NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.048

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsAntibodies, lectins, microbial adhesins, viral agglutinins, and other proteins with carbohydrate-binding modules, collectively termed glycan-recognizing probes (GRPs), are widely used in glycan analysis because their specificities enable them to discriminate among a diverse variety of glycan structures. The native multivalency of many of these molecules promotes high-affinity avidity binding to the glycans and cell surfaces containing those glycans. This chapter describes the variety of commonly used GRPs, the types of analyses to which they may be applied, and cautionary principles that affect their optimal use.
BACKGROUND
The first evidence that glycans were antigenic arose from the discovery of the human blood group ABO antigens (Chapter 14). A key tool in these studies were plant lectins that by the mid-1940s had found widespread use in typing blood, because some were relatively specific for blood types and they could be easily purified and are stable (Chapter 31). The discovery of the blood groups and the antibodies and lectins binding them indicated that such proteins could also be generally useful in identifying specific glycan sequences.
Hundreds of different plant and animal lectins and other proteins with carbohydrate-binding molecules (CBMs) have now been characterized. Thus, although monoclonal antibodies (mAbs) are often more specific for glycan determinants and bind with higher affinity, many plant and animal lectins and CBMs also have useful specificities for determinants beyond monosaccharides, have cloned sequences, are usually less expensive and commercially available, and have well-characterized binding specificities. GBPs are also found in many other organisms (Chapters 31–36), including viruses and bacteria (Chapter 37), and reagents from these organisms are also being used in the field. The availability of GBPs and mAbs has helped to catapult the field of glycobiology into the modern era.
LECTINS MOST COMMONLY USED IN GLYCAN ANALYSIS
Many of the lectins currently used as tools in glycobiology originate from plants, but some also come from animals (e.g., snails) or mushrooms. Most were characterized initially by hapten inhibition assays, in which monosaccharides, their derivatives, or small oligosaccharides block binding to cells or other glycan-coated targets. Such small-sized molecules that compete with binding of a lectin or antibody to a larger ligand are termed haptens. Lectins are often grouped by specificity depending on the monosaccharide(s) that can inhibit their binding at millimolar concentrations and their distinct preference for α- or β-anomers of the sugar. However, lectins within a particular specificity group may also differ in their affinities for different glycans. The binding affinity (Kd) of lectins for complex glycans is often in the range of 1–10 µm, but for monosaccharides the affinity may be in the mm range. For complex glycoconjugates with multiple determinants or multivalency, the binding avidity of lectins may approach nanomolar values. For example, concanavalin A (ConA) is an α-mannose/α-glucose-binding lectin that recognizes N-glycans and is not known to bind common O-glycans on animal cell glycoproteins. However, it binds oligomannose-type N-glycans with much higher affinity than complex-type biantennary N-glycans, and it does not recognize more highly branched complex-type N-glycans (Figure 48.1). Other lectins, such as L-phytohemagglutinin (L-PHA) and E-PHA from Phaseolus vulgaris, as well as lentil lectin (LCA) from Lens culinaris, also recognize specific determinants of N-glycans. Some animal lectins that are widely used include those from invertebrates, such as Helix pomatia agglutinin (HPA) from the snail. In fact, these lectins and other lectins are used most frequently to explore structural features of glycans on glycoproteins, glycolipids, and cells (Figures 48.2–48.4) (see also Chapters 31 and 32).
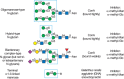
FIGURE 48.1.
Examples of N-glycans recognized by concanavalin A (ConA) from Canavalia ensiformis and Galanthus nivalis agglutinin (GNA). The determinants required for binding are indicated in the boxed areas. Hapten sugars that can competitively inhibit binding of (more...)
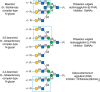
FIGURE 48.2.
Examples of types of N-glycans recognized by L-PHA, E-PHA, and DSA. The determinants required for binding are indicated in the boxed areas. Hapten sugars that can competitively inhibit binding of the lectin to the indicated glycans are shown on the right (more...)

FIGURE 48.3.
Examples of types of glycan determinants bound with high affinity by different plant and animal lectins. The determinants required for binding are indicated in the boxed areas. Hapten sugars that can competitively inhibit binding of the lectin to the (more...)

FIGURE 48.4.
Examples of types of glycan determinants bound with high affinity by different plant lectins. The determinants required for binding are indicated in the boxed areas. Hapten sugars that can competitively inhibit binding of the lectin to the indicated glycans (more...)
GENERATION OF MONOCLONAL ANTIBODIES TO GLYCAN ANTIGENS
A large number of mAbs have been generated against specific glycan determinants. Several approaches to obtain these antibodies have been described.
