycosyltransferases. Among several other known examples, flagellin filament proteins of Caulobacter crescentus are glycosylated intracellularly with pseudaminic acid by the hitherto unknown FlmG glycosyltransferase. This modification is important for successful cell surface flagellation.
INTERMEDIATES IN ASSEMBLY OF EXPORTED GLYCOCONJUGATES OR POLYSACCHARIDES
The biosynthesis of several polysaccharides and precursors of secretory pathway glycans are mediated by membrane-associated glycosyltransferases whose catalytic domains are situated at the cytoplasm-facing surface. This includes early steps in the synthesis of glucoceramides, GPI anchors, and dolichyl-linked N-glycosylation precursors (Figure 18.1M). However, these precursors are ultimately “flipped” to the other side of the membrane where they are extended and, in the case of the GPI-anchor and N-glycan precursors, are transferred to proteins within the lumen of the ER in the secretory pathway (Chapter 9). Cytosolic-oriented glycosyltransferases also include transmembrane proteins that polymerize hyaluronic acid (Chapter 16), cellulose, chitin, and lipopolysaccharides, in which the products are directly translocated across the plasma membrane (Figure 18.1N). The glycan products of these membrane-associated glycosyltransferases normally exit the cytoplasmic space but, if this did not occur, they could serve as the origin of novel, cytoplasmic, nonprotein-linked glycoconjugates.
NUCLEAR AND CYTOPLASMIC LECTINS AND ENZYMES
The occurrence of cytoplasmic or nuclear glycoproteins or glycolipids (oriented toward the cytoplasm) underlies hypotheses for the parallel existence of carbohydrate-binding proteins (or lectins) within these same compatments. O-β-GlcNAc is a prominent nucleocytoplasmic modification, and the heat-shock chaperone protein CBP70 reportedly recognizes O-β-GlcNAc-modified nuclear, cytoplasmic, and mitochondrial proteins. Furthermore, recent evidence shows that the 14-3-3 family of proteins are O-GlcNAc lectins. Early experiments demonstrating that BSA-based neoglycoproteins derivatized with L-Rha, D-GlcNAc, D-Glc, lactose, Man-6-PO4, and L-Fuc all bind to nuclei at threefold higher affinity than underivatized BSA (see below) reinforced the possible existence of cytoplasmic and nuclear lectins. However, little is known about the molecular nature of these putative carbohydrate-binding proteins.
The galectin family of lectins in animals, and the discoidin family of cytoplasmic lectins in the amoebozoan Dictyostelium, are high-abundance proteins that have carbohydrate-binding specificity generally directed toward β-linked Gal and/or GalNAc (Chapter 36). Large pools of these proteins are soluble in the cytoplasm, but cell biological studies show these proteins also reside at the cell surface and in the pericellular matrix. These proteins lack typical amino-terminal signal peptides and exit the cytoplasm by a poorly characterized, posttranslational, unconventional, secretory mechanism that may avoid premature association with glycoproteins in the secretory pathway. Biochemical and genetic studies have uncovered primarily extracellular functions for these lectins (Chapter 36); strikingly, the soluble galectins appear to act as “cytoplasmic sentinels” regulating cellular response to endomembrane damage. On lysosomal damage, galectins-3 and -8 recognize exposed cytoplasmic glycoconjugates. These galectins recruit autophagy and ESCRT proteins to either repair or remove the damaged lysosome. Concurrently, galectin-9 will interact with and activate AMP-dependent protein kinase, and galectin-8 acts to inhibit mTOR activity.
Galectin-3 (CBP35) and possibly galectin-1 are present in the nucleus as part of the hnRNP complex, where they appear to be required for normal mRNA splicing in extracts. However, at present there is only limited evidence for a functional role of the carbohydrate-binding activity of galectin-3 in mRNA splicing. In addition, a cytoplasmic function in regulating apoptosis has been implicated for galectin-1, although a role for the carbohydrate-binding activity has not been established. Galectin-3 is a regulator of mitotic spindle by interacting with the O-GlcNAcylated form NuMA1 protein to stabilize the microtubule-organizing center. Thus, these findings demonstrate that galectins have numerous important cellular functions and potentially other functions waiting to be discovered.
Several filamentous fungi express cytoplasmic lectins with glycan-binding specificities that do not seem to be expressed in the fungi themselves (Chapter 23). These lectins are toxic to predators like roundworms, mosquitoes, and amoebas, and represent a form of innate immunity. In a potentially related observation, several galectins show selective reactivity to glyco-epitopes on pathogenic bacteria. It is interesting to speculate that the cytoplasm represents a “safe harbor” to stow carbohydrate reactive proteins as a defense for other cells in the community should damage cause their release from individual members. Thus, the occurrence of a lectin does not necessarily imply the existence of a cognate glycan in the same compartment.
Numerous other soluble cytoplasmic proteins have been assigned sugar-binding activity that point to the potential significance of cytoplasmic glycoconjugates. In many cases, these must be identified based on biochemical or genetic studies because of the many paths by which these activities seem to have evolved. In plants, cytoplasmic mannose- and GlcNAc-binding lectins are induced by various kinds of biotic and abiotic effectors. Further studies are warranted to evaluate the functional significance of the glycan-binding activities of these interesting proteins.
Indirect evidence for the existence of intracellular lectins comes from evidence that sugars may also serve as nuclear localization signals. Molecules larger than ∼40 kDa do not diffuse freely through nuclear pores and must be specifically and actively transported into and out of the nucleus. The synthetically generated neoglycoproteins BSA-Glc, BSA-Fuc, and BSA-Man are rapidly transported into the nucleus of permeabilized or microinjected living HeLa cells, whereas bovine serum albumin (BSA, ∼66 kDa) itself is not. Like the classical basic peptide–mediated NLS pathway, the sugar-mediated nuclear transport requires energy and is blocked by the lectin WGA, which binds O-GlcNAc at nuclear pores. However, unlike the basic peptide system, the sugar-mediated pathway does not require cytosolic factors and is not blocked by sulfhydryl-reactive chemicals. Additional evidence shows that BSA-GlcNAcβ1-4GlcNAc is rapidly localized to purified nuclei in vitro by a pathway distinct from the classically defined NLS systems. Validation of these fascinating results awaits characterization of the components involved, and identification of natural counterparts to the neoglycoproteins.
A final line of evidence for nucleocytoplasmic glycans is the colocalization of glycosidases. In addition to the lysosomal hexosaminidases Hex A and Hex B, mammals possess two neutral hexosaminidases Hex C and Hex D. Hex C is the O-GlcNAcase (OGA) that catalyzes the removal of O-βGlcNAc. Like OGA, HexD has a neutral pH optimum and is localized to the nucleus and cytoplasm; however, HexD has a preference for galactosaminide substrates. Isoforms of the Neu4 sialidase have been localized at the outer member of the mitochondrion. As described above, processing of products of ERAD have been ascribed to resident cytoplasmic NGLY1 and ENGase, but if the translocated proteins are not degraded, ENGase provides a mechanism to generate mono-β-GlcNAcylation at Asn residues (Figure 18.1H), and the cytoplasmically localized α-mannosidase MAN2C1 might process high-mannose N-glycans to simplified core structures.
CONCLUSION
Overall, there are many tantalizing clues for the existence and importance of glycoconjugates with simple or complex glycans within the nucleus and cytoplasm, in addition to the ubiquitous O-βGlcNAc (Chapter 19) and the analogous O-fucose outside of animals. Well-characterized examples involve novel linkages to the protein via tyrosine in animal and yeast glycogenin, via hydroxyproline in protist Skp1, and via arginine in bacteria (Table 18.1). The known glycosyltransferases that mediate these modifications are traditional cytoplasmically localized proteins that evolved from the same evolutionary lineages that generated the enzymes of the secretory pathway. At present, these modifications appear to be directed to specific protein targets.