- Whole cells, historically tumor cells, have been used to immunize mice to generate specific mAbs to various glycoprotein and glycolipid antigens, including the Tn antigen (GalNAcα1-Ser/Thr) and the stage-specific embryonic antigen-1 (SSEA-1), now known as the Lewis x (Lex) antigen (Figure 48.5). In this approach, hybridomas are screened for mAbs that recognize the immunizing cell but not other types of cells.
- Glycan-protein conjugates (glycans coupled to carrier proteins such as bovine serum albumin [BSA] or keyhole limpet hemocyanin [KLH]) have been used to generate mAbs to specific structures. (BSA is not glycosylated, but the unusual glycans on KLH may serve as adjuvants to enhance immune responses.) Coupling of polysaccharides to protein carriers typically leads the generation of antibodies recognizing internal structural features, whereas coupling of small glycans typically yields antibodies that bind to terminal structural features. A common variation of this approach is to immunize mice directly with total cells, or a preparation of glycoproteins (such as a membrane fraction) or even a purified glycoprotein, glycolipid, or glycosaminoglycan. For example, antibodies to the plant glycoprotein horseradish peroxidase are used to detect the presence of the unusual modifications nonhuman Xylβ1-2Man-R and Fucα1-3GlcNAc-R in the core regions of complex-type N-glycans. Immunization with glycan conjugates has also been used to generate polyclonal antisera in rabbits and chickens. Antibodies to specific glycan determinants can be purified from such antisera by affinity chromatography on immobilized glycans. Knockout mice lacking specific glycoconjugates are also useful for generating antibodies to common self-antigenic structures. For example, antibodies to the common glycolipid sulfatide (3-O-sulfate-Galβ1-ceramide) were obtained from mice that lacked the cerebroside sulfotransferase after immunization with sulfatide. If desired, recombinant single-chain antibodies to glycan determinants can be produced after cloning the VH and VL domains of antibodies from specific hybridomas.
- A novel approach to obtain mAbs to specific glycan antigens has been to use mice infected with specific parasites or bacteria followed by preparing hybridomas from splenocytes of the infected animals. This approach has been used to generate a variety of mAbs to pathogen-specific glycan antigens.
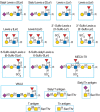
FIGURE 48.5.
Examples of different mammalian glycan antigens recognized by specific monoclonal antibodies. The antigens have the structures shown within the boxed area and are named as indicated. Usually, the antigen shown in the box can be linked to almost any glycan (more...)
Screening for antibodies to the desired antigen usually involves enzyme-linked immunoabsorbent assay (ELISA)-type assays with immobilized target glycans, which may vary from small glycans to large polysaccharides. In addition, there is an emerging technology using phage display to identify single-chain variable fragments (scFvs) of antibodies that can also bind to glycan antigens. However, in many cases, it is difficult to define the specific epitopes recognized by these antibodies because isolated glycans are often characterized by microheterogeneities and related glycan antigens for comparison are often unavailable. Recently developed approaches, such as glycan microarrays and related techniques, especially when combined with advances in the chemical synthesis of glycans, help to better define the specificities of mAbs (Chapter 29).
Anti-glycan antibodies are widely used in glycobiology, and some common mammalian antigens they recognize are shown in Figures 48.5 and 48.6. Most of the murine mAbs to glycan antigens are of the IgM isotype. However, if the animal is well-immunized and serum titers are high, IgGs are frequently generated (∼35% of the plant-glycan-directed mAbs are IgG). The higher valency of IgM antibodies that form pentamers or hexamers can affect the binding specificity. Some antibodies to glycan antigens are commercially available, whereas others are obtainable only from individual laboratories or stock centers. Some antibodies against mammalian antigens are shown in Figures 48.5 and 48.6, and they recognize terminal glycan determinants, although subterminal sequences may also be required for binding and or in some cases may decrease binding. Many of the antibodies generated to date against plant glycans recognize internal structural features of these polysaccharides. In addition, the context of expression (free glycan vs. glycoprotein vs. glycolipid) or the class of glycan (N- vs. O-linked) may play a significant role in determining specificity and affinity. For example, antibodies to the 6-sulfo-SLex antigen require the fucose, sialic acid, and GlcNAc-6-O-sulfate residues. In contrast, the MECA-79 antibody recognizes extended core-1 O-glycans that contain internal GlcNAc-6-O-sulfate residues; it does not require either the fucose or sialic acid for recognition, but it does require the core-1 O-glycan. Several antibodies against the plant cell wall homogalacturonans or xylans bind to internal backbone residues. There are also many mAbs that recognize different glycolipids and glycosaminoglycans.
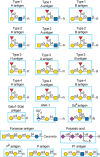
FIGURE 48.6.