Cytoplasmic glycosylation is also a strategy for pathogens to control host-cell responses, and these mechanisms also frequently involve novel linkages and target single proteins. These examples clearly establish the importance of cytoplasmic and nuclear glycosylation in protein-specific regulation, which contrasts with the relatively broad distribution and heterogeneity of glycans on cell surface, extracellular matrix, and blood proteins. However, much remains to be explored to establish the generality of this concept. Significantly, there exists substantial indirect evidence for much more extensive complex cytoplasmic glycosylation in animal cells, and cell biologists continue to illustrate novel mechanisms of protein compartmentalization that could allow a protein that is glycosylated in one place to be transferred to another, as more commonly occurs in prokaryotes. Nevertheless, proof of the implied prevalence of familiar or unfamiliar glycans will require detailed structural evidence in conjunction with supporting biosynthetic, cell biological, and functional studies. Given that much of what is known about cytoplasmic glycosylation has only recently emerged, it is indeed likely that much remains to be discovered in this realm, and that these pathways are much more common in both eukaryotes and prokaryotes than is currently appreciated. This promises to be an exciting and important area of research in the future.
ACKNOWLEDGMENTS
The authors appreciate helpful comments from Priya Umapathi.
FURTHER READING
- Hart GW, Haltiwanger RS, Holt GD, Kelly WG. 1989. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem 58: 841–874. doi:10.1146/annurev.bi.58.070189.004205 [PubMed: 2673024] [CrossRef]
- Chandra NC, Spiro MJ, Spiro RG. 1998. Identification of a glycoprotein from rat liver mitochondrial inner membrane and demonstration of its origin in the endoplasmic reticulum. J Biol Chem 273: 19715–19721. doi:10.1074/jbc.273.31.19715 [PubMed: 9677401] [CrossRef]
- Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN. 2004. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta 1673: 3–12. doi:10.1016/j.bbagen.2004.02.013 [PubMed: 15238245] [CrossRef]
- Monsigny M, Rondanino C, Duverger E, Fajac I, Roche AC. 2004. Glyco-dependent nuclear import of glycoproteins, glycoplexes and glycosylated plasmids. Biochim Biophys Acta 1673: 94–103. doi:10.1016/j.bbagen.2004.03.015 [PubMed: 15238252] [CrossRef]
- Funakoshi Y, Suzuki T. 2009. Glycobiology in the cytosol: the bitter side of a sweet world. Biochim Biophys Acta 1790: 81–94. doi:10.1016/j.bbagen.2009.03.024 [PubMed: 18952151] [CrossRef]
- Haudek KC, Spronk KJ, Voss PG, Patterson RJ, Wang JL, Arnoys EJ. 2010. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim Biophys Acta 1800: 181–189. doi:10.1016/j.bbagen.2009.07.005 [PMC free article: PMC2815258] [PubMed: 19616076] [CrossRef]
- Lannoo N, Van Damme EJ. 2010. Nucleocytoplasmic plant lectins. Biochim Biophys Acta 1800: 190–201. doi:10.1016/j.bbagen.2009.07.021 [PubMed: 19647040] [CrossRef]
- Fredriksen L, Moen A, Adzhubei AA, Mathiesen G, Eijsink VG, Egge-Jacobsen W. 2013. Lactobacillus plantarum WCFS1 O-linked protein glycosylation: an extended spectrum of target proteins and modification sites detected by mass spectrometry. Glycobiology 23: 1439–1451. doi:10.1093/glycob/cwt071 [PubMed: 24000282] [CrossRef]