Additional examples of different mammalian glycan antigens recognized by specific monoclonal antibodies. The antigens have the structures shown within the boxed area and are named as indicated. In some cases, the importance of the reducing terminal end (more...)
USES OF ANTIBODIES, LECTINS, AND CBMs IN GLYCAN IDENTIFICATION
Some of the important uses of lectins, CBMs, and antibodies are illustrated in Figure 48.7. Antibodies, lectins, and CBMs each have distinct advantages. Lectins are usually less expensive than antibodies. The genes encoding many CBMs have been cloned, enabling facile heterologous expression to obtain needed quantities of the protein. However, antibodies, lectins, and CBMs independently may be needed to bind specifically to certain determinants. For example, no lectin specific for the SLea antigen has been identified. Conversely, no mAbs have been identified that bind general determinants, such as α2-6-linked sialic acid and α2-3-linked sialic acid, whereas lectins have this specificity (e.g., Sambucus nigra agglutinin [SNA] and Maackia amurensis lectin [MAL], respectively). However, mAbs that are specific to some restricted determinants, such as Lex and SLea antigens, often cannot distinguish their presentation on O-glycans, N-glycans, or glycolipids. In addition, although available antibodies thus far cannot distinguish N-glycan structural motifs, such features are well recognized by some plant lectins. For example, the plant lectin ConA does not bind mucin-type O-glycans in animal cells, but binds only to some specific classes of N-glycans (Figure 48.1). Additionally, E-PHA binds “bisected” complex-type N-glycans (Figure 48.2) and does not bind any known glycolipid or O-glycan. Great care should be taken to use lectins and antibodies at appropriate concentrations in which their specificity can be exploited. At high lectin concentrations, glycans with very low affinity binding may interact and confound interpretations. But in some cases this is advantageous, as when using concentrated lectins to a wide range of glycosylated proteins or glycopeptides differing in structures. Thus, antibodies, CBMs, and lectins each offer distinct advantages in defining glycan structures.

FIGURE 48.7.
Examples of different uses of plant and animal lectins, carbohydrate-binding molecules (CBMs), and antibodies in glycobiology. Many plant and animal lectins are multivalent as shown, and antibodies are always multivalent, whereas CBMs are monovalent. (more...)
The glycan determinants bound with highest affinity by each of these probes have been identified by a combination of approaches, including affinity chromatography, glycan synthesis, and binding to specific glycoconjugates and cells. A good example of this is the phytohemagglutinin L-PHA, which is often used by immunologists as a mitogen to stimulate quiescent T cells to divide. L-PHA originates from the red kidney bean P. vulgaris, which also contains isolectins to L-PHA, notably E-PHA. L-PHA binds to certain branched, complex-type N-glycans containing the pentasaccharide sequence Galβ1-4GlcNAcβ1-2(Galβ1-4GlcNAcβ1-6)Manα1-R (the so-called “2-6-branch”), as shown in the boxed portion of the glycan in Figure 48.2. Curiously, the only monosaccharide that effectively inhibits either L-PHA or E-PHA is GalNAc, although this monosaccharide is not part of the N-glycan determinants recognized by these lectins (Figure 48.2). The binding of L-PHA is used to identify specific types of branched N-glycans in cells. L-PHA binding is dramatically decreased in mice genetically null for the branching β1-6 N-acetylglucosaminyltransferase (MGAT5 and -5B.) (Chapter 9). The expression of L- or E-PHA-binding glycoproteins is increased in many tumor cells (see Chapter 47). Similar studies show E-PHA binds bisected complex-type N-glycans containing the GlcNAcβ1-4Man-R in the core, and such structures are produced by MGAT3, and E-PHA-binding glycans are also elevated in some tumor cells.
Thus, using a variety of lectins and antibodies, it is possible to deduce many aspects of glycan structures. Microarrays in which a variety of lectins and antibodies are printed on a slide can also give valuable information about the glycosylation status of cells and glycoconjugates. This approach is especially sensitive in regard to defining whether biological samples differ in glycosylation. For example, such approaches have been adapted to study differential glycosylation of prion glycoproteins using a panel of biotinylated lectins in ELISA-type formats.
USES OF ANTIBODIES AND LECTINS IN GLYCAN PURIFICATION
There are several approaches to using antibodies and lectins in glycan purification, including affinity chromatography or affinity binding and immunoprecipitation or lectin-induced precipitation. The proteins may be covalently coupled to a carrier such as Sepharose or biotinylated and captured on streptavidin-Sepharose. In addition, antibodies may be noncovalently captured on protein A (or G)-Sepharose. These bound antibodies and lectins can then be used to isolate glycoconjugates expressing specific glycan determinants. ConA-Sepharose is commonly used to isolate glycoproteins as it shows little binding to nonglycosylated proteins. However, it does not bind all glycoproteins because it recognizes specific N-glycan structures. ConA-Sepharose has also been used to isolate free oligo- and polymannose-, hybrid-, and complex-type biantennary N-glycans.
When combined in a serial format, multiple lectins can be used in affinity chromatography to isolate most of the major glycan structures present in animal cells, with glycans being separated as classes that share common determinants. An example of serial lectin affinity chromatography is shown in Figure 48.8. Coupling with ion-exchange chromatography and high-performance liquid chromatography (HPLC) can yield highly pure glycans with predicted structures that can then be confirmed by mass spectrometry of native and permethylated derivatives (Chapters 50 and 51).
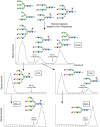
FIGURE 48.8.
An example of the use of different immobilized plant lectins in serial lectin affinity chromatography of complex mixtures of glycopeptides. In this example, a mixture of glycopeptides is applied to a column of immobilized concanavalin A (ConA), and the (more...)
Mixed-bed lectin chromatography using a combination of different immobilized lectins is useful for simultaneously separating all types of glycoconjugates from nonglycosylated material (e.g., glycopeptides from peptides). Combinations of affinity chromatography and other chromatographic techniques can be very useful in identifying and separating glycopeptides. Thus, the ability of glycans to be recognized by lectins dependent on specific structural features in the glycans is a powerful tool for glycan identification and isolation. In some approaches, the glycans are tagged at the reducing end by fluorophores and radioisotopes or may be obtained by metabolic radiolabeling from cells or tissues grown in the presence of radiolabeled sugar precursors, such as [2-3H]mannose or [6-3H]glucosamine. Glycan fractionation shown on immobilized lectins in Figure 48.8 is currently not possible with antibodies because no antibodies are known that can distinguish such core structural features in glycans.
When intact glycoproteins or complex polysaccharides (e.g., those found in plant cell walls [Chapter 24]) are analyzed for their interactions with plant lectins or antibodies, the interpretation of data may be complicated by the multivalency of the glycoprotein/polysaccharide and the density of the immobilized lectin/antibody. For example, glycoproteins containing multiple high-mannose-type N-glycans bind so tightly to immobilized ConA that it is difficult to elute the bound glycoprotein, even with extremely high concentrations of hapten and under harsh conditions. Lower densities of ConA conjugation reduce its avidity for the glycoproteins and promote hapten dissociation of bound ligands with lower concentrations of sugars. When used in combination, multiple lectins, such as ConA, AAL (Aleuria aurantia lectin), LCA, and RCA (Ricinus communis agglutinin), can be used to isolate most glycoproteins containing N-and O-glycans from animal cells. This is a potentially powerful approach for glycoproteomics, or the identification of glycoproteins and their glycosylation status.
Lectins and antibodies can also be used in western blotting approaches to characterize protein and lipid glycosylation, in which biotinylated lectin or antibody probes are applied to material transferred to nitrocellulose or other supports after electrophoresis or chromatography. The bound lectins/antibodies are visualized by binding streptavidin-alkaline phosphatase and conversion of luminescent substrates. In these approaches, the concentrations of lectins and antibodies used must be low enough both to reduce false-positive, nonspecific binding and to allow for inhibition by appropriate haptens to confirm sugar binding. Removal of N-glycans by PNGaseF, or of sialic acid by neuraminidase, can eliminate binding to specific lectins and antibodies in western blotting, thus indicating the lectin/antibody-bound determinant.
USES OF ANTIBODIES, LECTINS, AND CBMs IN CHARACTERIZING CELL-SURFACE GLYCOCONJUGATES
The major approaches for using antibodies, CBMs, and lectins to characterize cell-surface glycoconjugates are histochemistry (lectins) and immunohistochemistry (antibodies), flow cytometry with cell sorting, and cell agglutination. In histochemistry and immunohistochemistry, tissues are prepared and fixed as usual for histological staining, and then incubated with appropriate biotinylated or peroxidase-labeled lectins or antibodies (glycolipids are extracted during standard paraffin embedding procedures and require frozen sections for sensitive detection). The bound lectins, CBMs, or antibodies are then visualized by means of secondary reagents, such as streptavidin-peroxidase or labeled secondary antibody. These approaches often yield information that is difficult to obtain by any other approach. For example, they can reveal the spatial orientation of different glycans, their relative abundance, and whether they are intracellular and/or extracellular. Three important controls in such studies are (1) use of lectins, CBMs, or antibodies at limiting concentrations to avoid nonspecific binding; (2) confirmation of the specificity of binding by appropriate inhibition by haptens or by destruction of the predicted target glycans with glycosidases; and (3) use of multiple lectins, CBMs, or antibodies to provide further confirmation of the conclusions.