- Peschke M, Hempel F. 2013. Glycoprotein import: a common feature of complex plastids? Plant Signal Behav 8: e26050. doi:10.4161/psb.26050 [PMC free article: PMC4091080] [PubMed: 24220152] [CrossRef]
- Boudière L, Michaud M, Petroutsos D, Rébeillé F, Falconet D, Bastien O, Roy S, Finazzi G, Rolland N, Jouhet J, et al. 2014. Glycerolipids in photosynthesis: composition, synthesis and trafficking. Biochim Biophys Acta 1837: 470–480. doi:10.1016/j.bbabio.2013.09.007 [PubMed: 24051056] [CrossRef]
- Bullard W, Lopes da Rosa-Spiegler J, Liu S, Wang Y, Sabatini R. 2014. Identification of the glucosyltransferase that converts hydroxymethyluracil to base J in the trypanosomatid genome. J Biol Chem 289: 20273–20282. doi:10.1074/jbc.m114.579821 [PMC free article: PMC4106341] [PubMed: 24891501] [CrossRef]
- Naegeli A, Michaud G, Schubert M, Lin CW, Lizak C, Darbre T, Reymond JL, Aebi M. 2014. Substrate specificity of cytoplasmic N-glycosyltransferase. J Biol Chem 289: 24521–24532. doi:10.1074/jbc.m114.579326 [PMC free article: PMC4148877] [PubMed: 24962585] [CrossRef]
- Jank T, Belyi Y, Aktories K. 2015. Bacterial glycosyltransferase toxins. Cell Microbiol 17: 1752–1765. doi:10.1111/cmi.12533 [PubMed: 26445410] [CrossRef]
- Lassak J, Keilhauer EC, Fürst M, Wuichet K, Gödeke J, Starosta AL, Chen JM, Søgaard-Andersen L, Rohr J, Wilson DN, et al. 2015. Arginine-rhamnosylation as new strategy to activate translation elongation factor P. Nat Chem Biol 11: 266–270. doi:10.1038/nchembio.1751 [PMC free article: PMC4451828] [PubMed: 25686373] [CrossRef]
- West CM, Blader IJ. 2015. Oxygen sensing by protozoans: how they catch their breath. Curr Opin Microbiol 26: 41–47. doi:10.1016/j.mib.2015.04.006 [PMC free article: PMC4623824] [PubMed: 25988702] [CrossRef]
- Westphal N, Theis T, Loers G, Schachner M, Kleene R. 2017. Nuclear fragments of the neural cell adhesion molecule NCAM with or without polysialic acid differentially regulate gene expression. Sci Rep 7: 13631. doi:10.1038/s41598-017-14056-x [PMC free article: PMC5648764] [PubMed: 29051583] [CrossRef]
- Yoshida Y, Kuroiwa H, Shimada T, Yoshida M, Ohnuma M, Fujiwara T, Imoto Y, Yagisawa F, Nishida K, Hirooka S, et al. 2017. Glycosyltransferase MDR1 assembles a dividing ring for mitochondrial proliferation comprising polyglucan nanofilaments. Proc Natl Acad Sci 114: 13284–13289. doi:10.1073/pnas.1715008114 [PMC free article: PMC5740636] [PubMed: 29180407] [CrossRef]
- Johannes L, Jacob R, Leffler H. 2018. Galectins at a glance. J Cell Sci 131: jcs208884. doi:10.1242/jcs.208884 [PubMed: 29717004] [CrossRef]
- Curtino JA, Aon MA. 2019. From the seminal discovery of proteoglycogen and glycogenin to emerging knowledge and research on glycogen biology. Biochem J 476: 3109–3124. doi:10.1042/bcj20190441 [PubMed: 31689353] [CrossRef]
- Sernee MF, Ralton JE, Nero TL, Sobala LF, Kloehn J, Vieira-Lara MA, Cobbold SA, Stanton L, Pires DEV, Hanssen E, et al. 2019. A family of dual-activity glycosyltransferase-phosphorylases mediates mannogen turnover and virulence in Leishmania parasites. Cell Host Microbe 26: 385–399. doi:10.1016/j.chom.2019.08.009 [PubMed: 31513773] [CrossRef]