Lectins and antibodies to glycans have also been widely used in flow cytometry and cell sorting. In such studies, cells are incubated with low, nonagglutinating levels of lectins or antibodies that are biotinylated and conjugated to fluorescently labeled streptavidin or directly fluorescently labeled. Cells with bound lectins or antibodies can then be identified by their fluorescence in the flow cytometer, and the degree of fluorescence can be correlated with the number of binding sites. A key consideration in flow cytometry is to avoid high concentrations of lectins or antibodies that cause agglutination of cells, which must be ruled out by direct microscopic visualization. Lectins and antibodies can also be used to identify specific membrane localization of different glycans, using confocal microscopy and electron microscopy.
Lectins and antibodies are useful for characterizing cell-surface glycans when limited numbers of cells are available. Studies on glycosylation of embryonic stem cells have been greatly aided by using panels of specific lectins to identify unique glycan determinants and changes in their expression during cellular differentiation. A recent variation of this approach is to use a microarray of immobilized lectins, which are probed with fluorescently labeled glycoproteins in extracts of cells. Such assays can reveal minor differences in protein glycosylation between different samples and give insight into the glycan structures that are present.
One of the oldest uses of lectins is in the agglutination and precipitation of glycoconjugates, cells, and membrane vesicle preparations. The easiest assay for many soluble lectins that are multivalent is agglutination of target cells, such as erythrocytes, leukocytes, or even bacteria or fungi. Agglutination can often be easily observed without a microscope, but it is also measurable in instruments such as aggregometers. In these assays, a lectin solution is serially diluted and the reciprocal of the final dilution that gives measurable cell agglutination is taken to define the activity. Bacterial agglutination by plant and animal lectins is often used to explore the surface glycocalyx and changes in glycocalyx upon culture conditions and to define phenotypes of different strains or serotypes. Lectin precipitation and aggregation can be used to define the glycan composition and overall architecture of polysaccharides, as has been done for bacterial, algal, plant, and animal polysaccharides.
USES OF ANTIBODIES AND LECTINS FOR GENERATING ANIMAL CELL GLYCOSYLATION MUTANTS
An important use of lectins and antibodies has been in the selection of cell lines (e.g., Chinese hamster ovary [CHO] cells) that express altered cell-surface glycans. The common lectins that have been used are ConA, WGA (wheat germ agglutinin), L-PHA, LCA, PSA (Pisum sativum agglutinin), E-PHA, ricin, modeccin, and abrin. The latter three lectins are heterodimeric, disulfide-bonded proteins that are classified as type-II ribosome-inactivating proteins; they contain an A subunit that constitutes an enzyme called RNA-N-glycosidase, which inactivates the 28S ribosome, and a B subunit that is a galactose-binding lectin (Chapter 31). Other plant lectins such as ConA, WGA, or LCA (which lack an enzymatic or toxic A subunit) are still toxic to animal cells via poorly understood mechanisms, whereas yet others, such as soybean agglutinin (SBA) and peanut agglutinin (PNA), are not highly toxic to animal cells in culture. One mechanism of toxicity of plant lectins is the induction of apoptosis, perhaps by blocking receptor or nutrient transport functions in cells, or by cross-linking apoptotic receptors. Noncytotoxic lectins and antibodies to specific glycans can be rendered toxic by conjugation to ricin A subunit or other toxic proteins. Using such agents, both loss-of-function (e.g., loss of a glycosyl-transferase or glycosidase) and gain-of-function (e.g., activation of a latent transferase gene) mutants have been obtained. More details about glycosylation mutants of cultured cells are presented in Chapter 49.
USES OF ANTIBODIES AND LECTINS FOR CLONING GLYCOSYLTRANSFERASE GENES BY EXPRESSION
The specificity of lectins and antibodies to particular glycans has made them especially useful in cloning genes encoding glycosyltransferases or other proteins required for proper glycosylation, such as nucleotide sugar transporters. For example, CHO cells and the African green monkey kidney cell line COS lack glycans with terminal α-galactose residues. Consequently, the cells do not bind the plant lectin GSI-B4 (Figure 48.3). When transfected with a cDNA library prepared from cells that do express terminal α-galactose residues (such as murine teratocarcinoma cells F9), cells that have taken up a plasmid encoding the cognate α1-3 galactosyltransferase and express terminal α-residues will bind to GSI-B4 and can be identified with plates coated with the lectin. Isolation of plasmids from the bound cells and repeated recloning and reexpression by this technique (called expression cloning) led to the identification of a specific gene encoding the murine α1-3 galactosyltransferase (Ggta1). Similar approaches using an antibody to the Lewis x antigen (SSEA-1) led to cloning of the Lewis blood group FuT-III.
Related approaches using antibodies and lectins to other glycan structures and wild-type CHO cells and CHO mutants led to the identification of the genes encoding many other glycosyltransferases, including some involved in extending glycosphingolipids and nucleotide sugar transporters, such as the transporter for CMP-NeuAc. Such approaches based on lectin selection are also useful with yeast. For example, the gene encoding a yeast N-acetyl-glucosaminyltransferase (GlcNAcT) was identified by expression cloning using a GlcNAcT-deficient yeast. The mannan chains of the yeast Kluyveromyces lactis normally contain some terminal N-acetylglucosamine residues that are bound by the plant lectin GSL-II, which binds terminal N-acetylglucosamine residues (Figure 48.4). A mutant lacking the GlcNAcT and lacking terminal N-acetylglucosamine residues on mannoproteins was identified. Transformation of yeast with DNA containing the gene encoding the GlcNAcT led to yeast clones that were bound by the fluorescently labeled GSL-II. This strategy was used to identify the gene encoding the GlcNAcT. The transporters for UDP-Gal in Leishmania parasites and in the plant Arabidopsis were also identified by expression cloning in Lec8 CHO cells; these cells have a mutation in their endogenous UDP-Gal transporter, and consequently lack galactose-containing glycans on their surface. For the UDP-Gal transporter from Arabidopsis, Lec8 cells were cotransfected with a cDNA library encoding the putative Arabidopsis UDP-Gal transporter, along with a glucuronosyltransferase that can lead to synthesis of the unsulfated version of the HNK epitope (Figure 48.6), GlcAβ1-3Galβ-R that is bound by a specific antibody. This identification strategy led to the expression cloning of several UDP-Gal transporters from Arabidopsis.
CHO and COS cell lines have also been useful in characterizing the activities of novel glycosyltransferase genes that were identified by other approaches. For example, candidate fucosyltransferase genes encoding α1-2 or α1-3 fucosyltransferases have been expressed in CHO and COS cells, which lack these enzymes. Expression of these enzymes then leads to surface expression of antigens recognized by specific antibodies and lectins, such as the H-antigen (for the α1-2 FucT) or Lex and SLex (for the α1-3 FucT).
USES OF ANTIBODIES AND LECTINS IN ASSAYING GLYCOSYLTRANSFERASES AND GLYCOSIDASES
Lectins and antibodies to glycan antigens have been useful in assaying specific glycosyltransferases and glycosidases in various formats (Figure 48.7). Immobilized lectins have been used to isolate products of glycosyltransferase assays, such as chitin polysaccharides on WGA or glycosylated peptides on mixed-bed lectin columns. α1-3 fucosyltransferases that synthesize the Lex and SLex antigens have been assayed based on capture of the product on immobilized antibodies to these antigens or binding of antibody to the immobilized fucosylated product in an ELISA-type format, or in flow cytometry using beads containing the acceptor glycan that is modified by an enzyme to a new structure recognized by an antibody or lectin. Likewise, α2-3 sialyltransferases and α2-6 sialyltransferases have been assayed using immobilized acceptors in an ELISA-type format and in BIAcore formats (Chapter 29), and their products have been measured with MAL (which binds to the α2-3-sialylated product) or SNA (which binds to the α2-6-sialylated product). Similarly, α1-3 galactosyltransferases have been assayed using immobilized acceptors in an ELISA-type format, and the product has been measured with GSI-B4 or Viscum album agglutinin, which binds to the α1-3 galactosylated product. The glycoprotein-specific β1-4 N-acetylgalactosaminyltransferase has been assayed using glycoprotein acceptors in solution, capture by a specific monoclonal antibody in a microtiter plate, and measurement of product by an ELISA-type assay with a Wisteria floribunda agglutinin (WFA) that binds to terminal β1-4-linked GalNAc residues generated by the enzyme. Conversely, glycosidases can be assayed by measuring the gain in binding of lectins or antibodies. For example, the lectin PNA, which binds to nonsialylated Galβ1-3GalNAcα1-Ser/Thr in O-glycans, can be used to measure bacterial sialidases by agglutination of treated erythrocytes. Many microbial hydrolases have evolved to attack their substrates in structurally complex contexts (e.g., plant cell walls [Chapter 24]). The number and diversity of glycan-directed probes now allows the detailed characterization of such enzymes acting on biologically relavent structures, yielding new insights into their activities. It is easy to envision other ways in which lectins, CBMs, and antibodies can be used to probe the products of specific glycosidases and glycosyltransferases, given the specificities of the glycan-directed probes described here.
ACKNOWLEDGMENTS
The authors acknowledge helpful comments and suggestions from Felix Broecker.