- Speciale I, Duncan GA, Unione L, Agarkova IV, Garozzo D, Jiménez-Barbero J, Lin S, Lowary TL, Molinaro A, Noel E, et al. 2019. The N-glycan structures of the antigenic variants of chlorovirus PBCV-1 major capsid protein help to identify the virus-encoded glycosyltransferases. J Biol Chem 294: 5688–5699. doi:10.1074/jbc.ra118.007182 [PMC free article: PMC6462530] [PubMed: 30737276] [CrossRef]
- Yoshida Y, Mizushima T, Tanaka K. 2019. Sugar-recognizing ubiquitin ligases: action mechanisms and physiology. Front Physiol 10: 104. doi:10.3389/fphys.2019.00104 [PMC free article: PMC6389600] [PubMed: 30837888] [CrossRef]
- Jia J, Claude-Taupin A, Gu Y, Choi SW, Peters R, Bissa B, Mudd MH, Allers L, Pallikkuth S, Lidke KA, et al. 2020. Galectin-3 coordinates a cellular system for lysosomal repair and removal. Dev Cell 52: 69–87. doi:10.1016/j.devcel.2019.10.025 [PMC free article: PMC6997950] [PubMed: 31813797] [CrossRef]
- Koh E, Cho HS. 2021. NleB/SseKs ortholog effectors as a general bacterial monoglycosyltransferase for eukaryotic proteins. Curr Opin Struct Biol 68: 215–223. doi:10.1016/j.sbi.2021.02.004 [PubMed: 33761453] [CrossRef]
- Sun TP. 2021. Novel nucleocytoplasmic protein O-fucosylation by SPINDLY regulates diverse developmental processes in plants. Curr Opin Struct Biol 68: 113–121. doi:10.1016/j.sbi.2020.12.013 [PMC free article: PMC8222059] [PubMed: 33476897] [CrossRef]
- West CM, Malzl D, Hykollari A, Wilson IBH. 2021. Glycomics, glycoproteomics, and glycogenomics: an inter-taxa evolutionary perspective. Mol Cell Proteomics 20: 100024. doi:10.1074/mcp.r120.002263 [PMC free article: PMC8724618] [PubMed: 32994314] [CrossRef]
- MONOGLYCOSYLATION OF NUCLEOCYTOPLASMIC PROTEINS
- COMPLEX GLYCOSYLATION OF NUCLEOCYTOPLASMIC PROTEINS
- POSSIBILITIES OF “CONVENTIONAL” SECRETORY-TYPE GLYCANS ON NUCLEOCYTOPLASMIC GLYCOPROTEINS
- VALIDATED CYTOPLASMIC GLYCOSYLTRANSFERASES WHOSE TARGETS ARE EXPORTED FROM THE CELL
- INTERMEDIATES IN ASSEMBLY OF EXPORTED GLYCOCONJUGATES OR POLYSACCHARIDES
- NUCLEAR AND CYTOPLASMIC LECTINS AND ENZYMES
- CONCLUSION
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Nucleocytoplasmic Glycosylation.[Essentials of Glycobiology. 2015]Review Nucleocytoplasmic Glycosylation.West CM, Hart GW. Essentials of Glycobiology. 2015
- Review Nucleocytoplasmic Glycosylation.[Essentials of Glycobiology. 2009]Review Nucleocytoplasmic Glycosylation.Hart GW, West CM. Essentials of Glycobiology. 2009
- Review Molecular Functions of Glycoconjugates in Autophagy.[J Mol Biol. 2016]Review Molecular Functions of Glycoconjugates in Autophagy.Fahie K, Zachara NE. J Mol Biol. 2016 Aug 14; 428(16):3305-3324. Epub 2016 Jun 23.
- Review Nucleocytoplasmic O-glycosylation in protists.[Curr Opin Struct Biol. 2019]Review Nucleocytoplasmic O-glycosylation in protists.West CM, Kim HW. Curr Opin Struct Biol. 2019 Jun; 56:204-212. Epub 2019 May 22.
- Rapid assays for lectin toxicity and binding changes that reflect altered glycosylation in mammalian cells.[Curr Protoc Chem Biol. 2014]Rapid assays for lectin toxicity and binding changes that reflect altered glycosylation in mammalian cells.Stanley P, Sundaram S. Curr Protoc Chem Biol. 2014 Jun 3; 6(2):117-133. Epub 2014 Jun 3.
- Nucleocytoplasmic Glycosylation - Essentials of GlycobiologyNucleocytoplasmic Glycosylation - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