FURTHER READING
- Hakomori S. 1984. Tumor-associated carbohydrate antigens. Annu Rev Immunol 2: 103–126. [PubMed: 6085749]
- Merkle RK, Cummings RD. 1987. Lectin affinity chromatography of glycopeptides. Methods Enzymol 138: 232–259. [PubMed: 3600324]
- Osawa T, Tsuji T. 1987. Fractionation and structural assessment of oligosaccharides and glycopeptides by use of immobilized lectins. Annu Rev Biochem 56: 21–42. [PubMed: 3304133]
- Osawa T. 1988. The separation of immunocyte subpopulations by use of various lectins. Adv Exp Med Biol 228: 83–104. [PubMed: 3051929]
- Esko JD. 1992. Animal cell mutants defective in heparan sulfate polymerization. Adv Exp Med Biol 313: 97–106. [PubMed: 1442273]
- Kobata A, Endo T. 1992. Immobilized lectin columns: Useful tools for the fractionation and structural analysis of oligosaccharides. J Chromatogr 597: 111–122. [PubMed: 1517308]
- Cummings RD. 1994. Use of lectins in analysis of glycoconjugates. Methods Enzymol 230: 66–86. [PubMed: 8139516]
- Knox JP. 1997. The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int Rev Cytol 171: 79–120. [PubMed: 9066126]
- Lis H, Sharon N. 1998. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem Rev 98: 637–674. [PubMed: 11848911]
- Bush CA, Martin-Pastor M, Imberty A. 1999. Structure and conformation of complex carbohydrates of glycoproteins, glycolipids, and bacterial polysaccharides. Annu Rev Biophys Biomol Struct 28: 269–293. [PubMed: 10410803]
- Morgan WT, Watkins WM. 2000. Unravelling the biochemical basis of blood group ABO and Lewis antigenic specificity. Glycoconj J 17: 501–530. [PubMed: 11421345]
- Rüdiger H, Gabius HJ. 2001. Plant lectins: Occurrence, biochemistry, functions and applications. Glycoconj J 18: 589–613. [PubMed: 12376725]
- Goldstein IJ. 2002. Lectin structure-activity: The story is never over. J Agric Food Chem 50: 6583–6585. [PubMed: 12381155]
- Madera M, Mechref Y, Novotny MV. 2005. Combining lectin microcolumns with high-resolution separation techniques for enrichment of glycoproteins and glycopeptides. Anal Chem 77: 4081–4090. [PMC free article: PMC1472620] [PubMed: 15987113]
- Paschinger K, Fabini G, Schuster D, Rendic D, Wilson IB. 2005. Definition of immunogenic carbohydrate epitopes. Acta Biochim Pol 52: 629–632. [PubMed: 16175237]
- Akama TO, Fukuda MN. 2006. N-Glycan structure analysis using lectins and an α-mannosidase activity assay. Methods Enzymol 416: 304–314. [PubMed: 17113875]
- Lehmann F, Tiralongo E, Tiralongo J. 2006. Sialic acid-specific lectins: Occurrence, specificity and function. Cell Mol Life Sci 63: 1331–1354. [PMC free article: PMC7079783] [PubMed: 16596337]
- Maeda Y, Ashida H, Kinoshita T. 2006. CHO glycosylation mutants: GPI anchor. Methods Enzymol 416: 182–205. [PubMed: 17113867]
- Patnaik SK, Stanley P. 2006. Lectin-resistant CHO glycosylation mutants. Methods Enzymol 416: 159–182. [PubMed: 17113866]
- Varki NM, Varki A. 2007. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab Invest 87: 851–857. [PMC free article: PMC7100186] [PubMed: 17632542]
- Cummings RD. 2009. The repertoire of glycan determinants in the human glycome. Mol Biosyst 5: 2–12. [PubMed: 19756298]
- Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, Bootten T, Albert A, Davis RH, Chennareddy C, et al. 2010. A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153: 514–525. [PMC free article: PMC2879786] [PubMed: 20363856]
- Lam SK, Ng TB. 2011. Lectins: Production and practical applications. Appl Microbiol Biotechnol 89: 45–55. [PMC free article: PMC3016214] [PubMed: 20890754]
- Pedersen HL, Fangel JU, McCleary B, Ruzanski C, Rydahl MG, Ralet M-C, Farkas V, von Schantz L, Marcus SE, Andersen MCF, et al. 2012. Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J Biol Chem 287: 39429–39438. [PMC free article: PMC3501085] [PubMed: 22988248]
- Gilbert HJ, Knox JP, Boraston AB. 2013. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr Opinin Struct Biol 23: 669–677. [PubMed: 23769966]
- Ribeiro JP, Mahal LK. 2013. Dot by dot: Analyzing the glycome using lectin microarrays. Curr Opin Chem Biol 17: 827–831. [PMC free article: PMC3823826] [PubMed: 23856055]
- Smith DF, Cummings RD. 2013. Application of microarrays for deciphering the structure and function of the human glycome. Mol Cell Proteomics 12: 902–912. [PMC free article: PMC3617337] [PubMed: 23412570]
- Akkouh O, Ng TB, Singh SS, Yin C, Dan X, Chan YS, Pan W, Cheung RC. 2015. Lectins with anti-HIV activity: A review. Molecules 20: 648–668. [PMC free article: PMC6272367] [PubMed: 25569520]
- Broecker F, Anish C, Seeberger PH. 2015. Generation of monoclonal antibodies against defined oligosaccharide antigens. Methods Mol Biol 1331: 57–80. [PubMed: 26169735]
- Oliveira C, Varvalho V, Domingues L, Gama FM. 2015. Recombinant CBM-fusion technology—Applications overview. Biotech Adv 22: 358–369. [PubMed: 25689072]
- Pattathil S, Avci U, Zhang T, Cardenas CL, Hahn MG. 2015. Immunological approaches to biomass characterization and utilization. Front Bioeng Biotechnol 3: 173. [PMC free article: PMC4623462] [PubMed: 26579515]
- Blackler RJ, Evans DW, Smith DF, Cummings RD, Brooks CL, Braulke T, Liu X, Evans SV, Müller-Loennies S. 2017. Single-chain antibody-gragment M6P-1 possesses a mannose 6-phosphate monosaccharide-specific binding pocket that distinguishes N-glycan phosphorylation in a branch-specific manner. Glycobiology 26: 181–192. [PMC free article: PMC4691289] [PubMed: 26503547]
- Walker JA, Pattathil S, Bergeman LF, Beebe E, Deng K, Mirzai M, Northen TR, Hahn MG, Fox BG. 2017. Determination of glycoside hydrolase specificities during hydrolysis of plant cell walls using glycome profiling. Biotechnol Biofuels 2: 31. [PMC free article: PMC5288845] [PubMed: 28184246]
- BACKGROUND
- LECTINS MOST COMMONLY USED IN GLYCAN ANALYSIS
- GENERATION OF MONOCLONAL ANTIBODIES TO GLYCAN ANTIGENS
- USES OF ANTIBODIES, LECTINS, AND CBMs IN GLYCAN IDENTIFICATION
- USES OF ANTIBODIES AND LECTINS IN GLYCAN PURIFICATION
- USES OF ANTIBODIES, LECTINS, AND CBMs IN CHARACTERIZING CELL-SURFACE GLYCOCONJUGATES
- USES OF ANTIBODIES AND LECTINS FOR GENERATING ANIMAL CELL GLYCOSYLATION MUTANTS
- USES OF ANTIBODIES AND LECTINS FOR CLONING GLYCOSYLTRANSFERASE GENES BY EXPRESSION
- USES OF ANTIBODIES AND LECTINS IN ASSAYING GLYCOSYLTRANSFERASES AND GLYCOSIDASES
- ACKNOWLEDGMENTS
- FURTHER READING
- Review Glycan-Recognizing Probes as Tools.[Essentials of Glycobiology. 2022]Review Glycan-Recognizing Probes as Tools.Cummings RD, Etzler M, Hahn MG, Darvill A, Godula K, Woods RJ, Mahal LK. Essentials of Glycobiology. 2022
- Review Antibodies and Lectins in Glycan Analysis.[Essentials of Glycobiology. 2009]Review Antibodies and Lectins in Glycan Analysis.Cummings RD, Etzler ME. Essentials of Glycobiology. 2009
- Review Principles of Glycan Recognition.[Essentials of Glycobiology. 2015]Review Principles of Glycan Recognition.Cummings RD, Schnaar RL, Esko JD, Drickamer K, Taylor ME. Essentials of Glycobiology. 2015
- Review Microbial Lectins: Hemagglutinins, Adhesins, and Toxins.[Essentials of Glycobiology. 2015]Review Microbial Lectins: Hemagglutinins, Adhesins, and Toxins.Nizet V, Varki A, Aebi M. Essentials of Glycobiology. 2015
- Review Microbial Lectins: Hemagglutinins, Adhesins, and Toxins.[Essentials of Glycobiology. 2022]Review Microbial Lectins: Hemagglutinins, Adhesins, and Toxins.Lewis AL, Kohler JJ, Aebi M. Essentials of Glycobiology. 2022
- Glycan-Recognizing Probes as Tools - Essentials of GlycobiologyGlycan-Recognizing Probes as Tools - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

